Abstract
Background and Aim
Dehydroepiandrosterone (DHEA) has a protective role against several types of cancer, although its mechanisms of action are still unknown, it may be related to the antioxidant effect of DHEA. We hypothesized that DHEA has a preventive effect on the formation of the 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) DNA adduct in pancreatic cancer patients.
Methods
Serum DHEAs were quantified by the ELISA method in 50 pancreatic cancer patients with histopathological diagnosis of adenocarcinoma and 50 matched controls. The amount of 8‐OHdG was assessed in peripheral blood leukocyte extracted DNA using a 32P‐DNA postlabeling technique.
Results
Pancreatic cancer patients had lower serum DHEA levels than healthy controls, although it did not differ significantly. Instead, the 8‐OHdG DNA adduct was significantly higher in the case than in the control (P = <0.001). Remarkably, the negative correlation between 8‐OHdG and DHEA was distinguished between cases (P = 0.025, r = −0.315) but not in controls (P = 0.078, r = −0.250). In the crude and corrected estimate for pancreatic cancer risk, a significant protective effect of DHEA against pancreatic cancer was found with increasing DHEA when 8‐OHdG is greater than its median (adjusted OR = 0, 79, 95% confidence intervals [CI]: 0.66–0.94). Similarly, a lower risk of pancreatic cancer was observed in the third tertile of DHEA (adjusted OR = 0.05, 95% CI: 0.004–0.69).
Conclusions
These results indicate that serum DHEA reduces the risk of pancreatic cancer with an anti‐DNA damage effect. Hence, the influence of DHEA to prohibit the accumulation of 8‐OHdG may be one of its physiological functions.
Keywords: 8‐hydroxy‐2‐deoxyguanosine, dehydroepiandrosterone, DNA damage, pancreatic cancer
Regardless of dehydroepiandrosterone (DHEA)'s association with a range of beneficial effects in humans, including decreased cardiovascular disease, weight loss, decreased serum cholesterol, and immune system activation, the functional effects of DHEA as an anticarcinogenic are not well evaluated. In this article, we sought to examine the relationship between serum DHEA level and DNA 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) adducts with pancreatic cancer risk. We showed a negative association between serum DHEA level and 8‐OHdG adducts in pancreatic cancer patients.
Introduction
Pancreatic cancer with poor prognosis is currently a major health problem in developing countries. 1 Exposure to occupational and environmental carcinogens and an individual's genetic background are some risk factors for pancreatic cancer. 2 , 3 , 4 , 5 Changes in redox metabolism in the metabolic reprogramming of nearly all cancers were associated with the accumulation of reactive oxygen species (ROS). 6 ROS are free radicals that are an extremely dynamic material for cell damage and are produced during the transport of electrons from the mitochondria of living cells. 7 , 8 At high concentrations, ROS induce oxidative damage, especially in DNA, then causing mutations that ultimately lead to cancer. 6
There are several restructured bases that have been recognized in relation to ROS attacks. However, 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) is the fundamental adduct as a marker of oxidative DNA damage and oxidative stress and some attention is focused on the 8‐OHdG‐rich lesions that are most involved in cancer. 9 , 10 Although various types of DNA products appear during oxidative DNA damage, particular interest is focused on nucleobase modulation and especially on abundant 8‐OHdG lesions. Dehydroepiandrosterone (DHEA), an adrenal steroid, is abundant in human plasma and declines markedly with age in humans of both sexes, and it is functionally important in tumor suppression by inhibiting the generation of superoxide anion radicals. 11 , 12 , 13 DHEA and its sulfate ester (DHEA‐S) take care of chronic diseases related to aging such as cardiovascular disease and cancer. However, despite some offers for the anticancer effect of DHEA for over a decade, the particular mechanism of how it works is still not well known. 13 , 14 , 15
DHEA has been shown to deteriorate atypical hyperplasia and reduce the incidence of invasive colon cancer. 16 Similarly, the growth of pancreatic cancer cell lines and pancreatic tumorigenesis in nude mice were inhibited by DHEA. 14 Previous studies have revealed the inhibition of tumor cell proliferation following the use of DHEA and its analogs that have been attributed to obstruction in the function of the pentose phosphate (PPP) pathway and mainly by disturbance of glucose‐6‐phosphate dehydrogenase (G6PD) enzyme. 11 , 12 Therefore, the decline in circulating levels of these hormones that occur with age is a major concern that could be studied in cancers such as pancreatic cancer. 17 , 18
In the present work, we examined the association of serum DHEA level with 8‐OHdG DNA adduct and their correlation with the natural characteristics of pancreatic cancer patients.
