Abstract
Background:
Lopinavir, ritonavir, atazanavir, and saquinavir had been reportedly used or suggested for coronavirus disease 2019 (COVID-19) treatment. They may cause electrocardiography changes. We aim to evaluate risk of PR prolongation, QRS widening, and QT prolongation from lopinavir, ritonavir, atazanavir, and saquinavir.
Methods:
In accordance with preferred reporting items for systematic reviews and meta-analyses guidelines, our search was conducted in PubMed Central, PubMed, EBSCOhost, and ProQuest from inception to June 25, 2020. Titles and abstracts were reviewed for relevance. Cochrane Risk of Bias Tool 2.0 and Downs and Black criteria was used to evaluate quality of studies.
Results:
We retrieved 9 articles. Most randomized controlled trials have low risk of biases while all quasi-experimental studies have a positive rating. Four studies reporting PR prolongation however only 2 studies with PR interval >200 ms. One of which, reported its association after treatment with ritonavir-boosted saquinavir treatment while another, during treatment with ritonavir-boosted atazanavir. No study reported QRS widening >120 ms with treatment. Four studies reporting QT prolongation, with only one study reaching QT interval >450 ms after ritonavir-boosted saquinavir treatment on healthy patients. There is only one study on COVID-19 patients reporting QT prolongation in 1 out of 95 patients after ritonavir-boosted lopinavir treatment.
Conclusion:
Limited evidence suggests that lopinavir, ritonavir, atazanavir, and saquinavir could cause PR prolongation, QRS widening, and QT prolongation. Further trials with closer monitoring and assessment of electrocardiography are needed to ascertain usage safety of antivirals in COVID-19 era.
Keywords: atazanavir, lopinavir, PR prolongation, QRS widening, QT prolongation, ritonavir, saquinavir
1. Introduction
The rapid spread of coronavirus disease 2019 (COVID-19) infection has been declared to be on an international scale by the World Health Organization since March 11, 2020.[1] Many therapeutic modalities using chloroquine, hydroxychloroquine, azithromycin, and antivirals have been recommended in order to treat this infection.[2] Among all of the antivirals available, ritonavir-boosted lopinavir (LPV/r), favipiravir, and remdesivir were commonly used all over the world for off-label use as first line therapy in patients with COVID-19.[2–4] Other antivirals such as atazanavir (ATV), saquinavir (SQV), nelfinavir, zanamivir, and efavirenz were suggested to be effective inhibitors for severe acute respiratory syndrome coronavirus 2.[5,6] Meanwhile, there have been >300 clinical trials going on for mentioned antivirals along with agents such as oseltamivir, emtricitabine, tenofovir, darunavir.[3,6] Despite the lacking evidence to support the effectivity of regimens that might protect against COVID-19 infection, the use of such antiviral agents has spread very quickly due to rapid progression of the epidemic and high mortality rate in susceptible population.[2] However, the risk of prolong QT interval from some antivirals has been evident in many studies.[7,8] The risk of QT interval prolongation from nelfinavir, efavirenz, darunavir, emtricitabine, tenofovir has been reviewed by previous article.[8] QT prolongation due to oseltamivir has also been evaluated from a Cochrane review, while no available study exists reporting such risk in zanamivir yet.[9] Based on latest available study, only one case report available reporting QT interval prolongation due to a treatment using favipiravir.[10,11] The risk of acute QT interval prolongation along with PR interval, QRS wave abnormality from the use of lopinavir (LPV), ritonavir (RTV), ATV, and SQV remains unclear.[2,8,12,13] The effect of QT prolongation from these drugs was theoretically due to alteration in human ether-a-go-go related gene ion channels.[8,14–16] We determined to evaluate these ECG changes because of reports and their suggested role in treating COVID-19 infections.[2–6] This is crucial as high risk population especially with genetic predisposition (congenital long QT syndrome) and consumption of other pro-arrhythmic drugs may further developed malignant arrhythmia such as Torsades de Pointes or ventricular fibrillation.[2]
2. Methods
A structured search of literature was conducted to identify research on the current evidence for the risk of acute ECG abnormalities from LPV, RTV, ATV, and SQV using preferred reporting items for systematic reviews and meta-analyses statement guideline, with a pre-determined search strategy.[17] The search was conducted in PubMed Central, PubMed, EBSCOhost, and ProQuest. The search done for article from inception to June 25, 2020. We used MeSH terms and complemented the search strategy using [All Field] and a combination by using keywords as following: prolong QT, prolong PR, wide QRS, electrocardiogram abnormality, lopinavir, ritonavir, saquinavir, atazanavir. We also applied other similar terms added to our search strategy in order to include more related results into our findings.
We imported our results into Endnote X9 after conducting the search strategy. Duplicates were removed leaving articles to be reviewed for relevance based on the following criteria: human study published in English; outcome of PR interval, QRS wave, and QT interval were provided as a change in millisecond. We exclude studies that only provide outcome of >1 month in ECG abnormalities because our aim is to find the acute effect in ECG abnormalities and duration for treatment in COVID-19 is commonly up to 14 days.[4] Studies that did not report which antiviral caused the ECG abnormalities and the dosage used were excluded. We also excluded case reports, observational studies, research letters, review articles, and studies which cannot be retrieved.
We used Cochrane Risk of Bias Tool 2.0 in order to evaluate the bias.[18] The tool covers for 6 domains of risk including random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; and selective reporting.[19] The risk of bias in each of these domains is scored as “low,” “high,” or “unclear.”[19] Methodological quality for quasi-experimental studies was evaluated using the relevant items from Downs and Black Criteria.[20] Based on previous study which utilized this criteria, using the 10-item criteria, studies were given a positive (>50% criteria met), neutral (50% criteria met), or negative (<50% criteria met) rating.[21]
2.1. Ethics approval
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
2.2. Patient and publication consent statement
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the patient consent statement indicated in each case.
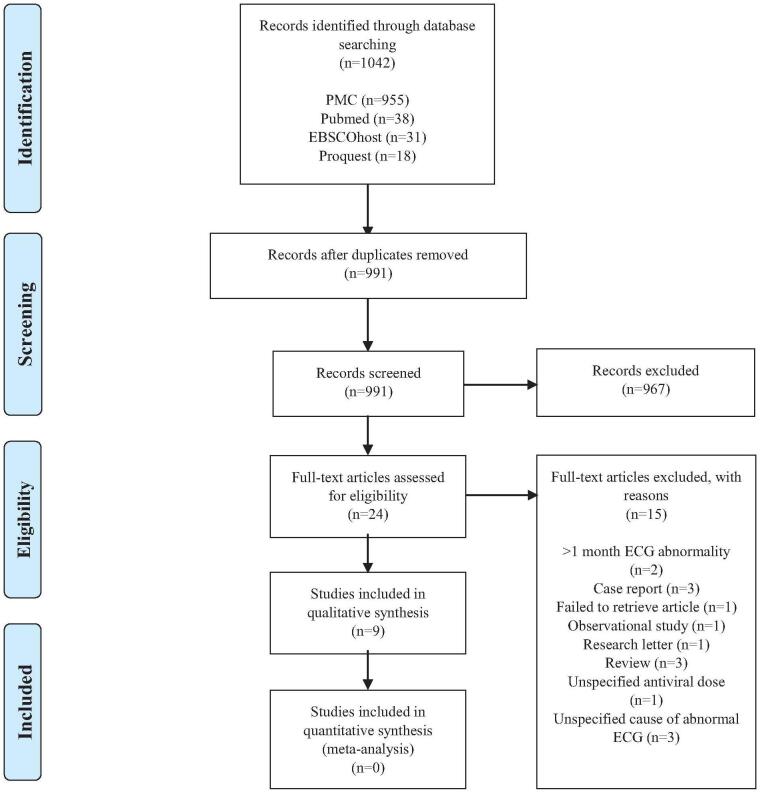
3. Results
The search strategy identified 1042 articles (Table 1). After removing duplicates, 991 articles were left to be reviewed. Titles and abstract were retrieved and reviewed for relevance resulting 24 articles. From these studies, we identified 3 case reports, 2 studies that only provide outcome of >1 month in ECG abnormalities after antiviral administration, 3 studies that did not report which antiviral causing the ECG abnormalities, 1 study did not report how much the dose used in the antivirals, 1 observational study, 1 research letter, 3 review papers, and 1 study which cannot be retrieved. We decided to exclude these 15 articles and assessed the remaining 9 articles to be reviewed qualitatively (Fig. 1). We found no comparable data that can be extracted for quantitative analysis and thus did not perform meta-analysis.
Table 1.
