An opioid-sparing protocol for postoperative pain management in less invasive cranial neurosurgery significantly lowered opioid usage while reducing pain scores.
Keywords: Postoperative, Surgery, Neurosurgery, Cranial, NSAIDs, Opioid-sparing, Multimodal, Functional, Opioid
Abstract
Introduction:
Opioid overuse in postoperative patients is a worrisome trend, and potential alternatives exist which warrant investigation. Nonsteroidal anti-inflammatory drug use in treating postoperative cranial surgery pain has been hampered by concern for inadequate pain control and increased risk of hemorrhagic complications. A safe and effective alternative to opioid-based pain management is critical to improving postoperative care.
Objective:
The objective of this retrospective study was to determine whether an NSAID-based opioid-sparing pain management protocol (OSP) is effective in analgesic control of less invasive cranial surgery patients at 6-, 12-, and 24-hour postoperatively. Secondary aims included investigating differences in hemorrhagic complications.
Methods:
Five hundred sixty-six consecutive patients who underwent cranial surgery before and after implementation of the celecoxib-based OSP were eligible. Propensity score matching was used to match patients in each cohort.
Results:
The opioid-sparing cohort had lower pain scores at 6 hours (3.45 vs 4.19, P = 0.036), 12 hours (3.21 vs 4.00, P = 0.006), and 24 hours (2.90 vs 3.59, P = 0.010). Rates of postoperative hemorrhage were not significantly different (5% intervention vs 8% control, P = 0.527). The opioid-sparing pain management protocol provided comparable or better pain control in the first 24 hours after less invasive cranial surgery. Hemorrhage rates did not change with the use of an NSAID-based OSP.
Conclusion:
An effective alternative to the current standard opioid-based pain management is feasible for less invasive cranial surgery. Determinations of hemorrhage risk and more complex cranial surgery will require larger prospective randomized trials.
1. Introduction
Management of postoperative pain after cranial surgery remains a major challenge. Although surgical techniques and perioperative care have improved greatly,14 these patients continue to receive the same postoperative analgesia as decades ago,53 with opioids predominating. Given the need for accurate neurological assessments after surgery, opioids are often used sparingly for fear of sedation.25
Current postoperative analgesic strategies in cranial surgery patients include intravenous morphine or hydromorphone, administered as needed or through patient-controlled analgesia, along with acetaminophen, hydrocodone, or tramadol as adjuncts.27,34,70 Craniotomies were previously considered less painful compared with other types of surgery,21 presumably because of the lack of pain receptors in the brain. Recent studies suggest 69% and 48% of patients report significant uncontrolled pain during the first and second postoperative days, respectively, after undergoing various cranial procedures despite typical postoperative analgesic regimens.22,28
Multimodal analgesia medications including nonsteroidal anti-inflammatory drugs (NSAIDs) are a fundamental part of enhanced recovery after surgery protocols.56 Among the goals of enhanced recovery after surgery, which include lower postoperative morbidity, cost-savings, and improved quality of life, is the reduced the use of opioids. Although NSAIDs have been effective in postoperative pain control in many types of surgery,45,46 results in cranial surgery are less clear.35,48,79 Nonsteroidal anti-inflammatory drugs as a class are generally avoided because of the presumed risk of postoperative hemorrhagic complications which are often devastating in cranial surgery38,78,75. Some observational studies in these patients have suggested increased bleeding risks,43,55 raising safety concerns for their use in adults44 and children.61 Furthermore, prior publications attempting to study NSAIDS in postoperative cranial surgery patients include analgesic regimens with a combination of NSAIDS and opioids together, limiting the ability to delineate the efficacy of NSAIDs alone.22 Thus, there is impetus to study the safety and efficacy of opioid-sparing analgesia regimens in postoperative cranial surgery patients to develop up to date evidence-based analgesic protocols.
At the authors' institution, an NSAID-based opioid-sparing protocol (OSP) was initiated in January 2019 for treatment of postoperative pain in less invasive cranial surgery cases. Owing to hypothetical increased postoperative hemorrhage risk, we elected to initiate the OSP in less invasive, and henceforth lower risk, cranial surgery patients including burr holes (neurostimulation, shunts, subdural hematoma evacuations) and supratentorial or infratentorial craniotomies (epilepsy, hematoma evacuations, and simple tumors defined as less than 5 centimeters in size and well circumscribed). Before January 2019, all patients were managed by an opioid-based regimen. The primary aim of this study was to establish noninferiority of using an OSP by comparing the OSP cohort with a matched opioid-based control group.
2. Methods
This was a retrospective study evaluating the efficacy of using an OSP compared with a standard opioid-based analgesia protocol (OP) in postoperative cranial surgery patients. The primary aim of the study was to determine noninferiority of the OSP in postoperative pain control with a margin of 1 point as evaluated by the Defense and Veterans Pain Rating Scale (DVPRS) at 6, 12, and 24 hours after surgery compared with an OP. Secondary aims of the study included investigating differences in postoperative hemorrhagic complications, opioid usage, rates of urinary retention, length of stay, 30-day return to emergency room, and readmission rates. The study was approved by the institution's review board (IRB # 19092301).
2.1. Data source and variable selection
Data were collected from all patients who underwent cranial surgery procedures by one surgeon between July 2015 and November 2019. Starting on January 2019, an OSP was used for postoperative analgesia in all patients. The exclusion criteria were patients with NSAID allergies, end stage renal disease, chronic kidney disease with baseline serum creatinine greater than 1.5 mg/dL, and liver disease. All patients were preoperatively familiarized with the new protocol and the underlying rationale.
The OSP cohort was paired with a cohort before the onset of the OSP (July 2015–June 2018). During the time period between the 2 cohorts (July–December 2018), patients were transitioned to the OSP with protocol adjustments based on feedback. As such, patients from this time period were not included in the study. The analgesia regimen for the OP cohort included oral acetaminophen, hydrocodone, and intravenous morphine.
