Abstract
Background:
We tested the workflow and comparability of compression garments (CG) automatically knitted from 3D-body-scan data (3DBSD) versus manually measured data for scar treatment. Industry 4.0 has found its way into surgery, enhancing the trend toward personalized medicine, which plays an increasingly important role in CG scar therapy. Therefore, we conducted a study to evaluate the workflow from 3DBSD to fast and precisely knitted CG and compared it with standard of care.
Methods:
A randomized controlled crossover feasibility study was conducted as part of the individual medical technology research project “Smart Scar Care.” Objective and patient-reported outcome measures were documented for 10 patients with hypertrophic burn scars at baseline and after wearing CG automatically knitted from 3DBSD versus CG from manually measured data for one month.
Results:
The “scan-to-knit” workflow and the study design were feasible in 10 of 10 patients. No adverse effects were found. 3DBSD showed a bias of half a centimeter compared with manually measured data and wider limits of agreement. With respect to fit, comfort, suitability, Vancouver Scar Scale, Patient and Observer Scar Assessment Scale, stiffness and microcirculation, this was a promising pilot study. Stiffness and blood flow were increased in scars compared with normal skin. The highest rank correlations were found between pain and itch, stiffness and Patient and Observer Scar Assessment Scale, Vancouver Scar Scale, and pain.
Conclusions:
These results indicate that automatically knitted CG using 3DBSD could become an alternative to the standard of care, especially with regard to economical and faster patient care. The produced scan data opens the door for objective scar science.
INTRODUCTION
Skin scarring can occur after trauma, burns, and surgery and be associated with pain, tightness, pruritus, and limited mobility. Methods of prevention and treatment of small scars include tension reduction, compression, silicone, pulsed-dye or CO2 laser, scar-massage, corticosteroids, 5-fluoruracil, bleomycin, and fat grafting.1,2 However, post-burn scarring regularly involves wide body surface areas and includes various qualities of scar formation; thus, treatments designed to reduce scarring in burn patients are of special interest.3 Burn garments are used to reduce scar height, increase scar pliability, and improve clinical appearance. Recent data indicate that early application of pressure on scars results in decreased contraction; reduced scar thickness, hardness, and strength; and improved biomechanics.4,5 However, it usually takes days to weeks before a compression garment is delivered. Custom modifications are often required to improve its fit. Measurements are typically taken by putting a measuring tape on the sensitive scarred skin. This can be painful and there is a risk of infection to residual wounds. Moreover, patients’ compliance depends on satisfaction with the garment. Compliance with wearing the garment can be improved by optimizing its pressure, heat and moisture transport characteristics, fit, comfort, and suitability. Hence, personalization and reproducibility are key aspects of creating a compression garment. Therefore, we developed a fast and precise automatic “scan-to-knit” technology using Industry 4.0 that allows for the creation and customization of an individual compression garment with “no touch technique.” Industry 4.0 represents automation and data exchange in manufacturing technologies.
The aims of this study were to determine if 3D-body-scan data and manually measured data (MMD) of patients with post-burn scars are comparable, if the “scan-to-knit” workflow works and if individually automated, knitted compression garments (CG) are a potential alternative to standard care. To this end, we conducted a randomized controlled feasibility study.
METHODS
Trial Design and Ethical Approval
The study was conducted as a randomized controlled crossover study in a German University Hospital from September 2018 to December 2018 after approval by the ethics committee (authorization number: 18-174). It represents the clinical part of an individual medical technology research project (named “Smart Scar Care”) that was performed from August 2016 until July 2019. A process was developed to provide burn patients with a compression garment within 24 hours, which was adapted to their individual body dimensions. For this purpose, the individual dimensions of the patient were recorded using a 3D-body-scanner (Artec Eva, Artec 3D, Luxembourg). The scan data were loaded into a configurator system that allows for the simulation of the body and the garment, with options for modifying geometry, material, and compression pressure. The configuration data were then interpreted and transferred to a flat-knitting machine (CMS ADF-3, Stoll, Reutlingen, Germany) (Fig. 1).6 The automated procedure was expected to enhance precision and reproducibility by reducing human subjectivity in the measurement process.
Fig. 1.
Workflow of the “Smart Scar Care” process.
This study was performed in accordance with the standards set by the Declaration of Helsinki and the relevant regulations of HIPAA. It complies with the reporting standards established by the CONSORT guidelines (Consolidated Standards of Reporting Trials). The protocol is registered at ClinicalTrials.gov (NCT03664505).
Patients and Acquisition
Ten patients with hypertrophic burn scars on the upper extremity were included in the study. For inclusion, exclusion, and treatment discontinuation criteria, see Table 1.
