Abstract
Background:
Vitamin E has antioxidant properties, which help in scavenging free radicals, thereby reducing oxidation of lipids and proteins. This study aims to evaluate the efficacy of oral vitamin E supplementation in preventing retinopathy of prematurity (ROP) in very low birth weight (VLBW) infants with respiratory distress syndrome (RDS) and decreasing oxidative stress 15 and 28 days post-intervention.
Methods:
Ninety VLBW infants were randomly assigned to two groups:
-
1.
Treatment (treatment group (T), n = 48) or
-
2.
Placebo (control group (C), n = 42).
Each group received 25 IU of vitamin E (T) or placebo (C).
Results:
The incidence of ROP in groups T and C was 12.5% (n=6) and 31% (n = 13), respectively (RR: 0.40; 95% CI: 0.10–0.96). There were no differences in mortality between groups. As expected, the vitamin E concentration was significantly increased 28 days post-intervention in group T.
Conclusion:
Oral supplementation with vitamin E may effectively prevent ROP development in VLBW infants with RDS. Oxidative damage markers were significantly lower, whereas total antioxidant capacity was increased in group T. However, levels of other antioxidants as vitamin A and C were not measured in two groups.
Keywords: antioxidant vitamins, oxidative damage, retinopathy of prematurity, total antioxidant capacity, vitamin E
1. Introduction
The survival rate of very low birth weight (VLBW) infants has markedly increased in the last two decades.[1] Retinopathy of prematurity (ROP) continues to be prevalent among VLBW infants.[1] ROP can be mild, with no visual defects, or aggressive, with retinal detachment and blindness.[1]
Ludwig[2] reported a frequency of ROP of 30.2% in newborns with birth weights between 750 and 999 g in the USA versus ROP incidence in Switzerland, which was only 9.3% in VLBW infants.[3] In Mexico, the reported incidence is 59.1% among premature neonates weighing 751–1000 g at birth.[4]
In addition to prematurity, oxidative stress contributes to the pathogenesis of ROP.[5–7] Oxidative stress is associated with tissue damage caused by excessive generation of reactive oxygen species (ROS).[6,7] The delivery of a fetus from a relatively hypoxic intrauterine environment, with a partial pressure of oxygen (PO2) of 20–25 mm/Hg, to a normal extrauterine environment, with a PO2 of nearly 100 mm/Hg, can increase oxidative stress.[5,6] It has been suggested that ROP could result from different types of ROS and/or high concentrations of polyunsaturated fatty acids in photoreceptors.[8] The initial stimulus that activates the inflammatory process in very low birth weight and ROP infants may be the increased free radicals induced by oxygen therapy, barotrauma, volutrauma, infectious agents, antioxidant deficiency, and/or other stimuli.[9–11] The association between oxygen therapy and ROP has been recognized for a long time.[4,7,8]
Respiratory distress syndrome (RDS) is a risk factor for ROP.[4–7] Respiratory management of RDS includes oxygen therapy and surfactant administration.[12] This therapy can increase the risk of ROP. In this regard, several approaches have been implemented to decrease ROP, particularly the use of antioxidants. Vitamin E is an important antioxidant that can scavenge free radicals, reducing lipid peroxidation and oxidant injury in VLBW infants.[11] However, the results of trials evaluating the efficacy of vitamin E in the prevention of ROP have not been encouraging.[13–17] In fact, research on the roles of vitamins and antioxidants, including vitamin E, in preventing ROP were halted because of complications involving sepsis and necrotizing enterocolitis.[18] These complications were attributed to excipients of oral vitamin E products, which included polyethylene glycol, propylene glycol, ethanol and, polysorbate 80.[11] Since these substances, may induce adverse effects in premature newborns.[11,18] In a meta-analysis by Fang et al. (2016),[19] the use of vitamin E supplementation at any stage did not affect ROP rates.
In Mexico, pediatricians and neonatologists advocate supplementation with vitamin E free of possibly toxic vehicles for ROP prevention in premature newborns. However, to date, there have been no reports on the efficacy of this intervention. Thus, the present study's objective was to evaluate the effect of vitamin E oral supplementation at a dosage of 12.5 IU every 12 h (25 IU/day) from 72 h after birth to 28 days in the prevention of ROP in VLBW infants with RDS.
2. Methods
2.1. Study design and participants
The study was a randomized, double-blind clinical trial conducted in the intensive care unit of the Instituto Nacional de Perinatología (INPer) in Mexico City, from March 1, 2013, to October 1, 2015. The study protocol was approved by the Internal Review Board of INPer (Registration ID: 212250-10231), and signed informed consent was obtained from the children's parents. This trial was retrospectively registered as NCT03274596 at http://clinicaltrials.gov/.
The inclusion criteria were VLBW infants with an RDS diagnosis defined as labored breathing, such as tachypnoea, nasal flaring, chest retractions, or grunting.[20] All VLBW infants included were treated with the intubation-surfactant-extubation technique. Subsequently, they received continuous positive airway pressure (CPAP). Some neonates required mechanical ventilation, after which they received CPAP via the nasal route or a nasal cannula. All the patients were continuously monitored using a transcutaneous oxygen monitor to maintain O2 saturation between 90 and 93%. At all times from birth, a controlled oxygen supply was provided using an air-oxygen blender in accordance with the institution's neonatal resuscitation guidelines.
