Abstract
Over-expression of vitronectin (VN) is associated with tumorigenesis. The present study aimed to evaluate the prognostic value of VN expression in gastric cancer.
The least absolute shrinkage and selection operator analysis was performed to screen the hub gene from The Cancer Genome Atlas gastric cancer patients with complete follow-up data, and 347 patients were finally included. Moreover, 102 patients were enrolled from the Affiliated Fuzhou First Hospital of Fujian Medical University. VN expression in paired gastric cancer and adjacent gastric normal tissues was detected using immunohistochemistry, and the clinicopathological significance of VN expression was evaluated. The prognostic significance of VN expression in gastric cancer patients was evaluated using by Kaplan–Meier method and Cox regression analysis and confirmed using Oncomine.
VN was the prognosis relative gene which screened by The Cancer Genome Atlas dataset. Moreover, we identified the VN expression in an external dataset by immunohistochemistry. The result demonstrated that VN expression was remarkedly elevated in gastric cancer tissues (P < .001). High VN expression correlated with higher pathological Tumor-Node-Metastasis stage, and poorer survival outcomes. Cox regression analysis showed that VN expression was independently predictive of overall survival (OS) and disease-free survival (P = .004, P < .001, respectively). A prognostic risk score for OS was built based on VN expression. A meta-analysis from Oncomine datasets revealed that significantly lower VN mRNA levels in gastric cancer correlated with poorer OS.
VN expression could be a prognostic marker of gastric cancer.
Keywords: biomarker, gastric cancer, prognosis, vitronectin
1. Introduction
Gastric cancer, a common cancer worldwide, is a leading cause of cancer-related death, especially in East Asian countries.[1] Despite significant improvements in surgery, chemotherapy, radiotherapy, and neoadjuvant therapy, the prognosis of patients with gastric cancer patients remains unfavorable.[2,3] The pathogenesis of gastric cancer has not been well clarified. Besides, gastric cancer is also biologically and genetically heterogeneous. Thus, it is difficult to accurately predict the oncological outcomes of gastric cancers.[4] Currently, many efforts have devoted to identifying biomarkers in a basis of clinical information and comprehensive genome analysis.[5] Identifying robust biomarkers for gastric cancer has become of great importance.
Vitronectin (VN), a member of the integrin family, is an abundant glycoprotein found in serum and the extracellular matrix. VN can bind to its receptor, integrin αVβ3, and is implicated in the process of inflammation, cell adhesion, and spreading.[6] Importantly, VN is involved in the pathogenesis of several tumors, including melanoma,[7] prostate,[8] and breast[9] cancer. Additionally, serum VN has been proposed to be a biomarker of diagnosis and prognosis of tumors, such as hepatitis B-related hepatocellular carcinoma,[10] glioma,[11] and breast cancer.[12] To date, studies on the association between VN and gastric cancer are relatively rare, and the prognostic significance of VN in gastric cancer remains unclear.
Therefore, we calculated VN expression in gastric cancers by immunohistochemical staining. We next evaluated the clinicopathological significance of VN expression in gastric cancer and built a prognostic model for overall survival (OS). Besides, we evaluated the prognostic implication of VN expression and further confirmed our findings by bioinformatics analysis.
2. Materials and methods
2.1. Screening of VN in the TCGA database
The mRNA expression profiles and the corresponding clinical information from the patients with gastric cancer were acquired from The Cancer Genome Atlas (TCGA) database. Least absolute shrinkage and selection operator Cox regression model was applied to determine the ideal coefficient for each prognostic feature and estimate the likelihood deviance.
2.2. Patients and specimens
Totally 102 gastric cancer patients treated with operation at the Affiliated Fuzhou First Hospital of Fujian Medical University between 2013 and 2015 were enrolled in this study. All patients have not been treated by systemic chemotherapy or radiotherapy before the operation. Gastric cancer and adjacent normal tissue samples were collected after resection and used for further immunohistochemistry. The present study was approved by the Ethics and Research Committees of the Affiliated Fuzhou First Hospital of Fujian Medical University.
