Abstract
Introduction:
Pulmonary vein isolation (PVI) is the cornerstone of atrial fibrillation (AF) catheter ablation. However, a PVI alone has been considered insufficient for persistent AF. This study aimed to evaluate the efficacy of persistent AF ablation targeting complex fractionated atrial electrogram (CFAE) areas within low voltage zones identified by high-resolution mapping in addition to the PVI.
Methods:
We randomized 50 patients (mean age 58.4 ± 9.5 years old, 86.0% males) with persistent AF to a PVI + CFAE group and PVI only group in a 1:1 ratio. CFAE and voltage mapping was performed simultaneously using a Pentaray Catheter with the CARTO3 CONFIDENSE module (Biosense Webster, CA, USA). The PVI + CFAE group, in addition to the PVI, underwent ablation targeting low voltage areas (<0.5 mV during AF) containing CFAEs.
Results:
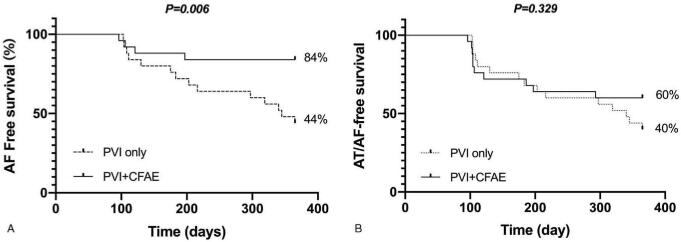
The mean persistent AF duration was 24.0 ± 23.1 months and mean left atrial dimension 4.9 ± 0.5 cm. In the PVI + CFAE group, AF converted to atrial tachycardia (AT) or sinus rhythm in 15 patients (60%) during the procedure. The PVI + CFAE group had a higher 1-year AF free survival (84.0% PVI + CFAE vs 44.0 PVI only, P = .006) without antiarrhythmic drugs. However, there was no difference in the AF/AT free survival (60.0% PVI + CFAE vs 40.0% PVI only, P = .329).
Conclusion:
Persistent AF ablation targeting CFAE areas within low voltage zones using high-density voltage mapping had a higher AF free survival than a PVI only. Although recurrence with AT was frequent in the PVI+CFAE group, the sinus rhythm maintenance rate after redo procedures was 76%.
Keywords: atrial fibrillation, complex fractionated atrial electrogram, low voltage areas, substrate, voltage mapping
1. Introduction
Pulmonary vein isolation (PVI) is the cornerstone strategy for catheter ablation of atrial fibrillation (AF) in those who do not respond to antiarrhythmic drugs.[10] However, catheter ablation of persistent AF (PeAF) and long-standing PeAF has had less favorable outcomes and additional substrate modification methods are often added to the PVI. Although substrate modification is a rational approach when considering the AF mechanism, the procedural success rates and clinical results vary widely.[6,11,16,19,27] However, recent advances in three-dimensional (3D) reconstruction/mapping technologies have provided standardized complex fractionated atrial electrogram (CFAE) maps. In addition, they also have improved the tissue contact and reduced the interelectrode distance, allowing for multipoint recording with a high spatial resolution and improved signal fidelity.
In this study, we evaluated the effects of a substrate modification strategy targeting CFAE areas within low voltage zones identified by the latest 3D mapping technology in persistent and long-standing persistent AF patients.
2. Methods
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Checklist S1 and Protocol S1. This clinical trial was designed and reported according to recommendations of the Consolidated Standard of Reporting Trials (CONSORT) statement[15] (ClinicalTrials.gov Number, NCT 03046043).
2.1. Study population
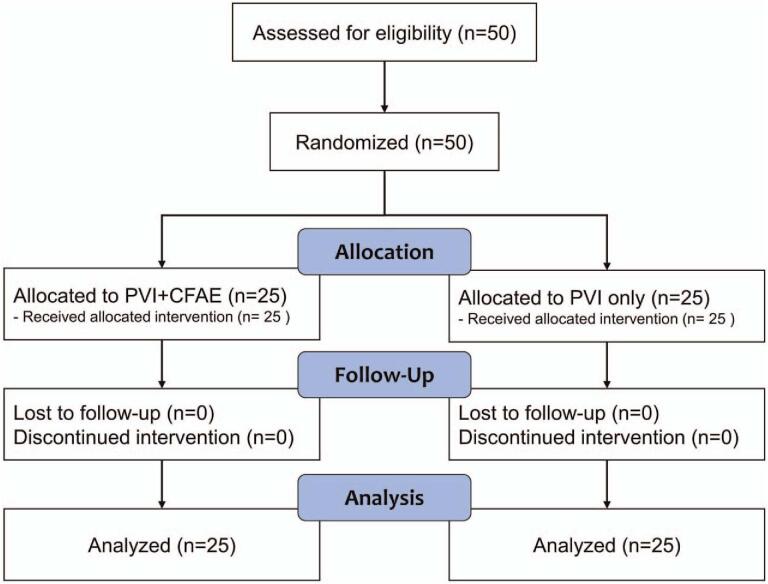
Fifty drug-refractory, symptomatic persistent and long-standing persistent AF patients scheduled for catheter ablation of AF at our hospital from January to October 2018 were consented for enrollment. Persistent AF was defined as continuous AF lasting more than 7 days. Long-standing PeAF was defined as continuous AF lasting for ≥1 year.[10] All patients gave their written informed consent for the study and the study protocol was approved by the Institutional Review Board of Keimyung University Dongsan Hospital (March 2017; DSMC 2017-02-034-001). The flowchart of our study according to the CONSORT guidelines is shown in Figure 1.