Search strategy completed using MeSH terms and [all field].
| Database | Search terms | Studies found |
| PMC | (((((((((((((((((((((((((((((((((((“QT Prolongation”[All Fields] OR “QT Prolonged”[All Fields]) OR “Prolong QT”[All Fields]) OR “QTc Prolongation”[All Fields]) OR “QTc Prolonged”[All Fields]) OR “Prolong QTc”[All Fields]) OR “QT Interval”[All Fields]) OR “QT Abnormality”[All Fields]) OR “Abnormal QT”[All Fields]) OR “QT Lengthening”[All Fields]) OR “Long QT”[All Fields]) OR “QTc Interval”[All Fields]) OR “QTc Abnormality”[All Fields]) OR “Abnormal QTc”[All Fields]) OR “QTc Lengthening”[All Fields]) OR “Long QTc”[All Fields]) OR “PR Interval”[All Fields]) OR “PR prolongation”[All Fields]) OR “Abnormal PR”[All Fields]) OR “PR lengthening”[All Fields]) OR “Long PR”[All Fields]) OR “QRS Wave”[All Fields]) OR “QRS complex”[All Fields]) OR “QRS widening”[All Fields]) OR “Wide QRS”[All Fields]) OR “QRS Abnormality”[All Fields]) OR “QRS Abnormalities”[All Fields]) OR “Abnormal QRS”[All Fields]) OR “Electrocardiogram Abnormality”[All Fields]) OR “Electrocardiogram Abnormalities”[All Fields]) OR “Abnormal Electrocardiogram”[All Fields]) OR “ECG abnormality”[All Fields]) OR “ECG abnormalities”[All Fields]) OR “Abnormal ECG”[All Fields]) OR “ECG Pattern”[All Fields]) OR “Long QT Syndrome”[MeSH Terms]) AND (((((((((((“lopinavir”[MeSH Terms] OR “ritonavir”[MeSH Terms]) OR “saquinavir”[MeSH Terms]) OR “atazanavir sulfate”[MeSH Terms]) OR (“lopinavir”[MeSH Terms] OR “lopinavir”[All Fields])) OR (“ritonavir”[MeSH Terms] OR “ritonavir”[All Fields])) OR (“saquinavir”[MeSH Terms] OR “saquinavir”[All Fields])) OR (“atazanavir sulfate”[MeSH Terms] OR (“atazanavir”[All Fields] AND “sulfate”[All Fields]) OR “atazanavir sulfate”[All Fields] OR “atazanavir”[All Fields])) OR (“saquinavir”[MeSH Terms] OR “saquinavir”[All Fields] OR “saquinivir”[All Fields])) OR “Saquinavir Mesylate”[All Fields]) OR (“saquinavir”[MeSH Terms] OR “saquinavir”[All Fields] OR “invirase”[All Fields])) OR (“ritonavir”[MeSH Terms] OR “ritonavir”[All Fields] OR “Atazanavir Sulfate”[All Fields]) OR (“atazanavir sulfate”[MeSH Terms] OR (“atazanavir”[All Fields] AND “sulfate”[All Fields]) OR “atazanavir sulfate”[All Fields])) | 955 |
| PubMed | (((((((((((((((((((((((((((((((((((“QT Prolongation”[All Fields] OR “QT Prolonged”[All Fields]) OR “Prolong QT”[All Fields]) OR “QTc Prolongation”[All Fields]) OR “QTc Prolonged”[All Fields]) OR “Prolong QTc”[All Fields]) OR “QT Interval”[All Fields]) OR “QT Abnormality”[All Fields]) OR “Abnormal QT”[All Fields]) OR “QT Lengthening”[All Fields]) OR “Long QT”[All Fields]) OR “QTc Interval”[All Fields]) OR “QTc Abnormality”[All Fields]) OR “Abnormal QTc”[All Fields]) OR “QTc Lengthening”[All Fields]) OR “Long QTc”[All Fields]) OR “PR Interval”[All Fields]) OR “PR prolongation”[All Fields]) OR “Abnormal PR”[All Fields]) OR “PR lengthening”[All Fields]) OR “Long PR”[All Fields]) OR “QRS Wave”[All Fields]) OR “QRS complex”[All Fields]) OR “QRS widening”[All Fields]) OR “Wide QRS”[All Fields]) OR “QRS Abnormality”[All Fields]) OR “QRS Abnormalities”[All Fields]) OR “Abnormal QRS”[All Fields]) OR “Electrocardiogram Abnormality”[All Fields]) OR “Electrocardiogram Abnormalities”[All Fields]) OR “Abnormal Electrocardiogram”[All Fields]) OR “ECG abnormality”[All Fields]) OR “ECG abnormalities”[All Fields]) OR “Abnormal ECG”[All Fields]) OR “ECG Pattern”[All Fields]) OR “Long QT Syndrome”[MeSH Terms]) AND (((((((((((“lopinavir”[MeSH Terms] OR “ritonavir”[MeSH Terms]) OR “saquinavir”[MeSH Terms]) OR “atazanavir sulfate”[MeSH Terms]) OR (“lopinavir”[MeSH Terms] OR “lopinavir”[All Fields])) OR (“ritonavir”[MeSH Terms] OR “ritonavir”[All Fields])) OR (“saquinavir”[MeSH Terms] OR “saquinavir”[All Fields])) OR (“atazanavir sulfate”[MeSH Terms] OR (“atazanavir”[All Fields] AND “sulfate”[All Fields]) OR “atazanavir sulfate”[All Fields] OR “atazanavir”[All Fields])) OR (“saquinavir”[MeSH Terms] OR “saquinavir”[All Fields] OR “saquinivir”[All Fields])) OR “Saquinavir Mesylate”[All Fields]) OR (“saquinavir”[MeSH Terms] OR “saquinavir”[All Fields] OR “invirase”[All Fields])) OR (“ritonavir”[MeSH Terms] OR “ritonavir”[All Fields] OR “Atazanavir Sulfate”[All Fields]) OR (“atazanavir sulfate”[MeSH Terms] OR (“atazanavir”[All Fields] AND “sulfate”[All Fields]) OR “atazanavir sulfate”[All Fields])) | 38 |
| EBSCOhost | (TI “Long QT Syndrome” OR TI “QT Prolongation” OR TI “QT Prolonged” OR TI “Prolong QT” OR TI “QTc Prolongation” OR TI “QTc Prolonged” OR TI “Prolong QTc” OR TI “QT Interval” OR TI “QT Abnormality” OR TI “Abnormal QT” OR TI “QT Lengthening” OR TI “Long QT” OR TI “QTc Abnormality” OR TI “QTc Interval” OR TI “Abnormal QTc” OR TI “QTc Lengthening” OR TI “Long QTc” OR TI “PR Interval” OR TI “PR prolongation” OR TI “Abnormal PR” OR TI “PR lengthening” OR TI “Long PR” OR TI “QRS Wave” OR TI “QRS complex” OR TI “QRS widening” OR TI “Wide QRS” OR TI “QRS Abnormality” OR TI “QRS Abnormalities” OR TI “Abnormal QRS” OR TI “Electrocardiogram Abnormality” OR TI “Electrocardiogram Abnormalities” OR TI “Abnormal Electrocardiogram” OR TI “ECG abnormality” OR TI “ECG abnormalities” OR TI “Abnormal ECG” OR AB “Long QT Syndrome” OR AB “QTc Abnormality” OR AB “QTc Interval” OR AB “Abnormal QTc” OR AB “QTc Lengthening” OR AB “Long QTc” OR AB “PR Interval” OR AB “PR prolongation” OR AB “Abnormal PR” OR AB “PR lengthening” OR AB “Long PR” OR AB “QRS Wave” OR AB “QRS complex” OR AB “Long QT Syndrome” OR AB “QT Prolongation” OR AB “QT Prolonged” OR AB “Prolong QT” OR AB “QTc Prolongation” OR AB “QTc Prolonged” OR AB “Prolong QTc” OR AB “QT Interval” OR AB “QT Abnormality” OR AB “Abnormal QT” OR AB “QT Lengthening” OR AB “Long QT” OR AB “QRS widening” OR AB “Wide QRS” OR AB “QRS Abnormality” OR AB “QRS Abnormalities” OR AB “Abnormal QRS” OR AB “Electrocardiogram Abnormality” OR AB “Electrocardiogram Abnormalities” OR AB “Abnormal Electrocardiogram” OR AB “ECG abnormality” OR AB “ECG abnormalities” OR AB “Abnormal ECG”) AND (AB Lopinavir OR AB “Lopinavir/Ritonavir” OR AB Ritonavir OR AB Saquinavir OR AB “atazanavir sulfate” OR TI Lopinavir OR TI “Lopinavir/Ritonavir” OR TI Ritonavir OR TI Saquinavir OR TI “atazanavir sulfate”) | 31 |
