Abstract
Previous studies had shown that an increased aspartate aminotransferase to alanine aminotransferase ratio (AST/ALT ratio) was associated with cardiovascular disease. This study aimed to assess the relationship between AST/ALT ratio and all-cause and cardiovascular mortality in patients with hypertension.
By March 31, 2020, a cohort of 14,220 Chinese hypertensive patients was followed up. The end point was all-cause and cardiovascular death. Hazard ratios (HRs) and 95% CIs were calculated for mortality associated with AST/ALT ratio, using Cox proportional hazards models and competing risk model.
In an average of 1.7 years of follow-up, 1.39% (n = 198) of patients died, 55.5% (n = 110) of whom from cardiovascular disease. AST/ALT ratio was associated with increased risk of all-cause death (HR:1.37, 95% CI:1.15–1.63) and cardiovascular death (HR:1.32, 95% CI:1.03–1.68) after adjustment for other potential confounders. Compared with low AST/ALT ratio (Tertile 1), high AST/ALT ratio was associated with high cause mortality (Tertile 2: HR:1.35, 95% CI:0.86–2.10; Tertile 3: HR:2.10, 95% CI:1.37–3.21; P for trend <.001). Compared with low AST/ALT ratio (Tertile 1), a statistically significant increased risk of cardiovascular mortality was also observed (Tertile 2: HR:1.27, 95% CI:0.70–2.29; Tertile 3: HR:1.92, 95% CI:1.09–3.37; P for trend <.001). High AST/ALT ratio was also associated with high cardiovascular mortality (Tertile 2: HR:1.27, 95% CI:0.70–2.29; Tertile 3: HR:1.92, 95% CI:1.09–3.37; P for trend <.001).
Present study indicated that increased AST/ALT ratio levels were predictive of all-cause and cardiovascular mortality among Chinese hypertensive patients.
Trial registration: CHICTR, CHiCTR1800017274. Registered 20 July 2018.
Keywords: all-cause mortality, aspartate aminotransferase/alanine aminotransferase ratio, cardiovascular mortality, hypertension
1. Introduction
It is well known that aminotransferase, which was composed of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), is the main marker of liver damage. The former only exists in the liver, while the latter distributes in the liver and myocardial tissue. AST/ALT ratio was first put forwarded in 1957.[1] Since then, the AST/ALT ratio had often been identified as markers of various chronic liver diseases, including alcoholic and non-alcoholic liver diseases,[2] autoimmune liver diseases,[3] and hepatitis C.[4] To our knowledge, liver disease and heart disease often affect each other. Previous studies had shown that heart-liver interaction was found in patients with heart failure,[5] atrial fibrillation[6] and myocardial infarction.[7] Therefore, it is reasonable to suspect that the elevated AST/ALT ratio might reflect cardiovascular disease (CVD).
Despite great progress in medicine, CVD is still a global problem, with high morbidity and mortality.[8] Understandably, it would be essential to identify subjects with high risk for CVD in the future through routine examination. The current findings suggested that higher AST/ALT ratio could be used as a predictor of CVD. For instance, Ewid et al[9] conducted a study which evaluated the functional severity of chronic heart failure with reduced left ventricular ejection, found that 0.9 was the best predictive cut-off value of the AST/ALT ratio. At the same time, a study demonstrated that the best cut-off value in predicting the cardiometabolic risk in the AST/ALT ratio was 1.[10] In addition, a prospective cohort study[11] involving 29,316 UK primary care patients aged 25 to 84 years with no history of CVD found that an elevated AST/ALT ratio was significantly associated with increased risk of developing CVD, especially in males.
At present, however, no study had reported the association between AST/ALT ratio and death in hypertensive patients. Therefore, our aim was to assess whether the AST/ALT ratio was a predictor for all-cause mortality and cardiovascular mortality in patients with hypertension.
2. Methods
2.1. Study design and patients
The present study was established in China Hypertension Registry Study, a large observational population-based cohort study, with the objective of establishing a national registry of patients with hypertension, investigating the prevalence and treatment of hypertension in China and assessing the related factors affecting its prognosis. The detailed inclusion criteria and exclusion criteria had already been published in previous article.[12] To be brief, the China H-type Hypertension Registration Study was a non-intervention, prospective, observational, and real-world study. The purpose of this study was to establish a national registry of patients with H-type hypertension, investigate the prevalence and treatment of H-type hypertension in China and assess the related factors affecting its prognosis. The Ethics Committee of the Anhui Medical University Biomedical Institute approved the study. All patients signed informed consent. The inclusion criteria were as follows: 18 years of age or older, Hypertensive patients diagnosed according to Chinese guidelines for the prevention and treatment of hypertension, and signing the informed consent. The exclusion criteria were as follows: psychological or nervous system impairment resulting in an inability to demonstrate informed consent; unable to be followed up according to the study protocol, or plans to relocate in the near future; and those patients who are not suitable for inclusion or for long-term follow-up as assessed by study physicians. The primary indicators were the control rate of hypertension, blood pressure decrease, homocysteine (HCY) decrease and stroke. Secondary indicators included compound cardio cerebrovascular events, myocardial infarction (fatal/nonfatal), ischemic stroke, hemorrhagic stroke, vascular death, tumor, and all hospitalizations.