2.3. Immunohistochemistry
The streptavidin-biotin complex method[13] was used to evaluate VN expression. Briefly, anti-vitronectin (Bioss, China) was used as the primary antibody. Sections were scored by 2 experienced pathologists using an Olympus BX53 microscope (Olympus Co., Tokyo, Japan, magnification ×200). The percentage of positive cells was determined and the total score was described in a previous study.[14]
2.4. Validation of VN by bioinformatics analysis
Next, we further validate the expression of VN in gastric cancer patients using publicly available databases. Additionally, Oncomine database[15] was utilized to evaluate VN expression and survival based on publicly available gastric cancer tissues and gastric cells. Besides, the prognostic value of VN expression in gastric cancer was evaluated using the R2 Platform (http://r2.amc.nl).
2.5. Statistical analysis
Chi-square or Student t tests were used to calculate statistical significance for categorial or continuous variables. Patient survival rates were analyzed with the Kaplan–Meier method. OS was calculated from surgery to death. Disease-free survival (DFS) was estimated from surgical resection to tumor relapse. Cox regression analysis was conducted to determine independent prognostic predictors. According to the absolute value of coefficients in the Cox regression model, a risk score system was constructed to calculate the corresponding risk scores for OS in a gastric cancer patient. Time-dependent receiver operating characteristics analysis was used to measure the performance of the model. Statistic analyses were performed using SPSS 24.0 software (SPSS Inc., Chicago, IL). P < .05 was defined as statistical significance.
3. Results
3.1. Screening of VN in the TCGA database
First, we attempted to validate the expression of VN in the TCGA for gastric cancer. Patients with complete follow-up data were screened out, and 347 patients were finally included. We briefly introduce the method of the genes inclusion and exclusion. Firstly, we annotated the geneset from the TCGA. And then, genes with the same name were combined and averaged. Finally, we removed the non-coding genes and a total of 15,734 genes were included. By using least absolute shrinkage and selection operator, 4 genes were screened out, including VN, ZNF702P, WASH2P, and ACACA. The results demonstrated that VN expression was the most significant factor associated with prognosis of gastric cancer, as shown in Fig. 1A and B. Kaplan–Meier survival analysis revealed that high expression of VN was associated with worse overall survival (P < .01, Fig. 1C). Moreover, the clinicopathological characteristics from the TCGA dataset were enrolled. The result demonstrated that the VN high expression was associated with pathology N stage (P = .049), pathology M stage (P < .001), and pathology TNM stage (P = .002), as shown in Table 1.
Figure 1.
(A and B) LASSO analysis. (C) Kaplan–Meier survival analysis revealed that high expression of VN was associated with worse overall survival (P < .01). LASSO = least absolute shrinkage and selection operator.