Table 1.
Inclusion, Exclusion, and Treatment Discontinuation Criteria
| Inclusion Criteria | Exclusion Criteria | Treatment Discontinuation Criteria |
|---|---|---|
| Burn injury occurred from 1 month until 2 years before the study started | Patient uses immune suppressant medication | Severe pain caused by the burn garment |
| BSA was 1%–30% and included the upper extremity | Patient suffers from diseases influencing wound or scar healing | Pressure marks caused by the burn garment |
| Scars have hypertrophic parts | Circulatory disturbances caused by the burn garment | |
| Patient has worn a standard burn garment for at least 1 month | ||
| Patient is aged 18 years or older |
Treatment and admission were as usual. Patients were recruited after prescreening by mail and phone call. They were included after giving informed consent.
Interventions
The patients’ visits were at days 0, 7, 28, 35, 56, and 63 (Touchpoints 1-6; TP1-6) (Table 2). In short, at TP1 randomization was performed, the first measurements were taken manually at standard arm measurement points7 and also using 3D-scan technology. (See figure 1, Supplemental Digital Content 1, which displays measure data collection (A: standard measurement points with c: circumference and l: distance of the corresponding circumference to the wrist, B: Manual measure, C: 3D-scan, D: 3D-scan image from the arm). http://links.lww.com/PRSGO/B703.)
Table 2.
Time Schedule
| Touchpoints | 0 | 1 | 2 | 3 | 4 | 5 | 6 | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Time point | Before inclusion | Day 0 | Day 7 | Day 28 | Day 35 | Day 56 | Day 63 | Day 93 |
| Monitoring | Initialization of the study | On-site monitoring visits | Close-out visit | |||||
| Prescreening (check inclusion criteria) | X | |||||||
| Information letter to the patient by mail | X | |||||||
| Information call of the patient | X | |||||||
| Inclusion (informed consent, check exclusion criteria, randomization, and allocation) | X | |||||||
| Intervention: | ||||||||
| – Measure (manual and 3D scan) | X | X | X | X | X | X | ||
| – Measure (manual and 3D scan) used for compression garment | X | X | ||||||
| – Wearing standard compression | X | X | X | |||||
| – Wearing compression garment (depending on assignment) | X | X | X | |||||
| – Wearing compression garment (other group) | X | X | X | |||||
| Analysis: | ||||||||
| – CRFs | X | X | X | X | X | X | ||
| – Stiffness | X | X | X | X | X | X | ||
| – Microcirculation | X | X | X | X | X | X | ||
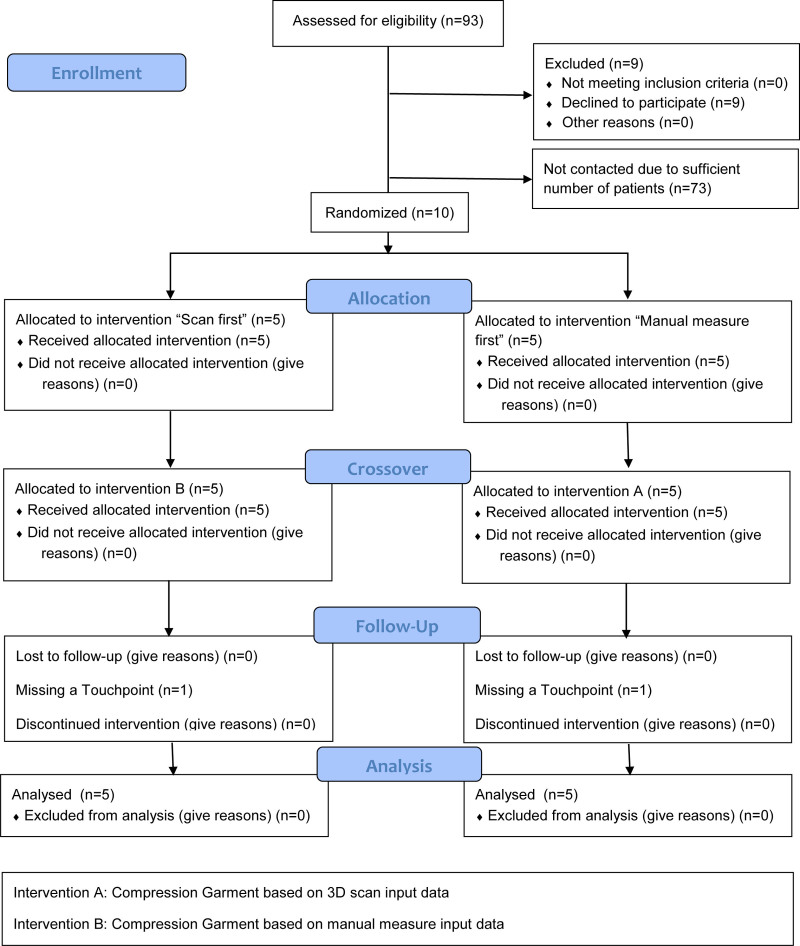
Assessed for eligibility (n = 93).