2.2. Laboratory assays
After an overnight fast, venous blood was withdrawn in the morning for all the participants. Subsequently, the blood samples of all subjects would be collected, frozen, and transported to the Shenzhen Biotech Laboratory in Shenzhen for analysis. Serum concentrations of fasting blood glucose, total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol, ASTALT, total bilirubin, direct bilirubin, gamma-glutamyltransferase (GGT), albumin (ALB), uric acid, HCY, and creatinine were measured using automatic clinical analyzers (Beckman Coulter, USA). Estimated glomerular filtration rate (eGFR) was estimated using the newly developed Chronic Kidney Disease (CKD) Epidemiology Collaboration equation.[13]
2.3. Covariates
Participants reported on their lifestyle, medical conditions, and demographic information in baseline assessment. Medical history, health status, and medication intake were queried by trained health professionals. The anthropometric examinations included weight, height, waist circumference, and hip circumference. Body mass index (BMI) was calculated as the body weight in kilograms divided by the square of the height in meters (kg/m2). After 10 minutes of rest for all subjects, a professional clinician would measure systolic blood pressure (SBP), diastolic blood pressure, and pulse rate by electronic sphygmomanometers. Categorical variables consisted of smoking (no, yes), drinking (no, yes), self-reported history of diseases (any hypertension, diabetes, stroke, CKD, and hyperlipidemia), and medication use (antihypertensive drugs, glucose-lowering drugs, lipoprotein-lowering drugs, and antiplatelet drugs).
2.4. Exposure variable, outcomes, and follow-up
In our study, the exposure variable was the AST/ALT ratio. The primary endpoints included all-cause death and cardiovascular death. Cardiovascular death included sudden cardiac death, death due to myocardial infarction, heart failure, stroke, or cardiovascular invasive procedures, death due to cardiovascular hemorrhage (refers to a death related to hemorrhage such as a nonstroke intracranial hemorrhage, nonprocedural, or nontraumatic vascular rupture (eg, aortic aneurysm), or pulmonary hemorrhage from a pulmonary embolism),[14] and death due to other known vascular causes.[15] All follow-up was performed by professional clinical study coordinator. Participants were local residents, so door-to-door and telephone were main methods of follow-up. The task of follow-up was to observe whether there had end-point events. If participants were observed dead, it would be verified through hospital medical records, death registration, disease report card, insurance files, etc. So far (average follow-up 1.7 years), only 7 patients lost follow-up. Throughout the study period, we collected the information on death and its specific cause from local death and disease registries of the National Disease Surveillance Point System and National Health Insurance System. In addition, we also collected and adjudicated the medical records of subjects who visited the emergency department or hospitalized. Meanwhile, we calculated the sample size. Total population of Wuyuan County (Jiangxi province) is 34,6200 and the average mortality rate of Chinese population is 5.95%. Assuming that the power of test was 0.95, permissible error was 0.1 and the required sample size was 5967. Considering the 20% lost to follow-up rate, the sample size needed was 7160. In this way, the sample size of this study was adequate.
2.5. Statistical analysis
In our study, continuous variables were presented as mean ± standard deviation. Categorical variables were presented as frequency and percentage. Baseline characteristics of patients were stratified in three groups according to the tertiles of AST/ALT ratio and in two groups according to the status of patients. In order to compare differences among the different AST/ALT ratio groups (tertiles), t-test, chi-square test, and one-way analysis of variance were used. Survival analysis was analyzed by Kaplan–Meier curves and the statistical significance estimated by the log-rank test. The independent association of the AST/ALT ratio (included as either continuous or categorical variable) with risk of both all-cause and cardiovascular mortality were performed by Cox proportional-hazards model and competing risk model. Meanwhile, AST/ALT ratio variable was transformed into cubic splines to evaluate the association between AST/ALT ratio and all-cause and cardiovascular death. Variables for adjustment were carefully chosen, given the number of events available, clinical importance, and published studies.[16–18] Three models were conducted: model 1, with no adjustment; model 2, with adjusting for age and sex; and model 3, with adjusting for other confounding factors based on model 2. In addition, we also performed smooth curve fitting (penalized spline method) and subgroup analysis. All data analysis used the statistical package R (https://www.R-project.org; Version 4.1.1,Vienna, Austria) and Empower (R) (www.empowerstats.com; X&Y Solutions, Inc., Boston, MA). Two-tailed P < .05 was defined as statistically significant.
3. Results
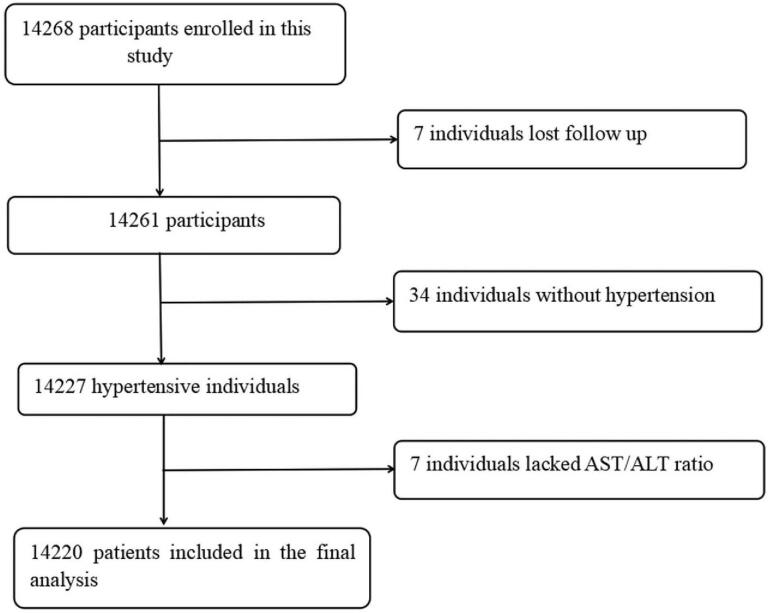
From March 2018 to March 2020, a total of 14,268 patients were followed up. Excluding those who were lost to follow-up (n = 7), those without hypertension (n = 34), and those AST and ALT data were missing (n = 7)), 14,420 people were included in the analysis finally (Fig. 1). In an average of 1.7 years of follow-up, 1.39% (n = 198) of patients died, 55.5% (n = 110) of whom from cardiovascular causes.