Figure 1.
Flow diagram of the study.
Eligible participants were provided unique identification numbers according to study criteria and were assigned to the PVI only group or PVI plus CFAE ablation group (PVI+CFAE). Randomization was done by a clinical research coordinator and a blocked randomization sequence was created with a computerized random number generator and applied with a 1:1 assignment to each group. The study coordinator nurse had informed the investigators of the number.
2.2. Sample size calculation
To date, there are no studies regarding ablation targeting CFAE areas only within low voltage zones. However, Rolf et al reported that the 12-month atrial tachyarrhythmia-free survival was 27% for patients with a PVI only and 70% for patients with additional low voltage area ablation.[22] Based on this study results, we hypothesized that the difference between the 2 groups in the one-year atrial tachyarrhythmia free survival was 43%. When the power was 80%, and the significance level 0.05, the required number of subjects was 17.3. Considering the dropout rate of 20%, the number of patients required to achieve the purpose of this study was 21.5. Therefore, we assigned 25 patients to each group.
2.3. Ablation procedure
All antiarrhythmic agents were stopped at least 5 half-lives before the procedure except for amiodarone, which was stopped at least 8 weeks pre-ablation. Multi-slice computed tomography scans and transesophageal echocardiography were performed in all patients prior to the procedure. The ablation procedure was performed under conscious sedation with remifentanil and midazolam. Three multipolar catheters were inserted through the left femoral region and placed in the right atrium, coronary sinus, and His bundle. Two 8.5 Fr non-steerable long sheaths (SwartzTM Braided Transseptal Guiding Introducer SL1, St. Jude Medical, St. Paul, MN) were placed in the right atrium through the right femoral region and inserted into the left atrium (LA) using one or 2 transseptal punctures. After the transseptal puncture, an activated clotting time of over 350 seconds was maintained with intermittent boluses of intravenous heparin. The anatomical structure was identified through the LA and PV angiography. Thereafter, a 3.5 mm irrigated ablation catheter with contact force monitoring (ThermoCool SmartTouch SF) and a Pentaray Catheter (Biosense Webster, Inc., Diamond Bar, CA, USA) were introduced through the sheaths.
Ablation was performed with a SmartTouch SF ablation catheter in a power-controlled mode using a power of 30 to 35 W for a duration of 25 to 30 seconds. Ablation of the posterior wall was performed with a power of 20 to 25 W for 15 to 20 seconds. The circumferential PVI was performed with a point-by-point ablation at the junction between the LA and PVs and an automated annotation program (VisiTagTM module) was used. The endpoint of the PVI was bidirectional conduction block of the PVs identified by a multipolar catheter. Entrance block was defined as the absence of any electrical activity within the PV antrum. If remnant potentials were present, the dissociation was demonstrated by local PV capture without conduction to the LA to prove exit block.[2]
In the PVI only group, electrical cardioversion to sinus rhythm was performed if the patient was still in AF after the PVI. If AF organized into atrial tachycardia (AT) during the PVI, the AT was mapped and ablated. If the patient had a history of typical atrial flutter, linear ablation targeting the cavo-tricuspid isthmus (CTI) was conducted. Non-inducibility tests were not performed in this group of patients.