Case report forms (CRFs) were completed and objective data were collected. Next, second manual measurements were taken. Depending on the randomization result, the CG were knitted using 3D-scan data (group “Scan first”) or MMD (group “Manual measure first”). The patients underwent the same procedures at the following touchpoints and received the garments at TP2 and TP4, wearing them for 1 month. (See figure 2, Supplemental Digital Content 2, which displays CG created and used in this study (A: Individualized compression garment using 3D-scan data, B: Left-sided compression garment using MMD and right-sided using 3D-scan data, meshes are visible in detail). http://links.lww.com/PRSGO/B704.) In short, there were three periods of treatment with assessments in the middle and at the end of each. Treatment was unspecified in the first period and subsequently randomized to different sequences of treatments.
Safety
All products were Conformité Européenne (CE)-certified, made from the same materials and used as intended by the manufacturer. Safety was ensured by testing the compression levels of the individually produced burn garments on Hohenstein system compression test device (HOSY)8 before fitting them to the patients. An independent monitor regularly visited the study site and controlled each step, as well as the entire documentation, during an initiation visit, on-site visits and a close-out visit.
Outcomes
The patients reported patient scar assessment scale (PSAS) scores and values of numeric rating scales with 0–10 points that were summarized for fit, comfort, and suitability. A higher number meant a worse subjective outcome. Subsequently, a clinical investigator with experience in the assessment and treatment of burn scars and in conducting clinical trials documented the observer scar assessment scale (OSAS), Vancouver scar scale (VSS; 0–18 points)9 and possible adverse effects on the observer case report form. (See figure 3, Supplemental Digital Content 3, which displays A: Case report form, B: Overview of the subjective data, C: Overview of the objective data. http://links.lww.com/PRSGO/B705.) Patient and observer scar assessment scale (POSAS) is defined as the sum of PSAS and OSAS questionnaires (12-120 points).10
Scar stiffness (N/m) was assessed by measuring skin elasticity. To this end, a special device (ElastiMeter, Delfin Technologies, Kuopio, Finland)11 was briefly pressed on four representative hypertrophic scar areas. Elasticity was then determined by a force sensor and the mean value was calculated. Microcirculation was assessed by applying an optical fiber probe of a combined laser Doppler and spectrophotometer (Oxygen-to-see, Lea Medizintechnik, Gießen, Germany)12 onto the same areas for 1 minute. The mean values for oxygen saturation, blood flow, and postcapillary venous filling pressure were calculated in a depth of 2 and 6 mm.
Sample Size
In this feasibility study, successful applications were counted and their proportion estimated in a 95% CI. In case of 100% success, the interval reaches from about 1– 3/n to 100%. In total, 10 patients were included in the study and were assessed at six touchpoints so that CIs could range from about 70%–100% of patients or from about 92%–100% of assessments.
Clinically relevant patient-reported outcomes would require a higher sample size. Replication of prior effects on fit indicated that 12 patients were needed for 80% power at 5% significance level, as the SD of individual differences was 1.2 points on a numerical rating scale when the mean difference was 0.8 points. Thus, not even a large effect could have been detected in this pilot study, let alone the minimum clinically interesting difference of perhaps 0.5 SD or 0.6 points.
Randomization, Allocation, Implementation and Blinding
The balanced pseudorandomized allocation sequence was generated in a reproducible way as permuted blocks of confidential length. It was kept in opaque envelopes by a person with no other role in the study. There were 10 envelopes, five containing the allocation to use of 3D-scan data first and five containing the allocation to use of MMD first. The envelopes were labeled with consecutive numbers and opened by a trustee in this order, whenever a patient was included in the study. The patients were blinded to the assignment; they were not told which compression garment was based on 3D-scan data or on MMD.
Statistical Methods
Agreement of measurements by scanner and by hand was described by mean difference “bias” with 95%-CI and by limits of agreement (LOA) for raw data and for their logarithms. The difference of treatment effects was estimated by analysis of covariance with fixed factor treatment and covariable time. Random patient effects were included if estimable. VSS, POSAS, and its subscales were logit-transformed so that residuals could be normally distributed. Missingness of 2 assessments threatened the balance of the design, but was mitigated by multiple imputation and use of Rubin’s rule. For statistical analysis, the software R with packages crossdes, nlme, and mice was used. Correlations between subjective and objective assessments were partial rank correlations. Individual mean ranks were subtracted; thus, the interpretation is: What to expect during the course of an individual history?