The exclusion criteria were preterm newborns with congenital malformations, Rh incompatibility and isoimmunization, non-immune or immune hydrops fetalis, or grade III/IV intraventricular hemorrhage, and neonates born to mothers with thrombocytopenic purpura and/or any immunodeficiency.
2.2. Randomization and allocation concealment
The VLBW infants were randomly assigned to a treatment group (group T) or a placebo control group (group C) using a random digit table. Pharmacy staff not involved in the patients’ care were responsible for allocating, enrolling, and assigning participants to interventions. Group T received orally administered vitamin E (25 IU) in two 12.5 IU doses every 12 h. Group C received a placebo comprising 0.1 mL of distillate water every 12 h. Both groups received the assigned treatment from 72 h after birth until 28 days of age. Both interventions were presented in the same packaging, appearance, and solution (0.1 mL) amount, administered in a sterile and graduated plastic syringe, which was protected from light using aluminum foil. The research team and participants’ parents were blinded to the group allocation until after the final analysis.
Nutritional management in both groups was similar. From birth, the infants received a protein solution (2.4 g/kg/day). Then, 12–24 h later, they received total parenteral nutrition (3.5 g/kg/day protein, 3 g/kg/day lipids and 4 mg/kg/min glucose, up to 10 mg/kg/min). Trophic feeding of the VLBW was initiated 36–48 h after birth.
According to institutional guidelines, prophylactic surfactant was administrated to premature infants less than 28 weeks of gestation that had not received prenatal steroids. Rescue surfactant was administrated to newborns < 32 weeks of gestation who received antenatal steroids but had risk factors for developing RDS. Caffeine was started in all newborns < 32 weeks of gestation, within the first 24 h of life, with an impregnation dose of 20 mg/kg and a maintenance dose of 5 mg/kg per day until term-corrected gestational age (38 weeks of gestation). All infants with patent ductus arteriosus with hemodynamic repercussions received Ibuprofen treatment orally at 10 mg/kg per day as initial dose and subsequently 5 mg/kg every 24 h for two days. Neither group received erythropoietin. All infants who remained intubated on day 14 of life received only one steroid scheme as follows: Dexamethasone 0.075 mg/kg/dose every 12 h for three days, continued with 005 mg/kg/dose every 12 h for three days, then 0.025 mg/kg/dose every 12 h for two days and finally 0.01 mg/kg/dose every 12 h for two days and it was suspended.
2.3. Vitamin E preparation
The pharmacologist team at the institution prepared a vitamin E formulation at a 25 IU/0.2 mL concentration under laminar flow hood and sterile conditions. The preparation was as follows: 2800 IU of vitamin E contained in a volume of 1.75 mL (7 capsules of 400 IU, vitamin E (dl-alpha-tocopherol); Eternal, Bayer, Mexico) and 1.75 mL of glycerol at 1% were mixed in a 1:1 ratio and gauged to 22.5 mL of sterilized water. The solution was mixed until a uniform preparation in a sterilized Erlenmeyer flask, which contained 224 doses of vitamin E (12.5 IU/0.1 mL per dose), was attained. Only the required doses for each day were placed in a sterile and graduated plastic syringe protected from light (stored at 4°C) using aluminum foil. Until the administration, the rest of the prepared solution was discarded. This process was repeated every day in order to maintain the stability of vitamin E.
2.4. Primary endpoint
The primary endpoint was the efficacy and safety of vitamin E in preventing ROP in VLBW infants with RDS. ROP was defined according to the international ROP classification.[20,21] An ophthalmological evaluation by a blinded-to-treatments ophthalmologist was performed 28 days after birth, and the incidence of any grade of ROP was compared between groups. The safety of vitamin E administration was evaluated according to the incidence of morbidity, including sepsis, necrotizing enterocolitis, gastrointestinal intolerance (defined as transitory dysfunction (<24 h) to ingest and digest the prescribed enteral feeding), and mortality. Additionally, each participant's evolution was evaluated by an external clinical monitor from epidemiological department of our institution that did not take part in this research, with constant comparison with our institutional endemic channel for each complication reported among participants of the study. As security criteria, any complication outside the endemic channels in both groups could be used to stop the study.
2.5. Secondary outcomes
As secondary outcomes, markers of oxidative stress were evaluated in both groups. A blood sample (0.3 mL) was collected by venipuncture before the administration of the intervention. Subsequent blood samples were obtained in both groups 15 and 28 days after vitamin E or placebo supplementation. Clinical status was monitored, and demographic variables, including gestational age, sex, weight, height, days of oxygen therapy, prenatal care, treatment with prenatal and postnatal steroids, type of mechanical ventilation, and surfactant administration, were recorded.