Methods
Study participants
During the year from 2012 to 2015, 50 cases of pancreatic cancer and 50 healthy volunteers were enrolled by referral subjects at the Endoscopic Ultrasound Center (EUS) in the hospital. Controls were those who had normal pancreases on EUS with no history or current diagnosis of liver or kidney failure or any cancer. Episode cases were those with a histopathological diagnosis of adenocarcinoma when tissue was taken directly from the pancreas. EUS was performed by using the Pentax linear EUS device (EG 3830 UT) for all potential cases and controls. If a solid or cystic lesion was distinguished in the pancreas by EUS, fine needle aspiration (FNA) biopsy was proposed. A pathologist evaluated the histopathology of all FNA biopsies and the patients' surgical tissues, if available. To be admitted to the study, all subjects completed a questionnaire. Paired blood samples were collected and processed according to the standardized protocol for each participant and stored at −80 °C. 19 On paper, informed consent was obtained in medical research involving all participants. This work was supported by the Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran, with an ethical number: IR. TUMS. DDRI.REC.1394.8.
Assessment of 8‐OHdG DNA adduct
DNA extraction from white blood cells (WBCs) in blood samples that were aspirated into EDTA glass tubes was performed manually using the Gentra Puregene Kit (Qiagen, Alameda, CA, USA) as directed by the manual reference and analyzed for purification and concentration by the NanoDrop‐1000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA). To evaluate the establishment of the 8‐OHdG adduct in peripheral WBCs, the P1 32P‐postlabeling nuclease procedure was used as described previously. 20 Briefly, the DNA was fragmented by the P1 nuclease and the obtained nucleosides were incubated by [γ‐32P] ATP (3000 Ci/mM) (Amersham, UK) and polynucleotide kinase T4 (10 U/μL) (Roche Diagnostics, Indianapolis, IN, USA) to make 32P‐labeled adducts, which are traced by thin layer chromatography with multidirectional anion exchange and finally visualized by means of autoradiography film (Packard Instruments). The lower detection limit of 8‐OHdG with this method is 0.1 adducts for 109 normal nucleotides with validation in previous studies. The amount of 8‐OHdG DNA adduct was evaluated based on the relative adduct labeling (RAL) values. 20 , 21 , 22
Measurement of DHEA serum levels
The highly specific ELISA method from Demeditec Diagnostics GmbH, Germany, was used to determine serum DHEA. Results are expressed as μg/dL, with standard range: 0.37–30 ng/mL.
Statistical analysis
STATA software, version 12.0 (Stata Corp, College Station, TX, USA) was used in statistical surveys. Data are described by frequency and percentage, as well as mean ± SD, whenever is needed. The Fisher exact probability test and the χ 2 test were used to estimate alterations in frequencies between cases and controls by simple eventuality table analysis. Continuous variables were well weighted with Mann–Whitney t test or U test and Spearman rank‐order correlation. The postulation of normality was calculated using the Shapiro–Wilk test. Unconditional logistic regression models used to show odds ratios (ORs) with 95% confidence intervals (CI). P values <0.05 were well thought to be statistically significant.
Results
General information of study participants
Table 1 summarizes the exponential characteristics of the study participants. As shown in Table 1, it is sufficient to observe that cases and controls are balanced according to the potential risk factors for pancreatic cancer. Pancreatic cancer patients showed no noteworthy variations with healthy subjects on natural characteristics such as age, body mass index (BMI), smoking habits, and diabetes in this study. The median level of the 8‐OHdG DNA adduct was significantly higher in the cases than in the controls (235.3 [145.6–325.9] vs 122.8 [96.5–224.6]), P < 0.001). However, the median level of serum DHEA was lower in cases and did not differ significantly from healthy controls (0.5 [0.3–1.1] vs 0.9 [0.3–1.5], P = 0.081). There was a negative association between serum DHEA level and 8‐OHdG DNA adduct (r = −0.367, P < 0.001), so we examined the relationship between 8‐OHdG and DHEA with cancer of the pancreas as a continuous variable.