Table 1.
Association between vitronectin expression and clinicopathological characteristics in TCGA gastric cancer patients.
| VN expression | |||
| Variables | Low (N = 328) | High (N = 19) | P value |
| Gender (%) | .084 | ||
| Male | 208 (63.4) | 16 (84.2) | |
| Female | 120 (36.6) | 3 (15.8) | |
| Age (mean ± SD, yrs) | 65.4 ± 10.4 | 62.4 ± 10.0 | .212 |
| Grade type (%) | .679 | ||
| G1 | 9 (20.0) | 0 (0.0) | |
| G2 | 114 (35.6) | 8 (42.1) | |
| G3 | 197 (33.3) | 10 (52.6) | |
| Unknown | 8 (2.4) | 1 (5.3) | |
| Pathological T stage (%) | .653 | ||
| T1 | 15 (4.6) | 0 (0.0) | |
| T2 | 67 (20.4) | 5 (26.3) | |
| T3 | 154 (47.0) | 7 (36.8) | |
| T4 | 88 (26.8) | 7 (36.8) | |
| Unknown | 4 (1.2) | 0 (0.0) | |
| Pathological N stage (%) | .049 | ||
| N0 | 101 (30.8) | 4 (21.1) | |
| N1 | 91 (27.7) | 3 (15.8) | |
| N2 | 69 (21.0) | 3 (15.8) | |
| N3 | 61 (18.6) | 9 (47.7) | |
| Unknown | 6 (1.8) | 0 (0.0) | |
| Pathological M stage (%) | <.001 | ||
| M0 | 300 (91.5) | 12 (63.2) | |
| M1 | 28 (8.5) | 7 (36.8) | |
| TNM stage (%) | .002 | ||
| I | 43 (13.1) | 2 (10.5) | |
| II | 106 (32.3) | 3 (15.8) | |
| III | 138 (42.1) | 7 (36.8) | |
| IV | 28 (8.5) | 7 (36.8) | |
| Unknown | 13 (4.0) | 0 (0.0) | |
3.2. VN expression
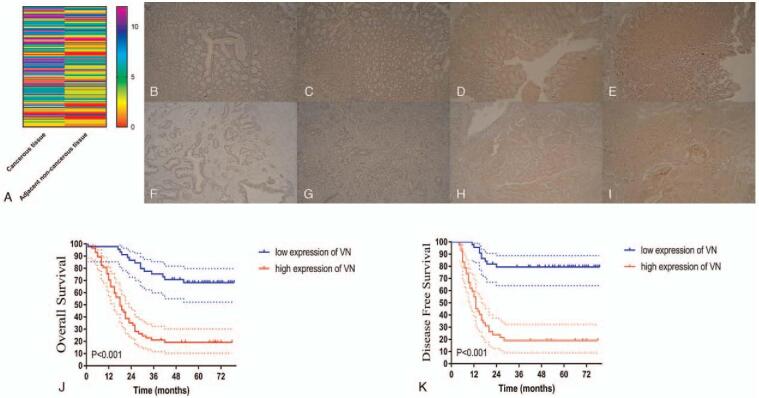
Totally 102 patients were analyzed (including 81 men and 21 women) in the present study. The age of patients was 63.4 ± 10.9 years. The baseline clinicopathological characteristics were listed in Table S1, Supplemental Digital Content. Immunohistochemical staining revealed that higher VN expression in the gastric cancer tissues than in normal tissues (Fig. 2A–I, P < .001).
Figure 2.
(A) The immunohistochemical score of VN. Representative figures of VN expression gastric cancer (B–E) and adjacent non-cancerous (F–I) tissues. The immunohistochemical score of VN. (J) Overall survival. (K) Disease-free survival stratified by VN expression. VN = vitronectin.
X-tile analysis[16] was conducted to determine the cut-off values of the VN immunohistochemical score that yielded the biggest survival difference concerning OS and DFS. As shown in Figure S1, Supplemental Digital Content, an optimal cutoff value was identified as 4 of VN expression for both OS and DFS. Accordingly, 45 patients were classified as the low (n = 45, 44.1%) and high (n = 57, 55.9%) VN groups.
3.3. Clinicopathological significance of VN in gastric cancer
As demonstrated in Table 2, high VN expression was significantly correlated with younger age (P = .021), more advanced pathological T and N stage (both P < .001), number of metastatic lymph node (P < .001), pathological M stage (P = .001), and pathological TNM stage (P < .001). Besides, no statistical association was observed between the expression of VN and sex, American Society of Anesthesiologists (ASA) score, operation time, estimated blood loss, tumor location, histopathology, primary tumor size, differentiation, Borrmann type, vascular and neural invasion (all P > .05).
Table 2.
Association between vitronectin expression and clinicopathological characteristics in gastric cancer patients.