Propensity score matching was used to ensure consistency between the 2 cohorts. Preoperative variables included age, sex, body mass index, prior surgeries, prior opioid use, and medical comorbidities including diabetes mellitus, hypertension (HTN), depression, and anxiety. Operative data included type and length of surgery. All operations included cranial access, opening of dura, and surgery involving the parenchyma of the brain. Postoperative data included opioid usage in morphine equivalent units, postoperative pain scores (DVPRS) at 6, 12, and 24 hours, postoperative hemorrhage, antiseizure medication use, steroid use, urinary retention, length of stay, 30-day emergency room visits, and 30-day readmission.
To determine postoperative opioid requirements, cumulative morphine equivalents were recorded at each time point (6-hour, 12-hour, and 24-hour postoperatively and at discharge). Oral morphine milligram equivalents were calculated at various time points during a patient's hospital stay using published conversion factors from the Centers of Disease Control and clinical calculator for IV to oral dose conversions.20,41 At the authors' institution, DVPRS pain scores (0–10) are evaluated every 1 hour by nursing staff and re-evaluated immediately before and 1 hour after administration of medications. Averages of the DVPRS pain scores were computed at 6, 12, and 24 hours. Postoperative hemorrhage was evaluated by computed-tomography (CT) or magnetic resonance imaging (MRI) of the brain. Urinary retention was defined as failure to void more than 8 hours after removal of catheter in patients with intraoperative Foley placement or failure to void more than 8 hours after surgery in patients without a catheter. Patients with greater than 400 mL of fluid on routine ultrasound of their bladder were catheterized.2
2.2. Cohort
A total of 139 and 427 patients were initially identified in the OSP and OP groups, respectively. Emergency operations were excluded because postoperative pain management was not under the direct care of the attending neurosurgeon and the OSP was not incorporated in their postoperative care. All patients with extended intubation after surgery were also excluded as accurate pain assessment with the DVPRS was not possible. After exclusions, 94 and 261 patients remained in the OSP and OP groups for propensity score matching.
Propensity scores were used to match the OP cohort with the OSP intervention cohort to reduce baseline differences. Prior studies have demonstrated that this approach allows for analysis of observational data on a level similar to randomized control trials.5,6,63,69 The score was calculated as the conditional probability of being in the intervention group based on the following variables: age, sex, body mass index, preoperative opioid use, procedure length, bone flap removal, and comorbidities including diabetes mellitus, depression, and anxiety. A continuous variable between 0 and 1 was calculated and served as an index. Next, the conditional strategy of matching was used with a 1:1 matching algorithm with nearest neighbor to pair comparable control and OSP cohorts using a caliper setting of 0.1. After propensity score matching, a final total of 93 and 91 patients in the OSP and OP cohorts were included for final analysis.
2.3. Intervention
Implementation of OSP included 3 initiatives: clinical staff education, patient education, and an opioid-sparing analgesic protocol.
Clinical staff education included educational meetings with members of the surgical care team including nursing, ancillary, pharmacy, and intensivist care providers to facilitate input. All protocol medications were available in an automated dispensing cabinet on the nursing unit to ensure timely treatment of pain. The finalized OSP protocol was presented to all staff at formal educational events before initiation.
Patient education involved preoperative counseling on expectations of pain after surgery. Patients were educated on the use of NSAIDs and acetaminophen as first-line treatments for pain. Emphasis was placed on the goal of analgesia as controlling the pain rather than eliminating it. In addition, patients were counseled that opioid medications were available as alternatives if first-line pharmacotherapy did not provide adequate pain relief.
If indicated, ASMs and dexamethasone were administered at the discretion of the attending surgeon before surgery. A dose of fentanyl (25–75 μg) and ondansetron (4 mg) was routinely administered at the end of the procedure by the anesthesiologist in both groups.
The OSP and OP pharmacological protocols are outlined in Figure 1. Postoperative pain scores between 1 and 3 (of 10) were treated by verbal reassurance, ice packs, and repositioning alone. If pain remained severe (score 6–10) despite administration of oral acetaminophen, oral celecoxib, and IV ketorolac, escalation to an opioid regimen ensued after communication with the attending neurosurgeon. The control group (OP) received oral acetaminophen, oral hydrocodone, and oral tramadol or IV morphine, respectively, for escalating pain scores.
Figure 1.
(A) Opioid-sparing management protocol (OSP). All acetaminophen was given orally. (B) Opioid protocol (OP).
2.4. Statistics
For continuous variables, Student t tests or Mann–Whitney U tests were used. Categorical variables were compared using the χ2 or Fisher exact test as appropriate. Logistic regression analysis was used to identify variables associated with opioid escalation during hospitalization or at discharge in the OSP cohort. An independent t test was used to compare mean pain scores among the OP and OSP cohorts, including 95% confidence interval for determination of noninferiority with a margin of 1 point on the DVPRS. All results were analyzed using SPSS, version 26.0 (IBM, Armonk, NY). The level of significance was set at P < 0.05, and all P values were 2-tailed.
3. Results
3.1. Baseline characteristics
Prepropensity and postpropensity score–matching characteristics are shown in Table 1. Before matching, preoperative opioid use and HTN were more prevalent (25% vs 4%, P < 0.001% and 31% vs 18%, P < 0.001, respectively), whereas anxiety was less prevalent (23% vs 16%, P = 0.003) in the OSP as compared with the OP cohort. After matching, there was no statistically significant difference noted between the cohorts.
Table 1.