Table 1.
Comparison of the cases and controls for participants' characteristics
| Participants' characteristics | Cases (n = 50) | Controls (n = 50) | P‐value |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 22 (44%) | 23 (46%) | 0.841 |
| Female | 28 (56%) | 27 (54%) | |
| Age (mean ± SD) | 66.38 ± 9.84 | 68.94 ± 12.43 | 0.256 |
| Smoking status, n (%) | |||
| No | 37 (74%) | 34 (68%) | 0.509 |
| Yes | 13 (26%) | 16 (32%) | |
| Diabetes mellitus, n (%) | |||
| No | 36 (72%) | 42 (84%) | 0.148 |
| Yes | 14 (28%) | 8 (16%) | |
| BMI (mean ± SD) | 22.86 ± 4.04 | 23.74 ± 5.53 | 0.367 |
| 8‐OHdG, median (IQR) | 235.3 (145.6–325.9) | 122.8 (96.5–224.6) | <0.001* |
| DHEA, median (IQR) | 0.5 (0.3–1.1) | 0.9 (0.3–1.5) | 0.081 |
P values < 0.05.
8‐OHdG, 8‐hydroxy‐2′‐deoxyguanosine; BMI, body mass index; DHEA, dehydroepiandrosterone; IQR, interquartile range.
As shown in Table 2, 8‐OHdG DNA adduct is negatively associated with serum DHEA level in all participants and patients (r = −0.367, P < 0.001 and r = −0.315, P = 0.025); however, no significant correlation of these variables was observed in healthy participants (r = −0.250, P = 0.078). Figure 1 illustrates the association between 8‐OHdG and DHEA in cases versus controls.
Table 2.
The inverse association between 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) and dehydroepiandrosterone (DHEA) with pancreatic cancer as a continuous variable
| Variables | Correlation | P‐value | |
|---|---|---|---|
| 8‐OHdG and DHEA | Totally | −0.367 | <0.001* |
| Cases | −0.315 | 0.025* | |
| Controls | −0.250 | 0.078 |
P values < 0.05.
Figure 1.
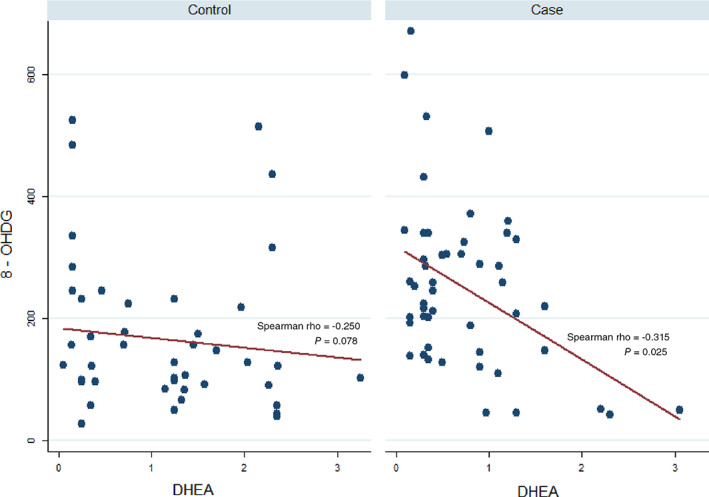
Scatter plot for the association between dehydroepiandrosterone (DHEA) and 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) in cases versus controls. A significant correlation was observed between these values among cases (P = 0.025) but not in controls (P = 0.078).
Pancreatic cancer risk assessment in correlation with DHEA
Table 3 contains a description of the crude and corrected estimates of pancreatic cancer risk in association with DHEA between two median levels of 8‐OHdG and based on the logistic regression model. When 8‐OHdG was below its median, DHEA had no limits to the development of pancreatic cancer. Conversely, when 8‐OHdG is above its median, the increase in DHEA has a significant protective effect against pancreatic cancer (adjusted OR = 0.79, 95% CI: 0.66–0.94). When we looked at the DHEA tertiles, an expected trend toward a reduced risk of pancreatic cancer was notable; therefore, the third tertile of DHEA compared to the first was associated with a lower risk of pancreatic cancer (adjusted OR = 0.05, 95% CI: 0.004–0.69).