2.6. Biochemical analysis
In order to detect oxidative damage, biochemical analysis of plasma and red blood cell lysates was performed, as described previously.[22] Briefly, to detect the total antioxidant capacity (TAC) of plasma, we used the cupric ion reducing antioxidant capacity spectrophotometric method, with absorbance measured at 450 nm.[23] Malondialdehyde (MDA) concentrations in erythrocyte lysates were measured using the spectrophotometric method at 586 nm,[24] and the resulting values were expressed as pmol of carbocyanine/mg dry weight. The assay described by El-Saadani was used to assess lipid peroxides in the erythrocyte lysate, and lipid peroxidation was measured spectrophotometrically at 360 nm.[25] Protein damage was evaluated by determining the carbonyl content in plasma following treatment with 2,4-dinitrophenylhydrazine, which reacts with carbonyl groups to form stable hydrazones.[26] These were then measured spectrophotometrically at 370 nm. All experiments were performed in duplicate. Unless otherwise specified, all reagents used in this study were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Vitamin E in human serum was measured using high-performance liquid chromatography (Flexar LC, PerkinElmer, Norwalk, U.S).[27]
2.7. Sample size
Thirty-nine participants per group were required to probe the hypothesis that vitamin E decreased the incidence of any stage of ROP in VLBW infants with RDS from 40% to 15%, with a power of 80% and an alpha error of.05 in a one-tailed test.
2.8. Statistical analysis
Mann–Whitney U-tests were conducted to compare continuous variables according to the distribution of the variables. Chi-square tests were performed to determine significant differences in categorical values between groups. Student's t-tests were used when needed. Significance was defined at P < .05. Relative risks (RRs), with 95% confidence intervals (CIs) were calculated. SPSS, version 15 (SPSS, Chicago, IL, USA) was used for the statistical analyses. Oxidative damage data were expressed as the mean ± standard deviation (SD), and values were examined using Prism 5.0 software (GraphPad, San Diego, CA, USA).
3. Results
3.1. Flowchart of the participants in the study and clinical characteristics of the mothers and newborns
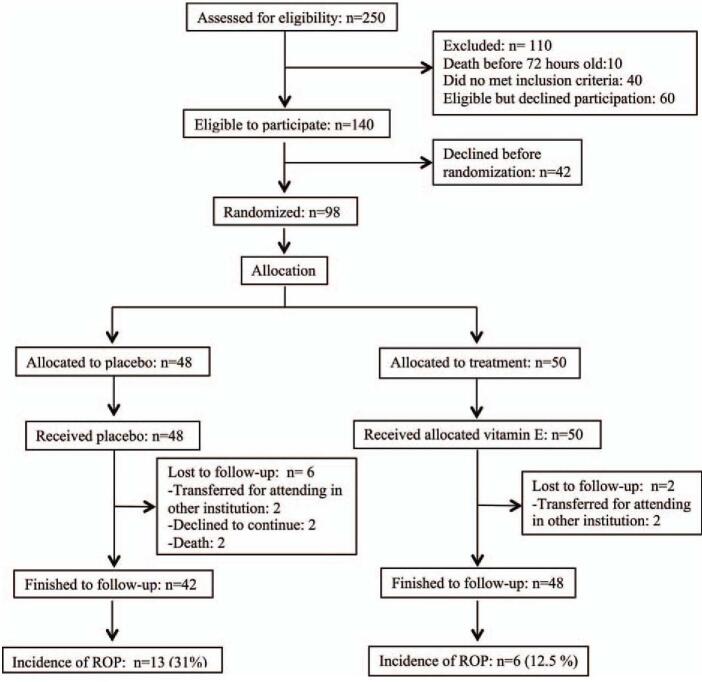
During the study period, 240 VLBW infants were born. Of these, 140 patients were eligible to participate in the study. Parents declined participation before randomizing the 42 newborns; therefore, 98 patients whose parents consented to participate in the study were randomly allocated (Fig. 1).
Figure 1.
Flowchart of the participants in the study.
The clinical characteristics of the mothers and newborns at the time of admission to the study are presented in Table 1. There were no significant between-group differences in maternal or neonatal morbidity or demographic characteristics and pharmacologic interventions upon admission to the study.
Table 1.
Maternal and newborns characteristics at the time of admission to the study.