| ProQuest | (ti(“Long QT Syndrome”) OR ti(“QT Prolongation”) OR ti(“QT Prolonged”) OR ti(“Prolong QT”) OR ti(“QTc Prolongation”) OR ti(“QTc Prolonged”) OR ti(“Prolong QTc”) OR ti(“QT Interval”) OR ti(“QT Abnormality”) OR ti(“Abnormal QT”) OR ti(“QT Lengthening”) OR ti(“Long QT”) OR ti(“QTc Abnormality”) OR ti(“QTc Interval”) OR ti(“Abnormal QTc”) OR ti(“QTc Lengthening”) OR ti(“Long QTc”) OR ti(“PR Interval”) OR ti(“PR prolongation”) OR ti(“Abnormal PR”) OR ti(“PR lengthening”) OR ti(“Long PR”) OR ti(“QRS Wave”) OR ti(“QRS complex”) OR ti(“QRS widening”) OR ti(“Wide QRS”) OR ti(“QRS Abnormality”) OR ti(“QRS Abnormalities”) OR ti(“Abnormal QRS”) OR ti(“Electrocardiogram Abnormality”) OR ti(“Electrocardiogram Abnormalities”) OR ti(“Abnormal Electrocardiogram”) OR ti(“ECG abnormality”) OR ti(“ECG abnormalities”) OR ti(“Abnormal ECG”) OR ab(“Long QT Syndrome”) OR ab(“QTc Abnormality”) OR ab(“QTc Interval”) OR ab(“Abnormal QTc”) OR ab(“QTc Lengthening”) OR ab(“Long QTc”) OR ab(“PR Interval”) OR ab(“PR prolongation”) OR ab(“Abnormal PR”) OR ab(“PR lengthening”) OR ab(“Long PR”) OR ab(“QRS Wave”) OR ab(“QRS complex”) OR ab(“Long QT Syndrome”) OR ab(“QT Prolongation”) OR ab(“QT Prolonged”) OR ab(“Prolong QT”) OR ab(“QTc Prolongation”) OR ab(“QTc Prolonged”) OR ab(“Prolong QTc”) OR ab(“QT Interval”) OR ab(“QT Abnormality”) OR ab(“Abnormal QT”) OR ab(“QT Lengthening”) OR ab(“Long QT”) OR ab(“QRS widening”) OR ab(“Wide QRS”) OR ab(“QRS Abnormality”) OR ab(“QRS Abnormalities”) OR ab(“Abnormal QRS”) OR ab(“Electrocardiogram Abnormality”) OR ab(“Electrocardiogram Abnormalities”) OR ab(“Abnormal Electrocardiogram”) OR ab(“ECG abnormality”) OR ab(“ECG abnormalities”) OR ab(“Abnormal ECG”)) AND (ab(Lopinavir) OR ab(Ritonavir) OR ab(Saquinavir) OR ab(“atazanavir sulfate”) OR ti(Lopinavir) OR ti(Ritonavir) OR ti(Saquinavir) OR ti(“atazanavir sulfate”)) | 18 |
Figure 1.
Flow diagram of the identification and selection of studies included in the analysis. ECG = electrocardiogram, PMC = PubMed Central.
This systematic review identifies 4 randomized trials and 5 quasi-experimental studies gathered from 5 countries consisting 541 participants (Table 2). We found 4 studies evaluating the risk of ECG abnormalities after first dose of antiviral therapy.[22–25] One study evaluate the risk of QRS widening and PR prolongation after 3 days treatment with LPV/r.[25] Meanwhile, another study evaluate the risk of QT prolongation after 7 days treatment with LPV/r.[26] The same study evaluate such risk after 10 days treatment with RTV.[26] One study evaluate the risk of PR prolongation and QRS widening after 12 to 20 days treatment with ATV, LPV, and RTV.[27] One study evaluate the risk of PR and QT prolongation after 3 days treatment with ritonavir-boosted SQV (SQV/r).[28] Another study evaluate the risk of PR and QT prolongation along with QRS widening after 14 days treatment with SQV/r.[29] We only found one study that report the risk of QT prolongation in COVID-19 patients. This study evaluate such risk after 14 days treatment with LPV/r.[30]
Table 2.
Characteristic of included studies.
| Author (year) | Study design | Country of study origin | Subjects | Antiviral agent of interest | Other treatment used | Baseline ages |
| Non-COVID19 participants | ||||||
| Baker et al (2006)[26] | Quasi-experimental | Virginia, United States | N (male vs female) = 26 vs 23Opioid dependence | LPV/r (400 mg/100 mg, twice daily for 7 days, n = 9)RTV (100 mg, twice daily for 10 days, n = 10) | All subjects received: buprenorphine/naloxone 16.3 ± 1.1 mgOther Group of study:Delavirdine (n = 10)Efavirenz (n = 10)Nelfinavir (n = 10) | 35.8 ± 7.9 |
| Busti et al (2006)[22] | Quasi-experimental | Texas, United States | N (all male) = 21HIV (+) patients | ATV (300 mg once daily for 1 month, n = 2)ATV/r (300 mg/100 mg once daily for 1 month, n = 19) | Other drug used (% Subjects):Lamivudin (33%)Stavudine (19%)Didanosine enteric release (9.5%)Abacavir (19%)Tenofovir (43%)Lamivudine/Zidovudine (19%)Lamvudine/Abacavir/Zidovudine (19%)Efavirenz (24%) | 48.7 ± 7.9 |
| Sarapa et al (2008)[23] | Randomized, crossed-over, placebo and positive-controlled trial | Texas, United States | N (male vs female) = 33 vs 32Healthy | Group 1 (n = 65) = RTV (100 mg, single dose) | Group 2 (n = 65) = Moxifloxacin (400 mg, single dose)Group 3 (n = 65) = Placebo | 32.7 (18–55) |
| Rathbun et al (2009)[27] | Quasi-experimental | Oklahoma, United States | N (male vs female) = 6 vs 2HIV (+) patients | Group of Arm A:Day 1–6 = ATV/r(300 mg/100 mg, daily)Day 7–16 = ATV (300 mg daily) and LPV/r(400 mg/100 mg, twice daily)Day 17–20 = ATV (300 mg daily) and LPV/r (800 mg/200 mg, daily)Group of Arm B:Day 1–6 = LPV/r (400 mg/100 mg, twice daily)Day 7–12 = LPV/r (400 mg/100 mg, twice daily) and ATV (300 mg, daily) | All subjects continued pre-existing NRTIs:Tenofovir/emtricitabine (n = 7)Zidovudine/Lamivudine (n = 2)Abacavir/Lamivudine (n = 1)Didanosine (n = 1)Tenofovir (n = 1)Other treatment used:TMP/SMX (n = 3)Ranitidine (n = 2)Triamcinolone nasal spray (n = 1)Mirtazepine (n = 1)Methylphenidate (n = 1)Fish oil (n = 3)Azithromycin (n = 1)Fluconazole (n = 1)Paroxetine (n = 1)Dapsone (n = 1)Lisinopril (n = 2)Enalapril (n = 1)Hydrochlorothiazide (n = 1)Atorvastatin (n = 2)Alprazolam (n = 1)Gemfibrozil (n = 1)Trazodone (n = 1) | 45.2 (37–52) |
| Byakika-Kibwika et al (2011)[24] | Quasi-experimental | Kampala, Uganda | N (male vs female) = 31 vs 41HIV (+) patients | LPV/r (400 mg/100 mg, for 1 month) | All subjects received:Artemeter-lumefantrine (80 mg/480 mg, single dose)TMP/SMX | LPV/r Arm = 38 (33–41)ART naïve arm = 34 (28–39) |
| Zhang et al (2012)[28] | Randomized, double-blind, crossed-over, placebo and positive-controlled trial | Strasbourg, France | N (male vs female) = 35 vs 21Healthy | Group A:SQV/r 1000 mg/100 mg, twice daily for 3 days)Group BSQV/r (1500 mg/100 mg, twice daily for 3 days) | Group CMoxifloxacin 400 mg on day 3 onlyGroup DPlacebo | 34 (18–54) |
| Boffito et al (2015)[29] | Quasi-experimental | London, United Kingdom | N (all male) = 21HIV (+) patients | Day 1–7 = SQV/r (500 mg/100 mg, twice daily)Day 8–14 = SQV/r (1000 mg/100 mg, twice daily) | All subjects received: Tenofovir/Emtricitabine | 33 ± 9 |
| Vicente et al (2019)[25] | Randomized, double-blind, placebo-controlled trial | Wisconsin, United States | N (male vs female) = 38 vs 22Healthy | LPV/r (800 mg/200 mg, twice daily, for 3 days) (n = 50) | Ranolazine 1500 mg (twice daily, for 3 days) (n = 10)Verapamil 480 mg (day 1, day 2) + 120 mg (Day 3) (n = 10)Chloroquine 1000 mg (day 1) + 1500 mg (day 2) + 1000 mg (day 3) (n = 50) | 31.7 ± 8.7 |
| COVID-19 participants | ||||||
| Cao et al (2020)[30] | Randomized, controlled trial | Hubei, China | Study Group:N (male vs female) = 61 vs 38Control Group: N (male vs female) = 59 vs 41COVID-19 | LPV/r (400 mg/100 mg, twice daily, for 14 days) | Study group received (as needed):Supplemental oxygen (n = 72)Noninvasive and invasive ventilation (n = 10)Antibiotic agents (n = 94)Vasopressor (n = 17)Renal replacement therapy (n = 3)Extracorporeal membrane oxygenation (n = 2)Control group received (as needed):Supplemental oxygen (n = 67)Noninvasive and invasive Ventilation (n = 19)Antibiotic agents (n = 95)Vasopressor (n = 27)Renal replacement therapy (n = 6)Extracorporeal membrane oxygenation (n = 2) | Study vs Control = 58.0 (50.0–68.0) vs 58.0 (48.0–68.0) |