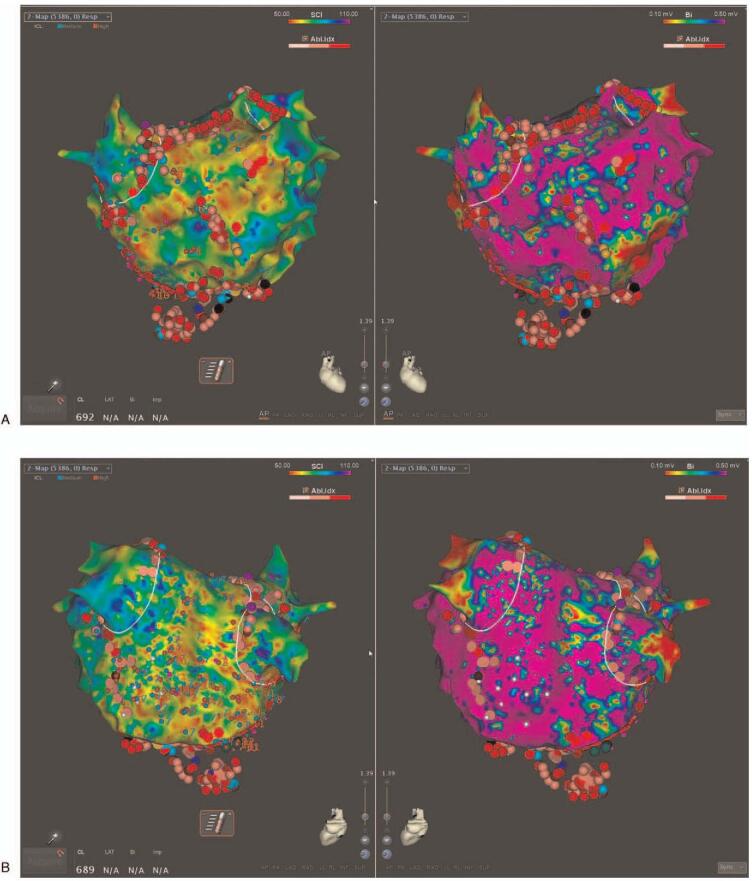
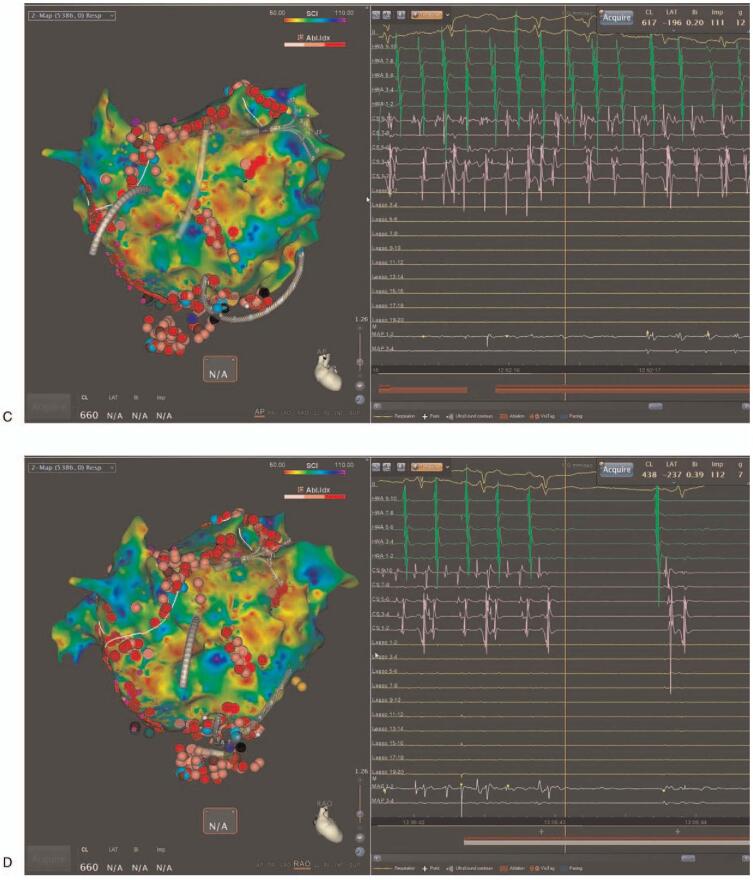
In the PVI+CFAE ablation group, ablation targeting the CFAE areas within low voltage zones was performed in addition to the PVI. High-density voltage and CFAE mapping were performed during an AF state using a Pentaray catheter with the CARTO3 CONFIDENSE module (Biosense Webster, Inc., Diamond Bar, CA, USA) before the ablation start. Automatic characterization of the CFAE signals was performed with a CARTO CFAE software module (Biosense Webster, Inc., Diamond Bar, CA, USA). Further, visually detected CFAE areas by the operator were manually annotated. A low voltage zone was defined as an area with bipolar peak-to-peak voltage amplitudes of <0.5 mV. The CFAEs were defined by the system based on the peak-to-peak (P-P) interval in milliseconds. The minimal amplitude threshold and maximal amplitude threshold were set to ± 0.05 millivolt (mV) and ± 0.15 mV, respectively, and the minimal interval and maximal interval between 2 consecutive peaks was set to 50 to 120 m/sec. The CFAE areas were identified and displayed in a color-coded manner according to the parameters using an algorithm with the interval confidence level (ICL) and shortest complex interval (SCI) parameters. Areas with the shortest SCI and a high ICL within low voltage areas were targeted first, followed by longer SCI regions (up to 120 ms). Radiofrequency energy was delivered at target sites for 30 to 60 seconds until the local electrograms were completely eliminated (Fig. 2 A and B). If AF persisted after the LA ablation, the CFAE areas within the low voltage zones in the right atrium (RA) were mapped and ablated. If AF organized into atrial tachycardia (AT) during the CFAE area ablation, the AT was mapped and ablated (Fig. 2 C and D). If AF persisted even after ablation targeting the CFAE areas with low voltage zones, electrical cardioversion was performed.