Figure 1.
Flow chart of participants.
3.1. Baseline characteristics of patients
The baseline clinical and biochemical characteristics of patients stratified by the tertiles of AST/ALT ratio were illustrated in Table 1. Overall, mean age of participants was 63.80 ± 9.36 years and 47.23% of the population was male. Patients with high level of AST/ALT ratios were older, had lower BMI, and diastolic blood pressure. Moreover, patients with higher AST/ALT ratio had lower levels of eGFR, TC, TGs, and LDL-C. There was no difference between drinking status, self-reported history of stroke and CKD, and taking antiplatelet drugs.
Table 1.
Baseline characteristics of participants stratified according to the AST/ALT ratio categories.
| AST/ALT ratio | Total | T1 (≤1.2) | T2 (1.2–1.6) | T3 (≥1.6) | P- value |
| N | 14,220 | 4739 | 4726 | 4755 | |
| Male | 6716 (47.23%) | 2571 (54.25%) | 2064 (43.67%) | 2081 (43.76%) | <.001 |
| Age (yr) | 63.80 ± 9.36 | 59.94 ± 8.75 | 63.79 ± 8.50 | 67.68 ± 9.17 | <.001 |
| Current smoking | 3658 (25.73%) | 1310 (27.65%) | 1124 (23.79%) | 1224 (25.74%) | <.001 |
| Current drinking | 3063 (21.55%) | 1011 (21.35%) | 975 (20.63%) | 1077 (22.65%) | .053 |
| BMI (kg/m2) | 23.61 ± 3.74 | 25.35 ± 3.94 | 23.48 ± 3.25 | 22.00 ± 3.22 | <.001 |
| Mean SBP (mm Hg) | 148.39 ± 17.86 | 147.17 ± 17.04 | 148.67 ± 17.82 | 149.31 ± 18.63 | <.001 |
| Mean DBP (mm Hg) | 88.92 ± 10.75 | 91.02 ± 10.30 | 88.86 ± 10.53 | 86.90 ± 11.01 | <.001 |
| FBG, mmol/L | 6.18 ± 1.61 | 6.63 ± 2.13 | 6.08 ± 1.33 | 5.84 ± 1.07 | <.001 |
| TC, mmol/L | 5.16 ± 1.12 | 5.23 ± 1.17 | 5.18 ± 1.09 | 5.06 ± 1.08 | <.001 |
| TG, mmol/L | 1.81 ± 1.26 | 2.22 ± 1.52 | 1.72 ± 1.10 | 1.48 ± 1.00 | <.001 |
| LDL-C, mmol/L | 2.98 ± 0.81 | 3.11 ± 0.85 | 2.99 ± 0.79 | 2.84 ± 0.79 | <.001 |
| HDL-C, mmol/L | 1.57 ± 0.43 | 1.45 ± 0.37 | 1.58 ± 0.42 | 1.67 ± 0.45 | <.001 |
| TBIL, μmol/L | 14.59 ± 6.85 | 14.96 ± 6.67 | 14.34 ± 6.27 | 14.47 ± 7.53 | <.001 |
| DBIL, μmol/L | 5.55 ± 2.58 | 5.57 ± 2.19 | 5.45 ± 2.17 | 5.64 ± 3.22 | <.001 |
| GGT, U/L | 33.23 ± 43.00 | 44.94 ± 50.45 | 28.35 ± 32.65 | 26.41 ± 41.55 | <.001 |
| ALB, g/L | 46.63 ± 4.05 | 47.23 ± 3.95 | 46.68 ± 3.91 | 45.99 ± 4.20 | <.001 |
| UA, mg/dL | 7.04 ± 2.03 | 7.37 ± 2.08 | 6.89 ± 1.96 | 6.84 ± 2.00 | <.001 |
| HCY, μmol/L | 17.97 ± 11.13 | 17.30 ± 11.30 | 17.73 ± 10.86 | 18.86 ± 11.18 | <.001 |
| eGFR, mL/min/1.73 m2 | 88.18 ± 20.23 | 91.78 ± 19.89 | 88.65 ± 19.56 | 84.12 ± 20.48 | <.001 |
| Medical history, n (%) | |||||
| Diabetes | 2616 (18.40%) | 1328 (28.02%) | 768 (16.25%) | 520 (10.94%) | <.001 |
| Stroke | 981 (6.90%) | 346 (7.30%) | 322 (6.81%) | 313 (6.58%) | .370 |
| CKD | 702 (4.94%) | 236 (4.98%) | 227 (4.80%) | 239 (5.03%) | .870 |
| Dyslipidemia | 2087 (14.68%) | 994 (20.97%) | 640 (13.54%) | 453 (9.53%) | <.001 |
| Medication use, n (%) | |||||
| Antihypertensive drugs | 9218 (64.84%) | 3160 (66.71%) | 3049 (64.53%) | 3009 (63.28%) | .002 |
| Glucose-lowering drugs | 754 (5.30%) | 435 (9.18%) | 206 (4.36%) | 113 (2.38%) | <.001 |
| Lipid-lowering drugs | 504 (3.54%) | 215 (4.54%) | 164 (3.47%) | 125 (2.63%) | <.001 |
| Antiplatelet drugs | 543 (3.82%) | 196 (4.14%) | 180 (3.81%) | 167 (3.51%) | .284 |
As shown in Table S1, Supplemental Digital Content, compared with survivors, the decedents were more likely to be older, and had higher level of AST/ALT ratio, SBP, HCY, uric acid, and GGT at baseline. In addition, they tend to be thinner and had lower DBP, TC, TG, LDL-C, and eGFR. Finally, people who died had a higher proportion of history of diabetes, stroke, or kidney disease, while antihypertensive and hypoglycemic drugs were less frequently used.