All studies in this systematic review also included other treatments beside antiviral agents of interest. However, care in COVID-19 patients using hydroxycholoroquine and azithromycin was not seen in all studies found for evaluation in this qualitative synthesis. The use of chloroquine was only mentioned by one study.[25] This study evaluated ECG changes in healthy patients after administration of LPV/r combined with ranolazine, verapamil, and chloroquine.[25] Other study by Baker et al[26] was done in opioid dependent patients receiving brupenorphine/naloxone to investigate the effect of QT interval prolongation upon combination with one of antiretroviral agents (efavirenz, nelfinavir, delavirdine, RTV, LPV/r). Monotherapy using brupenorphine/naloxone did not prolong QT interval. Study by Busti et al[22] in human immunodeficiency virus (HIV)(+) patients combined ATV or ritonavir-boosted atazanavir (ATV/r) with other antiretroviral agents as part of their treatment regimen including lamivudine in 7 patients, stavudine in 4 patients, didanosine enteric release in 2 patient, abacavir in 4 patients, tenofovir in 9 patients, lamivudine/zidovudine in 4 patients, lamivudine/abacavir/zidovudine in 4 patients, and efavirenz in 5 patients. Study by Rathbun et al[27] in HIV(+) patients combined ATV, ATV/r, or LPV/r with pre-existing nucleoside/nucleotide reverse transcriptase inhibitors regimen including tenofovir/emtricitabine in 7 patients, zidovudine/lamivudine in 2 patients, abacavir/lamivudine in 1 patient, didanosine in 1 patient, and other treatments outside the regimen were also used. All HIV(+) patients in study by Byakika-Kibwika et al[24] received artemeter-lumefantrine to investigate the effect of QT interval prolongation upon combination with LPV/r. All HIV(+) patients in study by Boffito et al[29] received tenofovir/emtricitabine as part of their treatment regimen. Study by Cao et al[30] in COVID-19 patients combined LPV/r with standard care including vasopressors, renal replacement therapy, noninvasive mechanical ventilation, extracorporeal membrane oxygenation, antibiotic agent, and glucocorticoid therapy as needed. Study by Zhang et al[28] and Sarapa et al[23] in healthy patients did not combined another treatment while administering the antiviral agents. In addition, these studies used moxifloxacin on the comparator group.
We found 4 studies that report PR prolongation.[22,24,27,28] Of these, only 2 studies had PR interval >200 ms.[22,28] One of which reported after third day use of SQV/r in healthy patients while another was evident in HIV(+) patients during treatment on day 16 and 20 using ATV/r.[22,28] No study reported QRS widening >120 ms with treatment. Four studies reporting QT prolongation.[22,24,28,30] Of these, only 2 studies with QT interval >450 ms.[28,30] One of which reported after the third day use of SQV/r in healthy patients, while another was evident in 1 out of 95 COVID-19 patients after 14 days treated with LPV/r.[28,30]
Clinical events related to ECG abnormalities such as arrhythmias and syncopes were reported by 3 studies.[27,28,30] One study in HIV(+) patients reported left bundle branch block after 10 days of ATV (300 mg daily) and LPV/r (400 mg/100 mg twice daily) coadministration in 1 male patient.[27] ECG abnormality remain persisted for 1 month despite antiretroviral discontinuation.[27] Meanwhile, another male patient was reported to have first degree atrioventricular block after 6 days of concurrent ATV and LPV/r using the same dose.[27] After 1 week of ATV discontinuation, patient's ECG reverted to normal sinus rhythm.[27] Another study also reported that there were 23 events of syncope or presyncope recorded from 16 healthy participants administered with SQV/r.[28] It was then estimated that 17 out of 23 events occurred while receiving SQV/r regimens.[28] Furthermore, based on a study in COVID-19 patients, there was one case of unconsciousness recorded in LPV/r group.[30] However, there was not enough evidence reported to suggest that this event was induced by antiviral agents.[30]
The complete summary of every study reporting the effect of LPV, RTV, ATV, and SQV in changing ECG will be reviewed below.
3.1. Effect of antiviral agents of interest on PR interval
We obtained 6 studies which consist of 228 participants that reported about the risk of antivirals in changing PR interval (Table 3).[22,24,25,27–29] Based on small study done in HIV patients, it was reported that a first dose treatment using ATV/r (300 mg/100 mg) did not prolong PR interval after 2 hours. However, after 1 month observation, PR interval changes were statistically significant.[22] Upper value of 1 standard deviation also showed that PR interval exceeded 200 ms.[22] One study in HIV patients treated with nucleoside reverse transcriptase inhibitors (NRTI), reported that combination of daily ATV 300 mg, and twice daily LPV/r (400–800 mg/100–200 mg) for 12 to 20 days prolonged PR interval significantly.[27] In 30 days follow-up, the interval returns similarly to baseline.[27] Meanwhile, in population receiving the first dose of artemeter-lumefantrine with LPV/r 400 mg/100 mg could shortened PR interval statistically compared with population not receiving any ART.[24] In another study of HIV patients receiving SQV/r (1000 mg/100 mg) twice daily, PR interval could be prolonged 25 ms after 4 hours on the third day of treatment and it was estimated that 40% of the population have PR interval >200 ms.[28] Increasing the dose of SQV to 1500 mg while still maintaining the same dose of RTV could further prolong 34 ms in PR interval after 5 hours on the third day of treatment. In this study, population with PR interval >200 ms was observed in 40% of participants in the 1000/100 mg group and 47% of participants in the 1500/100 mg group.[28] However, the author concluded only 2 events of abnormal ECG (PR > 200 ms) that possibly associated with SQV/r treatment after further assessment.[28] In other HIV population receiving emtricitabine and tenofovir, additional twice daily treatment using SQV/r (500 mg/100 mg) for the first week and continued with twice daily regimen of SQV/r (1000 mg/100 mg) during second week did not prolong PR interval >200 ms, even during the tenth day when the maximum changes happened.[29] Another study in healthy patients reported that treatment of twice daily using LPV/r 800 mg/200 mg on day 1 could prolong the PR interval for 14.8 ms (90% confidence interval: 10.3–19.4) and as for day 3, the prolongation was 33.5 ms (90% confidence interval: 22.7–44.4).[25]
Table 3.
Clinical events of ECG findings from included studies.