Figure 2.
Examples of electroanatomic maps of a low voltage complex fractionated atrial electrogram (CFAE) ablation. (A) Antero-posterior view and (B) Postero-anterior view of the left atrium (LA) after ablation. The left side of Figure A/B is a CFAE map and the right side a voltage map. (C) During an LA roof area CFAE ablation, atrial fibrillation converted into atrial tachycardia (AT). (D) During a CS os ablation, the AT terminated, and sinus rhythm was restored in the patient. The CFAE area was tagged with an azure dot. The ablation points are the pink and red dots. The blue dot is the AT termination site.
2.4. Outcomes
The primary outcome of our study was the freedom from any atrial tachyarrhythmia recurrence at the 1-year follow-up without antiarrhythmic drug treatment after a single ablation procedure. The secondary endpoints were the procedure time, fluoroscopy time, and complications.
2.5. Follow-up
Anti-arrhythmic agents were discontinued after a 3-month blanking period. An electrocardiogram (ECG) and 24-hour Holter monitoring were obtained right after the procedure and at 3, 6, and 12 months after the ablation. ECG and Holter monitoring were also performed whenever the patient had symptoms suggesting an arrhythmia recurrence. Recurrence of an atrial tachyarrhythmia was defined as the detection of AF or AT lasting more than 30 seconds on the 12-lead ECG or 24-hour Holter monitoring after the 3-month blanking period.
2.6. Statistical analysis
For the statistical analysis, a Student t test or Mann–Whitney U test was used for the comparison of the continuous variables between the 2 groups and a Chi-Squared or Fisher exact test was used for the categorical data. Kaplan–Meier survival estimates were used to compare the degree of recurrence of AF or the incidence of atrial arrhythmias. The data analyses were performed with IBM SPSS Statistics version 23 software (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Patient characteristics
The mean age of total patients was 58.4 ± 9.5 years and 43 (86%) patients were male. The mean AF duration was 24.0 ± 23.1 months and mean LA dimension 4.9 ± 0.5 cm. The baseline characteristics between the PVI only group and PVI + CFAE group did not differ. The results are summarized in Table 1.
Table 1.
Baseline characteristics of the patients.
| Total (n = 50) | PVI + CFAE (n = 25) | PVI (n = 25) | P value | |
| Age | 58.4 ± 9.5 | 58.8 ± 9.3 | 57.9 ± 9.8 | .600 |
| Female | 7 (14.0) | 5 (20.0) | 2 (8.0) | .417 |
| Male | 43 (86.0) | 20 (80.0) | 23 (92.0) | |
| Height | 167.6 ± 9.1 | 167.4 ± 9.0 | 167.9 ± 9.3 | .698 |
| Weight | 75.5 ± 13.4 | 72.8 ± 11.7 | 78.2 ± 14.8 | .139 |
| BSA | 1.9 ± 0.2 | 1.8 ± 0.2 | 1.9 ± 0.2 | .258 |
| BMI | 26.8 ± 3.5 | 25.9 ± 3.0 | 27.6 ± 3.7 | .320 |
| Risk factor | ||||
| HF | 8 (16.0) | 4 (16.0) | 4 (16.0) | 1.000 |
| HTN | 25 (50.0) | 14 (56.0) | 11 (44.0) | .396 |
| DM | 9 (18.0) | 5 (20.0) | 4 (16.0) | 1.000 |
| History of a CVA | 10 (20.0) | 6 (24.0) | 4 (16.0) | .480 |
| Peripheral Vascular Disease | 1 (2.0) | 1 (4.0) | 0 (0.0) | 1.000 |
| CHA2DS2-Vasc Score | 1.6 ± 1.4 | 1.8 ± 1.4 | 1.4 ± 1.4 | .220 |
| AF Duration (Month) | 24.3 ± 22.9 | 21.4 ± 26.2 | 27.1 ± 19.2 | .250 |
| Echocardiography | ||||
| EF | 58.7 ± 10.6 | 59.4 ± 11.4 | 57.9 ± 10.0 | .547 |
| LAD | 4.9 ± 0.5 | 4.9 ± 0.4 | 5.0 ± 0.6 | .083 |
| LAV | 120.5 ± 36.0 | 118.6 ± 33.3 | 122.4 ± 39.2 | .839 |
3.2. Procedural results
In the PVI only group, in one patient the AF terminated into sinus rhythm by a PVI only. Additional CTI linear ablation for CTI dependent macroreentrant atrial flutter was performed in 4 patients. In the PVI+CFAE group, additional RA ablation was performed in 64% (16 of 25) of the patients. Termination of AF during ablation was observed in 60% (15 of 25) of the patients (directly to sinus rhythm: 1 patient, conversion to AT: 14 patient). The most common site for AF termination was the LA septum (7 of 15). In 5 patients, the AF organized into AT during the ablation in the RA. Overall, 32% (8 of 25) of the patients were converted to sinus rhythm by ablation. Non-inducibility was achieved in 56% of the patient. No major procedure related complications occurred. One patient in the PVI+CFAE group developed a groin hematoma after the procedure. The procedure time, fluoroscopy time, and ablation time were significantly longer in the PVI+CFAE group. The major procedural results are summarized in Table 2.
