Abstract
Transfusion-related immunomodulation (TRIM) in the intensive care unit (ICU) is difficult to define and likely represents a complicated set of physiologic responses to transfusion, including both proinflammatory and immunosuppressive effects. Similarly, the immunologic response to critical illness in both adults and children is highly complex and is characterized by both acute inflammation and acquired immune suppression. How transfusion may contribute to or perpetuate these phenotypes in the ICU is poorly understood, despite the fact that transfusion is common in critically ill patients. Both hyperinflammation and severe immune suppression are associated with poor outcomes from critical illness, underscoring the need to understand potential immunologic consequences of blood product transfusion. In this review we outline the dynamic immunologic response to critical illness, provide clinical evidence in support of immunomodulatory effects of blood product transfusion, review preclinical and translational studies to date of TRIM, and provide insight into future research directions.
Defining transfusion-related immunomodulation (TRIM) in critically ill patients is complicated by the underlying dynamic nature of the immune response to critical illness, variation in the timing of transfusion, number of transfusions received, variation in blood products transfused, and a myriad of additional immunomodulatory treatments often received in the intensive care unit (ICU). Early reports of TRIM in the 1970s stemmed from the observation that red blood cell (RBC) transfusion was associated with fewer episodes of organ rejection in renal transplant patients, implying an immunosuppressive effect of transfusion.1 On the other hand, subsequent studies have attributed a number of proinflammatory effects to blood transfusion.2–4 These seemingly incompatible findings may be explained by blood product–specific, host-related, and other contextual influences at the time of transfusion including the highly dynamic immunologic response to acute illness itself. Changes in blood banking practices over the last decade, including the adoption of near-universal prestorage leukoreduction, have likely changed the epidemiology and indeed the pathophysiology of TRIM. Furthermore, inflammation and immune function in pediatric and adult critical illness is now known to be more complex and dynamic than previously thought. Together, these observations provide impetus for a reevaluation of TRIM, both to place transfusion immunobiology in a modern ICU context and to highlight important research priorities in this rapidly changing field.
The dynamic immune response to critical illness
The need for a more comprehensive understanding of the immunologic effects associated with transfusion in critically ill children is underscored by recent data that suggest that the immunobiology of critical illness is far more variable than previously thought. Many of the events inciting critical illness are rooted in an amplified inflammatory response—or systemic inflammatory response syndrome. It is increasingly apparent, however, that this initial response is commonly accompanied by an overly robust compensatory anti-inflammatory response syndrome, which leads to a significant critical illness-induced immune suppression. Restoration of immunologic homeostasis, with resolution of both the systemic inflammatory response syndrome and the compensatory anti-inflammatory response syndrome states, is an important goal of treatment (Fig. 1). This is evidenced by the fact that high systemic levels of proinflammatory mediators such as interleukin (IL)-6 and IL-8 and elevations in anti-inflammatory cytokines such as IL-10 have all been associated with increased risks for secondary infection or death from critical illness in both adults and children.5–10
Fig. 1.
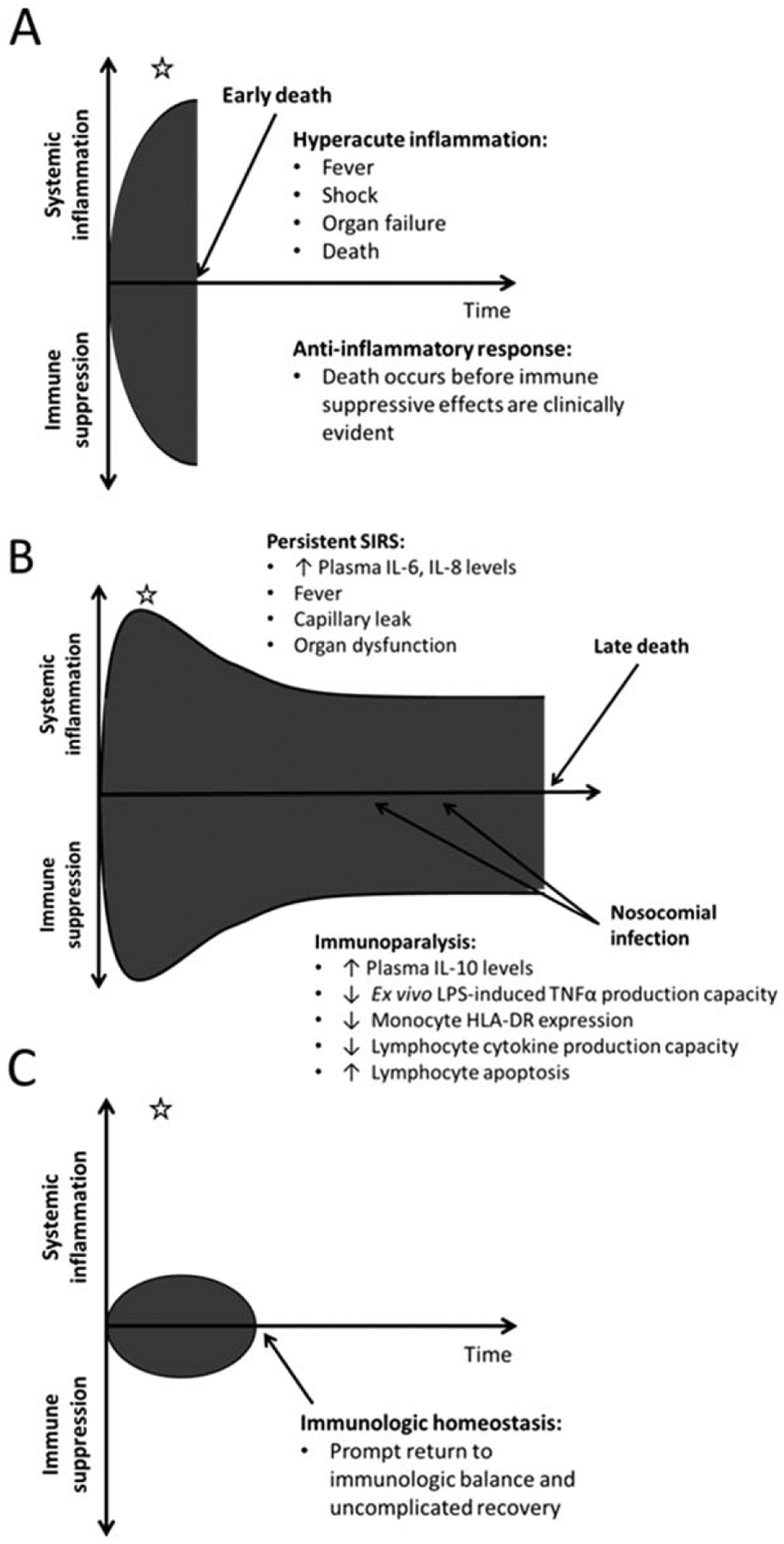
The dynamic immune response to critical illness. Some patients experience severe hyperacute inflammation that results in early death before the immunosuppressive effects of the compensatory anti-inflammatory response can become clinically evident (A). Those who survive the initial proinflammatory insult can develop severe persistent immune suppression that is associated with increased risks for nosocomial infection and late death (B). An important goal of critical care management is to promote prompt restoration of immunologic homeostasis through resolution of systemic inflammation and normalization of immune function (C). The immunologic effects of a transfusion at a given point in time (white star) have the potential to be quite variable depending on the context, with some subjects being too ill to detect an effect (A), others being too well to have a clinical impact (C), and others in a highly dynamic state where pro- or anti-inflammatory effects of transfusion could greatly influence overall immune function. SIRS = systemic inflammatory response syndrome.