| Characteristic | Group CN = 42 | Group TN = 48 | P-value |
| Maternal age (years) | 30.1 ± 8.1 | 28.6 ± 6.4 | .32 |
| Maternal infections | 29 (69%) | 34 (70.8%) | .37 |
| -Urinary tract infections | 20 (47.6%) | 19 (36.6%) | .30 |
| -Cervico-vaginal infections | 9 (21.4%) | 12 (25.0%) | .30 |
| Chronic hypertension | 1 (2.4%) | 1 (2.1%) | 1.0 |
| Preeclampsia | 14 (33.3%) | 19 (39.6%) | .90 |
| Prenatal care | 40 (95.2%) | 43 (89.6%) | .42 |
| Prenatal steroids | 26 (61.9%) | 31 (64.6%) | .82 |
| Male | 23 (54.8%) | 22 (45.8%) | .52 |
| Female | 19 (45.2%) | 26 (54.2%) | .52 |
| Weight (g) | 1164 ± 203 | 1087 ± 194 | .07 |
| Gestational age (weeks) | 30.1 ± 2 | 30.0 ± 2 | .78 |
| Nosocomial sepsis | 28 (66.7%) | 29 (60.4%) | .66 |
| Nosocomial pneumonia | 1 (2.4%) | 2 (4.2%) | .90 |
| Apnea of prematurity | 26 (61.9%) | 30 (62.5%) | .92 |
| Barotrauma | 2 (4.8%) | 5 (10.4%) | .44 |
| Renal failure | 1 (2.4%) | 3 (6.2%) | .62 |
| Neonates managed only with Continuous positive airway pressure | 19 (45.2%) | 29 (60.4%) | .21 |
| Mechanic ventilation (days) | 5.0 ± 7.1 | 4.5 ± 7.6 | .74 |
| FiO2 maximum concentration (%) | 51.6 ± 10 | 52.3 ± 10 | .75 |
| Oxygen requirements (h) | 512.9 ± 29 | 517.3 ± 23 | .94 |
| Parenteral nutrition (days) | 14 ± 5.5 | 13.2 ± 6.3 | .50 |
| Hospital stay (days) | 38.5 ± 11.6 | 42.6 ± 19.4 | .20 |
| Prophylactic surfactant | 16 (38.0%) | 13 (27.0%) | .37 |
| Early surfactant | 6 (14.2%) | 6 (12.5%) | .95 |
| Ibuprofen | 13 (30.9%) | 12 (25%) | .69 |
| Steroid scheme | 28 (66.7%) | 31 (64.5%) | .83 |
3.2. Vitamin E supplementation was associated with a lower incidence of ROP
The clinical outcomes of group T and group C are shown in Table 2. The total incidence of ROP was 21.1% (19/90), and the incidence of ROP was significantly lower in group T (12.5%; 6/48) than group C (31%; 13/42) in the per-protocol analysis. However, the reduced incidence was observed only in stage I of ROP. In the analysis based on intention to treat, there were no significant differences between-group in the incidence of ROP; C group n = 13/48 (27%) and T group n = 6/50 (12%) P = .10 relative risk; 0.44 (CI 95% 0.18 − 1.07). There were no between-group differences in other clinical outcomes, including sepsis or necrotizing enterocolitis, which were considered markers of vitamin E supplementation safety. Additionally, there were three cases of intrahepatic cholestasis in each group.
Table 2.
Relative risks (RR), with 95% confidence intervals (CIs) for clinical outcomes in new-borns in the control (group C) and treatment (group T) groups at 28 days of age.
| Outcome | Group Cn = 42 | Group Tn = 48 | P-value | Relative risks | 95% Confidence intervals |
| Retinopathy of prematurity | 13 (31%) | 6 (12.5%) | .03 | 0.40 | 0.17–0.97 |
| Retinopathy of prematurity severity | 12 = Stage I | 5 = Stage I | .03 | 0.40 | 0.17–0.97 |
| 1 = Stage II | 1 = Stage II | .5 | 0.87 | 0.05–13.5 | |
| Bronchopulmonary Dysplasia | 27 (64.3%) | 31 (64.6%) | .97 | 1.00 | 0.74–1.37 |
| Ductus arteriosus | 19 (45.2%) | 16 (33.3%) | .28 | 0.78 | 0.51–1.2 |
| Intraventricular haemorrhage | 5 (11.9%) | 4 (8.3%) | .72 | 0.81 | 0.38–1.75 |
| Sepsis | 22 (52.4%) | 28 (58.3%) | .72 | 1.11 | 0.76–1.6 |
| Gastrointestinal intolerance | 3 (7.1%) | 7 (14.6%) | .32 | 1.30 | 0.86–2.16 |
| Necrotizing enterocolitis | 0 (0%) | 1 (2.1%) | .91 | 2.60 | 0.11–61.7 |
The basal vitamin E concentration was similar in group T and group C (1.03 ± 1.0 vs 1.09 ± 1.5, P = .97). On day 15, the vitamin E concentration was higher in group T than group C, but the difference was not significant (1.52 ± 0.7 vs 1.20 ± 1.5, P = .27). On day 28, the vitamin E concentration was significantly higher in group T than group C (1.71 ± 1.1 vs 1.37 ± 1.5, P = .05). In addition, there was no significant difference in calorie intake (130 ± 10 kcal in group C and 127 ± 18.4 kcal in group T, P = .18).
3.3. Oxidative stress markers
In terms of oxidative damage, group T showed a significant decrease as compared to group C. TAC was higher in group T than in group C (P < .05) (Fig. 2).
Figure 2.