| PR interval | QRS Wave | QT interval | ||||||||||
| Author | Antiviral | Before treatment | During treatment | After treatment | Before treatment | During treatment | After treatment | Before treatment | During treatment | After treatment | Clinical events related to ECG abnormalities induced by antiviral agents | Incidence for ECG abnormality |
| Baker et al (2006)[26] | LPV/r (400 mg/100 mg, twice daily for 7 days, n = 9) | N.R | N.R | N.R | N.R | N.R | N.R | Baseline QTcB for LPV/r = 407 (SE: 5.8)Baseline QTcB for RTV = 409 (SE: 5.8)After receiveing Brupenorphine/Naloxone for 2 weeks = 410 ± 2.6 | N.R | QTcB 2 hour post-dose on day 7 for LPV/r = 413 (SE: 6.1)Mean change from baseline = 6.14 (95% CI: –5.97–18.25), P > .05 | N.R | 0% |
| RTV (100 mg, twice daily for 10 days, n = 10) | QTcB 2 hour post-dose on day 10 for RTV = 419 (SE: 5.9)Mean change from baseline = 9.38 (95% CI: –2.36–21.12), P > .05 | |||||||||||
| Busti et al (2006)[22] | ATV (300 mg once daily for 1 month, n = 2) or ATV/r (300 mg/100 mg once daily for 1 month, n = 19) | 176 ± 30 | 2 hours after first dose (±SD) = 173 ± 33, P = .11 | After 1 month (± SD) = 184 ± 34, P = .005 | 87 ± 6.5 | 2 hours after first dose (± SD) = 90 ± 9.4, P = .005 | After 1 month (± SD) = 91 ± 7.2, P = .001 | QT interval (± SD) = 388 ± 28.4QTcB (± SD) = 404.6 ± 16.4QTd (± SD) = 28.5 ± 13 | QT interval:2 hour after first dose (±SD) = 372 ± 25.8, P = .002QTcB interval:2 hours after first dose (± SD) = 407.8 ± 15.2, P = .084QTd Interval:2 hours after first dose (n = 16, ± SD) = 23.4 ± 14, P = .197 | QT interval:After 1 month (± SD) = 386 ± 28.6, P = .74QTcB interval:After 1 month (± SD) = 403 ± 14.5, P = .434QTd Interval:After 1 month (± SD) = 26.3 ± 12.6, P = .865 | N.R | PR interval > 200 ms after 1 month of ATV/r = 16% (approximately based on upper value of one standard deviation) |
| Sarapa et al (2008)[23] | RTV (100 mg, single dose) | N.R | N.R | N.R | N.R | N.R | N.R | QTcF = 373.3–449.7QTcB = 371.7–458.0 | N.A | 6 hours after first dose:QTcF change = 0.16 (90% CI: –1.38–1.69), P > .05QTcB change = 0.73 (90% CI: –1.29–2.75), P > .0512 hours after first dose:QTcF change = –1.04 (90%CI: –2.98–0.90), P > .05QTcB change = –0.44 (90%CI: –2.76–1.87), P > .05 | N.R | 0% |
| Rathbun et al (2009)[27] | Group of Arm A:Day 1–6 = ATV/r (300 mg/100 mg, daily)Day 7–16 = ATV (300 mg daily) and LPV/r(400 mg/100 mg twice daily)Day 17–20 = ATV (300 mg daily) and LPV/r (800 mg/200 mg daily)Group of Arm B:Day 1–6 = LPV/r (400 mg/100 mg twice daily)Day 7–12 = LPV/r (400 mg/100 mg twice daily) and ATV (300 mg daily) | 145 ± 14 | Combined results of both group:Day 16, Arm A + Day 12, Arm B (± SD) = 159 ± 21, P < .05Day 20, Arm A + Day 12, Arm B (± SD) = 162 ± 24, P < .05 | Combined results of both group:30 days follow up (± SD) = 144 ± 13, P > .05 | 92 ± 10 | Combined results of both group:Day 12, Arm B + Day 16, Arm A (±SD) = 97 ± 10, P < .01Day 12, Arm B + Day 20, Arm A (± SD) = 97 ± 12, P < .01 | Combined results of both group:30 days follow up (± SD) = 91 ± 8, P > .05 | N.R | N.R | N.R | Arm A:Left bundle branch block after 10 days ATV and LPV/r coadministration (32-year-old man)Arm B:First degree atrioventricular block after 6 days ATV and LPV/r coadministration (40-year-old man) | Left bundle branch block after 10 days of ATV and LPV/r coadministration = 1 out of 8 patients (12.5%)First degree atrioventricular block after 6 days of ATV and LPV/r coadministration = 1 out of 8 patients (12.5%) |
| Byakika-Kibwika et al (2011)[24] | LPV/r (400 mg/100 mg, for 1 month) | N.R | Mean PR interval after first dose of artemeter-lumefantrine + LPV/r:LPV/r arm vs ART naïve arm (± SD) = 154 ± 18.4 vs 169 ± 15.9, P = .02 | N.R | N.R | Mean QRS wide after first dose of artemeter-lumefantrine + LPV/r:LPV/r arm vs ART naïve arm (±SD) = 87.4 ± 6.6 vs 82.8 ± 6.6, P = .06 | N.R | QTcB median:LPV/r arm vs ART naïve arm (IQR) = 415 (403–439) vs 395 (388–425) | QTcB median after first dose of artemeter-–lumefantrine dosing + LPV/r:LPV/r arm vs ART naive arm (IQR) = After 12h: 415 (404–439) vs 419 (403–427), P = .7 | QTcB median after first dose of Artemeter-Lumefantrine + LPV/r:LPV/r arm vs ART naive arm (IQR) = After 24h: 424 (401–434) vs 406 (393–411), P = .02After 48h: 411 (396–432) vs 409 (401–419), P = .7After 72h: 424 (416–441) vs 408 (392–417), P = .04 | N.R | 0% |
| Zhang et al (2012)[28] | Group A:SQV/r (1000 mg/100 mg, twice daily, for 3 days) | N.R | N.R | Mean maximum prolongation at 4-h post dose on day 3 = 25 msPR interval > 200, % Subjects in 3 days = 40% | N.R | N.R | N.R | QTc < 450 (female)QTc < 430 (male) | N.R | QTcS 12-h post dose on day 3, mean maximum increase = 18.9 (Upper 95% CI = 22.0)QTcS interval > 450–480, % Subjects in 3 days = 11% | 16 participants reported 23 events of syncopeor presyncope with 74% events (17 out of 23) were reported while receiving SQV/r regimens. | PR interval > 200 ms in 3 days after SQV/r (1000 mg/100 mg, twice daily) = 40%QTcS interval > 450–480 ms in 3 days after SQV/r (1000 mg/100 mg, twice daily) = 11% |
| Group BSQV/r (1500 mg/100 mg, twice daily, for 3 days) | Mean maximum prolongation at 5-h post dose on day 3 = 34 msPR interval >200, % Subjects in 3 days = 47% | QTcS 20-h post dose on day 3, mean maximum increase = 30.2 (Upper 95% CI = 33.4)QTcS interval >450–480, % Subjects in 3 days = 18%QTcS interval > 480 – 550, % Subjects in 3 days = 2% | PR interval > 200 ms in 3 days after SQV/r (1500 mg/100 mg, twice daily, for 3 days) = 47%QTcS interval > 450–480 ms in 3 days after SQV/r (1500 mg/100 mg, twice daily) = 18%QTcS interval > 480–550 ms in 3 days after SQV/r (1500 mg/100 mg, twice daily) = 2% | |||||||||
| Boffito et al (2015)[29] | Day 1–7 = SQV/r (500 mg/100 mg, twice daily)Day 8–14 = SQV/r (1000 mg/100 mg, twice daily) | N.R | Change of PR interval at every 4 hours post-dose:Day 3 (± SD) = 5 ± 9Day 4 (± SD) = 2 ± 9Day 7 (± SD) = 5 ± 6Day 10 (± SD) = 8 ± 8 | Change of PR interval at 4 hours hour post-dose on day 14 (95% CI) = 9 ± 7 | N.R | Change of QRS wave at 0 hour hour post-dose:Day 3 (± SD) = 1 ± 3Day 4 (± SD) = 1 ± 3Day 7 (± SD) = 2 ± 4Day 10 (± SD) = 3 ± 3 | Change of QRS wave at 0 hour hour post-dose on day 14 (± SD) = 2 ± 4 | N.R | Change of QTcF interval at 2 hours post-dose:Day 3 (± SD): 3 ± 7Day 4 (± SD): 1 ± 9Change of QTcF interval at 6 hours post-dose:Day 7 (±SD): 7 ± 7Day 10 (±SD): 12 ± 12 | Change of QTcF interval at 6 hours post-dose on day 14 (±SD) = 7 ± 8 | N.R | 0% |
| Vicente et al (2019)[25] | LPV/r (800 mg/200 mg, twice daily, for 3 days) (n = 50) | 162.3 ± 16.5 | Change of PR interval after first dose on day 1 = 14.8 [90% CI: 10.3–19.4] | Change of PR interval after first dose on day 3 = 33.5 [90% CI: 22.7–44.4] | 87.1 ± 5.8 | Change of QRS wave after first dose on day 1 = –0.2 [90% CI: –3.5–3.1] | Change of QRS wave after first dose on day 3 = 4.0 [90% CI: –0.4–8.5] | QTcF = 383.9 ± 17.1 | N.R | N.R | N.R | 0% |
| Cao et al (2020)[30] | LPV/r (400 mg/100 mg, twice daily, for 14 days) | N.R | N.R | N.R | N.R | N.R | N.R | N.R | Day 14 = 1.1% (1 out of 95 patients) of LPV/r treatment group has QT Prolongation | N.R | Unconsciousness (1.1%) | QT prolongation in LPV/r treatment group = 1 out of 95 patients (1.1%) |
3.2. Effect of antiviral agents of interest on QRS wave
We obtained 5 studies which consist of 172 participants that reported about the risk of antivirals in changing QRS wave length (Table 3).[22,24,25,27,29] One study in HIV patients already treated with antiviral drugs reported treatment using ATV/r (300 mg/100 mg) could prolong QRS wave length in 2 hours after first dose and persisted after 1 month.[22] Another study in HIV patients treated with NRTI, reported that combination of daily ATV 300 mg, and twice daily LPV/r (400–800 mg/100–200 mg) for 12 to 20 days prolonged QRS wave statistically significant.[27] In 30 days follow-up, the interval return similarly to baseline.[27] Meanwhile, in population receiving the first dose of artemeter-lumefantrine with LPV/r (400 mg/100 mg) had no effect on QRS wave.[24] In other HIV population receiving emtricitabine and tenofovir, additional twice daily treatment using SQV/r (500 mg/100 mg) for the first week and continued with twice daily regimen of SQV/r (1000 mg/100 mg) during second week did not prolong QRS wave >120 ms even during the tenth day when the maximum changes happened.[29] Another study in healthy patients reported that treatment of twice daily using LPV/r (800 mg/200 mg) on day 1 and day 3 had no effect on QRS wave.[25]