Critical illness-induced immune suppression, termed “immunoparalysis” in its most severe form, can affect both the innate (e.g., neutrophil, monocyte, macrophage) and the adaptive (e.g., lymphocyte) arms of the immune system. This form of acquired immunodeficiency can be quantified with laboratory measures that have been used in single- and multicenter studies of immune function in critical illness. From the innate immune perspective, the two assays most often associated with ICU outcomes are monocyte human leukocyte antigen (HLA)-DR expression and ex vivo lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production capacity. HLA-DR is an important antigen presenting molecule that should be highly expressed on more than 90% of circulating monocytes. In the immunoparalyzed state, monocyte HLA-DR expression is markedly down regulated.11–14 Another characteristic of the immunoparalyzed monocyte is diminished proinflammatory cytokine response to a standardized ex vivo LPS challenge. Healthy innate immune cells should produce large quantities of TNF-α upon ex vivo LPS stimulation. Markedly and persistently reduced whole blood ex vivo LPS-induced TNF-α production capacity predicts increased risks for secondary infection or death in the adult and pediatric ICU (PICU).6,8,12,15,16
Adaptive immune suppression has also been clearly identified as a risk factor for adverse ICU outcomes. Severe and prolonged lymphopenia, along with lymphocyte apoptosis in lymphoid organs such as the spleen, are strongly associated with adverse outcomes in adult and pediatric sepsis.17–19 Further, lymphocyte hyporeactivity to ex vivo stimulation, with reduced interferon (IFN)-γ production in response to incubation with phytohemagglutinin or anti-CD3, has been demonstrated in adult sepsis nonsurvivors as well as in children with adverse infectious outcomes from sepsis.17,20
Mechanisms of critical illness-induced immune suppression are unclear, but are almost certainly multifactorial with host, environmental, and treatment-related factors all likely contributors. For instance, while specific genotypes have not been identified that confer increased risk for immunoparalysis in the setting of critical illness, strong evidence for both heritable and epigenetic factors exist.15,21 Interplay between proinflammatory and anti-inflammatory mediators likely exists, wherein immunoparalysis has been reported in patients with many forms of “proinflammatory” critical illness including sepsis, trauma, multiple organ dysfunction syndrome (MODS), critical viral infections, cardiopulmonary bypass, and pancreatitis. It appears, however, that certain inflammatory insults, such as Staphylococcus aureus infection, confer particularly high risk for development of immune suppression.6 Additionally, many of the treatments employed in the PICU are intentionally immunosuppressive, including glucocorticoids, antineoplastic agents, and transplant rejection prophylaxis. Moreover, immunomodulatory side effects are common in the ICU pharmacopeia, including antimicrobials, catecholamines, sedatives, and diuretics, with the bulk of these effects being immunosuppressive.22
It is in this context that TRIM in the ICU must be considered, with weight being given to both proinflammatory and immunosuppressive effects of transfusion. Depending on the patient’s genetic background, diagnosis, comorbidities, severity of illness, and timing of transfusion, the immunologic effects of a given transfusion may be difficult to predict. However, it can be expected that ICU patients may be more sensitive to immunomodulatory mediators in blood products because of their background immune dysregulation. As transfusion remains a common occurrence in the PICU, particularly in children with high severity of illness, it is important to understand the contribution of transfusion to systemic inflammation and to critical illness-induced immune suppression.
Epidemiology of transfusion in the PICU
Published rates of RBC transfusion among critically ill children vary from 49% among children admitted to the PICU for more than 48 hours to between 6.8 and 17% when considering all PICU admissions.23–26 Compared to nontransfused patients, those receiving RBC transfusion tend to be sicker, with higher pediatric logistic organ dysfunction (PELOD) scores and incidence of multiple organ dysfunction being independently predictive of transfusion status.24,25
Regarding plasma transfusion, Karam and colleagues27 recently published the most comprehensive-to-date study of the epidemiology of plasma transfusion in critically ill children. Among all children admitted to 103 included PICUs over 6 study weeks, a relatively low percentage of children (3.4%) received plasma. However, the mortality rate for the 443 children who received at least one plasma transfusion was as high as 27%, suggesting that children who receive plasma represent a cohort with high severity of illness. Very little is known about the epidemiology and outcomes associated with platelet (PLT) transfusion in the PICU, representing an important gap in the literature.
Clinical evidence suggesting TRIM in the ICU
In multiple observational studies, RBC transfusion is independently associated with adverse outcomes in hospitalized patients.28 However, because sicker patients tend to receive transfusions, these observational studies are often limited by confounding by indication. Consequently, several prospective randomized controlled trials in adults across a variety of clinical settings have compared restrictive versus liberal transfusion strategies and have demonstrated either improved outcomes in restrictive-group subjects or lack of benefit in the liberally transfused.29–34 Several meta-analyses have since been performed and support the finding that a restrictive transfusion strategy appears to be safe in most settings, with no obvious benefit to liberal transfusion practices.35–38
Similar to adult studies, retrospective studies in critically ill children identify RBC transfusion as an independent risk factor for morbidity and mortality.39,40 In prospective, multicenter observational study by Bateman and colleagues,23 after controlling for age and severity of illness, RBC transfusion was independently associated with longer mechanical ventilation, cardiopulmonary dysfunction, PICU stay, and mortality. In 2007 Lacroix and coworkers41 published a prospective randomized controlled trial of transfusion strategies in pediatric critically ill patients that demonstrated that a restrictive transfusion strategy decreased RBC transfusions without increasing the incidence of new or progressive MODS.41 Subgroup analyses from this trial showed similar results in children after surgery or cardiac surgery and those with sepsis.42–44 Two additional prospective randomized trials in pediatric cardiac surgery, also examining hemoglobin (Hb) thresholds, found no benefit to liberal transfusions even in this high-risk population.45,46 Whether the lack of apparent benefit from liberal transfusion strategies stems from a lack of efficacy or from an increase in transfusion-related risk remains unclear and continues to be a topic of debate.47–50
Potential risk may arise from immunomodulation related to RBC transfusion. Multiple analyses link RBC transfusion to infection risk in the ICU, suggesting that RBC transfusion may be associated with immune suppression. Earlier adult studies identified an association between infections and nonleukoreduced RBC transfusions, with more recent studies—using leukoreduced transfusions—confirming these results.28,51–55 Variations in storage duration and volume transfused drew criticism of these studies.56 However, when volume of transfusion is controlled for, the association between postoperative infection and RBC transfusion is supported.57,58 Meta-analyses in postoperative and medical patients comparing transfused versus nontransfused adults demonstrates increased risk for nosocomial infections in transfused patients.59–62 Large meta-analyses of medical and surgical patients randomized in restrictive versus liberal RBC transfusion trials demonstrate significantly reduced risks for health care–associated infections in those managed with a restrictive transfusion strategy.37,38,63
Critically ill children are also at risk for hospital-acquired infections, and RBC transfusions may increase this risk.64,65 Critically ill children with sepsis managed with a restrictive transfusion strategy had significantly fewer cases of nosocomial infection than liberally managed patients.42 Children with significant burn injuries receiving high numbers of blood transfusions were at increased risk for development of sepsis.66 Additionally, transfused children after cardiac surgery had higher incidence of ventilator associated pneumonia and postoperative infections.67,68
RBC transfusions in critically ill children are also associated with MODS and transfusion-associated respiratory complications, including respiratory dysfunction, transfusion-related acute lung injury, and transfusion-associated circulatory overload.69–73 Like TRIM, these transfusion complications are likely the result of transfusion in a susceptible host under certain circumstances (two-hit phenomenon). They are also likely to be mediated by immunologic mechanisms.