RESULTS
Patients and Population Data
For recruitment and enrollment, see Figure 2. In total, three patients were men, seven women. None of them had a relevant preexisting condition. Population data are shown in Table 3.
Fig. 2.
Flow Diagram.
Table 3.
Population Data
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |
|---|---|---|---|---|---|---|
| Age (y) | 19 | 31.8 | 37 | 40.2 | 42.5 | 76 |
| Duration (mo) last operation to study | 3 | 4.3 | 9 | 9.1 | 13.3 | 17 |
| Duration (mo) first compression to study | 3 | 4 | 8.5 | 8.8 | 13 | 17 |
| TBSA | 1 | 1.5 | 4.5 | 8.9 | 12 | 30 |
| BSA IIa | 0 | 0 | 0 | 2.2 | 2.8 | 12 |
| BSA IIb | 0 | 0.3 | 1 | 3.7 | 7.5 | 12 |
| BSA III | 0 | 0 | 0 | 3 | 2.5 | 15 |
Population as minimum, 25%-, 50%-, 75%-quantiles, mean and maximum [(T)BSA: (Total) burned surface area (%)].
Patients 4, 5, 6, 7, and 10 were assigned to “Scan first,” and patients 1, 2, 3, 8, 9 to “Manual measure first.” The respective other intervention was used 1 month later. No treatment discontinuation was necessary.
Precision of the 3D-scan Data Compared with Manual Measure Data
3D-scan data showed a bias of about half a centimeter or 2% compared with manual measure data (Fig. 3) resulting in LOA of −4% to +9% for the difference between scan and manual measurement. Both manual measures did not differ on average and had narrower LOA of ±3%, ie, were clinically more precise (Table 4). Garment pressure measurements were consistent with compression class 2 according to RAL-GZ 387/2, ranging from 23 to 32 mm Hg.
Fig. 3.
Bland-Altman diagram of manual vs. 3D-scan measure. Bland-Altman diagram showing differences between 3D-scan data and manual measure data sorted by the different measure points.
Table 4.
Agreement of Measurements
| Bias | 95% Confidence Interval | Limits of Agreement | |
|---|---|---|---|
| Scan—Manual 1 | 0.51 | (0.44; 0.57) | [−0.89; 1.9] |
| Scan—Manual 2 | 0.56 | (0.49; 0.63) | [−0.92; 2.05] |
| Manual 1—Manual 2 | 0.06 | (0.03; 0.09) | [−0.63; 0.75] |
Bias: Mean of difference.
Feasibility
All treatments were feasible in 10 of 10 patients (CI 72%–100%). Two assessments could not be performed. Feasibility of 58/60 assessments (97%, CI 89%–99%) was not perfect because two patients did not present themselves at single, different time points. One received garments knitted using 4-week-old measurements. The other just received his garment by mail.
Subjective Data
Differences and 95% CI in the mean values between the groups “Standard,” “Scan first,” and “Manual measure first” are shown in Table 5. No significant differences were found, but tendencies emerged (See figure 3, Supplemental Digital Content 3, which displays A: Case report form, B: Overview of the subjective data, C: Overview of the objective data. http://links.lww.com/PRSGO/B705).
Table 5.