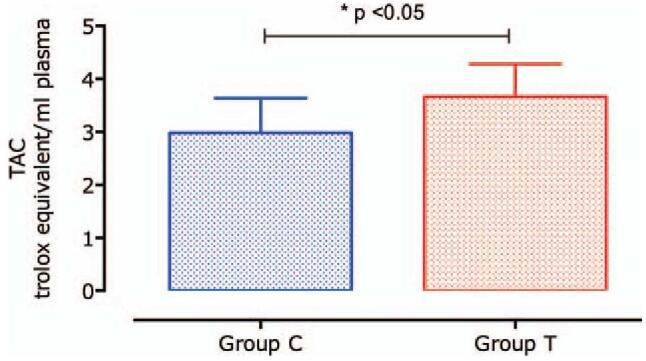
Total antioxidant capacity (TAC) comparison between group T and C. Data are expressed as mean ± standard deviation (SD).
Lipohydroperoxide (LHP) formation was lower, approximately 76.3% in group T (P < .0001) than in group C, as shown in Figure 3.
Figure 3.
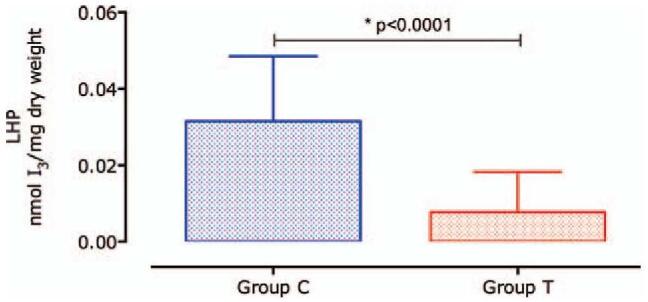
Concentration of Lipohydroperoxide. The generation of lipohydroperoxide (LHP) was 4.2 times higher in group C as compared with group T. Data are expressed as mean ± standard deviation (SD).
The production of a highly reactive MDA molecule, a consequence of the breakdown of LHP during the lipoperoxidation process, is 53.3% lower (P < .0001) in group T than in group C (Fig. 4).
Figure 4.
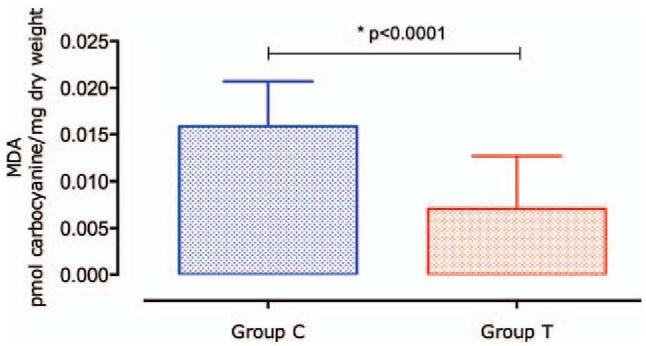
Concentration of malondialdehyde. The malondialdehyde (MDA) concentration was significantly higher in group C as compared with that in group T. Data are expressed as mean ± standard deviation (SD).
MDA generation is associated with protein injury, favoring new carbonyl formation groups (carbonylation) in the protein molecules.
Figure 5 shows a significant decrease (P < .001) in protein carbonylation in group T (2.87 ± 0.93 nmol DNPH/mg protein) as compared with that in group C (3.96 ± 1.32 nmol DNPH/mg protein).
Figure 5.
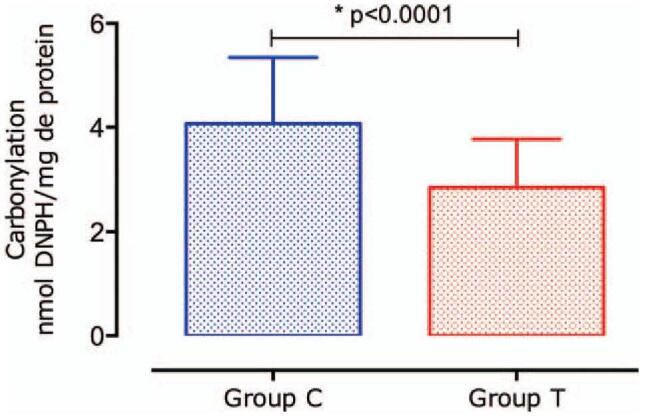
Protein carbonylation values. Carbonylation was significantly lower in group T than in group C. Data are expressed as mean ± standard deviation (SD).
4. Discussion
This study is the first to report the efficacy of vitamin E in ROP prevention among Mexican VLBW infants. The results showed that oral supplementation of 12.5 IU of vitamin E every 12 h, free of polyethylene glycol, propylene glycol, ethanol, or polysorbate 80, from 72 h after birth until 28 days of age in VLBW infants with RDS was effective in preventing ROP. They also revealed a significant decrease in carbonylation and lipid peroxidation (i.e., oxidative damage markers), as well as higher TAC in VLBW infants supplemented with vitamin E.
Emerging evidence suggests that lung and retinal injury that leads to broncho pulmonary dysplasia and ROP occur within hours to a few days after delivery.[28] During pregnancy, placental transfer of vitamin E into the fetus is limited,[29,30] and antioxidant enzymes are expressed at high concentrations only at the end of gestation, in preparation for the relatively hyperoxic extrauterine environment.[1,5] In premature newborns, antioxidant defenses are not appropriately expressed, and vitamin E is a good alternative to protect against peroxidation.[30] As a lipophilic compound, vitamin E accumulates in circulating lipoproteins, cell membranes, and fatty deposits, and it reacts with free radicals and molecular oxygen.