3.2. Survival analysis
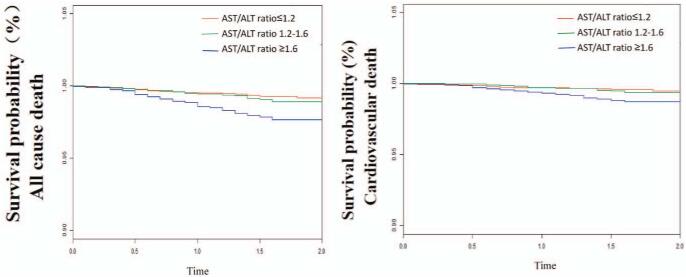
Figure 2 shows the cumulative survival probability of all-cause mortality and cardiovascular mortality respectively. It could be seen from the picture that the survival probability in patients with a high AST/ALT ratio was significantly reduced. Differences in survival rate were estimated by log-rank test and P values for both analyses were statistical significant (P < .001).
Figure 2.
Kaplan–Meier curves according to AST/ALT ratio categories. A: All-cause mortality. B: Cardiovascular mortality. ALT = alanine aminotransferase, AST = aspartate aminotransferase.
3.3. Multivariate regression analyses
We constructed 3 models to further test the independent association between the AST/ALT ratio and the risk of all-cause and cardiovascular death. Table 2 showed the association between AST/ALT ratio and all-cause mortality by Cox proportional regression model. In the unadjusted model (model 1), for every 1 increase in AST/ALT ratio, the risk of all-cause death increased by 75% (hazard ratio [HR]:1.75, 95% CI:1.52–2.01). After adjusting gender and age (model 2), the risk of all-cause increased by 52% (HR:1.52, 95% CI:1.30–1.79) with the AST/ALT ratio increased. Model 3 was fully adjusted in which the AST/ALT ratio was still significantly correlated with all-cause death (HR:1.37, 95% CI:1.15–1.63). Certainly, we also converted AST/ALT ratio from continuous variables to categorical variables (tertiles) for analysis. A statistically significant increased risk of all-cause death for the third AST/ALT ratio tertile (T3) compared to the first tertile (T1) (HR:2.10, 95% CI:1.37–3.21) was found. The relationship between AST/ALT ratio and cardiovascular mortality was evaluated by competing risk model (Table 3). In detail, for every 1 increase in AST/ALT ratio, the risk of all-cause death increased by 68% (HR:1.68, 95% CI:1.37–2.06) in the unadjusted model (model 1). After adjusting gender and age (model 2), the risk of all-cause increased by 46% (HR:1.46, 95% CI:1.17–1.83) with the AST/ALT ratio increased. Model 3 was fully adjusted in which the AST/ALT ratio was still significantly correlated with all-cause death (HR:1.32, 95% CI:1.03–1.68). A statistically significant was also observed in the increase risk of cardiovascular mortality for the third AST/ALT ratio tertile (T3) compared to the first tertile (T1) (HR:1.92, 95% CI:1.09, 3.37). The specific linear relationship between AST/ALT ratio and all-cause death and cardiovascular death was described in Figure S1, Supplemental Digital Content and Figure S2, Supplemental Digital Content by the method of cubic spline.
Table 2.
Association between AST/ALT ratio and risk of all-cause mortality.
| Model 1 | Model 2 | Model 3 | ||||
| AST/ALT ratio | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Continuous | 1.75 (1.52, 2.01) | <.001 | 1.52 (1.30, 1.79) | .015 | 1.37 (1.15, 1.63) | <.001 |
| Tertiles | ||||||
| T1 (≤1.2) | Ref. | Ref. | Ref. | |||
| T2 (1.2–1.6) | 1.53 (1.00, 2.36) | .052 | 1.30 (0.84, 2.01) | .237 | 1.35 (0.86, 2.10) | .193 |
| T3 (≥1.6) | 3.31 (2.25, 4.85) | <.001 | 2.32 (1.56, 3.44) | <.001 | 2.10 (1.37, 3.21) | <.001 |
| P for trend | <.001 | <.001 | <.001 | |||
| Categories | ||||||
| T1–T2 (<1.6) | Ref. | Ref. | Ref. | |||
| T3 (≥1.6) | 2.61 (1.97, 3.46) | <.001 | 1.99 (1.49, 2.65) | <.001 | 1.74 (1.28, 2.36) | <.001 |
Table 3.