Baseline and matched characteristics.
| Full cohort | Matched cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Opioid (n = 261) | OSP (n = 94) | Significance | Mean standard difference (d) | Opioid (n = 91) | OSP (n = 93) | Significance | Mean standard difference (d) | |
| Craniotomy | 25% | 20% | 0.057 | 0.119 | 23% | 20% | 0.665 | 0.072 |
| Preoperative opioid use | 25% | 4% | <0.001 | 0.619 | 12% | 4% | 0.056 | 0.292 |
| Age | 59.2 | 57.7 | 0.429 | 0.099 | 58.6 | 57.6 | 0.644 | 0.066 |
| Body mass index (BMI) | 28.3 | 28.1 | 0.769 | 0.030 | 28.3 | 28.2 | 0.922 | 0.015 |
| Time of procedure (h) | 3.3 | 2.7 | 0.075 | 0.501 | 2.9 | 2.7 | 0.364 | 0.176 |
| Type 2 diabetes | 13% | 14% | 0.839 | 0.029 | 10% | 14% | 0.396 | 0.123 |
| Hypertension | 31% | 18% | <0.001 | 0.303 | 22% | 18% | 0.534 | 0.099 |
| Depression | 15% | 14% | 0.482 | 0.028 | 15% | 14% | 0.789 | 0.028 |
| Anxiety | 16% | 23% | 0.003 | 0.176 | 23% | 24% | 0.927 | 0.023 |
| Sex (male) | 56% | 60% | 0.237 | 0.080 | 59% | 60% | 0.904 | 0.020 |
Bold values indicate statistically significant findings.
The OSP cohort included the following surgery types: 74 neurostimulation, 2 intracranial hematoma evacuation, 8 tumor resection, and 9 epilepsy surgery. The OP cohort included 63 neurostimulation, 4 burr hole biopsy, 3 shunt placement, 4 intracranial hemorrhage evacuation, 6 tumor resection, and 11 epilepsy surgery. All neurostimulation cases except for 19 in the OSP and 21 in the OP included deep brain stimulator electrode placements and were performed awake under monitored anesthesia care. The remainder of neurostimulation cases was performed under general anesthesia. All other operations were performed under general anesthesia.
There was no significant difference in antiseizure medication usage (P = 0.24) between OSP and OP cohorts. The OSP cohort had a significantly larger percentage of patients treated with dexamethasone (44% OSP vs 15% OP, P < 0.01).
3.2. Outcomes
After 1:1 matching, the pain outcomes were compared as listed in Table 2. At 6, 12, and 24-hour postoperatively, the mean pain scores were lower in the OSP group at the 6 hours (3.45 vs 4.19, P = 0.036), 12 hours (3.21 vs 4.00, P = 0.006), and 24 hours (2.90 vs 3.59, P = 0.010) period (Fig. 2). The 95% confidence interval at each period exceeded the noninferiority margin, demonstrating noninferiority and superiority.
Table 2.
Primary and secondary outcomes.
| Opioid (n = 91) | OSP (n = 93) | Significance | 95% CI | |
|---|---|---|---|---|
| Primary outcomes | ||||
| 6 hours pain | 4.19 | 3.45 | 0.036 | 0.05–1.44 |
| 12 hours pain | 4.00 | 3.21 | 0.006 | 0.23–1.34 |
| 24 hours pain | 3.59 | 2.90 | 0.010 | 0.17–1.21 |
| Postoperative hemorrhage | 8% | 5% | 0.527 | |
| Secondary outcomes | ||||
| 30-d emergency department visit | 5% | 6% | 0.786 | |
| 30-d readmission | 9% | 3% | 0.113 | |
| MEU 6 hours | 11.6 | 1.2 | <0.001 | |
| MEU 12 hours | 13.0 | 0.8 | <0.001 | |
| MEU 24 hours | 12.6 | 1.6 | <0.001 | |
| MEU discharge | 45.2 | 4.6 | <0.001 | |
| Urinary retention | 5% | 2% | 0.238 | |
| Length of stay (d) | 2.24 | 1.85 | 0.184 |
Bold values indicate statistically significant findings.
ED, emergency department; LOS, length of stay.
Figure 2.
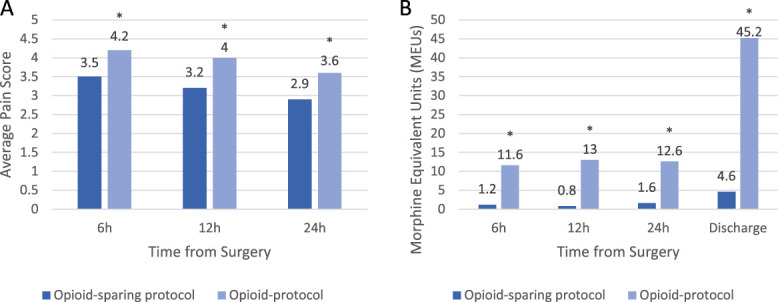
Pain and opioid drug usage after cranial neurosurgery. (A) Average postoperative pain scores assessed using DVPRS. (B) Cumulative opioid usage measured by morphine equivalent units (MEUs). DVPRS, Defense and Veterans Pain Rating Scale; OSP, opioid-sparing protocol; OP, opioid pain regimen.
The OSP cohort also had a statistically significant reduction in opioid usage (Table 2). In the OSP group, there were 9.6, 16.2, and 7.9-fold decreases in morphine equivalent units at 6, 12, and 24 hours (P < 0.001), respectively, when compared with the OP group (Fig. 2).
There was no significant difference in postoperative hemorrhage rates between the OSP and OP groups (5% vs 8%, P = 0.527). The OSP cohort had 5 (5%) hemorrhages (2 subdural and 3 intraparenchymal). The OP cohort had 7 (8%) hemorrhages (2 subdural, 1 epidural, and 4 intraparenchymal). The hemorrhages in both cohorts were comparable in size and severity. No hemorrhage led to surgical intervention or death.
Evaluation of secondary outcomes revealed the OSP cohort had a lower 30-day readmission rate (3% vs 9%, P = 0.113), slightly higher 30-day emergency department visit rate (6% vs 5%, P = 0.786), lower urinary retention rate (2% vs 5%, P = 0.238), and lower length of stay (1.85 days vs 2.24 days, P = 0.184) compared with the OP cohort. However, none of these values reached statistical significance.