3.3. Effect of antiviral agents of interest on QT interval
We obtained 7 studies which consist of 483 participants that reported about the risk of antivirals in changing QT interval (Table 3).[22–24,26,28–30] It was reported from a study done in patients with opioid dependence treated using brupenorphine/naloxone, addition of either twice daily regimen consisting LPV/r (400 mg/100 mg) for 7 days or treatment with RTV 100 mg for 10 days did not change QT interval significantly.[26] Another study in HIV patients already treated with antiviral drugs, found that treatment using ATV/r (300 mg/100 mg) was not associated with QT prolongation in 2 hours after first dose.[22] QT interval was similar throughout medication for 1 month.[22] Based on a study done in healthy patients, treatment using RTV 100 mg did not change QT interval significantly 6 to 12 hours after the first dose.[23] Meanwhile, in population receiving the first dose of artemeter-lumefantrine with LPV/r (400 mg/100 mg) revealed a QT interval prolongation with a statistical significance after 24 and 72 hours, compared with population not receiving any ART.[24] In another study of HIV patients receiving SQV/r (1000 mg/100 mg) twice daily could prolong 18.9 ms in QT interval after 12 hours on the third day of treatment and it was estimated that 11% population have QT interval of 450 to 480 ms.[28] Increasing the dose of SQV to 1500 mg while still maintaining the same dose of RTV could further prolong 30.2 ms in QT interval after 20 hours on the third day of treatment. In this group population that have QT interval of 450 to 480 ms was estimated up to 18%. Population with QT interval of 480 to 550 ms was 2%.[28] In this study, the authors suggested that both SQV and RTV dosing regimens are potentially proarrhythmic because these drugs prolong QT/QTc by >20 ms.
In other HIV population receiving emtricitabine and tenofovir, additional twice daily treatment using SQV/r (500 mg/100 mg) for the first week and continued with twice daily regimen of SQV/r (1000 mg/100 mg) during second week did not prolong QT interval >500 ms, even during the tenth day when the maximum changes happened.[29] This study only reported one individual who had an absolute QT interval of 454 ms on day 10 but eventually declined to 428 ms 2 hours after dosing.[29] We found only 1 study done in COVID-19 patients receiving twice daily of LPV/r (400 mg/100 mg) for 14 days in. This study revealed 1 out of 95 patients (1.1%) who was treated with such regimen had QT prolongation.[30]
3.4. Risk of bias for randomized trials
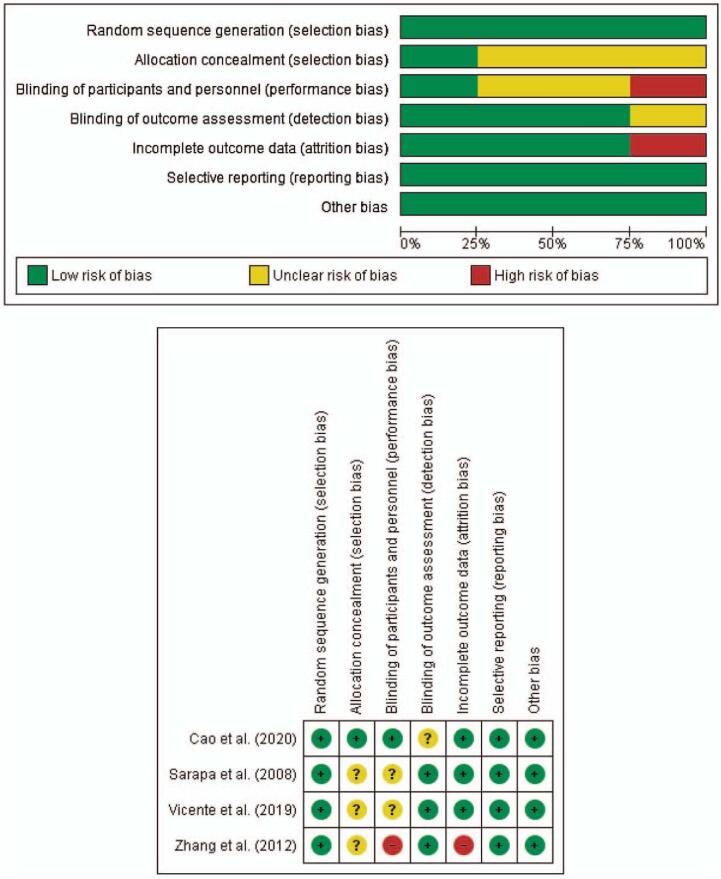
Figure 2 summarized the risk of bias according to authors’ judgments. All 4 randomized trials have a low risk of bias related to random sequence generation and reporting bias.[23,25,28,30] There are some concerns regarding the risk of bias for allocation concealment from 3 studies.[23,25,28] Related to performance bias, there are 2 studies[23,25] with some concerns and 1 study with high risk of bias.[28] There is some concern from 1 study[30] related to detection bias and high risk of bias from another study[28] related to attrition bias. There is no other bias identified throughout studies.
Figure 2.
A (Upper). Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies. Red = high risk of bias; Yellow = unclear risk of bias; Green = low risk of bias; B (Bottom). Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. Red (−) = high risk of bias; Yellow (?) = unclear risk of bias; Green (+) = low risk of bias.
3.4.1. Selection bias
Regarding random sequence generation, all studies were randomly assigned to each treatment planned. There was no information regarding whether the allocation was concealed but baseline imbalance suggested no problem based on 3 studies.[23,25,28]
3.4.2. Performance bias
There is low risk of performance bias in a study by Cao et al.[30] Although there was no blinding of participants and personnel in this study, there was no deviation from the intended intervention which arose because of trial context.[30] There were nearly 14% of LPV/r recipients who were unable to complete the full 14-day course of treatment, but this was primarily due to gastrointestinal adverse events caused by the drug.[30] The primary efficacy analysis was on an intention-to-treat basis and included all the patients who had undergone randomization.[30] A study by Sarapa et al[23] have some concerns regarding this bias because no blinding was done during the study and there was also no information on whether there were deviations from intended intervention because of the trial contex.[23] There is no information regarding the analysis used to estimate the effect of assignment to intervention, but there was not any report about participants excluded from the analysis and thus there would not be any substantial impact to analyze participants in the group to which they were randomized.[23] There was also some concern from a study by Vicente et al.[25] Despite being a double blind study, however there was no information regarding the analysis used to estimate the effect of assignment to intervention.[25] There was only 1 participant excluded from the analysis and thus there would not be any substantial impact to analyze participants in the group to which they were randomized.[25] Meanwhile, there is a high risk of performance bias from a study by Zhang et al.[28] This is also a double blind study. However, there was also no information regarding the analysis used to estimate the effect of assignment to intervention and there was >5% participants, excluded from the analysis and thus there might be a substantial impact to analyze participants in the group to which they were randomized.[28]
3.4.3. Detection bias
There is some concern related to the risk of detection bias from a study by Cao et al.[30] There is no information regarding whether the trained nurses in the study, being the assessors, have the knowledge of intervention.[30] The 7 category ordinal scale used to evaluate the outcome was subjective and further knowledge of intervention may result in misclassification, although such knowledge would not likely raise a strong belief that the intervention could either benefit or harm the patient.[30,31] The other 3 studies suggest a low risk of detection bias.[23,25,28] In the study by Sarapa et al,[23] the risk of bias was judged to be low as the authors have the knowledge of not measuring QT interval according to E14 guidance from the International Conference on Harmonization.[23,32] However, the authors had argued that instead of using third-party central ECG laboratories utilizing human observers, the 12 SL algorithm had a credibility for the measurement and that they carefully standardized experimental conditions and serial triplicate ECG recordings in healthy subjects to decrease the biological and measurement variability.[23] Detection bias was low in a study by Vicente et al[25] because the authors used 2 independent ECG readers blinded to treatment and time. They adjusted the measurements of ECG using high-resolution images using previously developed software.[25] Study by Zhang et al,[28] also had a low risk of detection bias because authors analyzed the continuous digital Holter readings by using a central laboratory.