Table 3.
Univariate and multivariate analyses of pancreatic cancer risk in relation to dehydroepiandrosterone (DHEA) level in two levels of 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) between cases and controls
| DHEA continuous scale | DHEA tertile 2/tertile 1 | DHEA tertile 3/tertile 1 | |
|---|---|---|---|
| 8‐OHdG < 178.6 (median) | |||
| Adjusted OR† (95% CI) | 1.002 (0.92–1.09) | 2.81 (0.39–20.09) | 0.46 (0.07–3.22) |
| Crude OR (95% CI) | 0.998 (0.92–1.07) | 1.71 (0.33–8.67) | 0.52 (0.11–2.48) |
| 8‐OHdG ≥ 178.6 (median) | |||
| Adjusted OR† (95% CI) | 0.79 (0.66–0.94)* | 0.48 (0.07–3.41) | 0.05 (0.004–0.69)* |
| Crude OR (95% CI) | 0.923 (0.83–1.01) | 1.44 (0.34–5.94) | 0.32 (0.06–1.60) |
P values < 0.05.
Adjusted for: age, gender, BMI, smoking status, and diabetes mellitus.
BMI, body mass index; CI, confidence interval; OR, odds ratio.
Discussion
According to the 2020 Worldwide Cancer Statistics and the American Cancer Society (ACS) website (Cancer.Net Editorial Board), pancreatic cancer is universally present as the ninth cancer in women and the tenth in men and accounts for 7% of all cancer deaths. However, there is no active treatment for pancreatic cancer and elimination of the tumor by surgery is not commonly suggested, even once removed, relapse is expected, so generation of a possible cancer approach is needed. 5 , 23 , 24
DHEA as a precursor of sex steroid hormones together with the physiological role has a protective effect against cancer. Previous studies have revealed that higher serum DHEA during adolescence reduces the occurrence of malignant tumors in both sexes much later in life. However, despite the association of DHEA with several favorable effects in humans, including the decrease of cardiovascular disease, weight loss, reduction of serum cholesterol, and activation of the immune system, the functional effects of DHEA as an anticarcinogenic are not well evaluated. On the mechanism of involvement of DHEA, cellular phase arrest of tumor cells and inhibition of tumor metastases is proposed. 25 High concentrations of DHEA prevent proliferation of endothelial cells, in vitro, and angiogenesis, in vivo, in various tumor cells such as cervical cancer and airway smooth muscle cells, which indicate its potential in cancer treatment. 26
Our results showed an inverse association between serum DHEA and 8‐OHdG DNA adducts in which pancreatic cancer patients had significantly higher levels of 8‐OHdG than those without and is compatible with the previous study. 3 Furthermore, the DHEA level was lower in cases, although this decrease was not significantly different from healthy controls. In studying the association between 8‐OHdG and DHEA and pancreatic cancer risk, we found that a higher serum level of DHEA has a significant protective effect against pancreatic cancer when the level of 8‐OHdG adducts is increased. A lower risk of pancreatic cancer has also been documented in the third tertile of serum DHEA.