Association between AST/ALT ratio and risk of cardiovascular mortality.
| Model 1 | Model 2 | Model 3 | ||||
| AST/ALT ratio | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Continuous | 1.68 (1.37, 2.06) | <.01 | 1.46 (1.17, 1.83) | <.01 | 1.32 (1.03, 1.68) | <.01 |
| Tertiles | ||||||
| T1 (≤1.2) | Ref. | Ref. | Ref. | |||
| T2 (1.2–1.6) | 1.46 (0.82, 2.57) | <.01 | 1.27 (0.71, 2.25) | .421 | 1.27 (0.70, 2.29) | .432 |
| T3 (≥1.6) | 3.01 (1.82, 5.00) | <.01 | 2.19 (1.30, 3.69) | .003 | 1.92 (1.09, 3.37) | .024 |
| P for trend | <.001 | .001 | .015 | |||
| Categories | ||||||
| T1–T2 (<1.6) | Ref. | Ref. | Ref. | |||
| T3 (≥1.6) | 2.46 (1.68, 3.58) | <.001 | 1.91 (1.30, 2.81) | .001 | 1.65 (1.09, 2.49) | .017 |
3.4. Subgroup analysis
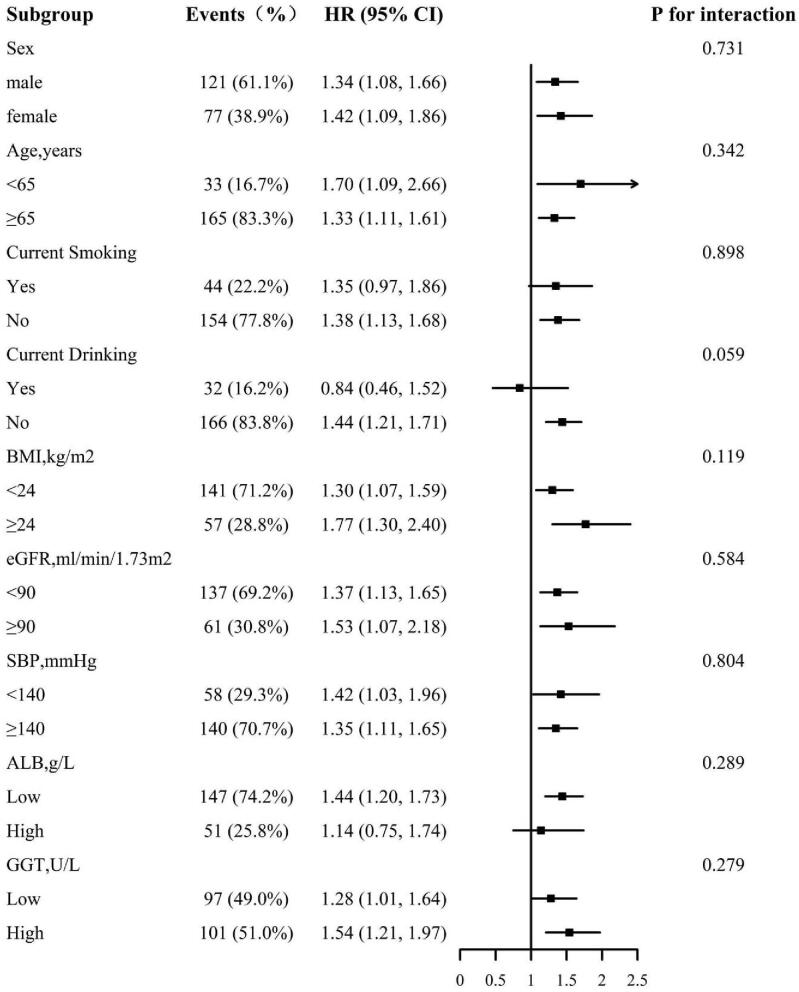
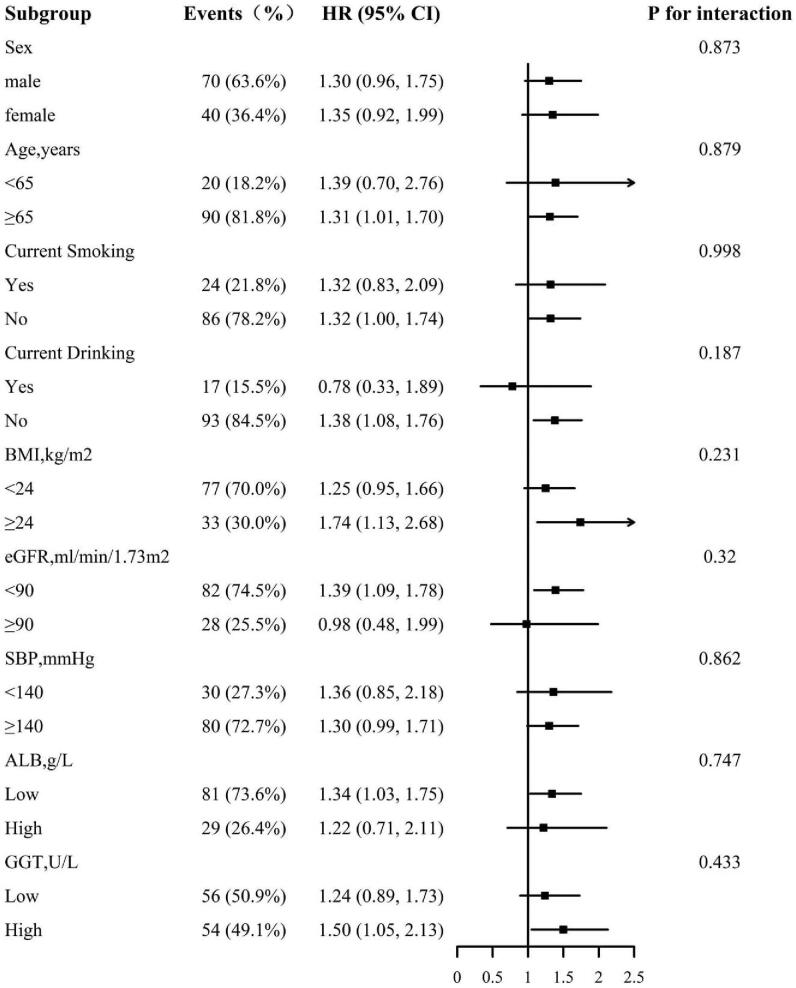
We further explored the role of other covariables on the association between all-cause death and cardiovascular death. Figures 3 and 4 show the association between all-cause and cardiovascular mortality in various subgroups respectively and the subgroup analysis were the followings: sex (male, female), age (<65, ≥65 years), smoking (yes, no), drinking (yes, no), BMI (<24, ≥24 kg/m2), eGFR (<90, ≥90 ml/min/1.73 m2), SBP (<140, ≥140 mm Hg), ALB (low, high), and GGT (low, high). No interaction was found in subgroup analysis. More specifically, the results of subgroup analysis were in good agreement with the main results.