Vit E protects polyunsaturated fatty acids from peroxidation.[30,31] Lipoperoxidation has been proposed as a crucial step in the pathogenesis of ROP, as the retina contains elevated levels of polyunsaturated fatty acids, such as docosahexaenoic acid and cis-arachidonic acid.[32,33] In addition to increased lipoperoxidation, protein changes may affect retinal functionality because of the consequences of oxidative damage.[28]
Results reported here demonstrate the antioxidant efficacy of supplementation with vitamin E in VLBW infants, resulting in a significant decrease in all oxidative stress biomarkers measured after 15 and 28 days of vitamin E supplementation.
In the present study, there was no statistically significant difference in the basal concentrations of vitamin E between groups, which could be attributed to similar concentrations at birth, and that participants in both groups received vitamin E (7.5 IU) as parenteral nutrition from 12 to 24 h of life.
The concentrations of vitamin E administered in the present study are considered in normal ranges, according to the American Academy of Pediatrics.[6] After 15 days of treatment, there were still no differences in serum vitamin E concentrations. Only after 28 days of treatment did the concentration significantly increase in the treated group, although the serum vitamin E concentrations did not exceed 3.5 mg/dL. The low levels of vitamin E can be attributed to poor oral absorption due to immaturity of the digestive tract, resulting in poor fat absorption in premature neonates.[34] The low levels found in this study also can be attributable to the preparation of solutions. Previous research reported that vitamin E supplementation increased levels (>3.5 mg/dL) and could possibly reduce the risk of severe ROP but increase the risk of sepsis,[18] our results avoiding potentially dangerous solvents decrease the potential toxicity of commercial vitamin E preparations.
In the present study, supplementation with vitamin E was not sufficient to prevent BPD, similar to findings reported by Brion et al.[11] We found no evidence of complications associated with sepsis or necrotizing enterocolitis. These findings agree with a previous study that examined the efficacy of vitamin E in preventing ROP, as well as the safety of vitamin E supplementation in 287 newborns <1,500 g (adjusted to plasma levels of 3 to 3.5 mg/dL),[33] and with a recent meta-analysis showing that the risk of any/severe ROP was significantly reduced with vitamin E (RR 0.30, 95% CI 0.14–0.62).[35]
In the present study, there were six intrahepatic cholestasis cases (three per group), which could be associated with parenteral nutrition.
The present study had several limitations. It was performed in a single center and consisted of a limited sample size of only Mexican VLBW infants with RDS. Future prospective and multi-center studies with larger sample sizes are required to corroborate these findings. Another limitation of the study was vitamin E's ability to prevent ROP only in the per-protocol analysis. Vitamin E showed a trend in the efficacy of intention-to-treat analysis, which was not significant. Another limitation was that this study used a vitamin E formulation prepared by our pharmacologist team rather than an available commercial formulation. It is necessary to adjust doses with this type of water-based preparations to reach higher serum concentrations. Other limitation was that levels of other antioxidants, as vitamin A and C have not been measured in two groups that could be confounding factors and results should be interpreted with caution.
Infants at risk of ROP suffer from numerous conditions, and are exposed to many forms of polypharmacy which likely add to the etiology of the disease. Pharmacologic interventions for treatment and/or prevention of ROP have predominantly targeted oxidants and VEGF, however given the multifactorial characteristic of ROP and complexity of the disease, the use of a single therapeutic agent may not be prudent.[36]
Antioxidant therapy may be useful in the management of neonates with problems caused by oxidative stress, but further biochemical investigations are required to identify the most effective antioxidant therapy. We propose that future studies explore the ability of antioxidants mixtures with different redox potentials to prevent ROP.
5. Conclusions
Oral supplementation with vitamin E may help prevent ROP development in VLBW infants with RDS without increasing adverse effects. Oxidative damage markers were significantly lower, whereas total antioxidant capacity was increased in the vitamin E group. However, levels of other antioxidants as vitamin A and C were not measured in two groups. More randomized clinical trials are necessary to confirm our findings.
Acknowledgments
This paper will be included in a thesis that will be submitted to the Graduate Council of the Escuela Superior de Medicina, Instituto Politécnico Nacional Mexico by Silvia Romero-Maldonado as part of requirements to obtain the Doctoral Degree in Medical Research Sciences.
This research was supported by The National Institute of Perinatology “Isidro Espinosa de los Reyes”.
Author contributions
Conceptualization: Silvia Romero-Maldonado, Araceli Montoya-Estrada, Aurora Belmont-Gómez, Javier Mancilla-Ramírez.
Data curation: Silvia Romero-Maldonado, Maricruz Tolentino-Dolores.
Formal analysis: Silvia Romero-Maldonado, Araceli Montoya-Estrada, Enrique Reyes-Muñoz, Mario David Sánchez-Mendez, Jorge A Cardona-Pérez.
Funding acquisition: Silvia Romero-Maldonado.