Effects on Subjective and Objective Endpoints
| Difference of Means | 95% CI | ||
|---|---|---|---|
| Fit | |||
| Manual—standard | 5.1 | −4.6 | 15 |
| Scan—standard | 6.6 | −2.9 | 16 |
| Scan—manual | 1.3 | −4.6 | 7.2 |
| Comfort | |||
| Manual—standard | −2.6 | −8.7 | 3.4 |
| Scan—standard | −4.4 | −10 | 1.6 |
| Scan—manual | −1.8 | −5.5 | 1.9 |
| Suitability | |||
| Manual—standard | −2.3 | −16 | 12 |
| Scan—standard | 4.3 | −9.7 | 18 |
| Scan—manual | 6.3 | −2.4 | 15 |
| logit.POSAS | |||
| Manual—standard | 0.35 | −0.46 | 1.2 |
| Scan—standard | 0.17 | −0.62 | 0.96 |
| Scan—manual | −0.19 | −0.67 | 0.3 |
| logit.VSS | |||
| Manual—standard | 0.71 | −0.16 | 1.6 |
| Scan—standard | 0.74 | −0.098 | 1.6 |
| Scan—manual | 0.023 | −0.5 | 0.55 |
| Stiffness | |||
| Manual—standard | 3.9 | −35 | 43 |
| Scan—standard | −9.7 | −47 | 28 |
| Scan—manual | −14 | −38 | 9.4 |
| sO2.superficial | |||
| Manual—standard | 0.21 | −8.3 | 8.7 |
| Scan—standard | 1 | −7.4 | 9.4 |
| Scan—manual | 0.83 | −4.4 | 6 |
| Flow.superficial | |||
| Manual—standard | −7 | −19 | 4.8 |
| Scan—standard | −1.6 | −13 | 10 |
| Scan—manual | 5.4 | −1.7 | 13 |
| rHb.superficial | |||
| Manual—standard | −1.1 | −8.6 | 6.5 |
| Scan—standard | −1.6 | −8.7 | 5.5 |
| Scan—manual | −0.14 | −4.7 | 4.4 |
| sO2.deep | |||
| Manual—standard | −3.4 | −10 | 3.3 |
| Scan—standard | −4.5 | −11 | 2 |
| Scan—manual | −1.5 | −5.7 | 2.8 |
| Flow.deep | |||
| Manual—standard | −11 | −37 | 14 |
| Scan—standard | −3.2 | −29 | 22 |
| Scan—manual | 8.9 | −6.9 | 25 |
| rHb.deep | |||
| Manual—standard | −2.5 | −7.8 | 2.9 |
| Scan—standard | −2.4 | −7.5 | 2.6 |
| Scan—manual | −0.09 | −3.2 | 3 |
Effects on subjective and objective endpoints (in case of POSAS and VSS as logarithm) in random effects ANCOVA adjusted for linear sequence effect using multiple imputation. Fit encompassed 6 items: limited mobility, pressure marks, pain, bad fit, inflexibility to the body, irregular compression; comfort 5 items: skin dryness, heat, touch stimulus, weight, difficult dressing; and suitability 10 items: restriction at work, home, hobby, social life, dressing, writing, using a computer, driving, eating, using a phone. [Manual: “Manual measure first” group, Standard: “Standard” group, Scan: “Scan first” group, CI: confidence interval, sO2: Oxygen saturation (%), Flow: Blood flow (AU), rHb: Relative hemoglobin (AU)].
There was a tendency for poorer fit after wearing the compression garment using 3D-scan data in comparison with manual data, independent of the allocation sequence. In comparison with standard treatment, CG after 3D-scan data tended to be assessed worse in period 1 and better in period 2. The garments using manual measurements tended to be rated as good as the standard in the first period, but better in the second period.
Comfort of the garments based on experimental measurements tended to be rated better than in the standard treatment. Moreover, after changing the garments at TP4, the rating seemed to improve.
Suitability was rated nearly the same as standard treatment in CG produced using 3D-scan data in period 1. Afterward, it tended to be rated better. Using data from manual measurements showed a tendency for better suitability.
Scar assessment using VSS(logit) showed a tendency for better results over time. Treatment with 3D-scan data-based garments tended to be rated poorer in period 1 in comparison with standard treatment.
POSAS(logit) also showed a tendency for better results over time. Differences between the groups were almost zero.
Stiffness of the Burned Skin
Scar stiffness and microcirculatory data did not show significant differences. A slight tendency for higher stiffness in manual measurement-based CG compared with garments based on 3D data was found.
Oxygen saturation and blood flow showed a tendency for lower values over time. Relative hemoglobin tended to lower values in the deeper skin over time (see SDC3, http://links.lww.com/PRSGO/B705).
Objective Data in Scars and in Normal Skin
Measurements at baseline are summarized in Table 6. Stiffness and blood flow showed differences between scars and healthy skin, while oxygen saturation and deep relative hemoglobin did not. Relative hemoglobin was slightly higher in superficial scars than in superficial healthy skin.
Table 6.