3.3. Escalation of pain medication
15 patients (16.3%) in the OSP cohort did required an escalation to opioids because of inadequate pain control. None of the assessed variables were associated with an increased likelihood of escalating to opioids (Table 3).
Table 3.
Predictors of escalation to opioids.
| Odds ratio | Significance | |
|---|---|---|
| Craniotomy | 8.6 | 0.010 |
| Preoperative opioid use | 0.001 | 0.999 |
| Age | 1 | 0.999 |
| Body mass index (BMI) | 0.975 | 0.605 |
| Procedure length | 1.165 | 0.586 |
| Type 2 diabetes | 0.542 | 0.626 |
| Hypertension | 0.660 | 0.695 |
| Depression | 2.377 | 0.347 |
| Anxiety | 3.541 | 0.121 |
| Sex | 0.777 | 0.699 |
Bold values indicate statistically significant findings.
4. Discussion
4.1. Pain in the postoperative cranial patient
Management of postoperative pain has gained significant interest in the context of the opioid crisis.32 More than 80% of postoperative patients receive opioids after low-risk surgery,80 and most patients discharged from hospitals with opioid prescriptions are surgical patients.17 Even in opioid-naïve patients undergoing short-stay surgery, the risk of developing opioid use disorder because of a prescription at discharge is alarmingly high.1 This trend fits with the 4-fold increase in opioid use in the United States in the past few decades76 and the concurrent increase in emergency department visits and deaths related to narcotic use.19 Despite the extensive use of narcotics in cranial surgery,27 an adequate postoperative pain management regimen remains elusive, and many patients suffer from uncontrolled pain in the acute (24–48 hours) period.
In postoperative cranial surgery patients, high levels of uncontrolled pain are associated with sympathetic-mediated HTN and increased risk of edema, hemorrhage, and mortality.10 Although opioids are highly efficacious in controlling somatic pain, their usage is associated with respiratory depression, urinary retention,16 constipation, nausea or vomiting resulting in increased intracranial pressure, sedation, and confusion which can mimic neurological compromise.4 Despite their widespread usage, they are not particularly suited for treatment of cranial surgery pain. This is because the pain generators in postoperative cranial surgery patients are unique because of the involvement of the dura mater.64,77
The dura is a highly vascular tissue innervated by meningeal branches of the trigeminal and vagus nerve as well as higher cervical nerves. It is routinely incised and coagulated during cranial surgery resulting in inflammation and exquisite pain37 after surgery. An anti-inflammatory regimen is thus better suited to treat this type of pain as supported by the results of this study. The OSP cohort had significantly improved pain scores compared with the OP cohort with less opioid usage, likely because of NSAIDs' ability to minimize inflammation and, therefore, the initiation of the pain signal.
4.2. Use of COX-2 inhibitors in cranial surgery
A number of postoperative analgesic regimens have been trialed in cranial surgery patients, including acetaminophen,4,9,53,67 scalp blocks with local anesthetics,30 subcutaneous sumatriptan,57 intraoperative and postoperative dexmedetomidine,52,58 and gabapentenoids,66 with varying results for reducing opioid use or minimizing side effects.
Celecoxib is a highly selective COX-2 inhibitor,47 making it suitable for treatment of cranial surgery pain. Nonsteroidal anti-inflammatory drugs vary in their inhibition of enzymes cyclooxygenase (COX)-1 and COX-2. Platelet dysfunction and increased risk of hemorrhage are mediated by COX-1 inhibitors, as they prevent formation of thromboxane A2, a potent platelet activator.39 By contrast, NSAIDs that inhibit COX-2 decrease prostaglandin I2, which itself is a platelet inhibitor.50 Thus, selective COX-2 inhibitors have minimal effect on the platelet function. Other adverse effects of NSAIDs such as renal injury and gastrointestinal bleeding are believed to be mediated by COX-1 inhibition75 and are decreased when using COX-2 inhibitors.49,71 Clinical usage of COX-2 inhibitors was initially slowed by concern for increased risk of cardiovascular events, but recent evidence suggests that certain COX-2 inhibitors were outliers for cardiovascular safety,13,29 and celecoxib specifically had a lower risk of stroke and myocardial infarction when compared with all NSAIDs.3,8,12,36
4.3. The opioid-sparing protocol components
Cranial surgery is an inherently anxiety-provoking experience, and experiencing excessive pain after surgery is one of the most significant patient concerns.51,60,72 Preoperative counseling and managing patients' expectations was a key component of the OSP. Although not formally evaluated, it is possible that patient education led to reduced postoperative anxiety and reduced the need for pain medications. Prior studies including preoperative counseling have shown similar results of reduced pain medication and better postoperative pain scores reported by patients.65,68 Although this was a potential confounding variable, patient education and counseling were unavoidable because generally the patient expect narcotic-based pain management after cranial procedure. Staff collaboration was equally important, involving the entire care team in patient-focused decision points allowed for clinical “pauses” and reassessments. This paradigm ensured medication escalation alone when absolutely necessary, rather than the all too common “reflexive” escalation to potent opioids.
The OSP protocol mandated scheduled dosing of celecoxib during the first 72 hours after surgery because most pain control issues occur during this period.77 Prior studies have shown mixed results with as-needed dosing of NSAIDs after surgery.4,15,33,74 Scheduled dosing offers the advantage of providing a prolonged therapeutic drug level for consistent analgesic control. This is particularly applicable to cranial surgery patients who often have difficulty requesting pain medications during the initial postoperative period.