Investigation: Mario David Sánchez-Mendez, Manuel Bernardo Salgado-Valladares, Aurora Belmont-Gómez, Nayelli Nájera.
Methodology: Araceli Montoya-Estrada, Alberto Martín Guzmán-Grenfell, Yessica Dorin Torres-Ramos, Maricruz Tolentino-Dolores, Manuel Bernardo Salgado-Valladares, Aurora Belmont-Gómez.
Project administration: Silvia Romero-Maldonado.
Validation: Araceli Montoya-Estrada.
Writing – original draft: Silvia Romero-Maldonado, Araceli Montoya-Estrada, Juan J Hicks.
Writing – review & editing: Enrique Reyes-Muñoz, Guillermo Ceballos.
Footnotes
Abbreviations: CIs = Confidence Intervals, CPAP = Continuous Positive Airway Pressure, INPerIER = Instituto Nacional de Perinatología. Isidro Espinosa de los Reyes, LHP = Lipohydroperoxide, MDA = Malondialdehyde, PO2 = Partial Pressure of Oxygen, RCTs = Randomized Controlled Trials, RDS = Respiratory Distress Syndrome, ROP = Retinopathy of Prematurity, ROS = Reactive Oxygen Species, RR = Relative Risks, TAC = Total Antioxidant Capacity, UCIREN = Newborn Intermediate Care Unit.
How to cite this article: Romero-Maldonado S, Montoya-Estrada A, Reyes-Muñoz E, Guzmán-Grenfell AM, Torres-Ramos YD, Sánchez-Mendez MD, Tolentino-Dolores M, Salgado-Valladares MB, Belmont-Gómez A, Najéra N, Ceballos G, Cardona-Pérez JA, Hicks JJ, Mancilla-Ramírez J. Efficacy of water-based vitamin E solution versus placebo in the prevention of retinopathy of prematurity in very low birth weight infants: A randomized clinical trial. Medicine. 2021;100:31(e26765).
This work was supported by Instituto National de Perinatología Isidro Espinosa de los Reyes, Mexico City. Grant: 212250-10231.
The authors have no conflicts of interest to disclose.
Ethics approval and consent to participate: The study was approved by the Ethics and Research Internal Review Board of the Instituto Nacional de Perinatología in Mexico City (register number: ID: 212250-10231) and signed informed consent was obtained from the children's parents.
Consent to publish: Not applicable.
Availability of data and materials: The datasets used during the present study are available from the corresponding author on reasonable request.
The datasets generated during and/or analyzed during the present study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Rogers S, Witz G, Anwar M, et al. Antioxidant capacity and oxygen radical diseases in the preterm newborn. Arch Pediatr Adolesc Med 2000;154:544–8. [DOI] [PubMed] [Google Scholar]
- [2].Ludwig CA, Chen TA, Hernandez-Boussard T, et al. The epidemiology of retinopathy of prematurity in the United States. Ophthalmic Surg Lasers Imaging Retina 2017;48:553–62. [DOI] [PubMed] [Google Scholar]
- [3].Gerull R, Brauer V, Bassler D, et al. Incidence of retinopathy of prematurity (ROP) and ROP treatment in Switzerland 206-2015: a population-based analysis. Arch Dis Child Fetal Neonatal Ed 2018;103:F337–42. [DOI] [PubMed] [Google Scholar]
- [4].Vázquez-Lara Y, Bravo-Ortiz JC, Hernández-Galván C, et al. Risk factors associated with retinopathy of prematurity in preterm infants treated at a tertiary level hospital. Bol Med Hos Infant Mex 2012;69:277–82. [Google Scholar]
- [5].Banjac L, Banjac G, Kotur-Stevuljević J, Spasojević-Kalimanovska V, Gojković T, Bogavac-Stanojević N, et al. Pro-oxidants and antioxidants in retinopathy of prematurity. Acta Clin Croat 2018;57:458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Peña-Bautista C, Durand T, Vigor C, Oger C, Galano JM, Cháfer-Pericás C. Non-invasive assessment of oxidative stress in preterm infants. Free Radic Biol Med 2019;142:73–81. [DOI] [PubMed] [Google Scholar]
- [7].Garg U, Jain A, Singla P, et al. Free radical status in retinopathy of prematurity. Indian J Clin Biochem 2012;27:196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Katz ML, Robison WJ, Jr. Autoxidative damage to the retina: potential role in retinopathy of prematurity. Birth Defects Orig Artic Ser 1988;24:237–48. [PubMed] [Google Scholar]
- [9].Penn JS. Oxygen-induced retinopathy in the rat: possible contribution of peroxidation reactions. Doc Ophthalmol 1990;74:179–86. [DOI] [PubMed] [Google Scholar]
- [10].Bjelakovic G, Nikolova D, Gludd LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012;14:CD007176. [DOI] [PubMed] [Google Scholar]
- [11].Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2003;4:CD003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reuter S, Moser Ch, Baack M. Respiratory distress in the newborn. Pediatr Rev 2014;35:417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dollory CT, Bulpitt CJ, Kohner EM. Oxygen supply to the retina from the retinal and choroidal circulations at normal and increased arterial oxygen tensions. Invest Ophthalmol 1969;8:588–94. [PubMed] [Google Scholar]