Table 2.
Procedural and clinical outcomes.
| Total (n = 50) | PVI + CFAE (n = 25) | PVI only (n = 25) | P value | |
| Procedure time (min) | 155.3 ± 45.0 | 190.4 ± 33.9 | 118.7 ± 17.1 | <.001 |
| Fluoroscopy time (min) | 17.3 ± 8.5 | 21.3 ± 7.7 | 13.3 ± 7.4 | <.001 |
| Ablation time (min) | 54.5 ± 24.9 | 70.7 ± 21.4 | 38.3 ± 16.0 | <.001 |
| 1-year AF-free, n (%) | 32 (64.0) | 21 (84.0) | 11 (44.0) | .006 |
| 1-year AT/AF free, n (%) | 25 (50.0) | 15 (60.0) | 10 (40.0) | .329 |
| Recurrent patients with AT | 7 (14.0) | 6 (24.0) | 1 (4.0) | .044 |
| Redo procedure, n (%) | 7 (14.0) | 3 (12.0) | 4 (16.0) | .687 |
| Patients with sinus rhythm after the initial procedure | 25 (50.0) | 15 (60.0) | 10 (40.0) | .329 |
| Patients with sinus rhythm at 1-year, n (%) | 33 (66.0) | 19 (76.0) | 16 (64.0) | .359 |
3.3. Clinical follow-up results
The PVI+CFAE group had a significantly higher 1-year AF-free survival without antiarrhythmic drugs than the PVI only group (84.0% in the PVI+CFAE group vs 44.0% in the PVI only group, P = .006, Fig. 3A). However, the AT/AF-free survival after a single procedure demonstrated no difference between the 2 groups (60.0% in the PVI+CFAE group vs 40.0% in the PVI only group, P = .329, Fig. 3B). Three patients in the PVI +CFAE group and 4 in the PVI only group received redo procedures during the follow-up period (re-isolation of PVs and CFAE ablation for the purpose of the achievement of non-inducibility, and the strategy used during the index procedure was not preserved). The sinus rhythm maintenance with a redo procedure at 1 year was 76.0% (19/25) and 64.0% (16/25) in the patients in the PVI+CFAE and PVI only groups, respectively (P = .359). AF termination during the procedure was not related to a low recurrence rate of atrial tachyarrhythmias (recurred in 6/15 in termination group vs 4/10 in the no-termination group). During one-year of the follow-up period, no patients experienced any late complications. One patient in the PVI+CFAE group received a permanent pacemaker implantation due to sick sinus syndrome after sinus rhythm restoration during the follow-up period.
Figure 2 (Continued).
Examples of electroanatomic maps of a low voltage complex fractionated atrial electrogram (CFAE) ablation. (A) Antero-posterior view and (B) Postero-anterior view of the left atrium (LA) after ablation. The left side of Figure A/B is a CFAE map and the right side a voltage map. (C) During an LA roof area CFAE ablation, atrial fibrillation converted into atrial tachycardia (AT). (D) During a CS os ablation, the AT terminated, and sinus rhythm was restored in the patient. The CFAE area was tagged with an azure dot. The ablation points are the pink and red dots. The blue dot is the AT termination site.
Figure 3.
Comparison of the Kaplan–Meier survival curves for the one-year freedom from atrial tachyarrhythmias after ablation between the 2 groups. (A) The pulmonary vein isolation (PVI) + complex fractionated atrial electrogram (CFAE) ablation group had a higher one-year atrial fibrillation free survival than the PVI only group. (B) However, there was no significant difference in the one-year atrial fibrillation / atrial tachycardia free survival between the 2 groups.