| VN expression | |||
| Variables | Low (N = 45) | High (N = 57) | P value |
| Gender (%) | .328 | ||
| Male | 38 (84.4) | 43 (75.4) | |
| Female | 7 (15.6) | 14 (24.6) | |
| Age (mean ± SD, yrs) | 66.2 ± 9.9 | 61.2 ± 11.2 | .021 |
| ASA | .222 | ||
| 1 | 22 (48.9) | 36 (63.2) | |
| 2 | 20 (44.4) | 20 (35.1) | |
| 3 | 3 (6.7) | 1 (1.8) | |
| Operative time, min | 209.4 ± 34.6 | 219.6 ± 54.0 | .272 |
| Estimated blood loss, mL | 322.9 ± 212.2 | 399.5 ± 242.6 | .098 |
| Tumor location (%) | .180 | ||
| Upper 1/3 | 8 (17.8) | 14 (24.6) | |
| Middle 1/3 | 6 (13.3) | 7 (12.3) | |
| Lower 1/3 | 30 (66.7) | 29 (50.9) | |
| More than 1/3 | 1 (2.2) | 7 (12.3) | |
| Histopathology (%) | .151 | ||
| Papillary | 10 (22.2) | 4 (7.0) | |
| Tubular | 26 (57.8) | 37 (64.9) | |
| Mucinous | 3 (6.7) | 4 (7.0) | |
| Signet-ring cell | 6 (13.3) | 12 (21.1) | .766 |
| Primary tumor size, cm | |||
| ≤5 | 40 (87.0) | 61 (89.7) | |
| >5 | 6 (13.0) | 7 (10.3) | |
| Tumor differentiation (%) | .307 | ||
| Well moderately differentiated | 20 (44.4) | 19 (33.3) | |
| Poorly differentiated and others | 25 (55.6) | 38 (66.7) | |
| Borrmann type (%) | .263 | ||
| I | 9 (20.0) | 5 (8.8) | |
| II | 16 (35.6) | 17 (29.8) | |
| III | 15 (33.3) | 25 (43.9) | |
| IV | 5 (11.1) | 10 (17.5) | |
| Pathological T stage (%) | <.001 | ||
| T1 | 13 (28.9) | 5 (8.8) | |
| T2 | 9 (20.0) | 4 (7.0) | |
| T3 | 13 (28.9) | 12 (21.1) | |
| T4 | 10 (22.2) | 35 (63.2) | |
| Pathological N stage (%) | .001 | ||
| N0 | 23 (51.1) | 13 (22.8) | |
| N1 | 11 (24.4) | 7 (12.3) | |
| N2 | 4 (8.9) | 17 (29.8) | |
| N3 | 7 (15.6) | 20 (35.1) | |
| Metastatic lymph nodes | 3.9 ± 6.0 | 9.3 ± 7.5 | <.001 |
| Pathological M stage (%) | .001 | ||
| M0 | 44 (97.8) | 42 (73.7) | |
| M1 | 1 (2.2) | 15 (26.3) | |
| TNM stage (%) | <.001 | ||
| I | 18 (40.0) | 6 (10.5) | |
| II | 14 (31.1) | 11 (19.3) | |
| III | 12 (26.7) | 25 (43.9) | |
| IV | 1 (2.2) | 15 (26.3) | |
| Vascular invasion (%) | 5 (11.1) | 7 (12.3) | 1.000 |
| Neural invasion (%) | 5 (11.1) | 8 (14.0) | .770 |
3.4. High VN expression in gastric cancer tissues correlates with worse survival
As shown in Fig. 2J and K, high VN expression was associated with significantly worse survival in gastric cancer patients. The OS and DFS rates among high VN gastric cancer patients were significantly lower than that of their counterparts (both P < .001).
3.5. High VN expression is predictive of poor survival in gastric cancer
Cox regression analysis was conducted to identify predictive prognostic factors in gastric cancer patients (Table 3). Univariate analysis revealed that operative time (P = .031), tumor differentiation (poorly, P = .020), primary tumor size (>5, P < .001), Borrmann type (III/IV, P < .001), pathological T, N, M stage (all P < .001), and VN expression (P < .001) were related to OS. Besides, primary tumor size (>5 cm, P < .001), Borrmann type (III/IV, P = .022), pathological T stage (P < .001), pathological N stage (P < .001), and VN expression (P < .001) were related to DFS.
Table 3.
Univariate and multivariate analyses of the risk factors of OS and DFS in patients with gastric cancer.