- [14].Cavallaro G, Filippi L, Banoli P, et al. The pathophisiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol 2014;92:02–20. [DOI] [PubMed] [Google Scholar]
- [15].Chen ML, Guol Smith LEH, et al. High or low oxygen saturation and severe retinopathy of prematurity: a meta-analysis. Pediatrics 2010;125:e1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Alajbegovic-Halimic J, Zvizdic D, Alimanovic-Halilovic-Halilovic E, et al. Risk factors for retinopathy of prematurity in premature born children. Med Arch 2015;69:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Sankar MJ, and Sankar J. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. RHL Commentary. The WHO Reproductive Health Library, Geneva: World Health Organization. 2011 available: https://extranet.who.int/rhl/topics/pregnancy-and-childbirth/antenatal-care/nutrition-during-pregnancy/vitamin-e-supplementation-prevention-morbidity-and-mortality-preterm-infants. [Google Scholar]
- [18].Johnson L, Frank W, Bowen, et al. Relationship of prolonged pharmacologic serum levels of vitamin E to incidence of sepsis and necrotizing enterocolitis in infants with birth weight 1,500 grams or less. Pediatrics 1985;75:619–38. [PubMed] [Google Scholar]
- [19].Fang JL, Sorita A, Carey WA, et al. Intervetions to prevent retinopathy of prematurity: a meta-analysis. Pediatrics 2016;137:e20153387. [DOI] [PubMed] [Google Scholar]
- [20].Reuter S, Moser C, Baack M. Respiratory distress in the newborn. Pediatr Rev 2014;35:417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].International Committee for the classification of retinopathy of prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthal 2005;123:1991–9. [DOI] [PubMed] [Google Scholar]
- [22].Montoya-Estrada A, Torres-Ramos YD, Flores-Pliego A, et al. Urban PM 2.5 activates GAPDH and induces RBC damage in COPD patients. Front Biosci 2013;5:638–49. [DOI] [PubMed] [Google Scholar]
- [23].Apak R, Guςlu K, Özyürek M, et al. Total antioxidant capacity assay of human serum using copper (II)-neocuproine as chromogenic oxidant: the CUPRAC method. Free Radic Res 2005;39:949–61. [DOI] [PubMed] [Google Scholar]
- [24].Gerard-Monnier D, Erdelmeier I, Reganrd K, et al. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol 1998;11:1176–83. [DOI] [PubMed] [Google Scholar]
- [25].El-Saadani M, Esterbauer H, El-Sayed M, et al. A spectrophotometric assay form lipid peroxides in serum lipoproteins using a commercially available reagent. J Lipid Res 1989;30:627–30. [PubMed] [Google Scholar]
- [26].Dalle-Done I, Rossi R, Giustarini D, et al. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 2003;329:23–38. [DOI] [PubMed] [Google Scholar]
- [27].Driskell WJ, Neese JW, Bryant CC, et al. Measurement of vitamin A and vitamin E in human serum by high-performance liquid chromatography. J Chromatogr 1982;231:439–44. [DOI] [PubMed] [Google Scholar]
- [28].Sapieha P, Joyal JS, Rivera JC, et al. Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life. J Clin Invest 2010;120:3022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maria Pacifici G. Effects of vitamin E in neonates and young infants. Int J Pediatr 2016;4:1745–57. [Google Scholar]
- [30].Didenco S, Gillingham MB, Go MB, et al. Increased vitamin E intake is associated with higher alpha-tocopherol concentration in the maternal circulation but higher alpha-carboxyethyl hydroxychroman concentration in the fetal circulation. Am J Clin Nutr 2011;93:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Debier C, Larondelle Y. Vitamins A and E: metabolism, roles and transfer to offspring. Br J Nutr 2005;93:153–74. [DOI] [PubMed] [Google Scholar]
- [32].Perrone S, Negro S, Tataranno ML, et al. Oxidative stress and antioxidant strategies in newborns. J Matern fetal Neonatal Med 2010;23:63–5. [DOI] [PubMed] [Google Scholar]
- [33].Phelps DL, Rosengaum AL, Isenberg SJ, et al. Tocopherol efficacy and safety for preventing retinopathy of prematurity: a randomized, controlled, double-masked trial. Pediatrics 1987;79:489–500. [PubMed] [Google Scholar]
- [34].Bell EF, Brown EJ, Milner R, et al. Vitamin E absorption in small premature infants. Pediatrics 1979;63:830–2. [PubMed] [Google Scholar]
- [35].Raghuveer TS, Zackula R. Strategies to prevent severe retinopathy of prematurity: a 2020 update and meta-analysis. Neoreviews 2020;21:e249–63. [DOI] [PubMed] [Google Scholar]
- [36].Beharry KD, Valencia GB, Lazzaro DR, Aranda JV. Pharmacologic interventions for the prevention and treatment of retinopathy of prematurity. Semin Perinatol 2016;40:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]