4. Discussion
Our study showed that ablation targeting the CFAE areas within low voltage zones guided by high-density mapping in addition to the PVI in patients with persistent and long-standing persistent AF had a significantly better one-year outcome in terms of the AF-free survival. However, the overall atrial tachyarrhythmia-free survival did not differ. Especially, recurrence with AT was more frequent in the additional CFAE ablation arm.
Previous studies reported a high recurrence rate after catheter ablation in patients with persistent and long-standing persistent AF.[25] To overcome this limitation, various ablation strategies have been attempted. Atrial fibrosis is the hallmark of structural remodeling and atrial fibrosis with its border zones has been identified as an important substrate for focal and reentrant activity maintaining AF.[8,18] The late gadolinium enhancement in the MRI has been reported to be associated with atrial fibrosis,[14] and there are several studies that have reported that the late gadolinium enhancement correlates with low voltage areas in electroanatomic mapping.[13,24] In lieu of this, one of the additional ablation strategies for AF is targeting areas of atrial fibrosis based on low voltage zones identified on voltage mapping.[7] Current approaches to low-voltage areas include a low voltage area isolation, linear ablation across a low-voltage area, and homogenization of a low voltage area.[23] Another approach is targeting specific electrogram characteristics within low voltage areas in addition to a PVI.[5,9] One recent study demonstrated that electrogram-guided ablation targeting only low-voltage areas of less than 0.4 mV in the LA and RA in addition to the PVI resulted in a 71.4% sinus rhythm maintenance rate after a single procedure during a mean follow-up of 21.1 ± 0.8 months.[5]
The multicenter randomized trial, Substrate and Trigger Ablation for Reduction of Atrial Fibrillation 2 (STAR-AF 2) study, failed to reveal the clinical significance of CFAE-targeted ablation in the patients with persistent AF.[26] However, the detection algorithm of the CFAEs in the STAR-AF2 study was based on the NaVX mapping system has a different algorithm than the CARTO system using the SCI and ICL software (CARTO CFAE software module, Biosense Webster, Inc., Diamond Bar, CA, USA).[16] In addition, a multielectrode mapping catheter with 1-mm electrodes (Pentaray catheter, Biosense Webster, Inc., Diamond Bar, CA, USA) used with the CARTO3 CONFIDENSE module can conduct high resolution mapping with an accurate electrogram and time annotation. Further, during the CFAE ablation, extensive ablation often occurs, and unnecessary ablation can affect both the atrial contraction and atrial compliance.[21]
Under this background, we hypothesized that ablation targeting CFAE areas only within low voltage zones using this latest high-density mapping technology with a multielectrode catheter and standardized CARTO CFAE mapping system could improve the results of the AF ablation in persistent and long-standing persistent AF patients. In our study, the one-year AF/AT-free survival rates of the PVI only group was 40% and that result demonstrated the need for an additional or alternative strategy to achieve better results. In the PVI+CFAE group, the 1-year AF-free survival rate was 84% and with a redo-procedure, 76% of patients were maintained in sinus rhythm without any anti-arrhythmic drugs. This result was better than some other studies, especially considering that the patients in this study had more long-standing persistent AF and a larger LA size than in those other studies.[5,12] In addition, entrainment mapping maneuvers with the current activation mapping technology allows precise activation mapping of ATs, hence, provides a clearer target for the procedure compared than for AF. Indeed, previous reports on RFCA of recurrent AT have shown favorable acute termination rates and a long-term arrhythmia freedom, which are in line with our study results.[1,3,4] Therefore, recurrence with ATs may be a step toward long-term sinus rhythm maintenance.[1]
One of the issues was the rhythm during the voltage/CFAE mapping. The voltages in the atrium are usually lower during AF than during sinus rhythm. Most of the previous studies performed voltage mapping during sinus rhythm. However, there has been no consensus for which rhythm is the most appropriate for substrate mapping. Selective ablation based on voltage mapping during AF had a relatively good procedural success rate[9] and another recent study reported that voltage mapping during AF is superior to mapping during sinus rhythm for localizing areas of delayed enhancement on the MRI.[20] Our study performed voltage/CFAE maps during AF. If the CFAE arm, the patients were in sinus rhythm at the beginning of the procedure, and the patients were induced into AF.
4.1. Limitations
Although randomization was performed, the small number of enrolled patients in a single center was the major limitation of our study. In addition, most patients were male, and there were several clinically important differences between groups, such as the body mass index and AF duration (even if they were not statistically significant). Ibutilide, which can improve the results of the CFAE ablation, was not available in our country.[17] As is well known, conventional CFAE ablation has many clinical limitations such as a long procedure/fluoroscopy time, frequent recurrences of AT, and requires multiple procedures for sinus rhythm maintenance.[28] Although we used the state of the art technology and equipment, these disadvantages of the CFAE ablation were still identified in our study.
