Abstract
The intricate sensorimotor neural circuits that control swallowing are heavily reliant on serotonin (5-hydroxytryptamine [5-HT]); however, the impact of 5-HT deficiency on swallow function remains largely unexplored. We investigated this using mice deficient in tryptophan-hydroxylase-2 (TPH2), the enzyme catalyzing the rate-limiting step in 5-HT synthesis. Videofluoroscopy was utilized to characterize the swallowing function of TPH2 knockout (TPH2−/−) mice as compared with littermate controls (TPH2+/+). Results showed that 5-HT deficiency altered all 3 stages of swallowing. As compared with controls, TPH2−/− mice had significantly slower lick and swallow rates and faster esophageal transit times. Future studies with this model are necessary to determine if 5-HT replacement may rescue abnormal swallowing function. If so, supplemental 5-HT therapy may have vast applications for a large population of patients with a variety of neurologic disorders resulting in life-diminishing dysphagia, particularly amyotrophic lateral sclerosis and Parkinson’s disease, for which 5-HT deficiency is implicated in the disease pathogenesis.
Keywords: serotonin deficiency, 5-hydroxytryptamine (5-HT), tryptophan hydroxylase (TPH), mouse model, videofluoroscopy, amyotrophic lateral sclerosis (ALS)
Dysphagia is a devastating symptom of many neurologic diseases and is often associated with complications such as malnutrition, aspiration pneumonia, decreased quality of life, and increased risk of mortality. Although the pathophysiology of dysphagia for each disease remains largely unknown, there is growing evidence that the intricate sensorimotor neural circuits involved in swallowing rely on the neurotransmitter serotonin (5-hydroxytryptamine [5-HT]).1–3 In particular, the nucleus tractus solitarius (NTS) within the brainstem central pattern generator for swallowing contains a large distribution of 5-HT1a receptors, suggesting that serotonergic neurons play a critical role in initiating swallowing.3 However, this role remains highly controversial, as previous studies found inhibitory,3 excitatory,4,5 and negligible6 actions of 5-HT in the NTS. In addition, the brainstem motor nuclei (ie, trigeminal, facial, nucleus ambiguus, dorsal motor nucleus of the vagus, and hypoglossal) that innervate the muscles involved in swallowing receive dense 5-HT innervation from the NTS and numerous other regions of the brain to facilitate the coordinated, sequential motor pattern of swallowing.7,8 This wide-spread serotonergic influence suggests that 5-HT deficiency may influence swallow function.
Previous studies utilized pharmacologic manipulation of the serotonergic system to investigate the effect of 5-HT on swallowing. However, a genetically modified animal model, particularly one in which central 5-HT is lacking, could provide the best evidence that 5-HT is necessary for the complex neural control of swallowing. A potential target is tryptophan hydroxylase (TPH), which is the rate-limiting enzyme for 5-HT synthesis from tryptophan that exists as 1 of 2 isoenzymes: TPH1 or TPH2.9,10 While TPH1 is primarily expressed in gastrointestinal tissue, TPH2 synthesizes 5-HT in the central nervous system.9,10 Here, we used TPH2 knockout (TPH2−/−) mice9,11 and videofluoroscopy to investigate the impact of central nervous system–derived 5-HT deficiency on swallowing.
Methods
Animals
TPH2−/− mice9,11 (n = 19: 9 females, 10 males) and littermate controls (TPH2+/+; n = 25: 13 females, 12 males) were used for this study. Pups were generated from heterozygous breeders maintained on a mixed C57BL/6J and 129/SvJ genetic background; heterozygous offspring (TPH2+/−) were excluded. Mice were group-housed by sex on a 12:12-hour light cycle with free access to food and water, except during swallow testing. All experiments were approved by the University of Missouri Institutional Animal Care and Use Committee.