Storage duration of the RBC unit may impact immune function in critically ill children, with longer storage duration of transfused RBCs associated with increased risks for postoperative infection, MODS, and hospital length of stay.74–76 While transfusion with shorter-storage RBCs has not been shown to improve outcomes in randomized trials in adults, premature neonates, or children with severe anemia, the ongoing Age of Blood in Children in Pediatric ICU (ABC-PICU) trial (NCT01977547) may shed light on this question in critically ill children.77–80 Notably, in the only studies to date evaluating immune function after RBC transfusion in critically ill or injured children, older prestorage leukoreduced RBCs were associated with persistent immune suppression and systemic inflammation compared to fresher RBCs.8,81
Taken together, these clinical studies strongly suggest some immunomodulatory effect of transfusion. Consequently, several preclinical and translational studies have been performed to try to define and clarify these effects.
Proinflammatory versus immune suppressive effects of transfusion
Preclinical studies demonstrate mixed immunomodulatory effects of RBC exposure, with results suggesting both proinflammatory and immune-suppressive effects (Table 1). Investigations to date are heterogeneous with variation in blood product studied and model utilized, limiting direct comparison of effects. In studies suggesting a proinflammatory effect using murine and in vitro neutrophil models, stored RBCs resulted in greater induction of proinflammatory cytokines compared to fresh RBCs, with effects more often seen when nonleukoreduced RBCs were used.76,82–85,87 Studies evaluating immune-suppressive effects are more heterogeneous. Studies to date have used various in vitro immune cell models (including monocytes, lymphocytes, natural killer [NK] cells, and neutrophils), neutrophil chemotaxis models, and murine tumor models.88–95 These studies likewise suggest that suppression of immune cell function may be associated with RBCs of longer storage duration or nonleukoreduced RBCs. For instance, murine tumor suppression models suggest that there is less tumor progression after exposure to fresh RBCs or leukoreduced RBCs when compared to stored RBCs.94,95 On the other hand, more recent studies suggest that even when leukoreduced, stored RBC products can directly suppress monocyte or lymphocyte function in vitro.90,93 However, findings are not consistent, as some murine and ex vivo studies report mixed or even no effect on inflammatory markers.76,86 In this way TRIM may be analogous to RBC alloimmunization, whereby immunologic consequences of transfusion are highly variable and likely host and/or context specific. Overall, the evidence to date suggests that RBC transfusion has important immune modulatory effects in vitro and in animal models, but the clinical impact of these experimental effects remains to be determined.
TABLE 1.
Selected preclinical studies evaluating RBC TRIM
| Model | Blood product(s) | Findings | Storage effect | Reference(s) |
|---|---|---|---|---|
| Proinflammatory effects | ||||
| Human cells | ||||
| In vitro neutrophil priming | RBCs, buffy coat–poor RBCs, LR | SN from RBCs but not buffy coat–poor or LR RBCs primed neutrophils, effect increased with storage time. | Yes | Sparrow et al.82 |
| In vitro neutrophil priming and in vitro monocyte | Washed RBCs vs. LR RBCs | Neutrophil CD11b expression and monocyte IL-8 production increased. Mitigated by LR but not by RBC washing. | Not evaluated | Cardo et al.83 |
| Animal models | ||||
| Murine transfusion | LR RBCs | Stored, but not fresh, RBCs induced IL-6, CXCL1, and MCP-1, reversed by concurrent fresh RBCs. | Yes | Hendrickson et al.84 |
| Murine hemorrhagic shock and transfusion | Non-LR RBCs | Unwashed, stored RBCs induced IL-6, KC, MIP-1α, and MIP-2. | Yes | Belizaire et al.85 |
| Mixed effects or no effect | ||||
| Human cells | ||||
| LPS-stimulated whole blood | Non-LR RBCs | RBC supernatant decreased LPS-induced TNF-α and increased IL-8 response. | Not evaluated | Patel et al.86 |
| Ex vivo T-cell activation | LR RBC supernatants, variable storage duration | Increased IL-6, IL-10, decreased TNF-α. No change in CD25 expression. | Yes | Karam et al.76 |
| Animal models | ||||
| Murine transfusion | LR RBCs | Stored but not fresh RBCs induced IL-6, KC, MCP-1, TNF-α, and MIP-1β and enhanced growth of Escherichia coli. No change in plasma IL-10 or IFN-γ. | Yes | Hod et al.87 |
| Immunosuppressive effects | ||||
| Human cells | ||||
| Anti-CD3–stimulated PBMNCs | RBCs and LR RBCs | Supernatant from fresh and stored RBCs with or without LR induced Tregs and decreased cytokine response in CD3-stimulated PBMNCs. | No | Baumgartner et al.88 |
| NK cells | RBCs | SN from stored but not fresh RBCs decreased NK-cell activity. | Yes | Ghio et al.89 |
| LPS-stimulated monocytes | LR RBCs | RBCs and RBC supernatant decreased LPS-induced TNF-α but not IL-10 production. | Yes | Muszynski et al.,90 Mynster et al.91 |
| Ex vivo neutrophils | Non-LR vs. LR RBCs | Inhibition of neutrophil chemotaxis via synthesis of TGF-β after exposure to non-LR RBCs. | Not evaluated | Ottonello et al.92 |
| Stimulated T cells | Older vs. fresh LR RBCs | Decreased T-cell proliferation, IL-10, IL-17a, IFN-γ, TNF-α, GM-CSF production with stored RBC exposure. | Yes | Long et al.93 |
| Animal models | ||||
| Murine tumor model | Whole blood vs. LR RBCs | Mice receiving whole blood but not LR blood had more tumors. Pre- but not post-storage LR removed the tumor enhancing effect of WB transfusion in rabbits. | Not evaluated | Bordin et al.94 |
| Rat mammary adenocarcinoma model and leukemia model | RBCs | Aged RBCs promoted cancer progression. | Yes | Atzil et al.95 |
CXCL1 = chemokine (C-X-C motif) ligand 1; KC = keratinocyte chemoattractant; LR = leukoreduced; MCP = monocyte chemoattractant protein; MIP = macrophage inflammatory protein; PBMNC = peripheral blood mononuclear cell; PHA = phytohemagglutinin; SN = supernatant; TGF = transforming growth factor; Treg = regulatory T cell; WB = whole blood.