5. Conclusion
Persistent AF ablation targeting CFAE areas only within the low voltage zones in addition to the PVI using a high-density mapping technology with a multielectrode catheter and the CARTO standardized CFAE mapping system had a higher AF free survival as compared to a PVI only. Although the recurrence of AF or AT did not differ in the PVI+CFAE group, the sinus rhythm maintenance rate after a redo procedure was 76%, which was higher than that in many studies including a similar patient group.
Acknowledgments
We thank Mr. John Martin for his linguistic assistance.
Author contributions
Conceptualization: Hyoung-Seob Park, Yun-Kyeong Cho, Hyuck-Jun Yoon, Hyungseop Kim, Seung-Ho Hur.
Data curation: Hyoung-Seob Park, Seongwook Han, Cheol Hyun Lee, In-Cheol Kim, Yun-Kyeong Cho, Hyuck-Jun Yoon, Hyungseop Kim, Chang-Wook Nam, Seung-Ho Hur, Yun Seok Kim, Woo Sung Jang.
Formal analysis: Jongmin Hwang, Seongwook Han, In-Cheol Kim, Jin wook Chung, Hyungseop Kim, Chang-Wook Nam, Seung-Ho Hur, Jin Young Kim, Yun Seok Kim, Woo Sung Jang.
Funding acquisition: Hyoung-Seob Park, Hyuck-Jun Yoon, Seung-Ho Hur.
Investigation: Hyoung-Seob Park, Seongwook Han, Cheol Hyun Lee, In-Cheol Kim, Yun-Kyeong Cho, Jin wook Chung, Jin Young Kim, Yun Seok Kim, Woo Sung Jang.
Methodology: Cheol Hyun Lee, Yun-Kyeong Cho, Hyuck-Jun Yoon, Jin wook Chung, Jin Young Kim, Woo Sung Jang.
Project administration: Seongwook Han, Hyuck-Jun Yoon, Hyungseop Kim, Chang-Wook Nam.
Resources: Cheol Hyun Lee, Chang-Wook Nam, Yun Seok Kim, Woo Sung Jang.
Supervision: Hyoung-Seob Park, Seongwook Han, Chang-Wook Nam, Seung-Ho Hur.
Validation: Jongmin Hwang.
Writing – original draft: Jongmin Hwang, Hyoung-Seob Park, Cheol Hyun Lee, In-Cheol Kim, Jin wook Chung, Jin Young Kim, Yun Seok Kim, Woo Sung Jang.
Writing – review & editing: Jongmin Hwang, Hyoung-Seob Park, Seongwook Han, Yun-Kyeong Cho, Hyuck-Jun Yoon, Hyungseop Kim, Chang-Wook Nam, Seung-Ho Hur.
Footnotes
Abbreviations: AF = atrial fibrillation, AT = atrial tachycardia, CFAE = complex fractionated atrial electrogram, CTI = cavo-tricuspid isthmus, ECG = electrocardiogram, ICL = interval confidence level, LA = left atrium, PeAF = persistent AF, PVI = pulmonary vein isolation, RA = right atrium, SCI = shortest complex interval.
How to cite this article: Hwang J, Park HS, Han S, Lee CH, Kim IC, Cho YK, Yoon HJ, Chung Jw, Kim H, Nam CW, Hur SH, Kim JY, Kim YS, Jang WS. Ablation of persistent atrial fibrillation based on high density voltage mapping and complex fractionated atrial electrograms: a randomized controlled trial. Medicine. 2021;100:31(e26702).
Conflicts of interest: Dr. Hyoung-Soeb Park received research funding from Biosense Webster Inc. For the remaining authors none were declared.
Source of funding: This work was supported by an Investigator-Initiated Study Grant from Biosense Webster Inc.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Values are presented as the mean ± standard deviation or n (%). BMI = body mass index, BSA = body surface area, CFAE = complex fractionate atrial electrogram, CVA = cerebrovascular disease, DM = diabetes mellitus, EF = ejection fraction, HF = heart failure, HTN = hypertension, LAD = left atrial dimension, LAV = left atrial volume, PVI = pulmonary vein isolation, SR = sinus rhythm.
AF = atrial fibrillation, AT = atrial tachycardia, CFAE = complex fractionate atrial electrogram, PVI = pulmonary vein isolation.