| OS (n = 102) | DFS (n = 86) | |||||||
| Variables | Univariate analysis | Multivariable analysis | Univariate analysis | Multivariable analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender (male vs female) | 1.072 (0.580–1.9982) | .824 | 0.875 (0.389–1.967) | .747 | ||||
| Age | 0.996 (0.973–1.020) | .730 | 0.981 (0.954–1.008) | .167 | ||||
| ASA | 0.932 (0.603–1.439) | .749 | 0.808 (0.477–1.369) | .428 | ||||
| Operative time (min) | 1.006 (1.001–1.012) | .031 | 1.000 (0.995–1.006) | .897 | 1.003 (0.996–1.011) | .386 | ||
| Estimated blood loss (ml) | 1.001 (1.000–1.002) | .206 | 1.001 (1.000–1.002) | .274 | ||||
| Tumor location | .284 | .233 | ||||||
| Upper 1/3 | Reference | Reference | ||||||
| Middle 1/3 | 0.512 (0.186–1.410) | .195 | 0.226 (0.051–1.005) | .051 | ||||
| Lower 1/3 | 0.806 (0.439–1.480) | .487 | 0.671 (0.344–1.306) | .240 | ||||
| More than 1/3 | 1.530 (0.592–3.952) | .380 | 0.585 (0.132–2.597) | .481 | ||||
| Histopathology | .381 | .065 | ||||||
| Papillary | Reference | Reference | ||||||
| Tubular | 1.691 (0.712–4.015) | .234 | 4.746 (1.130–19.931) | .033 | ||||
| Mucinous | 1.533 (0.432–5.433) | .508 | 2.697 (0.380–19.153) | .321 | ||||
| Signet-ring cell | 2.324 (0.890–6.063) | .085 | 7.084 (1.565–32.064) | .011 | ||||
| Tumor differentiation (poorly vs moderately/ well differentiated) | 1.928 (1.108–3.353) | .020 | 1.373 (0.736–2.561) | .319 | 1.732 (0.924–3.245) | .087 | ||
| Vascular invasion | 1.233 (1.560–2.715) | .603 | 1.366 (0.525–3.398) | .544 | ||||
| Neural invasion | 0.994 (0.472–2.092) | .987 | 1.174 (0.522–2.639) | .698 | ||||
| Primary tumor size | 4.269 (2.210–8.247) | <.001 | 1.500 (0.656–3.426) | .336 | 4.263 (2.035–8.928) | <.001 | 1.942 (0.753–5.010) | .170 |
| (> 5 vs ≤5 cm) | ||||||||
| Borrmann type | 3.049 (1.732–5.365) | <.001 | 1.643 (0.865–3.122) | .130 | 2.068 (1.111–3.850) | .022 | 0.804 (0.493–1.313) | .384 |
| (III/IV vs I/II) | ||||||||
| Pathological T stage | 2.566 (1.838–3.581) | <.001 | 1.714 (1.064–2.759) | .027 | 2.954 (1.963–4.445) | <.001 | 2.071 (1.220–3.517) | .007 |
| Pathological N stage | 1.780 (1.429–2.217) | <.001 | 1.001 (0.755–1.326) | .996 | 1.615 (1.265–2.062) | <.001 | 1.109 (0.837–1.468) | .472 |
| Pathological M stage | 18.561 (8.986–38.336) | <.001 | 8.935 (3.796–21.031) | <.001 | ||||
| VN expression | 4.909 (2.674–9.011) | <.001 | 2.758 (1.392–5.462) | .004 | 7.223 (3.438–15.174) | <.001 | 5.370 (2.459–11.725) | <.001 |
After adjustment for confounding factors, pathological T stage (HR = 1.714, P = .027), pathological M stage (HR = 8.935, P < .001), VN expression (HR = 2.758, P = .004) were independently predictive of OS. Additionally, pathological T stage (HR = 2.071, P = .007) and VN expression (HR = 5.370, P < .001) were found to be independently correlated with DFS.
3.6. Construction of a risk score for OS
According to the coefficients in Cox regression analysis, the risk score formula was calculated as follows: risk score = 0.783 × pathological T stage + 2.359 × pathological M stage + 0.990 × VN expressions. The patient-specific risk score was calculated, and the risk score distribution and patient survival status were demonstrated in Fig. 3A and B. Using the median risk score as the cutoff value, patients were separated into high- and low-risk groups using the median risk score, as shown in Fig. 3C. The current model had better predictive accuracy than any single parameter (Fig. 3D).
Figure 3.
Construction of a risk factor model for OS. (A) The risk score distribution. (B) The overall survival status. (C) The heatmap of 3 lncRNAs. (D) Time-dependent ROC analysis. OS = overall survival, ROC = receiver operating characteristics.