Studies investigating immunomodulatory effects of PLT and plasma products are fewer and mostly limited to preclinical models. Similar to RBC studies, PLT products have demonstrated both proinflammatory and immune-suppressive effects. This is perhaps not surprising given emerging evidence that PLTs themselves may play important roles in activating and modulating the innate inflammatory response.96,97 On the proinflammatory side, PLT products may increase immune cell cytokine production and neutrophil activity.98,99 By contrast, Aslam and colleagues100 demonstrated an immunosuppressive phenotype dependent on PLT major histocompatibility complex Class I expression with fresh but not 72-hour-stored PLTs in a murine transfusion model. Similarly, PLT products have been shown to suppress dendritic cell function in in vitro models.101 Limited data suggest that pathogen reduction technologies may mitigate some PLT-associated TRIM effects, although further study in this area is certainly needed.99,102 Data regarding immunomodulatory effects of plasma products are even fewer. In a single in vitro study, allogeneic fresh-frozen plasma suppressed innate immune cell function as measured by LPS-induced cytokine production.103
Clinical studies on immunologic effects of RBC transfusion have been executed in a variety of patient populations (Table 2). The majority of studies have limited sample size and are observational, describing effects of RBC transfusion in a “before–after” design. Contrasting results are reported, including both immune-stimulatory as well as immune-suppressive effects. On the proinflammatory side, nonleukoreduced RBC products were used in most studies demonstrating increases in posttransfusion cytokine levels.106–109 One observational study in neonates using leukoreduced RBC found mildly elevated plasma levels of select proinflammatory cytokines 2 to 4 hours posttransfusion.105 Notably, a relatively large randomized controlled trial comparing leukoreduced with white blood cell (WBC)-containing, buffy coat–depleted RBCs in adult cardiac surgery patients suggested that in the heavily transfused patients (needing > 4 units), WBC-containing blood products induced a stronger proinflammatory response.107 Taken together, these data suggest that proinflammatory responses observed after RBC transfusion may be largely mitigated by leukoreduction.
TABLE 2.
Selected clinical studies evaluating RBC TRIM
| Population | Study design | Number | Blood product(s) | Findings | Storage effect | Reference |
|---|---|---|---|---|---|---|
| Proinflammatory effects | ||||||
| Pediatric studies | ||||||
| Critically ill children | Observational pre- vs. posttransfusion | 100 | LR RBCs | Overall no change in markers of inflammation, but elevated CRP in children with elevated markers of hemolysis posttransfusion | No | L’Acqua et al.104 |
| Neonatal | Observational pre- vs. posttransfusion | 28 | LR RBCs | Increased plasma IL-1β, TNF-α, MCP-1, and IL-8 2 to 4 hr posttransfusion | Not evaluated | Keir et al.105 |
| Adult studies | ||||||
| Adult cardiac surgery | Observational | 114 | Non-LR RBCs | Higher postoperative plasma IL-6 and BPI levels in transfused vs. nontransfused | Not evaluated | Fransen et al.106 |
| Adult cardiac surgery | Randomized controlled trial | 346 | Buffy coat–poor vs. LR RBCs | IL-6 levels higher in non-LR group only in those with >3 RBC units | Not evaluated | Bilgin et al.107 |
| Nonhemolytic febrile reaction | Observational pre- vs. posttransfusion | 81 | Non-LR RBCs | IL-8 and IL-6 increased but not IL-1 and TNF-α | Not evaluated | Lin et al.108 |
| Critically ill adults | Observational before and after transfusion | 96 | LR vs non-LR RBCs | RBC but not LR RBC induced leukocytosis, associated with increased IL-8 levels in product | Yes | Izbicki et al.109 |
| Mixed effects or no effect | ||||||
| Pediatric studies | ||||||
| Neonatal | Observational | 9 | LR RBCs | No change in plasma IL-1β, IL-6, or IL-10 pre- vs. posttransfusion | Not evaluated | Locke et al.110 |
| Critically ill children | Observational pre- vs. posttransfusion | 31 | LR RBCs | No difference in plasma cytokines, Treg, or monocyte function pre- vs. 24 hr posttransfusion. Suppressed monocyte function after older vs. fresher RBCs. | Yes | Muszynski et al.81 |
| Adult studies | ||||||
| Healthy adult subjects | Observational | 14 | LR RBCs | No change in plasma IL-6 or CRP with fresh or stored RBCs. Increased in vitro proliferation of E. coli after stored RBCs | Not evaluated | Hod et al.111 |
| Adult cardiac surgery | Observational | 29 | LR RBCs | No difference in gene expression of 114 cytokines between transfused vs. nontransfused patients | Not evaluated | Sitniakowsky et al.112 |
| Immunosuppressive effects | ||||||
| Pediatric studies | ||||||
| Pediatric trauma | Observational fresh vs. older RBCs | 29 | LR RBCs | Suppressed monocyte function over time in patients transfused older vs. fresher RBCs | Yes | Muszynski et al.8 |
| Adult studies | ||||||
| Adult postoperative colorectal | RCT unfiltered whole blood vs. LR blood | 104 | Whole blood vs. LR blood | Whole blood but not LR blood decreased NK-cell function | Not evaluated | Jensen et al.51 |
| Adult abdominal surgery | RCT, liberal vs. restrictive transfusion | 20 | Non-LR RBCs | Higher IL-10 1 day postoperatively in liberal group | Not evaluated | Theodoraki et al.113 |
| Adult trauma | Observational fresh vs. older RBCs | 64 | LR RBCs | Decreased IL-12, IL-23, RORγt gene expression with older RBCs | Yes | Torrance et al.114 |
| Adult abdominal surgery | Observational | 119 | LR RBCs | Decreased TNF-α, IL-12, IL-23, RORγt gene expression; more infections in transfused | Yes | Fragkou et al.115 |
BPI = bactericidal permeability-increasing protein; CRP = C-reactive protein; LR = leukoreduced; MCP = monocyte chemoattractant protein; RORγt = retinoic acid-related orphan receptor-γt; Treg = regulatory T cell.