References
- [1].Ammar S, Hessling G, Reents T, et al. Arrhythmia type after persistent atrial fibrillation ablation predicts success of the repeat procedure. Circ Arrhythm Electrophysiol 2011;4:609–14. [DOI] [PubMed] [Google Scholar]
- [2].Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chae S, Oral H, Good E, et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol 2007;50:1781–7. [DOI] [PubMed] [Google Scholar]
- [4].Choi Y, Kim S, Baek JY, et al. Acute and long-term outcome of redo catheter ablation for recurrent atrial tachycardia and recurrent atrial fibrillation in patients with prior atrial fibrillation ablation. J Interv Card Electrophysiol 2020;doi: 10.1007/s10840-020-00795-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [5].Efremidis M, Vlachos K, Letsas KP, et al. Targeted ablation of specific electrogram patterns in low-voltage areas after pulmonary vein antral isolation in persistent atrial fibrillation: termination to an organized rhythm reduces atrial fibrillation recurrence. J Cardiovasc Electrophysiol 2019;30:47–57. [DOI] [PubMed] [Google Scholar]
- [6].Haissaguerre M, Hocini M, Sanders P, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol 2005;16:1138–47. [DOI] [PubMed] [Google Scholar]
- [7].Hansen BJ, Zhao J, Fedorov VV. Fibrosis and atrial fibrillation: computerized and optical mapping. JACC 2017;3:531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jadidi AS, Cochet H, Shah AJ, et al. Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: combined magnetic resonance imaging and high-density mapping. J Am Coll Cardiol 2013;62:802–12. [DOI] [PubMed] [Google Scholar]
- [9].Jadidi AS, Lehrmann H, Keyl C, et al. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol 2016;9:e002962. [DOI] [PubMed] [Google Scholar]
- [10].Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- [11].Kottkamp H, Berg J, Bender R, Rieger A, Schreiber D. Box isolation of fibrotic areas (BIFA): a patient-tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2016;27:22–30. [DOI] [PubMed] [Google Scholar]
- [12].Lee K-N, Choi J-I, Kim YG, et al. Comparison between linear and focal ablation of complex fractionated atrial electrograms in patients with non-paroxysmal atrial fibrillation: a prospective randomized trial. EP Europace 2019;21:598–606. [DOI] [PubMed] [Google Scholar]
- [13].Malcolme-Lawes LC, Juli C, Karim R, et al. Automated analysis of atrial late gadolinium enhancement imaging that correlates with endocardial voltage and clinical outcomes: A 2-center study. Heart Rhythm 2013;10:1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McGann C, Akoum N, Patel A, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol 2014;7:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moher D, Schulz KF, Altman D. Group ftC.The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285:1987–91. [DOI] [PubMed] [Google Scholar]
- [16].Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044–53. [DOI] [PubMed] [Google Scholar]
- [17].Oketani N, Seitz J, Salazar M, et al. Ablation of complex fractionated electrograms is useful for catheter ablation of persistent atrial fibrillation: protagonist point of view. Heart Rhythm 2016;13:2098–100. [DOI] [PubMed] [Google Scholar]
- [18].Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol 2011;58:2225–32. [DOI] [PubMed] [Google Scholar]
- [19].Porter M, Spear W, Akar JG, et al. Prospective study of atrial fibrillation termination during ablation guided by automated detection of fractionated electrograms. J Cardiovasc Electrophysiol 2008;19:613–20. [DOI] [PubMed] [Google Scholar]
- [20].Qureshi NA, Kim SJ, Cantwell CD, et al. Voltage during atrial fibrillation is superior to voltage during sinus rhythm in localizing areas of delayed enhancement on magnetic resonance imaging: an assessment of the posterior left atrium in patients with persistent atrial fibrillation. Heart Rhythm 2019;16:1357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reddy YNV, El Sabbagh A, Packer D, Nishimura RA. Evaluation of shortness of breath after atrial fibrillation ablation-Is there a stiff left atrium? Heart Rhythm 2018;15:930–5. [DOI] [PubMed] [Google Scholar]
- [22].Rolf S, Kircher S, Arya A, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:825–33. [DOI] [PubMed] [Google Scholar]
- [23].Sim I, Bishop M, O’Neill M, Williams SE. Left atrial voltage mapping: defining and targeting the atrial fibrillation substrate. J Interv Card Electrophysiol 2019;56:213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Spragg DD, Khurram I, Zimmerman SL, et al. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: Success and limitations. Heart Rhythm 2012;9:2003–9. [DOI] [PubMed] [Google Scholar]
- [25].Tilz RR, Rillig A, Thum AM, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol 2012;60:1921–9. [DOI] [PubMed] [Google Scholar]
- [26].Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- [27].Verma A, Sanders P, Champagne J, et al. Selective complex fractionated atrial electrograms targeting for atrial fibrillation study (SELECT AF): a multicenter, randomized trial. Circ Arrhythm Electrophysiol 2014;7:55–62. [DOI] [PubMed] [Google Scholar]
- [28].Wong KC, Paisey JR, Sopher M, et al. No benefit of complex fractionated atrial electrogram ablation in addition to circumferential pulmonary vein ablation and linear ablation: benefit of complex ablation study. Circ Arrhythm Electrophysiol 2015;8:1316–24. [DOI] [PubMed] [Google Scholar]