3.7. Verification of VN expression in publicly available databases
We further evaluated the role of VN expression in the prognosis of gastric cancer in the publicly available database Oncomine and the R2 platform. A meta-analysis was performed in 11 Gene Expression Omnibus datasets from Oncomine, the results showed significantly higher VN mRNA levels in gastric cancer tissues than in normal colorectal tissues (P < .001; Fig. 4A). As shown in Fig. 4B, in the “Tumor Gastric (Batch B)-Tan-56 MAS5.0-u133p2” data set (P = .118), patients with high VN expression tended to have a poorer OS, but it was not statistically significant (P = .099). Moreover, Kaplan–Meier curves using datasets “Tumor Gastric-Tan-56 fRMA-u133p2,” “Tumor Gastric-Tan-56 MAS5.0-u133p2,” and “Tumor Stomach adenocarcinoma-TCGA-415 rsem-tcgars” revealed that VN high expression correlated with a significantly poorer OS (all P < .001; Fig. 4C–E).
Figure 4.
Validation of VN in Oncomine and R2. (A) Meta-analysis of 11 GEO datasets from Oncomine showed significantly lower VN mRNA levels in CRC tissues (P < .001). (B) High expression of VN correlated with poorer overall survival, but without statistical significance (P = .099). (C–E) High expression of VN correlated with poorer overall survival rates (P < .05). GEO = Gene Expression Omnibus, VN = vitronectin.
4. Discussion
The clinical significance role of VN has been demonstrated in several tumors.[10–12] Nevertheless, its association with the prognosis of gastric cancer remains unclarified. Herein, by using immunohistochemistry, VN high expression was an independent prognostic biomarker for gastric cancer, and this phenomenon was further confirmed by bioinformatics approaches.
VN is implicated in the development of several cancers, such as melanoma,[7] prostate,[8] and breast cancer.[9] Yoo et al[17] has demonstrated that higher expression of VN could differentiate gastric cancer patients from normal patients. Herein, we first evaluated the prognostic value of VN in the TCGA database for gastric cancer, and the results demonstrated that VN expression was the most significant factor associated with prognosis of gastric cancer. Additionally, immunohistochemistry was used to explore the clinicopathological significance of VN in gastric cancers. The expression of VN was found significantly elevated in gastric cancer tissues, and associated with higher pathological TNM stage, suggesting that VN may be associated with the development of gastric cancer.
VN has also been reported to be associated with the prognosis of several cancers.[10–12] Herein, this issue was further explored in gastric cancer. Kaplan–Meier curve demonstrated a significantly poorer OS in patients with high VN expression. VN expression was found independently predictive of OS. Together, these findings suggested the prognostic value of VN in gastric cancer was in line with previous results. Besides, for the first time, we built a risk score model for OS in gastric cancer based on VN expression. Moreover, the result of the time-receiver operating characteristics demonstrated that the risk score had stronger predictive power compared with the VN expression, pathological T stage, and pathological M stage. The above result indicated that the expression value of the VN can act as an effective biomarker in gastric cancer. In addition, basing on the expression value of the VN and clinicopathological features can construct a useful risk model to predict the patients’ prognosis.
In the next step, using a publicly available database in silico, we found that higher VN mRNA levels in gastric cancer tissues correlated with a significantly poorer OS in gastric cancers. These bioinformatics findings were consistent with our aforementioned analysis.
Several limitations should be mentioned. Firstly, the main limitation was that our study was a retrospective study. Secondly, our results need to be verified in a larger sample size study. Nevertheless, these findings provided evidence of the prognostic value of VN in gastric cancer.
5. Conclusion
High VN expression was correlated with higher pathological TNM stage and impaired survival in with gastric cancer patients. VN may serve as a potential biomarker for prognostication of gastric cancer.
Author contributions
Conceptualization: Chao Gong, Haifeng Hong, Dekun Zhang.
Data curation: Chao Gong, Dekun Zhang.
Funding acquisition: Chao Gong, Dekun Zhang.
Methodology: Chao Gong, Jingfeng Xie.