This is the first study to evaluate the use of a scheduled NSAID such as celecoxib in cranial surgery patients. Two prior studies explored the use of parecoxib, another COX-2 inhibitor, in postoperative cranial surgery pain control. In a study by Williams et al.,79 a one-time dose of parecoxib or placebo along with acetaminophen was given at the conclusion of surgery. Intravenous morphine was used postoperatively. They found no difference in opioid consumption in the parecoxib group vs placebo. In another study by Jones et al.,35 administration of a one-time dose of parecoxib at the end of surgery failed to reduce postoperative opioid use. Although the study designs prevent direct comparison with our results, they do suggest that a single postoperative NSAID dose is not sufficient to reduce opioid usage.73 This is further supported by the orthopedic literature that has demonstrated analgesia from COX-2 inhibitors is dose dependent,62 and scheduled dosing is superior to a one-time dose in controlling postoperative pain.81
4.4. Safety of the opioid-sparing protocol in cranial surgery
Nonsteroidal anti-inflammatory drugs have previously been avoided in cranial surgery patients because of fear of hemorrhagic complications.38,75,78 This fear persists despite studies showing no difference in bleeding risk in adults44 and children11,61 undergoing cranial surgery. Similar investigations in orthopedics,81 spinal surgery,18 plastic surgery,59 general surgery,23 otolaryngology,7 and other major surgeries24 have affirmed the safety of postoperative NSAID use, as well as a meta-analysis of 27 studies with 2314 patients across a range of disciplines.26 Although not directly comparable, the pediatric literature has also shown safety in postoperative NSAID use in children undergoing surgery.31,40,42,54 This study is consistent with prior literature in demonstrating unchanged postoperative hemorrhage rates with NSAID use, although was not powered for significance. A much larger study will be able to detect a statistically significant difference in safety, if any, especially considering differences in types of cranial surgery. It may be that less invasive operations such as shunt placements inherently have a lower hemorrhage risk than extensive craniotomies, and larger cohorts are needed. Nonetheless, our results suggest that use of a celecoxib-based pain management regimen would not significantly increase hemorrhage risk.
4.5. Limitations
Although this study suggests the OSP is an effective alternative to standard opioid protocols, the results should be interpreted within the contexts of existing limitations. The sample size for the cohorts is relatively small, precluding us from reaching significance for certain secondary endpoints. We used a continuous cohort of patients to mitigate selection bias. Propensity score matching was also used to overcome the inherent limitations of retrospective studies and minimize preexisting differences between the 2 cohorts. This includes matching for complexity and invasiveness of surgery.
Less invasive cranial surgery such as burr holes or neurostimulation cases likely require less postoperative pain management than larger craniotomies which include temporalis muscle dissection or skull base tumors. This limits the generalizability of our results to all cranial surgery. Prior investigations of opioid-sparing protocols have not stratified their findings by the complexity of surgery. Thus, hemorrhagic complications and total pain medication usage may be higher in more complex cases.
More patients in the OSP group received dexamethasone than the OP. Although the anti-inflammatory action of dexamethasone may contribute to improved immediate pain relief in the OSP group, the one-time dosing used in most cases would preclude it from having a durable effect in our study, as was seen in the 12-hour and 24-hour pain scores.
Although most of our patients underwent relatively less complex surgeries, the OSP and OP cohorts were matched by procedure time and completion of a craniotomy through propensity score matching; thus, the reduction of pain scores is likely a true effect. In addition, the goal of the OSP is opioid reduction rather than elimination, which should still apply to larger craniotomies if hemorrhage risks remain the same. Many patients who would otherwise have been treated with the standard opioid protocol were able to successfully be managed with minimal or no narcotics. Our results suggest that the OSP is at least noninferior to a traditional opioid-based protocol for pain control using significantly less narcotics. Although this study provides encouraging safety and efficacy results, it ultimately serves as a benchmark for further randomized controlled studies with a wide spectrum of cranial procedure.
5. Conclusions
This is the first study to demonstrate an effective alternative to opioid-based analgesia in less invasive cranial surgery patients. The results demonstrate that the OSP significantly reduced opioid usage while decreasing postoperative pain scores in less invasive cranial surgery patients. Opioid-sparing protocol patients did not have increased hemorrhagic complications.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
The authors acknowledge the help and expertise of Skyler C. Boll, BS, Michael J. Forst, BS, and Josiah A. Baker, BS for their assistance with data collection.
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this article.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Ryan Khanna, Email: ryan_khanna@rush.edu.
Alvin Chidozie Onyewuenyi, Email: Alvin_Onyewuenyi@rush.edu.
Nicholas Panos, Email: Nicholas_Panos@rush.edu.
Rory Breslin, Email: Rory_Breslin@rush.edu.
Sepehr Sani, Email: sepehr_sani2@rush.edu.