Videofluoroscopic Swallow Study
We used our custom low-energy fluoroscope and established videofluoroscopic swallow study (VFSS) protocol12,13 to assess the swallowing function of each mouse at 6 and 12 months of age, representing young versus older adult mice prior to the onset of age-related swallowing impairment (presbyphagia).13 Briefly, after an overnight (~16 hour) water restriction, mice underwent videofluoroscopy in a custom chamber in lateral view while voluntarily drinking thin liquid contrast (chocolate-flavored iohexol) from a bowl. Fluoroscopic videos (30 frames per second) were digitally recorded when mice were observed drinking via a webcam. The oropharyngeal and esophageal stages of swallowing were recorded separately (Figure 1) by moving the mouse within the x-ray beam with a custom remote-controlled platform.
Figure 1.
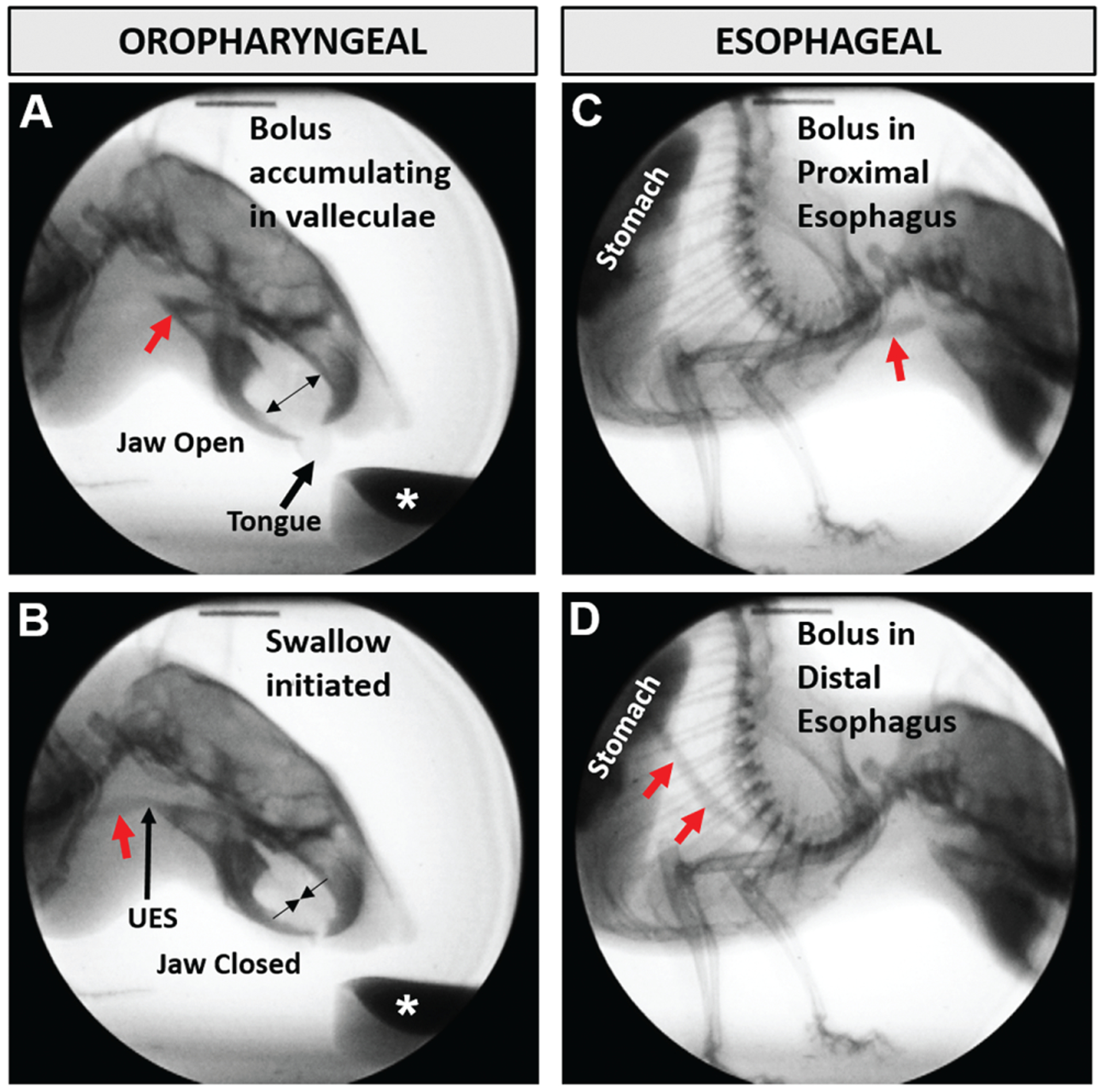
Videofluoroscopic images of a mouse drinking contrast solution (asterisk). A bolus (red arrow) is tracked through the (A, B) oropharyngeal and (C, D) esophageal stages of swallowing. UES, upper esophageal sphincter.