Figure 3.
The association between AST/ALT ratio and all-cause mortality in various subgroups age, sex, smoking, drinking, BMI, SBP, FBG, TC, TG, LDL-C, HDL-C, eGFR, ALB, GGT, antihypertensive drugs, glucose-lowering drugs, glucose-lowering drugs, and lipid-lowering drugs, except for the stratify. ALT = alanine aminotransferase, ALB = albumin, AST = aspartate aminotransferase, BMI = body mass index, eGFR = estimated glomerular filtration rate, FBG = fasting blood glucose, GGT = gamma-glutamyltransferase, HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein cholesterol, SBP = systolic blood pressure, TC = total cholesterol, TG = triglyceride.
Figure 4.
The association between AST/ALT ratio and cardiovascular mortality in various subgroups adjusted for age, sex, smoking, drinking, BMI, SBP, FBG, TC, TG, LDL-C, HDL-C, eGFR, ALB, GGT, antihypertensive drugs, glucose-lowering drugs, glucose-lowering drugs, and lipid-lowering drugs, except for the stratify. ALT = alanine aminotransferase, ALB = albumin, AST = aspartate aminotransferase, BMI = body mass index, eGFR = estimated glomerular filtration rate, FBG = fasting blood glucose, GGT = gamma-glutamyltransferase, HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein cholesterol, SBP = systolic blood pressure, TC = total cholesterol, TG = triglyceride.
4. Discussion
In present study, we reported that the AST/ALT ratio was associated with all-cause mortality and cardiovascular mortality independently. To our knowledge, this is the first large-scale study to investigate the relationship between AST/ALT ratio and all-cause and cardiovascular death in hypertensive patients.
There have been some small sample size researches and the results that were found were consistent with ours. For instance, a longitudinal cohort study[16] included 3494 Japanese community-checked subjects that showed a high AST/ALT ratio (>90%) was an independent predictor of all-cause and cardiovascular mortality. Feng et al[17] performed a multicenter retrospective study which consisted of 1579 patients on peritoneal dialysis. It reported that a higher AST/ALT ratio was independently associated with an increased risk of CVD mortality compared with their counterparts (HR = 1.43, 95% CI:1.08–1.41). Another research[18] which revealed the relationship between AST/ALT and all-cause death and cardiovascular death in people with diabetes found AST/ALT ratio was associated with a 83% increased risk of all-cause mortality (95% 1.14–2.93, P = .012) and a 160% increased risk of CVD mortality. Combined with the results of our research, we found that the AST/ALT ratio was related to all-cause death and cardiovascular death independently in different study populations, which might indicate that the AST/ALT ratio not only reflected liver damage, but also reflected system disorders.
In our study, the average age of the overall population was 63.80 ± 9.36 years, while the age of the high AST/ALT level group was 67.68 ± 9.17 years. Since liver function would deteriorates with age gradually,[19] age might explain the reason why the higher the AST/ALT level, the higher mortality of all-cause and cardiovascular. BMI was commonly used to classify overweight and obesity in adults.[20] Studies in the past had reported that lower body weight was significantly associated with higher mortality.[21] In our study, people with higher AST/ALT ratio had lower BMI, which was also observed in those who died. Furthermore, the higher value of AST/ALT ratio was indicative of more severe liver damage due to alcohol.[22] Although there was no statistically difference in drinking status, the proportion of alcohol consumption in people with high levels of AST/ALT was larger. However, age, BMI, and drinking had no interaction on the relationship between AST/ALT and all-cause death and cardiovascular death in our subgroup analysis. This might suggest a specific role for AST/ALT ratio in all-cause death and cardiovascular death.
Nevertheless, the possible biological mechanism is still under discussion. What we should pay attention to is that the AST/ALT ratio is not only a predictor of advanced alcoholic liver disease,[2] progressive liver damage,[23] and cirrhosis,[24] but also plays an important role in other diseases, such as heart failure,[9] insulin resistance,[25] metabolic syndrome,[26,27] arteriosclerosis,[28,29] and a prognostic factor in patients with upper tract urothelial cancer treated with surgery.[30] In addition, the AST/ALT ratio seemed to be related to increased oxidative stress, a potential factor of CVD. In animal models, as reported, mice with elevated AST/ALT ratio had a reduced ability to carry oxygen and in these animals, markers of oxidative stress were also increased.[31] Therefore, it is reasonable to guess that an increase in the AST/ALT ratio increased mortality through oxidative stress.