References
- [1].Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med 2012;172:425–30. [DOI] [PubMed] [Google Scholar]
- [2].Altschul D, Kobets A, Nakhla J, Jada A, Nasser R, Kinon MD, Yassari R, Houten J. Postoperative urinary retention in patients undergoing elective spinal surgery. J Neurosurg Spine 2017;26:229–34. [DOI] [PubMed] [Google Scholar]
- [3].Antman EM. Evaluating the cardiovascular safety of nonsteroidal anti-inflammatory drugs. Circulation 2017;135:2062–72. [DOI] [PubMed] [Google Scholar]
- [4].Artime C, Aijazi H, Zhang H, Syed T, Cai C, Gumbert S, Ferrario L, Normand K, Williams G, Hagberg C. Scheduled intravenous acetaminophen improves patient satisfaction with postcraniotomy pain management: a prospective, randomized, placebo-controlled, double-blind study. J Neurosurg Anesthesiol 2018;30:231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014;33:1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008;27:2037–49. [DOI] [PubMed] [Google Scholar]
- [7].Bailey R, Sinha C, Burgess LPA. Ketorolac tromethamine and hemorrhage in tonsillectomy: a prospective, randomized, double-blind study. Laryngoscope 1997;107:166–9. [DOI] [PubMed] [Google Scholar]
- [8].Bally M, Dendukuri N, Rich B, Nadeau L, Helin-Salmivaara A, Garbe E, Brophy JM. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. BMJ 2017;357:j1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ban VS, Bhoja R, McDonagh DL. Multimodal analgesia for craniotomy. Curr Opin Anaesthesiol 2019;32:592–9. [DOI] [PubMed] [Google Scholar]
- [10].Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiol 2000;93:48–54. [DOI] [PubMed] [Google Scholar]
- [11].Bauer DF, Waters AM, Tubbs RS, Rozzelle CJ, Wellons JC, Blount JP, Oakes WJ. Safety and utility of scheduled nonnarcotic analgesic medications in children undergoing craniotomy for brain tumor. Neurosurgery 2010;67:353–6. [DOI] [PubMed] [Google Scholar]
- [12].Beales ILP. Selective COX-2 inhibitors are safe and effective. BMJ 2020;368:1. [DOI] [PubMed] [Google Scholar]
- [13].Beales ILP. Time to reappraise the therapeutic place of celecoxib. Ther Adv Chronic Dis 2018;9:107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bernardo A. The changing face of technologically integrated neurosurgery: today's high-tech operating room. World Neurosurg 2017;106:1001–14. [DOI] [PubMed] [Google Scholar]
- [15].Boddu C, Genza A, McCann PD. Bridging multimodal pain management provides 48-hour pain control in patients undergoing total shoulder replacement. J Shoulder Elbow Surg 2018;27:S65–9. [DOI] [PubMed] [Google Scholar]
- [16].de Boer HD, Detriche O, Forget P. Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol 2017;31:499–504. [DOI] [PubMed] [Google Scholar]
- [17].Calcaterra SL, Yamashita TE, Min S-J, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med 2016;31:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cassinelli EH, Dean CL, Garcia RM, Furey CG, Bohlman HH. Ketorolac use for postoperative pain management following lumbar decompression surgery: a prospective, randomized, double-blinded, placebo-controlled trial. Spine 2008;33:1313–17. [DOI] [PubMed] [Google Scholar]
- [19].Center for Disease Control. Drug overdose deaths in the United States, 1999–2017. 2019. Available at https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed April 15, 2020. [Google Scholar]
- [20].Data resources|drug overdose|CDC injury center. 2019. Available at: https://www.cdc.gov/drugoverdose/resources/data.html. Accessed April 6, 2020. [Google Scholar]
- [21].Dunbar PJ, Visco E, Lam AM. Craniotomy procedures are associated with less analgesic requirements than other surgical procedures. Anesth Analg 1999;88:335–40. [DOI] [PubMed] [Google Scholar]
- [22].Dunn LK, Naik BI, Nemergut EC, Durieux ME. Post-craniotomy pain management: beyond opioids. Curr Neurol Neurosci Rep 2016;16:93. [DOI] [PubMed] [Google Scholar]
- [23].Firriolo JM, Nuzzi LC, Schmidtberg LC, Labow BI. Perioperative ketorolac use and postoperative hematoma formation in reduction mammaplasty: a single-surgeon experience of 500 consecutive cases. Plast Reconstr Surg 2018;142:632e–8e. [DOI] [PubMed] [Google Scholar]
- [24].Forrest JB, Camu F, Greer IA, Kehlet H, Abdalla M, Bonnet F, Ebrahim S, Escolar G, Jage J, Pocock S, Velo G, Langman MJS, Porro GB, Samama MM, Heitlinger E. Ketorolac, diclofenac, and ketoprofen are equally safe for pain relief after major surgery† †Declaration of interest. This study was funded by F. Hoffmann-La Roche Ltd. Br J Anaesth 2002;88:227–33. [DOI] [PubMed] [Google Scholar]
- [25].Gabriel RA, Swisher MW, Sztain JF, Furnish TJ, Ilfeld BM, Said ET. State of the art opioid-sparing strategies for post-operative pain in adult surgical patients. Expert Opin Pharmacother 2019;20:949–61. [DOI] [PubMed] [Google Scholar]
- [26].Gobble RM, Hoang HLT, Kachniarz B, Orgill DP. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg 2014;133:741–55. [DOI] [PubMed] [Google Scholar]
- [27].Goldsack C, Scuplak SM, Smith M. A double-blind comparison of codeine and morphine for postoperative analgesia following intracranial surgery. Anaesthesia 1996;51:1029–32. [DOI] [PubMed] [Google Scholar]
- [28].Gottschalk A, Berkow LC, Stevens RD, Mirski M, Thompson RE, White ED, Weingart JD, Long DM, Yaster M. Prospective evaluation of pain and analgesic use following major elective intracranial surgery. J Neurosurg 2007;106:210–16. [DOI] [PubMed] [Google Scholar]
- [29].Gudbjornsson B, Thorsteinsson SB, Sigvaldason H, Einarsdottir R, Johannsson M, Zoega H, Halldorsson M, Thorgeirsson G. Rofecoxib, but not celecoxib, increases the risk of thromboembolic cardiovascular events in young adults—a nationwide registry-based study. Eur J Clin Pharmacol 2010;66:619–25. [DOI] [PubMed] [Google Scholar]