By contrast, the studies that found no effect, mixed effects, or immune-suppressive effects associated with RBC transfusion largely employed leukoreduced RBC products.8,81,110–112,114,115 Immunosuppressive effects of transfusion have been studied most often in surgical patients.51,113,115 Whether these patients are more prone to immunosuppressive effects of RBC transfusion, or that other patient populations are understudied, is not clear. Different immunosuppressive effects of transfusion have been observed, including decreased ability of immune cells to produce proinflammatory cytokines, increased production of anti-inflammatory cytokines, and decreased function of T lymphocytes and NK cells.8,51,113–115 Effects of storage duration in the context of RBC TRIM are unclear, with some studies indicating a storage effect, while others do not. Among critically ill children, observational data suggest that transfusion with RBCs of longer storage duration may be immunosuppressive, although these studies are currently limited by small sample size.8,81 Further delineating potential storage-related effects on transfusion-related immune suppression remains an active area of investigation. Similarly, the precise mechanisms of immunosuppressive effects of RBC transfusion are not well understood and require further study.
Does TRIM exist in the ICU population?
Answering the question of whether or not TRIM exists is not an easy task. An in-depth review of epidemiologic, preclinical, and clinical studies reveal conflicting evidence for the existence of TRIM. Clearly one of the major obstacles to measuring effects of TRIM is the magnitude and variability of background immune modulation seen in ICU patients, independent of transfusion. Several (but not all) studies point to increased risk of infection after RBC transfusion, and while these data support an immunosuppressive TRIM effect, increased non–transferrin-bound iron may also provide an alternative explanation for increased infection after RBC transfusion, particularly after receipt of older units.87 Animal models show varied effects of TRIM depending on the model or immune cell tested. In our review, approximately twice as many animal studies showed immune-suppressive effects of transfusion compared to immune activation, although approximately one-third of published studies showed mixed or no immune effects of transfusion. Human studies showed a more balanced pro- vs. anti-inflammatory effect of transfusion, again with approximately one-third of published studies showing mixed or no immune effects of transfusion. Given the mixed results of both preclinical and clinical studies, it is tempting to conclude that TRIM does not exist or if it does occur the effects must be small compared to other insults patients face in the ICU setting. The answer is probably more complex, as there are likely both proinflammatory and immune-suppressive effects mediated by transfusion, and the balance of how these forces influence an individual patient will depend on the underlying physiology, age and comorbidities of the patient, the timing of transfusion, and the individual blood product(s) used. Understanding these interactions is important because RBC transfusion represents a potentially modifiable factor among the many potential mediators of immunologic dysregulation in the PICU.
Not all blood components are equivalent
Confounding most studies examining the potential immune modulatory activity of blood components is the growing recognition that blood donor variability (age, sex, race) and differences in blood component manufacturing can have a significant impact on the levels of pro- and anti-inflammatory molecules in transfused products.116–119 When different methods for the production of a RBC product were directly compared, enormous variability in immune modulatory agents including residual PLTs and WBCs, free Hb levels, extracellular vesicles, cell-free DNA, cytokines, growth factors, and lipid mediators were seen.117,119 While the length of storage of RBC products continues to be explored in randomized clinical trials, studies are now under way to examine the effect that donor factors and RBC component manufacturing may have on patient outcomes.120 In addition, plasma components have also been shown to have significant differences in levels of residual cells and extracellular vesicles depending on the method of manufacturing, and it is not inconceivable that similar observations will be made when different PLT products are evaluated.121 What is becoming clear is that it is no longer appropriate to consider all RBC, PLT, or plasma products used in transfusion as being equivalent, and care should be taken to understand which products are being used when evaluating the immune modulatory effect of transfused blood components.
Can studies be performed to measure immunologic consequences in the transfused critically ill child?
As noted, one of the biggest impediments to measuring immune-modulatory effects of transfusion is the heterogeneity of transfused patient populations and their underlying state of immune activation and competence. Indeed, a given blood product that may confer minimal risk in one critically ill child has the potential to confer substantial risk in another. Our understanding of how this risk is mediated, however, and how to mitigate it at the ICU bedside is rudimentary at best. Observational studies comparing transfused to nontransfused populations are limited by the fact that patients who receive transfusions are likely sicker than those who do not, introducing a confounding by indication bias which is difficult to overcome. However, the field of transfusion medicine may face an opportunity to study TRIM effectively in the near future. Further formal study of appropriate RBC transfusion “thresholds” for various patient populations are planned, as noted at the recent NIH State of the Science Symposium.122 Collection of biologic specimens in patients randomized to receive RBC transfusions or not would allow objective laboratory comparisons of immune function in these subjects, data that have been lacking to date. Incorporating comprehensive, longitudinal immunophenotyping into such a randomized trial would provide valuable information that could never be obtained through observational studies. Ideally, the design of these trials would be informed by greater understanding of TRIM mechanisms, including patient-related and individual blood product–related risk factors. This therefore demands a more comprehensive understanding of the fundamental biology of TRIM, importantly placing transfusion in the physiologic context of the critically ill child. Only in this way can we begin to identify which blood products may have the potential for harm in a given individual. A multidisciplinary approach, spanning the spectrum of basic, translational, and clinical research methodologies, may well be required to accomplish this important task.
How does one define TRIM in the PICU? The answer to this question will likely be complex. In fact, immunomodulation associated with transfusion may not be a single entity, but may represent multiple context-specific effects—both proinflammatory and/or immunosuppressive. As stated, much more work is needed to understand the full spectrum of immunologic consequences in the transfused critically ill child.
ABBREVIATIONS:
- ICU
intensive care unit
- LPS
lipopolysaccharide
- MODS
multiple organ dysfunction syndrome
- PICU
pediatric intensive care unit
- TRIM
transfusion-related immunomodulation
Footnotes
CONFLICT OF INTEREST
PCS is a consultant for Octapharma, Cerus, and Entegrion. The other authors have disclosed no conflicts of interest.