Measurements
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |
|---|---|---|---|---|---|---|
| Stiffness (scar) | 48.5 | 71.5 | 121 | 125.1 | 158.5 | 230.5 |
| Stiffness (healthy skin) | 37 | 53.5 | 77.5 | 76.3 | 99.3 | 111 |
| sO2.superficial (scar) | 42.3 | 56.4 | 61.6 | 63.6 | 71.4 | 89.5 |
| sO2.superficial (healthy skin) | 42 | 50.5 | 63.5 | 59.4 | 68 | 73 |
| Flow.superficial (scar) | 11.8 | 19.3 | 40.6 | 39.9 | 54.8 | 85.3 |
| Flow.superficial (healthy skin) | 10 | 15 | 19.5 | 29.1 | 29.8 | 90 |
| rHb.superficial (scar) | 45.5 | 61.1 | 70.8 | 68 | 73.4 | 82 |
| rHb.superficial (healthy skin) | 34 | 49 | 52.5 | 51.7 | 55.3 | 72 |
| sO2.deep (scar) | 67.8 | 71.8 | 75.4 | 77.1 | 82.4 | 90.5 |
| sO2.deep (healthy skin) | 50 | 75.3 | 83 | 78.6 | 85.8 | 92 |
| Flow.deep (scar) | 19.3 | 46.6 | 104 | 99 | 138.2 | 205 |
| Flow.deep (healthy skin) | 18 | 28.3 | 82 | 85.4 | 125.8 | 200 |
| rHb.deep (scar) | 30.8 | 36.3 | 38.9 | 41.6 | 45.4 | 62.5 |
| rHb.deep (healthy skin) | 17 | 32.5 | 40 | 38.2 | 45 | 51 |
Population as minimum, 25%-, 50%-, 75%-quantiles, mean, and maximum [sO2: Oxygen saturation (%), Flow: Blood flow (AU), rHb: Relative hemoglobin (AU)].
Correlations between Subjective and Objective Data
Pain and itch sensations showed a high correlation. Stiffness of the skin correlated with POSAS, VSS, and pain, but not exclusively with itch. Oxygen saturation, blood flow, and relative hemoglobin were only weakly correlated with pain or itch.
Harms and Safety
Treatment with CG was well tolerated by all patients. No pain or allergic reaction to the material or other adverse sensations were reported. No device-related issues were observed during or after the measurements.
Validation
We found that the study and the workflow “scan-to-knit” were feasible. However, a digital environment with internet connectivity and garment shipping are required. Manual and 3D-scan measurements were obtained by an experienced orthopedic technician. Post-scan processing of the 3D data (eliminating artifacts) was performed by a computer expert. The subsequent process until knitting of the garment was automatic (Fig. 4). (See Video 1 [online], which demonstrates the 3D-scan process and the 2D representation for knitting the 3D-scan geometry.)
Fig. 4.
Simulation of compression garment in comparison with scan data.
Video 1. Video 1 from “Smart Scar Care - Industry 4.0 in individualized Compression Garments: A Randomized Controlled Crossover Feasibility Study”.
DISCUSSION
New technologies like augmented reality, virtual reality, robotics, artificial intelligence, big data analytics, machine learning, and automation from diagnosis to treatment are becoming more and more available in various medical fields.13–15 Positive trends toward personalized medicine are also noted in the field of surgery. 3D-body-scan technology allows for the evaluation of anthropometry, eg, in body-contouring surgery and skin texture.16,17
We showed that data obtained by 3D-body-scan can be used to automatically produce a personalized compression garment. The “scan-to-knit” procedure was fast and precise, and the bias to manual measurements might be due to a tighter pull of the measuring tape and can be adjusted in future scans. In comparison with manual measurements, no interpersonal bias exists, but the use of the 3D-scanner needs training to reduce artifacts. In smaller areas, eg, the interdigital spaces, often virtual webs resulted due to interpolation errors during the scanning process (Fig. 5). A full-body scanner with more reliable 3D-scan requires considerable amounts of space; using one in a clinical setting is not realistic. Additionally, it is not possible for all patients to stand motionless. We used a height-adjustable positioning aid for the arm, to minimize movement artifacts based on Schwarz-Muller et al, who stabilized the posture during the scanning process with handles on telescopic tubes without compromising the reflected light.18 Today, new handheld 3D-scanners with integrated accelerometer, gyro, and compass improve the quality of the scan. They also offer onboard automatic processing.
Fig. 5.
Interdigital scan artifacts.
The 3D-scan data were used in a treatment configurator software, allowing for the customization of pressure, special zones eg, for silicone pads, closing mechanism, length, color, and yarn. (See figure, Supplemental Digital Content 4, which displays A: Configuration of style and material, B: Adjustment of compression, C: Adjustment of special zones. http://links.lww.com/PRSGO/B706.)
Throughout this study, we did not change these parameters. However, this personalization process might have improved subjective data. In this context, heat transmission, moisture, and pressure can be adjusted to meet individual needs and therefore may improve the patients’ compliance, which is the most important aspect in compression garment treatment, besides fitting, efficacy, and cost-effectiveness.4
Data transfer through a secure internet data tunnel from the clinic to the knitting machine via a computer-aided manufacturing system was robust. The data were finally compiled, using a 3D-knit interpreter for the special flat-knitting machine; this technology allows for the creation of a 3D-knit through adjustable quantity and design of the meshes to fit a predefined anatomical shape.
The process after the 3D-scan should be seamlessly digital. This has the benefits that compression therapy can be started without delay and that the garment will arrive in time to be worn in a postclinical rehabilitation facility.