3.4.4. Attrition bias
A study by Zhang et al[28] have a high risk of attrition bias. Data from participants excluded from the study were not provided and caused the extent of missing data to be >5%.[28] There was no analysis method to correct such bias and 7 participants were reported to have withdrawn consent or failed to cooperate but without further explaination.[28] The outcome for participants was nearly all available throughout the other 3 studies.[23,25,30]
3.4.5. Reporting bias
All randomized trials have a low risk of reporting bias because trials were analyzed in accordance with a prespecified plan.[23,25,28,30]
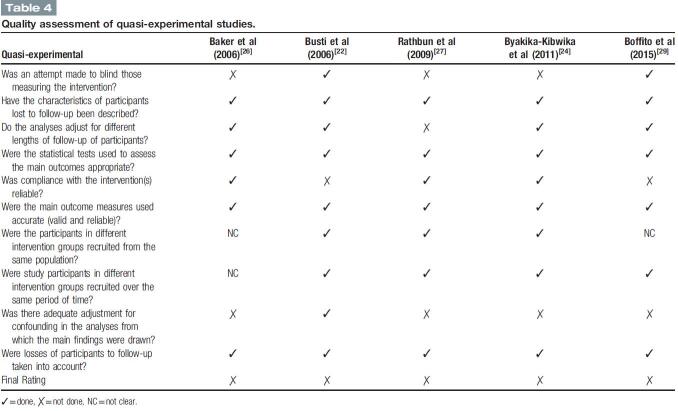
3.5. Risk of bias for quasi-experimental studies
All 5 quasi-experimental studies have a positive rating (Table 4). Only 2 studies made attempt to blind those measuring the intervention.[22,29] All 5 studies either have any or small losses to follow-up or have described the characteristic.[22,24,26,27,29] Only 1 study had a different follow-up length and did not make any adjustment accordingly.[27] All 5 studies have conducted appropriate statistical test to assess the outcomes.[22,24,26,27,29] There were 2 studies which have non-compliance participants with the treatment.[22,29] All 5 studies have clearly described outcome measures.[22,24,26,27,29] Two studies did not mentioned clearly whether the participants recruited from the same population.[26,29] One study did not mentioned clearly the period of time when the participants were recruited.[29] There was only one study that made adjustment for confounding in the study.[22] All 5 studies have a small proportion of loss to follow-up and therefore nearly all of the participants were taken into account throughout the studies.[22,24,26,27,29]
Table 4.
Quality assessment of quasi-experimental studies.
4. Discussion
We found a total of 9 studies related to the treatment using LPV, RTV, ATV, and SQV on mixed population including healthy participants, opioid dependence, HIV-seropositive, and COVID-19. Administration of these antivirals were short-term ranging from just a single dose and up to 1 month. These antivirals have been used commonly in HIV-seropositive patients with other antivirals combination.[33,34] LPV, ATV, and RTV are protease inhibitor (PI) commonly used for second-line treatment in HIV patients in combination with 2 NRTIs, at least one of which is new to the patient.[34] The standard adult dose of LPV/r is 400 mg/100 mg twice daily while a dose of 800 mg/200 mg once daily is indicated for therapy-naïve patients.[34,35] Standard dose of ATV/r is 300 mg/100 mg once daily.[34,36] SQV, which was the first Food and Drug Administration (FDA)-approved HIV PI, had a standard regimen of twice daily 1000 mg in combination with RTV 100 mg and currently this regimen was not preferred due to SQV slow bioavailability.[37,38]
Currently, 2 studies report PR interval >200 ms after PI administration.[22,28] One study support the notion of PR prolongation >200 ms due to SQV/r treatment.[28] The author concluded only 2 events of abnormal ECG (PR >200 ms) that possibly associated with SQV/r treatment after further assessment.[28] However, this study was done in healthy population and we did not find any study evaluating COVID-19 patients yet. In another study, upper value of one standard deviation for PR interval prolongation, after 1 month treatment with ATV/r exceeded 200 ms.[22] Meanwhile, a study using LPV/r in severe COVID-19 patients did not report PR prolongation in their observation.[30] The increase risk of PR interval abnormality as seen in healthy patients may suggest further trials to be done on COVID-19 patients.
Studies included in our reviews reported no QRS wave reaching >120 ms after PI administration.[22,24,25,27,29] QRS widening were evident during administration of ATV/r with or without LPV revealing the change after 12 to 20 days or during first-dose administration, respectively.[22,27] However, these changes did not reach >120 ms. We found no study that evaluate this risk in COVID-19 patients.
Currently only 1 study has been done in healthy patients suggesting the incidence of QT prolongation after SQV/r treatment.[28] In case of COVID-19, there is also only 1 study reporting the incidence of QT prolongation (without precise data on QT interval) treated with LPV/r.[30] Zhang et al,[28] reported a dosed dependent QT prolongation, estimating 11% incidence of QT prolongation ranged around 450 to 480 ms due to SQV/r standard dose treatment for 3 days. A 500 mg dose increment of SQV will add another 7% incidence and revealed another 2% incidence for QT prolongation of >480 ms. In COVID-19 patients we only found 1 study revealing only 1 out of 95 patients had the incident of QT prolongation after 14 days treatment with LPV/r. Other group consisting of 99 patients treated with standard care, did not report any incident of QT prolongation at all. However, the study by Cao et al[30] did not report detailed data about duration of prolongation, methods of ECG monitoring and assessment. We presumed that this factor may take into account when there is an intention to evaluate the incidence of ECG abnormalities thoroughly. This suggestion was based on the evidence that there seems to be a prolongation in QT interval for a short period of time after hours following administration of the first dose of combined QT prolonging drug with LPV/r.[24] Thus, future studies should be aware that ECG monitoring and assessment is crucial in COVID-19 patients especially after administering the first dose of combined QT prolonging drug with PI because concerns regarding QT prolongation may arise in the first few days.[24]
To our knowledge, this is the first systematic review of randomized-controlled trials and quasi-experimental studies evaluating the risk of PR prolongation, QRS widening, and QT prolongation from treatment using LPV, RTV, ATV, and SQV as these drugs have been suggested and reportedly used for treatment in COVID-19 patients. Small studies and limited number of trials limit a strong evidence suggesting the risk of these ECG abnormalities. The external evidence we used to compare the study from COVID-19 patients was limited and the study evaluating the risk of ECG abnormalities in COVID-19 population after treatment with LPV, RTV, ATV, and SQV was also limited. Based on the evidence we collected, future trials with larger participants and close ECG monitoring and assessment are necessary. Until then, baseline ECG should be recorded before treatment initiation using antiviral medications. Regular ECG re-evaluation during therapy is recommended.
5. Conclusion
Lopinavir, ritonavir, atazanavir, and saquinavir could cause PR prolongation, QRS widening, and QT prolongation. However, these findings were limited by small studies and limited number of trials. More future trials need to be done with closer monitoring and assessment of ECG to assure usage safety of antivirals in COVID-19 era.
Author contributions
Conceptualization: Denio A. Ridjab, Ignatius Ivan, Fanny Budiman, Dwi Jani Juliawati.
Data curation: Denio A. Ridjab, Ignatius Ivan, Fanny Budiman.
Formal analysis: Denio A. Ridjab, Ignatius Ivan, Fanny Budiman.
Funding acquisition: Denio A. Ridjab, Dwi Jani Juliawati.
Investigation: Denio A. Ridjab, Ignatius Ivan, Fanny Budiman, Dwi Jani Juliawati.
Methodology: Denio A. Ridjab, Ignatius Ivan, Fanny Budiman.
Project administration: Denio A. Ridjab.
Resources: Denio A. Ridjab, Ignatius Ivan.
Software: Denio A. Ridjab, Ignatius Ivan.
Supervision: Denio A. Ridjab, Dwi Jani Juliawati.
Validation: Denio A. Ridjab, Ignatius Ivan.
Visualization: Denio A. Ridjab, Ignatius Ivan, Fanny Budiman.
Writing – original draft: Denio A. Ridjab, Ignatius Ivan, Fanny Budiman.
Writing – review & editing: Denio A. Ridjab, Ignatius Ivan, Fanny Budiman, Dwi Jani Juliawati.