REFERENCES
- 1.Keown PA, Descamps B. Improved renal allograft survival after blood transfusion: a nonspecific, erythrocyte-mediated immunoregulatory process? Lancet 1979;1:20–2. [DOI] [PubMed] [Google Scholar]
- 2.Belizaire RM, Prakash PS, Richter JR, et al. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. J Am Coll Surg 2012;214:648–55; discussion 656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaz BH, Stowell SR, Hillyer CD. Transfusion-related acute lung injury: from bedside to bench and back. Blood 2011; 117:1463–71. [DOI] [PubMed] [Google Scholar]
- 4.Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion 2011;51:2549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doughty L, Carcillo JA, Kaplan S, et al. The compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failure. Chest 1998; 113:1625–31. [DOI] [PubMed] [Google Scholar]
- 6.Hall MW, Geyer SM, Guo CY, et al. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med 2013;41:224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) study. Arch Intern Med 2007;167:1655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muszynski JA, Nofziger R, Greathouse K, et al. Innate immune function predicts the development of nosocomial infection in critically injured children. Shock 2014;42:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong HR, Cvijanovich N, Wheeler DS, et al. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Respir Crit Care Med 2008;178: 276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green J, Doughty L, Kaplan SS, et al. The tissue factor and plasminogen activator inhibitor type-1 response in pediatric sepsis-induced multiple organ failure. Thromb Haemost 2002;87:218–23. [PubMed] [Google Scholar]
- 11.Döcke WD, Höflich C, Davis KA, et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clin Chem 2005;51:2341–7. [DOI] [PubMed] [Google Scholar]
- 12.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med 2011;37:525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monneret G, Lepape A, Voirin N, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 2006;32:1175–83. [DOI] [PubMed] [Google Scholar]
- 14.Volk HD, Reinke P, Krausch D, et al. Monocyte deactivation–rationale for a new therapeutic strategy in sepsis. Intensive Care Med 1996;22:S474–81. [DOI] [PubMed] [Google Scholar]
- 15.Cornell TT, Sun L, Hall MW, et al. Clinical implications and molecular mechanisms of immunoparalysis after cardiopulmonary bypass. J Thorac Cardiovasc Surg 2012;143: 1160–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Döcke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 1997;3:678–81. [DOI] [PubMed] [Google Scholar]
- 17.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011;306:2594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felmet KA, Hall MW, Clark RS, et al. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol 2005;174:3765–72. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Chang KC, Swanson PE, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol 2000;1:496–501. [DOI] [PubMed] [Google Scholar]
- 20.Muszynski JA, Nofziger R, Greathouse K, et al. Early adaptive immune suppression in children with septic shock: a prospective observational study. Crit Care 2014;18:R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westendorp RG, Langermans JA, Huizinga TW, et al. Genetic influence on cytokine production and fatal meningococcal disease. Lancet 1997;349:170–3. [DOI] [PubMed] [Google Scholar]
- 22.Hall MW. Immune balance in critical illness: SIRS, CARS, immunoparalysis. In: Zimmerman FA, editor. Pediatric critical care. 5th ed. Philadelphia: Elsevier; 2016. [Google Scholar]
- 23.Bateman ST, Lacroix J, Boven K, et al. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med 2008;178:26–33. [DOI] [PubMed] [Google Scholar]
- 24.Dallman MD, Liu X, Harris AD, et al. Changes in transfusion practice over time in the PICU. Pediatr Crit Care Med 2013; 14:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demaret P, Tucci M, Ducruet T, et al. Red blood cell transfusion in critically ill children (CME). Transfusion 2014;54: 365–75; quiz 364. [DOI] [PubMed] [Google Scholar]
- 26.Valentine SL, Lightdale JR, Tran CM, et al. Assessment of hemoglobin threshold for packed RBC transfusion in a medical-surgical PICU. Pediatr Crit Care Med 2014;15:e89–94. [DOI] [PubMed] [Google Scholar]
- 27.Karam O, Demaret P, Shefler A, et al. Indications and effects of plasma transfusions in critically ill children. Am J Respir Crit Care Med 2015;191:1395–402. [DOI] [PubMed] [Google Scholar]
- 28.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med 2008;36:2667–74. [DOI] [PubMed] [Google Scholar]
- 29.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011;365:2453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67. [DOI] [PubMed] [Google Scholar]
- 31.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17. [DOI] [PubMed] [Google Scholar]
- 32.Hébert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med 2001;29:227–34. [DOI] [PubMed] [Google Scholar]
- 33.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21. [DOI] [PubMed] [Google Scholar]
- 34.Walsh TS, Boyd JA, Watson D, et al. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med 2013;41:2354–63. [DOI] [PubMed] [Google Scholar]
- 35.Carson JL, Carless PA, Hébert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA 2013;309:83–4. [DOI] [PubMed] [Google Scholar]
- 36.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med 2012;157:49–58. [DOI] [PubMed] [Google Scholar]
- 37.Holst LB, Petersen MW, Haase N, et al. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015;350:h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014;127:124–31.e3. [DOI] [PubMed] [Google Scholar]
- 39.Goodman AM, Pollack MM, Patel KM, et al. Pediatric red blood cell transfusions increase resource use. J Pediatr 2003;142:123–7. [DOI] [PubMed] [Google Scholar]
- 40.Kneyber MC, Hersi MI, Twisk JW, et al. Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med 2007;33:1414–22. [DOI] [PubMed] [Google Scholar]
- 41.Lacroix J, Hébert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007;356:1609–19. [DOI] [PubMed] [Google Scholar]
- 42.Karam O, Tucci M, Ducruet T, et al. Red blood cell transfusion thresholds in pediatric patients with sepsis. Pediatr Crit Care Med 2011;12:512–8. [DOI] [PubMed] [Google Scholar]
- 43.Rouette J, Trottier H, Ducruet T, et al. Red blood cell transfusion threshold in postsurgical pediatric intensive care patients: a randomized clinical trial. Ann Surg 2010;251: 421–7. [DOI] [PubMed] [Google Scholar]
- 44.Willems A, Harrington K, Lacroix J, et al. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Crit Care Med 2010;38:649–56. [DOI] [PubMed] [Google Scholar]
- 45.Cholette JM, Rubenstein JS, Alfieris GM, et al. Children with single-ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red-cell transfusion strategy. Pediatr Crit Care Med 2011;12:39–45. [DOI] [PubMed] [Google Scholar]
- 46.de Gast-Bakker DH, de Wilde RB, Hazekamp MG, et al. Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients: a randomized controlled trial. Intensive Care Med 2013;39:2011–9. [DOI] [PubMed] [Google Scholar]
- 47.Istaphanous GK, Wheeler DS, Lisco SJ, et al. Red blood cell transfusion in critically ill children: a narrative review. Pediatr Crit Care Med 2011;12:174–83. [DOI] [PubMed] [Google Scholar]
- 48.Parker RI. Transfusion in critically ill children: indications, risks, and challenges. Crit Care Med 2014;42:675–90. [DOI] [PubMed] [Google Scholar]