We positively tested safety of the CG on HOSY. To determine the compression of a medical product, it is fixed and stretched to specified circumferences. Based on the force values determined at the body circumferences, the compression is calculated.7 However, simulation of function regarding pressure distribution and temperature has to be improved in future projects. Virtual fit simulation tools may help achieve this objective.19
A crossover design, in which each subject receives each treatment in a row, was used to eliminate interpersonal rating differences of the different CG. It has the advantage that individual subject differences are eliminated from the overall treatment effect, thereby increasing the statistical performance. We chose patients that were experienced with burn CG for high validity and reliability of subjective data. The assessment of fit, comfort, and suitability did not show significant differences between the groups. Because there are no validated scales for the measurements, we used appropriate subscale items.20 However, quality of life and compliance have to be evaluated in long-term studies.
Each patient wore the garment without adverse effects. There were no significant differences in objective parameters between the groups. However, the time period of the study was short, and it is known that the maturation of scars might take years21; so major changes could not be expected. The stiffness between scars and healthy skin showed significant differences, and it was correlated to pain, but not to itch, while pain and itch sensations correlated. Pain and stiffness seem to be significant predictors for reduced long-term scar quality.22 Reducing the stiffness of scars might therefore reduce pain. This underlines the importance of scar therapy.
In general, scar assessment is challenging due to their great variability.23–25 Until now, objective measurement tools for accurate and reproducible evaluation of scars are rare.26 The detailed 3D-data generated by this novel technology offers new possibilities and additional evidence in clinical scar studies. It might be a promising tool for analyzing controversial treatment methods in an objective way. However, the next steps should be to examine the influence of compression garment treatment using different compression levels, different yarns, and silicone pads on scars. Additionally, studies should be conducted to find out whether quality of life and compliance are higher when individualized CG are used that offer ongoing personalization, eg, by modifying heat transmission and moisture using different mesh pore volumes, diameters, and fiber surfaces to reduce sweating.
LIMITATIONS
The cohort was very small and with varying scar qualities on the upper extremity. This precluded the replication of effects known from the literature.27 However, this was a feasibility study with larger studies to follow. Personalization, such as adjusting compression zones, heat or moisture of the individual CG, is a major advantage of this novel technique. However, this was not performed during the study because it would have further affected the comparability of the outcome parameters. The time period for data collection was short; thus, scar quality might not have changed significantly. Blinding was difficult because patients might have seen the meshes on the CG. Nevertheless, after the study patients were asked whether they knew which group had these meshes and they were not sure. Scan artifacts were checked manually and interrupted the automatic workflow. In the future, posture normalization of body-scans might be possible with statistical shaping.28 Additionally, we did not record compliance.
CONCLUSIONS
This feasibility study demonstrates that individual CG automatically produced using Industry 4.0 are effective. This novel technology may be beneficial for clinical practice and scientific use, promising further evidence for use in scar therapy.
ACKNOWLEDGMENTS
The authors thank Jens Becker and Andreas Herzberg from Technische Orthopädie Lübeck for yielding their expert know-how and to obtain measurements. The authors also thank Sylvia Bothmer for her friendly assistance during the study. Furthermore, thanks go to all patients that took part in the study.
Supplementary Material
Footnotes
Published online 15 July 2021.
Presented at the 37th Meeting of German Society of Burn Surgery (DAV) in 2019.
Disclosure: Dominik Michel and Rainer Trieb are employed by the manufacturer of the configurator system. Sebastian Bannwarth, Simone Maly, Anika Dallmann, and Sebastian Klasen are employed by the manufacturer of the compression garments. Wolfgang Rempp is employed by the manufacturer of the flat-knitting machine. All the other authors have no financial interest to declare in relation to the content of this article. This study was funded by the Federal Ministry of Education and Research Germany (BMBF; funding announcement “Individualisierte Medizintechnik,” grant number 13GW0117F).