There are some contradictions on the inhibitory effects of DHEA in pancreatic tumorigenesis. 27 The impact of DHEA in rodents on the rapid shift of preneoplastic pancreatic foci to carcinogenic was reported by Tagliaferro et al. 27 However, Muscarella et al. observed that dietary supplementation with a small concentration of DHEA significantly inhibits the growth of pancreatic cancer cells in nude mice. 15
Thus, both aspects of DHEA are expected, as a positive impact of DHEA on breast cancer observed in prospective studies of premenopausal women who had lower DHEA levels at a younger age, while this level increased in older age due to oral intake. However, the hypothesis that higher serum DHEA levels during adolescence reduced the occurrence of malignancies in both sexes far later in life is still adequate; nonetheless, caution is advised in taking DHEA for prolonged periods. DHEA also inhibits dimethylbenz[a]anthracene (DMBA), a typical polycyclic aromatic hydrocarbon (PAH), which induced carcinogenic metabolites in rats. In fact, the anticancer effects of DHEA are mediated by obstructive binding of DMBA to the DNA, which occurs by blocking the PPP G6PD key enzymes. 11 , 28
In summary, we observed a negative relationship between serum DHEA and DNA adduct 8‐OHdG and proposed the antioxidant and anti‐DNA‐damaging effect of DHEA in pancreatic cancer patients, which highlighted our findings compared to all previous animal studies. Therefore, our findings underscore the importance of DHEA in preventing cancer development, although DHEA will not be a crucial treatment. This indicates the likelihood of biologically related molecules to DHEA in the treatment of pancreatic cancer. Further studies are needed to delineate the mechanism and scope of DHEA treatment for pancreatic cancer.
Acknowledgments
The authors of this article take this opportunity to appreciate the patients who have participated in this research program. This work was supported by the Digestive Disease Research Institute, Tehran University of Medical Sciences.
Declaration of conflict of interest: None of the authors have any conflicts of interest associated with this study.
Author contribution: All authors contributed to both the work and discussions.
References
- 1. Tsai HJ, Chang JS. Environmental risk factors of pancreatic cancer. J. Clin. Med. 2019; 8: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sollie S, Michaud DS, Sarker D et al. Chronic inflammation markers are associated with risk of pancreatic cancer in the Swedish AMORIS cohort study. BMC Cancer. 2019; 19: 858–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohamadkhani A, Pourshams A, Viti J et al. Pancreatic cancer is associated with peripheral leukocyte oxidative DNA damage. Asian Pac. J. Cancer Prev. 2017; 18: 1349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alimirzaie S, Mohamadkhani A, Masoudi S et al. Mutations in known and novel cancer susceptibility genes in young patients with pancreatic cancer. Arch. Iran. Med. 2018; 21: 228–33. [PubMed] [Google Scholar]
- 5. Vogtmann E, Han Y, Caporaso JG et al. Oral microbial community composition is associated with pancreatic cancer: a case‐control study in Iran. Cancer Med. 2020; 9: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arfin S, Jha NK, Jha SK et al. Oxidative stress in cancer cell metabolism. Antioxidants (Basel). 2021; 10: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohamadkhani A, Akbari MR, Ghanbari R, Naderi E, Rezanejad‐Asl P, Pourshams A. Direct sequencing of cyclooxygenase‐2 (COX‐2) revealed an intronic variant rs201231411 in Iranian patients with pancreatic cancer. Middle East J Dig Dis. 2015; 7: 14–8. [PMC free article] [PubMed] [Google Scholar]
- 8. Masoudi S, Hassanzadeh Nemati A, Fazli HR et al. An increased level of aryl hydrocarbon receptor in patients with pancreatic cancer. Middle East J. Dig. Dis. 2019; 11: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khadem‐Ansari M, Nozari S, Asoudeh M, Rasmi Y, Faridvand Y. Elevated serum 8‐hydroxy‐2′‐deoxyguanosine, nitrite, and nitrate in patients with stage I multiple MYELOMA. Int. J. Cancer Manag. 2016; 10: 1–6. [Google Scholar]