- 49.Secher EL, Stensballe J, Afshari A. Transfusion in critically ill children: an ongoing dilemma. Acta Anaesthesiol Scand 2013;57:684–91. [DOI] [PubMed] [Google Scholar]
- 50.Tyrrell CT, Bateman ST. Critically ill children: to transfuse or not to transfuse packed red blood cells, that is the question. Pediatr Crit Care Med 2012;13:204–9. [DOI] [PubMed] [Google Scholar]
- 51.Jensen LS, Andersen AJ, Christiansen PM, et al. Postoperative infection and natural killer cell function following blood transfusion in patients undergoing elective colorectal surgery. Br J Surg 1992;79:513–6. [DOI] [PubMed] [Google Scholar]
- 52.Jensen LS, Hokland M, Nielsen HJ. A randomized controlled study of the effect of bedside leucocyte depletion on the immunosuppressive effect of whole blood transfusion in patients undergoing elective colorectal surgery. Br J Surg 1996;83:973. [DOI] [PubMed] [Google Scholar]
- 53.Taylor RW, Manganaro L, O’Brien J, et al. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med 2002;30:2249–54. [DOI] [PubMed] [Google Scholar]
- 54.Vamvakas EC, Carven JH. Exposure to allogeneic plasma and risk of postoperative pneumonia and/or wound infection in coronary artery bypass graft surgery. Transfusion 2002;42:107–13. [DOI] [PubMed] [Google Scholar]
- 55.van de Watering LM, Hermans J, Houbiers JG, et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation 1998;97:562–8. [DOI] [PubMed] [Google Scholar]
- 56.Vamvakas EC. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev 2002;16:144–60. [DOI] [PubMed] [Google Scholar]
- 57.Tan TW, Farber A, Hamburg NM, et al. Blood transfusion for lower extremity bypass is associated with increased wound infection and graft thrombosis. J Am Coll Surg 2013; 216:1005–14.e2; quiz 1031–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woods BI, Rosario BL, Chen A, et al. The association between perioperative allogeneic transfusion volume and postoperative infection in patients following lumbar spine surgery. J Bone Joint Surg Am 2013;95:2105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bochicchio GV, Napolitano L, Joshi M, et al. Blood product transfusion and ventilator-associated pneumonia in trauma patients. Surg Infect (Larchmt) 2008;9:415–22. [DOI] [PubMed] [Google Scholar]
- 60.Hill GE, Frawley WH, Griffith KE, et al. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma 2003;54:908–14. [DOI] [PubMed] [Google Scholar]
- 61.Leal-Noval SR, Muñoz-Gómez M, Jiménez-Sánchez M, et al. Red blood cell transfusion in non-bleeding critically ill patients with moderate anemia: is there a benefit? Intensive Care Med 2013;39:445–53. [DOI] [PubMed] [Google Scholar]
- 62.Rachoin JS, Daher R, Schorr C, et al. Microbiology, time course and clinical characteristics of infection in critically ill patients receiving packed red blood cell transfusion. Vox Sang 2009;97:294–302. [DOI] [PubMed] [Google Scholar]
- 63.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA 2014;311:1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elward AM, Fraser VJ. Risk factors for nosocomial primary bloodstream infection in pediatric intensive care unit patients: a 2-year prospective cohort study. Infect Control Hosp Epidemiol 2006;27:553–60. [DOI] [PubMed] [Google Scholar]
- 65.White M, Barron J, Gornbein J, et al. Are red blood cell transfusions associated with nosocomial infections in pediatric intensive care units? Pediatr Crit Care Med 2010; 11:464–8. [DOI] [PubMed] [Google Scholar]
- 66.Jeschke MG, Chinkes DL, Finnerty CC, et al. Blood transfusions are associated with increased risk for development of sepsis in severely burned pediatric patients. Crit Care Med 2007;35:579–83. [DOI] [PubMed] [Google Scholar]
- 67.Kneyber MC, Grotenhuis F, Berger RF, et al. Transfusion of leukocyte-depleted RBCs is independently associated with increased morbidity after pediatric cardiac surgery. Pediatr Crit Care Med 2013;14:298–305. [DOI] [PubMed] [Google Scholar]
- 68.Székely A, Cserép Z, Sápi E, et al. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg 2009;87:187–97. [DOI] [PubMed] [Google Scholar]
- 69.Kleiber N, Lefebvre E, Gauvin F, et al. Respiratory dysfunction associated with RBC transfusion in critically ill children: a prospective cohort study. Pediatr Crit Care Med 2015;16:325–34. [DOI] [PubMed] [Google Scholar]
- 70.Lieberman L, Petraszko T, Yi QL, et al. Transfusion-related lung injury in children: a case series and review of the literature. Transfusion 2014;54:57–64. [DOI] [PubMed] [Google Scholar]
- 71.Mulder HD, Augustijn QJ, van Woensel JB, et al. Incidence, risk factors, and outcome of transfusion-related acute lung injury in critically ill children: a retrospective study. J Crit Care 2015;30:55–9. [DOI] [PubMed] [Google Scholar]
- 72.Roubinian NH, Looney MR, Kor DJ, et al. Cytokines and clinical predictors in distinguishing pulmonary transfusion reactions. Transfusion 2015;55:1838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev 2007;21:327–48. [DOI] [PubMed] [Google Scholar]
- 74.Cholette JM, Pietropaoli AP, Henrichs KF, et al. Longer RBC storage duration is associated with increased postoperative infections in pediatric cardiac surgery. Pediatr Crit Care Med 2015;16:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gauvin F, Spinella PC, Lacroix J, et al. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care patients. Transfusion 2010;50:1902–13. [DOI] [PubMed] [Google Scholar]
- 76.Karam O, Tucci M, Toledano BJ, et al. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red blood cells. Transfusion 2009;49:2326–34. [DOI] [PubMed] [Google Scholar]
- 77.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: the TOTAL randomized clinical trial. JAMA 2015;314:2514–23. [DOI] [PubMed] [Google Scholar]
- 78.Fergusson DA, Hébert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA 2012;308:1443–51. [DOI] [PubMed] [Google Scholar]
- 79.Lacroix J, Hébert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015;372:1410–8. [DOI] [PubMed] [Google Scholar]
- 80.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med 2015;372:1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muszynski JA, Frazier E, Nofziger R, et al. Red blood cell transfusion and immune function in critically ill children: a prospective observational study. Transfusion 2015;55:766–74. [DOI] [PubMed] [Google Scholar]
- 82.Sparrow RL, Patton KA. Supernatant from stored red blood cell primes inflammatory cells: influence of prestorage white cell reduction. Transfusion 2004;44:722–30. [DOI] [PubMed] [Google Scholar]
- 83.Cardo LJ, Wilder D, Salata J. Neutrophil priming, caused by cell membranes and microvesicles in packed red blood cell units, is abrogated by leukocyte depletion at collection. Transfus Apher Sci 2008;38:117–25. [DOI] [PubMed] [Google Scholar]
- 84.Hendrickson JE, Hod EA, Hudson KE, et al. Transfusion of fresh murine red blood cells reverses adverse effects of older stored red blood cells. Transfusion 2011;51:2695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belizaire RM, Makley AT, Campion EM, et al. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg 2012;73:S128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel MB, Proctor KG, Majetschak M. Extracellular ubiquitin increases in packed red blood cell units during storage. J Surg Res 2006;135:226–32. [DOI] [PubMed] [Google Scholar]