The biggest advantage of this study was hypertensive population, and the sample size was larger previous similar studies. In this era of stratified medicine, hypertensive patients with elevated AST/ALT ratio may represent a group of individuals at higher risk of mortality who deserve closer monitoring. Of course, some limitations should not be ignored. First, the AST and ALT values were only detected in baseline due to the short follow-up time, and if conditions permit, we plan to check again in subsequent follow-ups to obtain dynamic changes. Second, information about the history of acute or chronic liver disease and the use of related drugs were not collected. This made it impossible to exclude the possibility that liver diseases might cause changes in AST/ALT values. Third, the remaining influence cannot be ruled out even though the confounding factors have been adjusted. Finally, given that this study patients were hypertensive, our conclusions could not be generalized to other populations.
5. Conclusion
In summary, the findings of our study indicate that elevated AST/ALT ratio was an independent factor for risk of both all-cause and cardiovascular mortality in patients with hypertension.
Acknowledgments
The authors thank all the participating authors for their contributions to this article.
Author contributions
Conceptualization: Hui Liu, Congcong Ding, Lihua Hu, Minghui Li, Wei Zhou, Tao Wang, Lingjuan Zhu, Huihui Bao, Xiaoshu Cheng.
Data curation: Hui Liu, Congcong Ding, Lihua Hu, Minghui Li, Wei Zhou, Tao Wang, Lingjuan Zhu, Huihui Bao, Xiaoshu Cheng.
Formal analysis: Hui Liu, Congcong Ding, Lihua Hu, Minghui Li, Wei Zhou, Tao Wang, Lingjuan Zhu, Huihui Bao, Xiaoshu Cheng.
Project administration: Hui Liu, Congcong Ding, Lihua Hu, Minghui Li, Wei Zhou, Tao Wang, Lingjuan Zhu, Huihui Bao, Xiaoshu Cheng.
Writing – original draft: Hui Liu, Congcong Ding, Lihua Hu, Minghui Li, Wei Zhou, Tao Wang, Lingjuan Zhu, Huihui Bao, Xiaoshu Cheng.
Writing – review & editing: Hui Liu, Congcong Ding, Lihua Hu, Minghui Li, Wei Zhou, Tao Wang, Lingjuan Zhu, Huihui Bao, Xiaoshu Cheng.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CKD = chronic kidney disease, CVD = cardiovascular disease, eGFR = estimated glomerular filtration rate, GGT = gamma-glutamyltransferase, HR = hazard ratio, HCY = homocysteine, LDL-C = low density lipoprotein cholesterol, SBP = systolic blood pressure, TC = total cholesterol, TG = triglyceride.
How to cite this article: Liu H, Ding C, Hu L, Li M, Zhou W, Wang T, Zhu L, Bao H, Cheng X. The association between AST/ALT ratio and all-cause and cardiovascular mortality in patients with hypertension. Medicine. 2021;100:31(e26693).
This work was supported by the Science and Technology Innovation Platform Project of Jiangxi Province (Grant number: 20165BCD41005).
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Anhui Medical University Biomedical Institute.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Supplemental digital content is available for this article.
Values of continuous variables are presented as mean±SD, categorical variables are presented as n (%).
ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CKD = chronic kidney disease, DBIL = direct bilirubin, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, FBG = fasting blood glucose, GGT = glutamyltrantferase, HCY = homocysteine, HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein, SBP = systolic blood pressure, TBIL = total bilirubin, TC = total cholesterol, TG = triglyceride, UA = uric acid.
Model 1: adjusted for none.
Model 2: adjusted for age, sex.
Model 3: age, sex, smoking, drinking, BMI, SBP, FBG, TC, TG, LDL-C, HDL-C, eGFR, ALB, GGT, antihypertensive drugs, glucose-lowering drugs lipid-lowering drugs, antiplatelet drugs.
ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, eGFR = estimated glomerular filtration rate, FBG = fasting blood glucose, HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein cholesterol, SBP = systolic blood pressure, TC = total cholesterol, TG = triglyceride.
Model 1: adjusted for none.
Model 2: adjusted for age, sex.
Model 3: age, sex, smoking, drinking, BMI, SBP, FBG, TC, TG, LDL-C, HDL-C, eGFR, ALB, GGT, antihypertensive drugs, glucose-lowering drugs lipid-lowering drugs, antiplatelet drugs.
ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, eGFR = estimated glomerular filtration rate, FBG = fasting blood glucose, GGT = gamma-glutamyltransferase, HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein cholesterol, SBP = systolic blood pressure, TC = total cholesterol, TG = triglyceride.