- 10. Brancato B, Munnia A, Cellai F et al. 8‐Oxo‐7,8‐dihydro‐2′‐deoxyguanosine and other lesions along the coding strand of the exon 5 of the tumour suppressor gene P53 in a breast cancer case‐control study. DNA Res. 2016; 23: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gatto V, Aragno M, Gallo M et al. Dehydroepiandrosterone inhibits the growth of DMBA‐induced rat mammary carcinoma via the androgen receptor. Oncol. Rep. 1998; 5: 241–3. [DOI] [PubMed] [Google Scholar]
- 12. Luo S, Sourla A, Labrie C, Belanger A, Labrie F. Combined effects of dehydroepiandrosterone and EM‐800 on bone mass, serum lipids, and the development of dimethylbenz(A)anthracene‐induced mammary carcinoma in the rat. Endocrinology. 1997; 138: 4435–44. [DOI] [PubMed] [Google Scholar]
- 13. Ho HY, Cheng ML, Chiu HY, Weng SF, Chiu DT. Dehydroepiandrosterone induces growth arrest of hepatoma cells via alteration of mitochondrial gene expression and function. Int. J. Oncol. 2008; 33: 969–77. [PubMed] [Google Scholar]
- 14. Melvin WS, Boros LG, Muscarella P et al. Dehydroepiandrosterone‐sulfate inhibits pancreatic carcinoma cell proliferation in vitro and in vivo. Surgery. 1997; 121: 392–7. [DOI] [PubMed] [Google Scholar]
- 15. Muscarella P, Boros LG, Fisher WE, Rink C, Melvin WS. Oral dehydroepiandrosterone inhibits the growth of human pancreatic cancer in nude mice. J. Surg. Res. 1998; 79: 154–7. [DOI] [PubMed] [Google Scholar]
- 16. Osawa E, Nakajima A, Yoshida S et al. Chemoprevention of precursors to colon cancer by dehydroepiandrosterone (DHEA). Life Sci. 2002; 70: 2623–30. [DOI] [PubMed] [Google Scholar]
- 17. Regelson W, Kalimi M. Dehydroepiandrosterone (DHEA)–the multifunctional steroid. II. Effects on the CNS, cell proliferation, metabolic and vascular, clinical and other effects. Mechanism of action? Ann. N. Y. Acad. Sci. 1994; 719: 564–75. [DOI] [PubMed] [Google Scholar]
- 18. Chou HC, Ozawa S, Fu PP, Lang NP, Kadlubar FF. Metabolic activation of methyl‐hydroxylated derivatives of 7,12‐dimethylbenz[a]anthracene by human liver dehydroepiandrosterone‐steroid sulfotransferase. Carcinogenesis. 1998; 19: 1071–6. [DOI] [PubMed] [Google Scholar]
- 19. Mohamadkhani A, Poustchi H. Repository of human blood derivative biospecimens in biobank: technical implications. Middle East J. Dig. Dis. 2015; 7: 61–8. [PMC free article] [PubMed] [Google Scholar]
- 20. Reddy MV, Randerath K. Nuclease P1‐mediated enhancement of sensitivity of 32P‐postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986; 7: 1543–51. [DOI] [PubMed] [Google Scholar]
- 21. Peluso M, Hainaut P, Airoldi L et al. Methodology of laboratory measurements in prospective studies on gene‐environment interactions: the experience of GenAir. Mutat. Res. 2005; 574: 92–104. [DOI] [PubMed] [Google Scholar]
- 22. Jeng HA, Pan CH, Chao MR, Lin WY. Sperm DNA oxidative damage and DNA adducts. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015; 794: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takaoka M, Shimatani M, Ikeura T et al. Usefulness of half‐covered metallic stent placement in preventing acute cholecystitis complication in pancreatic cancer‐induced distal biliary stricture. JGH Open. 2020; 4: 1140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aguila EJT, Francisco CPD, Co JT. Pancreatic cancer masquerading as ischemic enteritis on endoscopy. JGH Open. 2021; 5: 157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez‐Marure R, Contreras PG, Dillon JS. Effects of dehydroepiandrosterone on proliferation, migration, and death of breast cancer cells. Eur. J. Pharmacol. 2011; 660: 268–74. [DOI] [PubMed] [Google Scholar]
- 26. Ortega‐Calderon YN, Lopez‐Marure R. Dehydroepiandrosterone inhibits proliferation and suppresses migration of human cervical cancer cell lines. Anticancer Res. 2014; 34: 4039–44. [PubMed] [Google Scholar]
- 27. Tagliaferro AR, Roebuck BD, Ronan AM, Meeker LD. Enhancement of pancreatic carcinogenesis by dehydroepiandrosterone. Adv. Exp. Med. Biol. 1992; 322: 119–29. [DOI] [PubMed] [Google Scholar]
- 28. Bhardwaj V, He J. Reactive oxygen species, metabolic plasticity, and drug resistance in cancer. Int. J. Mol. Sci. 2020; 21: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