Videos (n = 176: 44 mice × 2 anatomic views × 2 time points) were analyzed frame by frame by 2 independent blinded reviewers with Pinnacle Studio 14 video editing software (Pinnacle Systems, Inc, Mountain View, California). For each fluoroscopic view (ie, oropharyngeal vs esophageal stage of swallowing), five 2-second episodes of uninterrupted drinking were analyzed per mouse to quantify the VFSS metrics defined in Table 1. Discrepancies (<10%) were subjected to group consensus to resolve reviewer error.
Table 1.
VFSS Metrics for Objective Quantification of Dysphagia in Mice.
| Stage of Swallowing | VFSS Metrics | Operational Definitions |
|---|---|---|
| Oral | Lick rate | The number of lick cycles per second (30 frames) during uninterrupted drinking, starting with the jaw at maximum open position and the tongue protruded. |
| Pharyngeal | Swallow rate | The number of swallows occurring during each 2-second (60 frames) episode of uninterrupted drinking, beginning at the rest frame immediately preceding bolus transit from the valleculae (ie, anatomic swallow trigger point in mice). |
| Esophageal | Esophageal transit time | The time (in milliseconds) that it takes the bolus to be swallowed from the proximal esophagus into the stomach. |
Abbreviation: VFSS, videofluoroscopic swallow study.
Results
VFSS data were analyzed with a 3-factor (group, time point, sex) repeated measures analysis of variance design and Bonferroni post hoc comparisons (P ≤ .05, 2-sided) with SAS 9.4 (SAS Institute, Cary, North Carolina). As shown in Figure 2, 5-HT deficiency affected all 3 stages of swallowing. Compared with controls, TPH2−/− mice had significantly slower lick and swallow rates at 6 and 12 months of age, providing evidence of chronic oral and pharyngeal dysphagia, respectively. Sex differences were identified only for lick rate, which was impaired earlier for TPH2−/− males. The esophageal stage was affected only at 12 months of age, with TPH2−/− mice having significantly faster esophageal transit times as compared with controls. However, within-group comparisons over time revealed statistically longer esophageal transit only for controls, indicative of age-related effects. Indeed, significant age-related changes were observed for both groups, which was earlier than expected based on our previous work with a mouse model of primary aging.13 No other health- or weight-related issues were experienced by either group.
Figure 2.
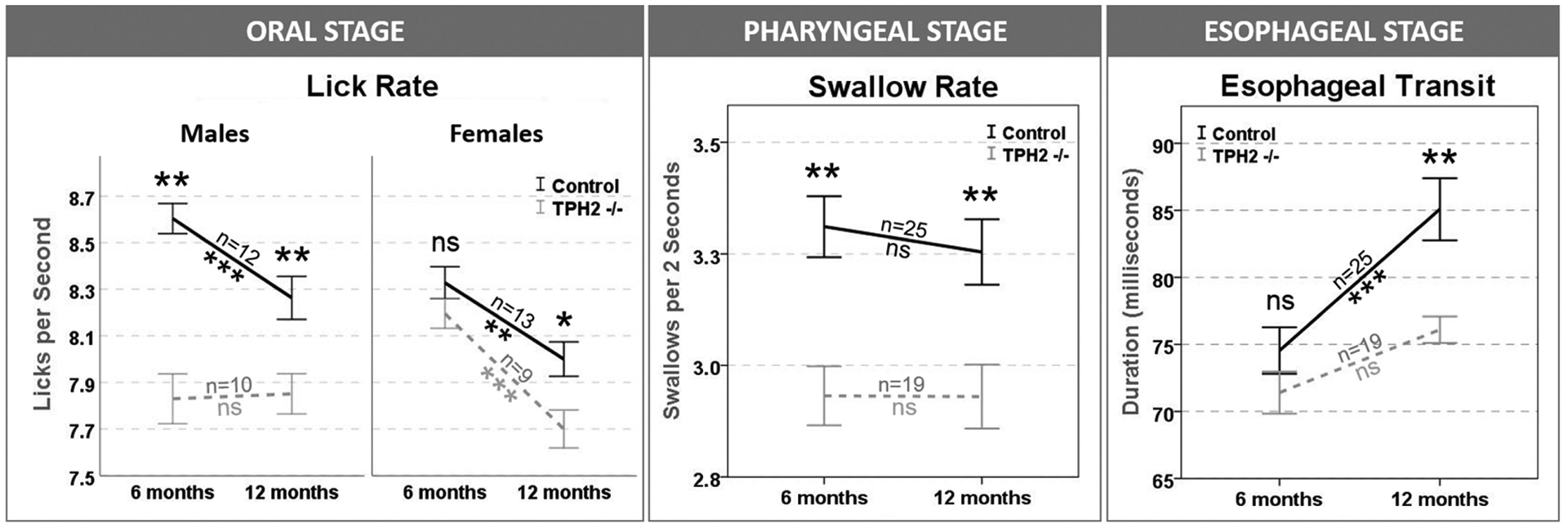
5-Hydroxytryptamine deficiency affected all 3 stages of swallowing. Lick rate was impaired earlier for TPH2−/− males. *P <.05. **P <.01. ***P ≤ .001. ns, nonsignificant; TPH2, tryptophan-hydroxylase-2. Error bars indicate standard error.