- [30].Guilfoyle MR, Helmy A, Duane D, Hutchinson PJA. Regional scalp block for postcraniotomy analgesia: a systematic review and meta-analysis. Anesth Analg 2013;116:1093–102. [DOI] [PubMed] [Google Scholar]
- [31].Gupta A, Daggett C, Drant S, Rivero N, Lewis A. Prospective randomized trial of ketorolac after congenital heart surgery. J Cardiothorac Vasc Anesth 2004;18:454–7. [DOI] [PubMed] [Google Scholar]
- [32].Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E. Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg 2017;125:1733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Horsley RD, Vogels ED, McField DAP, Parker DM, Medico C, Dove J, Fluck M, Gabrielsen JD, Gionfriddo MR, Petrick AT. Multimodal postoperative pain control is effective and reduces opioid use after laparoscopic roux-en-Y gastric bypass. Obes Surg 2019;29:394–400. [DOI] [PubMed] [Google Scholar]
- [34].Jellish WS, Leonetti JP, Sawicki K, Anderson D, Origitano TC. Morphine/ondansetron PCA for postoperative pain, nausea, and vomiting after skull base surgery. Otolaryngol Head Neck Surg 2006;135:175–81. [DOI] [PubMed] [Google Scholar]
- [35].Jones SJ, Cormack J, Murphy MA, Scott DA. Parecoxib for analgesia after craniotomy. Br J Anaesth 2009;102:76–9. [DOI] [PubMed] [Google Scholar]
- [36].Joshi GP, Gertler R, Fricker R. Cardiovascular thromboembolic adverse effects associated with cyclooxygenase-2 selective inhibitors and nonselective antiinflammatory drugs. Anesth Analg 2007;105:1793–804. [DOI] [PubMed] [Google Scholar]
- [37].Kemp WJ, 3rd, Tubbs RS, Cohen-Gadol AA. The innervation of the scalp: A comprehensive review including anatomy, pathology, and neurosurgical correlates. Surg Neurol Int 2011;2:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim SH, Lee JH, Joo W, Chough CK, Park HK, Lee KJ, Rha HK. Analysis of the risk factors for development of post-operative extradural hematoma after intracranial surgery. Br J Neurosurg 2015;29:243–8. [DOI] [PubMed] [Google Scholar]
- [39].Knijff‐Dutmer EaJ, Kalsbeek‐Batenburg EM, Koerts J, van de Laar MaFJ. Platelet function is inhibited by non‐selective non‐steroidal anti‐inflammatory drugs but not by cyclo‐oxygenase‐2‐selective inhibitors in patients with rheumatoid arthritis. Rheumatology 2002;41:458–61. [DOI] [PubMed] [Google Scholar]
- [40].Kokki H. Nonsteroidal anti-inflammatory drugs for postoperative pain. Pediatr Drugs 2003;5:103. [DOI] [PubMed] [Google Scholar]
- [41].Lexicomp Online. Opioid agonist conversion calculator. Lexicomp Inc, Hudson, OH. [Google Scholar]
- [42].Lieh-Lai MW, Kauffman RE, Uy HG, Danjin M, Simpson PM. A randomized comparison of ketorolac tromethamine and morphine for postoperative analgesia in critically ill children. Crit Care Med 1999;27:2786–91. [DOI] [PubMed] [Google Scholar]
- [43].Lillemäe K, Järviö JA, Silvasti-Lundell MK, Antinheimo JJ-P, Hernesniemi JA, Niemi TT. Incidence of postoperative hematomas requiring surgical treatment in neurosurgery: a retrospective observational study. World Neurosurg 2017;108:491–7. [DOI] [PubMed] [Google Scholar]
- [44].Magni G, La Rosa I, Melillo G, Abeni D, Hernandez H, Rosa G. Intracranial hemorrhage requiring surgery in neurosurgical patients given ketorolac: a case-control study within a cohort (2001–2010). Anesth Analg 2013;116:443–7. [DOI] [PubMed] [Google Scholar]
- [45].Martinez V, Beloeil H, Marret E, Fletcher D, Ravaud P, Trinquart L. Non-opioid analgesics in adults after major surgery: systematic review with network meta-analysis of randomized trials. Br J Anaesth 2017;118:22–31. [DOI] [PubMed] [Google Scholar]
- [46].Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth 2011;106:292–7. [DOI] [PubMed] [Google Scholar]
- [47].McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A 1999;96:272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Molnár C, Simon É, Kazup Á, Gál J, Molnár L, Novák L, Bereczki D, Sessler DI, Fülesdi B. A single preoperative dose of diclofenac reduces the intensity of acute postcraniotomy headache and decreases analgesic requirements over five postoperative days in adults: a single center, randomized, blinded trial. J Neurol Sci 2015;353:70–3. [DOI] [PubMed] [Google Scholar]
- [49].Moore RA, Derry S, McQuay HJ. Cyclo-oxygenase-2 selective inhibitors and nonsteroidal anti-inflammatory drugs: balancing gastrointestinal and cardiovascular risk. BMC Musculoskelet Disord 2007;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954–9. [DOI] [PubMed] [Google Scholar]
- [51].Munafò MR, Stevenson J. Anxiety and surgical recovery: reinterpreting the literature. J Psychosom Res 2001;51:589–96. [DOI] [PubMed] [Google Scholar]
- [52].Naik BI, Nemergut EC, Kazemi A, Fernández L, Cederholm SK, McMurry TL, Durieux ME. The effect of dexmedetomidine on postoperative opioid consumption and pain after major spine surgery. Anesth Analg 2016;122:1646–53. [DOI] [PubMed] [Google Scholar]
- [53].Nair S, Rajshekhar V. Evaluation of pain following supratentorial craniotomy. Br J Neurosurg 2011;25:100–3. [DOI] [PubMed] [Google Scholar]
- [54].Özkiriş M, Kapusuz Z, Yildirim YS, Saydam L. The effect of paracetamol, metamizole sodium and ibuprofen on postoperative hemorrhage following pediatric tonsillectomy. Int J Pediatr Otorhinolaryngol 2012;76:1027–9. [DOI] [PubMed] [Google Scholar]
- [55].Palmer JD, Sparrow OC, Iannotti F. Postoperative HematomaA 5-year survey and identification of avoidable risk factors. Neurosurgery 1994;35:1061–5. [DOI] [PubMed] [Google Scholar]
- [56].Parks L, Routt M, De Villiers A. Enhanced Recovery After Surgery. J Adv Pract Oncol 2018;9:511–519. [PMC free article] [PubMed] [Google Scholar]