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Khansa I, Harrison B, Janis JE. Evidence-based scar management: how to improve results with technique and technology. Plast Reconstr Surg. 2016;138(3 suppl):165S–178s. [DOI] [PubMed] [Google Scholar]
- 2.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254:217–225. [DOI] [PubMed] [Google Scholar]
- 3.Finnerty CC, Jeschke MG, Branski LK, et al. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet. 2016;388:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeBruler DM, Baumann ME, Blackstone BN, et al. Role of early application of pressure garments following burn injury and autografting. Plast Reconstr Surg. 2019;143:310e–321e. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, Willard JJ, Supp DM, et al. Burn scar biomechanics after pressure garment therapy. Plast Reconstr Surg. 2015;136:572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artschwager A, Surc D, Bauer B. Smart scar care schlussbericht. Deutsche Institute für Textil- und Faserforschung Denkendorf. 2019;1:1-38. [Google Scholar]
- 7.Brorson H, Höijer P. Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J Plast Surg Hand Surg. 2012;46:410–415. [DOI] [PubMed] [Google Scholar]
- 8.Hegarty-Craver M, Kwon C, Oxenham W, et al. Towards characterizing the pressure profiles of medical compression hosiery: an investigation of current measurement devices and techniques. The Journal of The Textile Institute. 2015;106(7):757–767. [Google Scholar]
- 9.Nedelec B, Shankowsky HA, Tredget EE. Rating the resolving hypertrophic scar: comparison of the vancouver scar scale and scar volume. J Burn Care Rehabil. 2000;21:205–212. [DOI] [PubMed] [Google Scholar]
- 10.van der Wal MB, Tuinebreijer WE, Bloemen MC, et al. Rasch analysis of the Patient and Observer Scar Assessment Scale (POSAS) in burn scars. Qual Life Res. 2012;21:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virén T, Iivarinen JT, Sarin JK, et al. Accuracy and reliability of a hand-held in vivo skin indentation device to assess skin elasticity. Int J Cosmet Sci. 2018;40:134–140. [DOI] [PubMed] [Google Scholar]
- 12.Kisch T, Helmke A, Schleusser S, et al. Improvement of cutaneous microcirculation by cold atmospheric plasma (CAP): results of a controlled, prospective cohort study. Microvasc Res. 2016;104:55–62. [DOI] [PubMed] [Google Scholar]
- 13.Mirnezami R, Ahmed A. Surgery 3.0, artificial intelligence and the next-generation surgeon. Br J Surg. 2018;105:463–465. [DOI] [PubMed] [Google Scholar]
- 14.Tepper OM, Rudy HL, Lefkowitz A, et al. Mixed reality with HoloLens: where virtual reality meets augmented reality in the operating room. Plast Reconstr Surg. 2017;140:1066–1070. [DOI] [PubMed] [Google Scholar]
- 15.Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019;20:e262–e273. [DOI] [PubMed] [Google Scholar]
- 16.Adler C, Steinbrecher A, Jaeschke L, et al. Validity and reliability of total body volume and relative body fat mass from a 3-dimensional photonic body surface scanner. PLoS One. 2017;12:e0180201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Niu J, Ran L, et al. Estimation of human body volume (BV) from anthropometric measurements based on three-dimensional (3D) scan technique. Aesthetic Plast Surg. 2017;41:971–978. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz-Müller F, Marshall R, Summerskill S. Development of a positioning aid to reduce postural variability and errors in 3D whole body scan measurements. Appl Ergon. 2018;68:90–100. [DOI] [PubMed] [Google Scholar]
- 19.Krzywinski S, Siegmund J. 3D product development for loose-fitting garments based on parametric human models. J Fashion Technol Textile Eng. 2017;0(3):1–5. [Google Scholar]
- 20.Miller A. Impact of seamless compression garments on limb functionality, comfort and quality of life. Br J Community Nurs. 2017;22(sup10):S26–S37. [DOI] [PubMed] [Google Scholar]
- 21.Oreopoulos DG. Humane medicine. Can Med Assoc J. 1983;128:1279–1280. [PMC free article] [PubMed] [Google Scholar]
- 22.Goei H, van der Vlies CH, Tuinebreijer WE, et al. Predictive validity of short term scar quality on final burn scar outcome using the patient and observer scar assessment scale in patients with minor to moderate burn severity. Burns. 2017;43:715–723. [DOI] [PubMed] [Google Scholar]
- 23.Anzarut A, Olson J, Singh P, et al. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. J Plast Reconstr Aesthet Surg. 2009;62:77–84. [DOI] [PubMed] [Google Scholar]
- 24.Engrav LH, Heimbach DM, Rivara FP, et al. 12-Year within-wound study of the effectiveness of custom pressure garment therapy. Burns. 2010;36:975–983. [DOI] [PubMed] [Google Scholar]
- 25.Steinstraesser L, Flak E, Witte B, et al. Pressure garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: randomized controlled trial with intraindividual comparison. Plast Reconstr Surg. 2011;128:306e–313e. [DOI] [PubMed] [Google Scholar]
- 26.Lee KC, Dretzke J, Grover L, et al. A systematic review of objective burn scar measurements. Burns Trauma. 2016;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. [DOI] [PubMed] [Google Scholar]
- 28.Danckaers F, Huysmans T, Hallemans A, et al. Posture normalisation of 3D body scans. Ergonomics. 2019;62:834–848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.