Footnotes
Abbreviations: ATV = atazanavir, ATV/r = ritonavir-boosted atazanavir, COVID-19 = coronavirus disease 2019, ECG = electrocardiography, HIV = human immunodeficiency virus, LPV = lopinavir, LPV/r = ritonavir-boosted lopinavir, RTV = ritonavir, SQV = saquinavir, SQV/r = ritonavir-boosted saquinavir.
How to cite this article: Ridjab DA, Ivan I, Budiman F, Juliawati DJ. Current evidence for the risk of PR prolongation, QRS widening, QT prolongation, from lopinavir, ritonavir, atazanavir, and saquinavir: a systematic review. Medicine. 2021;100:31(e26787).
Ethics Approval: For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
ATV = atazanavir; ATV/r = ritonavir-boosted atazanavir; CI = confidence interval; IQR = interquartile range; LPV = lopinavir; LPV/r = ritonavir-boosted lopinavir; N.R = not reported; p = P value; QTc = corrected QT interval; QTcB = corrected QT interval using Bazett formula (QT/RR0.5); QTcF = corrected QT interval using Fridericia formula (QT/RR0.33); RTV = ritonavir; SE = standard of error; SQV = saquinavir; SQV/r = ritonavir-boosted saquinavir; TMP/SMX = trimethoprim/sulfamethoxazole.
ATV = atazanavir; ATV/r = ritonavir-boosted atazanavir, CI = confidence interval; LPV = lopinavir; LPV/r = ritonavir-boosted lopinavir; N.A = not applicable; N.R = not reported; P = P value; QTc = corrected QT interval; QTcB = corrected QT interval using Bazett formula (QT/RR0.5); QTcF = corrected QT interval using Fridericia formula (QT/RR0.33); QTcS = corrected QT interval using study population-specific correction method (male, QT/RR0.319; female, QT/RR0.337); RTV = ritonavir; SD = standard deviation; SE = standard of error; SQV = saquinavir; SQV/r = ritonavir-boosted saquinavir.
References
- [1].Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol 2020;75:2352–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Asensio E, Acunzo R, Uribe W, Saad EB, Sáenz LC. Recommendations for the measurement of the QT interval during the use of drugs for COVID-19 infection treatment. Updatable in accordance with the availability of new evidence. J Interv Card Electrophysiol 2020;59:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Şimşek Yavuz S, Ünal S. Antiviral treatment of COVID-19. Turk J Med Sci 2020;50(SI-1):611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yousefifard M, Zali A, Ali KM, et al. Antiviral therapy in management of COVID-19: a system- atic review on current evidence. Arch Acad Emerg Med 2020;8:01–9. [PMC free article] [PubMed] [Google Scholar]
- [5].Hall DC, Ji H-F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Med Infect Dis 2020;35:01–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Copertino DC, Jr, Casado Lima BC, Duarte RR, et al. Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection. J Biomol Struct Dyn 2021;11:01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Michaud V, Dow P, Al Rihani SB, et al. Risk assessment of drug-induced long QT syndrome for some COVID-19 repurposed drugs. Clin Trans Sci 2021;14:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hunt K, Hughes CA, Hills-Nieminen C. Protease inhibitor–associated QT interval prolongation. Ann Pharmacother 2011;45:1544–50. [DOI] [PubMed] [Google Scholar]
- [9].Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in adults and children. Cochrane Database Syst Rev 2014;4:01–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chinello P, Petrosillo N, Pittalis S, et al. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl Trop Dis 2017;11:01–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Carpenter A, Chambers OJ, El Harchi A, et al. COVID-19 management and arrhythmia: risks and challenges for clinicians treating patients affected by SARS-CoV-2. Front Cardiovasc Med 2020;7:01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Crotti L, Arbelo E. COVID-19 treatments, QT interval and arrhythmic risk: the need for an international Registry on Arrhythmias. Heart Rhythm 2020;17:1423–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu J, Shah SK, Basu-Ray I, Garcia-Diaz J, Khalid K, Saeed M. QT prolongation in HIV-positive patients: Review article. Indian Heart J 2019;71:434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Recanatini M, Poluzzi E, Masetti M, Cavalli A, De Ponti F. QT prolongation through hERG K+ channel blockade: current knowledge and strategies for the early prediction during drug development. Med Res Rev 2005;25:133–66. [DOI] [PubMed] [Google Scholar]
- [15].Han S, Sun X, Zhang Z, Zhang L. The protease inhibitor atazanavir blocks hERG K+ channels expressed in HEK293 cells and obstructs hERG protein transport to cell membrane. Acta Pharmacol Sin 2015;36:454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Anson B, Weaver J, Ackerman M, et al. Blockade of HERG channels by HIV protease inhibitors. Lancet 2005;365:682–6. [DOI] [PubMed] [Google Scholar]
- [17].Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6:01–6. [PMC free article] [PubMed] [Google Scholar]
- [18].Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:01–8. [DOI] [PubMed] [Google Scholar]
- [19].Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, 2nd ed.Chichester (UK):2019. [Google Scholar]
- [20].Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Barwick MA, Bennett LM, Johnson SN, McGowan J, Moore JE. Training health and mental health professionals in motivational interviewing: a systematic review. Child Youth Serv Rev 2012;34:1786–95. [Google Scholar]
- [22].Busti A, Tsikouris J, Peeters M, et al. A prospective evaluation of the effect of atazanavir on the QTc interval and QTc dispersion in HIV-positive patients. HIV Med 2006;7:317–22. [DOI] [PubMed] [Google Scholar]
- [23].Sarapa N, Nickens DJ, Raber SR, Reynolds RR, Amantea MA. Ritonavir 100 mg does not cause QTc prolongation in healthy subjects: a possible role as CYP3A inhibitor in thorough QTc studies. Clin Pharmacol Ther 2008;83:153–9. [DOI] [PubMed] [Google Scholar]
- [24].Byakika-Kibwika P, Lamorde M, Lwabi P, et al. Cardiac conduction safety during coadministration of artemether-lumefantrine and lopinavir/ritonavir in hiv-infected ugandan adults. Chemother Res Pract 2011;2011:01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vicente J, Zusterzeel R, Johannesen L, et al. Assessment of multi ion channel block in a phase i randomized study design: results of the Ci PA phase I ECG biomarker validation study. Clin Pharmacol Ther 2019;105:943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baker JR, Best AM, Pade PA, McCance-Katz EF. Effect of buprenorphine and antiretroviral agents on the QT interval in opioid-dependent patients. Ann Pharmacother 2006;40:392–6. [DOI] [PubMed] [Google Scholar]
- [27].Rathbun CR, Liedtke MD, Blevins SM, et al. Electrocardiogram abnormalities with atazanavir and lopinavir/ritonavir. HIV Clin Trials 2009;10:328–36. [DOI] [PubMed] [Google Scholar]
- [28].Zhang X, Jordan P, Cristea L, et al. Thorough QT/QTc study of ritonavir-boosted saquinavir following multiple-dose administration of therapeutic and supratherapeutic doses in healthy participants. J Clin Pharmacol 2012;52:520–9. [DOI] [PubMed] [Google Scholar]
- [29].Boffito M, Jackson A, Pozniak A, et al. Effect of a modified saquinavir/ritonavir dosing regimen with lower dose lead-in phase on QTc interval, pharmacokinetics, antiviral activity and safety in treatment-Naïve HIV-1-infected patients. Drugs RD 2015;15:141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cao B, Wang Y, Wen D, et al. A Trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Peterson RL, Vock DM, Powers JH, et al. Analysis of an ordinal endpoint for use in evaluating treatments for severe influenza requiring hospitalization. Clin Trials 2017;14:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Food, Drug Administration, HHS. International conference on harmonisation; guidance on E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs; availability. Notice Fed Regist 2005;70:61134–5. [PubMed] [Google Scholar]
- [33].Boettiger DC, Nguyen VK, Durier N, et al. Efficacy of second-line antiretroviral therapy among people living with HIV/AIDS in Asia: results from the TREAT Asia HIV Observational Database. J Acquir Immune Defic Syndr 2015;68:186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach; 2013. Available at: http://www.ncbi.nlm.nih.gov/books/NBK195400/. Accessed July 1, 2020. [Google Scholar]
- [35].Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag 2008;4:1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Achenbach CJ, Darin KM, Murphy RL, Katlama C. Atazanavir/ritonavir-based combination antiretroviral therapy for treatment of HIV-1 infection in adults. Future Virol 2011;6:157–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cameron DW, Japour AJ, Xu Y, et al. Ritonavir and saquinavir combination therapy for the treatment of HIV infection. AIDS Lond Engl 1999;13:213–24. [DOI] [PubMed] [Google Scholar]
- [38].Lv Z, Chu Y, Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIVAIDS Auckl NZ 2015;7:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]