- 87.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 2010;115: 4284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baumgartner JM, Silliman CC, Moore EE, et al. Stored red blood cell transfusion induces regulatory T cells. J Am Coll Surg 2009;208:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghio M, Contini P, Negrini S, et al. Down regulation of human natural killer cell-mediated cytolysis induced by blood transfusion: role of transforming growth factor-β(1), soluble Fas ligand, and soluble Class I human leukocyte antigen. Transfusion 2011;51:1567–73. [DOI] [PubMed] [Google Scholar]
- 90.Muszynski J, Nateri J, Nicol K, et al. Immunosuppressive effects of red blood cells on monocytes are related to both storage time and storage solution. Transfusion 2012;52:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mynster T, Dybkjoer E, Kronborg G, et al. Immunomodulating effect of blood transfusion: is storage time important? Vox Sang 1998;74:176–81. [PubMed] [Google Scholar]
- 92.Ottonello L, Ghio M, Contini P, et al. Nonleukoreduced red blood cell transfusion induces a sustained inhibition of neutrophil chemotaxis by stimulating in vivo production of transforming growth factor-beta1 by neutrophils: role of the immunoglobulinlike transcript 1, sFasL, and sHLA-I. Transfusion 2007;47:1395–404. [DOI] [PubMed] [Google Scholar]
- 93.Long K, Meier C, Bernard A, et al. T-cell suppression by red blood cells is dependent on intact cells and is a consequence of blood bank processing. Transfusion 2014;54: 1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bordin JO, Bardossy L, Blajchman MA. Growth enhancement of established tumors by allogeneic blood transfusion in experimental animals and its amelioration by leukodepletion: the importance of the timing of the leukodepletion. Blood 1994;84:344–8. [PubMed] [Google Scholar]
- 95.Atzil S, Arad M, Glasner A, et al. Blood transfusion promotes cancer progression: a critical role for aged erythrocytes. Anesthesiology 2008;109:989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cognasse F, Nguyen KA, Damien P, et al. The inflammatory role of platelets via their TLRs and Siglec receptors. Front Immunol 2015;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stolla M, Refaai MA, Heal JM, et al. Platelet transfusion—the new immunology of an old therapy. Front Immunol 2015;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 2012;122:2661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loh YS, Johnson L, Kwok M, et al. Pathogen reduction treatment alters the immunomodulatory capacity of buffy coat-derived platelet concentrates. Transfusion 2014;54:577–84. [DOI] [PubMed] [Google Scholar]
- 100.Aslam R, Speck ER, Kim M, et al. Transfusion-related immunomodulation by platelets is dependent on their expression of MHC Class I molecules and is independent of white cells. Transfusion 2008;48:1778–86. [DOI] [PubMed] [Google Scholar]
- 101.Perros AJ, Christensen AM, Flower RL, et al. Soluble mediators in platelet concentrates modulate dendritic cell inflammatory responses in an experimental model of transfusion. J Interferon Cytokine Res 2015;35:821–30. [DOI] [PubMed] [Google Scholar]
- 102.Fast LD, DiLeone G, Marschner S. Inactivation of human white blood cells in platelet products after pathogen reduction technology treatment in comparison to gamma irradiation. Transfusion 2011;51:1397–404. [DOI] [PubMed] [Google Scholar]
- 103.Schneider SO, Rensing H, Gräber S, et al. Impact of platelets and fresh frozen plasma in contrast to red cell concentrate on unstimulated and stimulated cytokine release in an in vitro model of transfusion. Scand J Immunol 2009;70: 101–5. [DOI] [PubMed] [Google Scholar]
- 104.L’Acqua C, Bandyopadhyay S, Francis RO, et al. Red blood cell transfusion is associated with increased hemolysis and an acute phase response in a subset of critically ill children. Am J Hematol 2015;90:915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keir AK, McPhee AJ, Andersen CC, et al. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr Res 2013;73:75–9. [DOI] [PubMed] [Google Scholar]
- 106.Fransen E, Maessen J, Dentener M, et al. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest 1999;116:1233–9. [DOI] [PubMed] [Google Scholar]
- 107.Bilgin YM, van de Watering LM, Versteegh MI, et al. Effects of allogeneic leukocytes in blood transfusions during cardiac surgery on inflammatory mediators and postoperative complications. Crit Care Med 2010;38:546–52. [DOI] [PubMed] [Google Scholar]
- 108.Lin JS, Tzeng CH, Hao TC, et al. Cytokine release in febrile non-haemolytic red cell transfusion reactions. Vox Sang 2002;82:156–60. [DOI] [PubMed] [Google Scholar]
- 109.Izbicki G, Rudensky B, Na’amad M, et al. Transfusion-related leukocytosis in critically ill patients. Crit Care Med 2004; 32:439–42. [DOI] [PubMed] [Google Scholar]
- 110.Locke R, Paul D, Touch S, et al. Cytokine load in prestorage leukoreduced PRBC transfusions in premature infants. J Perinatol 2005;25:526–30. [DOI] [PubMed] [Google Scholar]
- 111.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood 2011;118:6675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sitniakowsky LS, Later AF, van de Watering LM, et al. The effect of RBC transfusions on cytokine gene expression after cardiac surgery in patients developing post-operative multiple organ failure. Transfus Med 2011;21:236–46. [DOI] [PubMed] [Google Scholar]
- 113.Theodoraki K, Markatou M, Rizos D, et al. The impact of two different transfusion strategies on patient immune response during major abdominal surgery: a preliminary report. J Immunol Res 2014;2014:945829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Torrance HD, Vivian ME, Brohi K, et al. Changes in gene expression following trauma are related to the age of transfused packed red blood cells. J Trauma Acute Care Surg 2015;78:535–42. [DOI] [PubMed] [Google Scholar]
- 115.Fragkou PC, Torrance HD, Pearse RM, et al. Perioperative blood transfusion is associated with a gene transcription profile characteristic of immunosuppression: a prospective cohort study. Crit Care 2014;18:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bakkour S, Acker JP, Chafets DM, et al. Manufacturing method affects mitochondrial DNA release and extracellular vesicle composition in stored red blood cells. Vox Sang 2016;111:22–32. [DOI] [PubMed] [Google Scholar]
- 117.Hansen AL, Kurach JD, Turner TR, et al. The effect of processing method on the in vitro characteristics of red blood cell products. Vox Sang 2015;108:350–8. [DOI] [PubMed] [Google Scholar]
- 118.Jordan A, Chen D, Yi QL, et al. Assessing the influence of component processing and donor characteristics on quality of red cell concentrates using quality control data. Vox Sang 2016;111:8–15. [DOI] [PubMed] [Google Scholar]
- 119.Radwanski K, Garraud O, Cognasse F, et al. The effects of red blood cell preparation method on in vitro markers of red blood cell aging and inflammatory response. Transfusion 2013;53:3128–38. [DOI] [PubMed] [Google Scholar]
- 120.Chasse M, McIntyre L, Tinmouth A, et al. Clinical effects of blood donor characteristics in transfusion recipients: protocol of a framework to study the blood donor-recipient continuum. BMJ Open 2015;5:e007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spinella PC, Frazier E, Pidcoke HF, et al. All plasma products are not created equal: characterizing differences between plasma products. J Trauma Acute Care Surg 2015; 78:S18–25. [DOI] [PubMed] [Google Scholar]
- 122.Spitalnik SL, Triulzi D, Devine DV, et al. 2015 proceedings of the National Heart, Lung, and Blood Institute’s state of the science in transfusion medicine symposium. Transfusion 2015;55:2282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