- [57].Patel KS, Laiwalla AN, DiCesare JAT, Garrett MC, Wang AC. Subcutaneous sumatriptan: association with decreases in postoperative pain and opioid use after elective cranial surgery. J Neurosurg 2020;1:1–9. [DOI] [PubMed] [Google Scholar]
- [58].Peng K, Jin X, Liu S, Ji F. Effect of Intraoperative Dexmedetomidine on Post-Craniotomy Pain. Clin Ther 2015;37:1114–1121.e1. [DOI] [PubMed] [Google Scholar]
- [59].Power I, Noble DW, Douglas E, Spence AA. Comparison of I.M. ketorolac trometamol and morphine sulphate for pain relief after cholecystectomy. Br J Anaesth 1990;65:448–55. [DOI] [PubMed] [Google Scholar]
- [60].Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother 2009;9:745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Richardson MD, Palmeri NO, Williams SA, Torok MR, O'Neill BR, Handler MH, Hankinson TC. Routine perioperative ketorolac administration is not associated with hemorrhage in pediatric neurosurgery patients. J Neurosurg Pediatr 2016;17:107–15. [DOI] [PubMed] [Google Scholar]
- [62].Rømsing J, Møiniche S. A systematic review of COX-2 inhibitors compared with traditional NSAIDs, or different COX-2 inhibitors for post-operative pain. Acta Anaesthesiol Scand 2004;48:525–46. [DOI] [PubMed] [Google Scholar]
- [63].Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- [64].Schueler M, Messlinger K, Dux M, Neuhuber WL, De Col R. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. PAIN 2013;154:1622–31. [DOI] [PubMed] [Google Scholar]
- [65].Sheldon RR, Weiss JB, Do WS, Forte DM, Carter PL, Eckert MJ, Sohn VY. Stemming the tide of opioid addiction—dramatic reductions in postoperative opioid requirements through preoperative education and a standardized analgesic regimen. Mil Med 2020;185:436–43. [DOI] [PubMed] [Google Scholar]
- [66].Shimony N, Amit U, Minz B, Grossman R, Dany MA, Gonen L, Kandov K, Ram Z, Weinbroum AA. Perioperative pregabalin for reducing pain, analgesic consumption, and anxiety and enhancing sleep quality in elective neurosurgical patients: a prospective, randomized, double-blind, and controlled clinical study. J Neurosurg 2016;125:1513–22. [DOI] [PubMed] [Google Scholar]
- [67].Sivakumar W, Jensen M, Martinez J, Tanana M, Duncan N, Hoesch R, Riva-Cambrin JK, Kilburg C, Ansari S, House PA. Intravenous acetaminophen for postoperative supratentorial craniotomy pain: a prospective, randomized, double-blinded, placebo-controlled trial. J Neurosurg 2018;130:766–22. [DOI] [PubMed] [Google Scholar]
- [68].Sjöling M, Nordahl G, Olofsson N, Asplund K. The impact of preoperative information on state anxiety, postoperative pain and satisfaction with pain management. Patient Educ Couns 2003;51:169–76. [DOI] [PubMed] [Google Scholar]
- [69].Staffa SJ, Zurakowski D. Five steps to successfully implement and evaluate propensity score matching in clinical research studies. Anesth Analg 2018;127:1066–73. [DOI] [PubMed] [Google Scholar]
- [70].Stoneham MD, Cooper R, Quiney NF, Walters FJM. Pain following craniotomy: a preliminary study comparing PCA morphine with intramuscular codeine phosphate. Anaesthesia 1996;51:1176–8. [DOI] [PubMed] [Google Scholar]
- [71].Teerawattananon C, Tantayakom P, Suwanawiboon B, Katchamart W. Risk of perioperative bleeding related to highly selective cyclooxygenase-2 inhibitors: a systematic review and meta-analysis. Semin Arthritis Rheum 2017;46:520–8. [DOI] [PubMed] [Google Scholar]
- [72].Theunissen M, Peters ML, Bruce J, Gramke H-F, Marcus MA. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain 2012;28:819–41. [DOI] [PubMed] [Google Scholar]
- [73].Tsaousi GG, Logan SW, Bilotta F. Postoperative pain control following craniotomy: a systematic review of recent clinical literature. Pain Pract 2017;17:968–81. [DOI] [PubMed] [Google Scholar]
- [74].Ulm MA, ElNaggar AC, Tillmanns TD. Celecoxib versus ketorolac following robotic hysterectomy for the management of postoperative pain: an open-label randomized control trial. Gynecol Oncol 2018;151:124–8. [DOI] [PubMed] [Google Scholar]
- [75].Umamaheswara Rao GS, Gelb AW. To use or not to use: the dilemma of NSAIDs and craniotomy. Eur J Anaesthesiol EJA 2009;26:625–6. [DOI] [PubMed] [Google Scholar]
- [76].United Nations. World drug report 2019. 2019. Available: https://wdr.unodc.org/wdr2019/. Accessed April 15, 2020. [Google Scholar]
- [77].Vacas S, Van de Wiele B. Designing a pain management protocol for craniotomy: A narrative review and consideration of promising practices. Surg Neurol Int 2017;8:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang C, Niu X, Ren Y, Lan Z, Zhang Y. Risk factors for postoperative intracranial hemorrhage after resection of intracranial tumor in 2259 consecutive patients. World Neurosurg 2019;129:e663–8. [DOI] [PubMed] [Google Scholar]
- [79].Williams DL, Pemberton E, Leslie K. Effect of intravenous parecoxib on post-craniotomy pain. Br J Anaesth 2011;107:398–403. [DOI] [PubMed] [Google Scholar]
- [80].Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD. Opioids prescribed after low-risk surgical procedures in the United States, 2004–2012. JAMA 2016;315:1654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhuang Q, Tao L, Lin J, Jin J, Qian W, Bian Y, Li Y, Dong Y, Peng H, Li Y, Fan Y, Wang W, Feng B, Gao N, Sun T, Lin J, Zhang M, Yan S, Shen B, Pei F, Weng X. Postoperative intravenous parecoxib sodium followed by oral celecoxib post total knee arthroplasty in osteoarthritis patients (PIPFORCE): a multicentre, double-blind, randomised, placebo-controlled trial [published correction appears in BMJ Open 2020;10:e030501corr1]. [DOI] [PMC free article] [PubMed] [Google Scholar]