Validation: Chao Gong, Yuqin Xue, Yudian Huang, Dekun Zhang.
Writing – original draft: Chao Gong, Haifeng Hong, Dekun Zhang.
Writing – review & editing: Chao Gong, Haifeng Hong, Jingfeng Xie, Yuqin Xue, Yudian Huang, Dekun Zhang.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: DFS = disease-free survival, OS = overall survival, TCGA = The Cancer Genome Atlas, VN = vitronectin.
How to cite this article: Gong C, Hong H, Xie J, Xue Y, Huang Y, Zhang D. Over-expression of vitronectin correlates with impaired survival in gastric cancers. Medicine. 2021;100:31(e26766).
This work was supported by the Startup Fund for scientific research, Fujian Medical University (Grant number: 2017XQ1207), and Young and middle-aged scientific research project in the health system of Fuzhou (2019-S-WQ1).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
SD = standard deviation, TCGA = The Cancer Genome Atlas, VN = vitronectin.
Bold value indicates statistical significance.
ASA = American Society of Anesthesiologists, SD = standard deviation, VN = vitronectin.
Bold value indicates statistical significance.
CI = confidence interval, DFS = disease-free survival, HR = hazard ratio, OS = overall survival, VN = vitronectin.
Bold value indicates statistical significance.
References
- [1].Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol 2014;20:13842–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol 2017;39:1010428317714626.1–7. [DOI] [PubMed] [Google Scholar]
- [4].Röcken C. Molecular classification of gastric cancer. Expert Rev Mol Diagn 2017;17:293–301. [DOI] [PubMed] [Google Scholar]
- [5].Matsuoka T, Yashiro M. Biomarkers of gastric cancer: current topics and future perspective. World J Gastroenterol 2018;24:2818–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Leavesley DI, Kashyap AS, Croll T, et al. Vitronectin--master controller or micromanager. IUBMB Life 2013;65:807–18. [DOI] [PubMed] [Google Scholar]
- [7].Ortega-Martínez I, Gardeazabal J, Erramuzpe A, et al. Vitronectin and dermcidin serum levels predict the metastatic progression of AJCC I-II early-stage melanoma. Int J Cancer 2016;139:1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Franzen CA, Todorović V, Desai BV, et al. The desmosomal armadillo protein plakoglobin regulates prostate cancer cell adhesion and motility through vitronectin-dependent Src signaling. PLoS One 2012;7:e42132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pola C, Formenti SC, Schneider RJ. Vitronectin-αvβ3 integrin engagement directs hypoxia-resistant mTOR activity and sustained protein synthesis linked to invasion by breast cancer cells. Cancer Res 2013;73:4571–8. [DOI] [PubMed] [Google Scholar]
- [10].Yang XP, Zhou LX, Yang QJ, Liu L, Cai Y, Ma SL. Diagnostic and prognostic roles of serum vitronectin in hepatitis B-related hepatocellular carcinoma. Cancer Biomark 2016;17:271–9. [DOI] [PubMed] [Google Scholar]
- [11].Chen MH, Lu C, Sun J, et al. Diagnostic and prognostic value of serum vitronectin levels in human glioma. J Neurol Sci 2016;371:54–9. [DOI] [PubMed] [Google Scholar]
- [12].Hao W, Zhang X, Xiu B, et al. Vitronectin: a promising breast cancer serum biomarker for early diagnosis of breast cancer in patients. Tumour Biol 2016;37:8909–16. [DOI] [PubMed] [Google Scholar]
- [13].Qiu JY, Liu P, Shi C, Han B. Low-grade myofibroblastic sarcomas of the maxilla. Oncol Lett 2015;9:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang Y, Xu Z, Sun Y, Chi P, Lu X. Knockdown of KLK11 reverses oxaliplatin resistance by inhibiting proliferation and activating apoptosis via suppressing the PI3K/AKT signal pathway in colorectal cancer cell. Onco Targets Ther 2018;11:809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 2004;6:01–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252–9. [DOI] [PubMed] [Google Scholar]
- [17].Yoo MW, Park J, Han HS, et al. Discovery of gastric cancer specific biomarkers by the application of serum proteomics. Proteomics 2017;17:1600032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.