References
- [1].de Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin Chim Acta 1957;2:70–4. [DOI] [PubMed] [Google Scholar]
- [2].Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol 2004;39:336–9. [DOI] [PubMed] [Google Scholar]
- [3].Nyblom H, Nordlinder H, Olsson R. High aspartate to alanine aminotransferase ratio is an indicator of cirrhosis and poor outcome in patients with primary sclerosing cholangitis. Liver Int 2007;27:694–9. [DOI] [PubMed] [Google Scholar]
- [4].Giannini E, Risso D, Botta F, et al. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med 2003;163:218–24. [DOI] [PubMed] [Google Scholar]
- [5].Samsky MD, Patel CB, DeWald TA, et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol 2013;61:2397–405. [DOI] [PubMed] [Google Scholar]
- [6].Deng W, Farricielli L. Hypoxic hepatitis and acute liver failure in a patient with newly onset atrial fibrillation and diltiazem infusion. BMJ Case Rep 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lofthus DM, Stevens SR, Armstrong PW, Granger CB, Mahaffey KW. Pattern of liver enzyme elevations in acute ST-elevation myocardial infarction. Coron Artery Dis 2012;23:22–30. [DOI] [PubMed] [Google Scholar]
- [8].Group, NIPPON DATA Research. Risk assessment chart for death from cardiovascular disease based on a 19-year follow-up study of a Japanese representative population. Circ J 2006;70:1249–55. [DOI] [PubMed] [Google Scholar]
- [9].Ewid M, Sherif H, Allihimy AS, et al. AST/ALT ratio predicts the functional severity of chronic heart failure with reduced left ventricular ejection fraction. BMC Res Notes 2020;13:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Long MT, Pedley A, Massaro JM, Hoffmann U, Fox CS. The association between non-invasive hepatic fibrosis markers and cardiometabolic risk factors in the Framingham heart study. PLoS One 2016;11:e157517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Weng SF, Kai J, Guha IN, Qureshi N. The value of aspartate aminotransferase and alanine aminotransferase in cardiovascular disease risk assessment. Open Heart 2015;2:e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yu Y, Hu L, Huang X, Zhou W, Bao H, Cheng X. BMI modifies the association between serum HDL cholesterol and stroke in a hypertensive population without atrial fibrillation. J Endocrinol Invest 2020;44:173–81. [DOI] [PubMed] [Google Scholar]
- [13].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rat. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). J Am Coll Cardiol 2015;66:403–69. [DOI] [PubMed] [Google Scholar]
- [15].Li Y, Qin X, Luo L, et al. Folic acid therapy reduces the risk of mortality associated with heavy proteinuria among hypertensive patients. J Hypertens 2017;35:1302–9. [DOI] [PubMed] [Google Scholar]
- [16].Yokoyama M, Watanabe T, Otaki Y, et al. Association of the aspartate aminotransferase to alanine aminotransferase ratio with bnp level and cardiovascular mortality in the general population: the Yamagata study 10-year follow-up. Dis Markers 2016;2016:4857917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Feng X, Wen Y, Peng F, Wang N, Zhan X, Wu X. Association between aminotransferase/alanine aminotransferase ratio and cardiovascular disease mortality in patients on peritoneal dialysis: a multicenter retrospective study. BMC Nephrol 2020;21:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zoppini G, Cacciatori V, Negri C, et al. The aspartate aminotransferase-to-alanine aminotransferase ratio predicts all-cause and cardiovascular mortality in patients with type 2 diabetes. Medicine (Baltimore) 2016;95:e4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cieslak KP, Baur O, Verheij J, Bennink RJ, van Gulik TM. Liver function declines with increased age. HPB (Oxford) 2016;18:691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Javed A, Jumean M, Murad MH, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis. Pediatr Obes 2015;10:234–44. [DOI] [PubMed] [Google Scholar]
- [21].Chung WS, Ho FM, Cheng NC, Lee MC, Yeh CJ. BMI and all-cause mortality among middle-aged and older adults in Taiwan: a population-based cohort stud. Public Health Nutr 2015;18:1839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gurung RB, Purbe B, Gyawali P, Risal P. The ratio of aspartate aminotransferase to alanine aminotransferase (AST/ALT): the correlation of value with underlying severity of alcoholic liver disease. Kathmandu Univ Med J (KUMJ) 2013;11:233–6. [DOI] [PubMed] [Google Scholar]
- [23].Giannini E, Botta F, Fasoli A, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci 1999;44:1249–53. [DOI] [PubMed] [Google Scholar]
- [24].Nyblom H, Bjornsson E, Simren M, Aldenborg F, Almer S, OlssonF R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int 2006;26:840–5. [DOI] [PubMed] [Google Scholar]
- [25].Simental-Mendia LE, Rodriguez-Moran M, Gomez-Diaz R, et al. Insulin resistance is associated with elevated transaminases and low aspartate aminotransferase/alanine aminotransferase ratio in young adults with normal weight. Eur J Gastroenterol Hepatol 2017;29:435–40. [DOI] [PubMed] [Google Scholar]
- [26].Zhao L, Cheng J, Chen Y, et al. Serum alanine aminotransferase/aspartate aminotransferase ratio is one of the best markers of insulin resistance in the Chinese population. Nutr Metab (Lond) 2017;14:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lin S, Tang L, Jiang R, et al. The relationship between aspartate aminotransferase to alanine aminotransferase ratio and metabolic syndrome in adolescents in northeast China. Diabetes Metab Syndr Obes 2019;12:2387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rief P, Pichler M, Raggam R, et al. The AST/ALT (De-Ritis) ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. Medicine (Baltimore) 2016;95:e3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu Y, Zhao P, Cheng M, et al. AST to ALT ratio and arterial stiffness in non-fatty liver Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis 2018;17:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee H, Choi YH, Sung HH, et al. De Ritis Ratio (AST/ALT) as a significant prognostic factor in patients with upper tract urothelial cancer treated with surgery. Clin Genitourin Cancer 2017;15:e379–85. [DOI] [PubMed] [Google Scholar]
- [31].Botezelli JD, Cambri LT, Ghezzi AC, Dalia RA, Voltarelli FA, de Mello MAR. Fructose-rich diet leads to reduced aerobic capacity and to liver injury in rats. Lipids Health Dis 2012;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.