Discussion
Results from this study showed that mice lacking central 5-HT have altered swallowing function as compared with controls. Specifically, 5-HT deficiency caused reduced lick and swallow rates, which is in agreement with other studies showing that 5-HT in the NTS has an excitatory/facilitatory effect on oropharyngeal swallowing.4,5 In addition, lick rate was impaired earlier for TPH2−/− males, which supports previous findings of sex differences in the 5-HT system of the brain.14,15 TPH2−/− mice failed to show age-related changes in esophageal transit times, congruent with previous research showing that low levels of 5-HT potentiate spontaneous contractions in the esophagus.16 However, it is unclear if faster esophageal transport in old age could be clinically beneficial versus a sign of dysphagia. Future work is needed to investigate the corresponding mechanisms of action for these findings, including any modulating effect of 5-HT deficiency on other neurotransmitter systems involved in swallowing, such as dopamine and glutamate.
In conclusion, 5-HT replacement or selective serotonin reuptake inhibitors, such as paroxetine or fluoxetine, may have vast applications for a large population of patients with a variety of neurologic disorders resulting in life-diminishing dysphagia. For example, 5-HT deficiency has been implicated in amyotrophic lateral sclerosis17–19 and Parkinson’s disease,20–22 both of which result in debilitating dysphagia. Furthermore, selective serotonin reuptake inhibitors have already shown promise in improving the swallowing function of patients after a stroke.23 Therefore, early 5-HT supplementation may alleviate or delay symptoms of dysphagia in neurologic diseases, providing patients with a better quality of life and a reduced risk of mortality.
Acknowledgments
We graciously acknowledge Mitchell Allen, Kaitlin Flynn, Derek Neubauer, and Sally Smith for assistance with data collection.
Funding source:
This study was funded in part by a grant from the National Institute On Deafness And Other Communication Disorders of the National Institutes of Health (Award Number R21DC016071; Teresa E. Lever) for personnel support during data analysis and manuscript writing. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Student support was provided by the University of Missouri School of Medicine Summer Research Fellowship Program (Joseph Sinnott).
Footnotes
This article was presented at the AAO-HNSF 2016 Annual Meeting & OTO EXPO; September 18–21, 2016; San Diego, California.
Competing interests: None.
References
- 1.Miller AJ. Deglutition. Physiol Rev. 1982;62:129–184. [DOI] [PubMed] [Google Scholar]
- 2.Jean A Brainstem organization of the swallowing network. Brain Behav Evol. 1984;25:109–116. [DOI] [PubMed] [Google Scholar]
- 3.Kessler JP, Jean A. Inhibition of the swallowing reflex by local application of serotonergic agents into the nucleus of the solitary tract. Eur J Pharmacol. 1985;118:77–85. [DOI] [PubMed] [Google Scholar]
- 4.Hashim M, Bieger D. Excitatory action of 5-HT on deglutitive substrates in the rat solitary complex. Brain Res Bull. 1987;18: 355–363. [DOI] [PubMed] [Google Scholar]
- 5.Bieger D Role of bulbar serotonergic neurotransmission in the initiation of swallowing in the rat. Neuropharmacology. 1981; 20:1073–1083. [DOI] [PubMed] [Google Scholar]
- 6.Jia YX, Jian QL, Toshifumi M, et al. Neurochemical regulation of swallowing reflex in guinea pigs. Gerontology. 2001;1: 56–61. [Google Scholar]
- 7.Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–1347. [DOI] [PubMed] [Google Scholar]
- 8.Sood S, Liu X, Liu H, Nolan P, Horner RL. 5-HT at hypoglossal motor nucleus and respiratory control of genioglossus muscle in anesthetized rats. Respir Physiol Neurobiol. 2003; 138:205–221. [DOI] [PubMed] [Google Scholar]
- 9.Alenina N, Kikic D, Todiras M, et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelosi B, Pratelli M, Migliarini S, Pacini G, Pasqualetti M. Generation of a Tph2 conditional knockout mouse line for time- and tissue-specific depletion of brain serotonin. PLoS One. 2015;10(8):e0136422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Magnusson J, Karsenty G, Cummings KJ. Time- and age-dependent effects of serotonin on gasping and autoresuscitation in neonatal mice. J Appl Physiol. 2013;114:1668–1676. [DOI] [PubMed] [Google Scholar]
- 12.Lever TE, Braun SM, Brooks RT, et al. Adapting human videofluoroscopic swallow study methods to detect and characterize dysphagia in murine disease models. J Vis Exp. 2015;97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lever TE, Brooks RT, Thombs LA, et al. Videofluoroscopic validation of a translational murine model of presbyphagia. Dysphagia. 2015;30:328–342. [DOI] [PubMed] [Google Scholar]
- 14.Kokras N, Pastromas N, Papasava D, et al. Sex differences in behavioral and neurochemical effects of gonadectomy and aromatase inhibition in rats. Psychoneuroendocrinology. 2018;87: 93–107. [DOI] [PubMed] [Google Scholar]
- 15.Kato R Serotonin content of rat brain in relation to sex and age. J Neurochem. 1960;5:202. [DOI] [PubMed] [Google Scholar]
- 16.Wells DW, Hill RB. The possible role of serotonin in the rhythmicity of the crop of Aplysia dactylomela. Comp Biochem Physiol C. 1985;80:337–345. [DOI] [PubMed] [Google Scholar]
- 17.Sandyk R Serotonergic mechanisms in amyotrophic lateral sclerosis. Int J Neurosci. 2006;116:775–826. [DOI] [PubMed] [Google Scholar]
- 18.Turner BJ, Lopes EC, Cheema SS. The serotonin precursor 5-hydroxytryptophan delays neuromuscular disease in murine familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:171–176. [DOI] [PubMed] [Google Scholar]
- 19.Dentel C, Palamiuc L, Henriques A, et al. Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: a link to spasticity. Brain. 2013;136(pt 2):483–493. [DOI] [PubMed] [Google Scholar]
- 20.Politis M, Loane C. Serotonergic dysfunction in Parkinson’s disease and its relevance to disability. ScientificWorldJournal. 2011;11:1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ. Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain. 2010;133:3434–3443. [DOI] [PubMed] [Google Scholar]
- 22.Mathur BN, Lovinger DM. Serotonergic action on dorsal striatal function. Parkinsonism Relat Disord. 2012;18(suppl 1): S129–S131. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Liu X, Luo X, et al. Effects of fluoxetine on post-stroke dysphagia: a clinical retrospective study. J Stroke Cerebrovasc Dis. 2018;27:3320–3327. [DOI] [PubMed] [Google Scholar]


