Abstract
Background:
Endometrial tissue plays an important role in the regulation of female fertility and there is evidence that endometrial pathology (including endometriosis) is closely related to endocrine disorders. On the other hand, various neuroendocrine changes can be significantly affected by psychosocial stress. In connection with these findings, we tested the relationship between neuroendocrine changes, sexual dysfunction, psychosocial/traumatic stress, and dissociative symptoms in women with endometriosis.
Methods:
A total of 65 patients with endometriosis were included in the study. Clinical examinations were focused on the biochemical analysis of neuroendocrine markers of endometriosis (cancer antigen 125 [CA 125] and cancer antigen 19-9 [CA 19-9]), estradiol, psychometric evaluation of sexual dysfunction, psychosocial/traumatic stress, and dissociative symptoms.
Results:
The results showed significant Spearman correlations between the values of the revised range of sexual difficulties for sexual dysfunction (Revised Female Sexual Distress Scale), psychosocial/traumatic stress (Trauma Symptoms Checklist) (R = 0.31), and dissociative symptoms (Somatoform Dissociation Questionnaire) (R = 0.33). Positive correlations were also found between CA 125 and CA 19-9 (R = 0.63), and between CA 125 and the results of the values of the revised scale of sexual difficulties for sexual dysfunction (Revised Female Sexual Distress Scale) (R = 0.29). Also psychosocial/traumatic stress (Trauma Symptoms Checklist) significantly correlated with CA 125 (R = 0.38) and with CA 19-9 (R = 0.33).
Conclusion:
These results represent the first findings regarding the relationship of the neuroendocrine markers CA 125 and CA 19-9 and sexual dysfunction with trauma/stress-related symptoms and dissociative symptoms in women with endometriosis.
Keywords: cancer antigen 19-9, cancer antigen 125, endometriosis, psychosocial and traumatic stress, sexual dysfunction, somatoform dissociation symptoms
1. Introduction
Endometriosis is a very serious disease affecting 5% to 20% of women of reproductive age.[1] Some Anglo-Saxon studies confirm that the time from the first symptoms to diagnosis often lasts between 8 to 12 years.[2] In women with endometriosis, the prevalence of fertility disorders occurs in up to 40% of cases and some form of sexual dysfunction is described in 50% of cases.[3–5] There is currently no evidence of the role of psychosocial and traumatic stress in the endometrial pathology, although it is known that various neuroendocrine and immune changes may be significantly related primarily with chronic stress, and therefore could also play a role in endometriosis-related neuroendocrine deficits.[5–7]
Cancer antigen 125 (CA 125) is a glycoprotein produced by epithelial tissue during the fetal period and in adulthood. It is an important marker of various gynecological diseases. In contrast, the oncofetal antigen – cancer antigen 19-9 (CA 19-9) is produced by glandular structures in some gynecological diseases and tumors.[8] There is evidence that neuroendocrine disorders may play a role in endometrial biology,[5,9–11] and research suggests that CA 125 and CA 19-9 molecules are important markers of endometrial pathology.
The current literature provides no evidence for relationships between trauma, stress-related symptoms, and dissociative symptoms with CA 125 and CA 19-9 as indicators of endometrial pathology. The aim of this study was to compare sexual dysfunction with other manifestations such as psychosocial/traumatic stress along with dissociative symptoms with blood marker values CA 125 and CA 19-9, which may be elevated in women with endometriosis.
2. Materials and methods
2.1. Study group
A total of 65 patients with a mean age (30.65 ± 4.15), age range (25–45) were selected from a total group of 100 patients with a histologically confirmed diagnosis of endometriosis for our prospective study at the Institute of Sexology of Charles University in Prague from June 2019 to June 2020. The average duration of endometriosis is 8.32 years, SD = 1.83. The correlation between the duration and stage of endometriosis and the selected factors (psychosocial trauma, stress, and sexual dysfunction) was not determined in our study. The most common symptoms were a dysmenorrhea, pain during sex, orgasmic disorders, lubrication disorder, sexual appetence disorders, fear of sex, urinary problems, and sleep disorders. Ten patients had a personal history of abortion and 18 patients of childbirth. Five patients had a personal history of depression. All 65 patients had one or more symptoms of endometriosis. The criteria for inclusion in our study were, in addition to the diagnosis of endometriosis, other symptoms such as dysmenorhoe, pain during sex, orgasmic disorders, lubrication disorder, sexual appetence disorders, fear of sex, urinary problems, and sleep disorders. These included pain (50 patients), dysmenorrhea (30 patients), dyschezia (5 patients), dyspareunia, and/or decreased sexual desire or fear of sex (28 patients). Most patients complained of abdominopelvic pain and dysmenorrhea since menarche, while dyspareunia has been reported practically since the beginning of sexual life. The time of onset of dyschezia varied. Two patients had long-term problems lasting more than 10 years, while 3 patients had dyschezia for approximately 6 months. In connection with these problems, patients also reported sleep disorders (12 patients), mood swings (21 patients), partner discomfort (42 patients), and fears of an unfulfilled sexual relationship with their partners (33 patients). The exclusion criteria were as follows: pregnancy, oncological disease, hormonal, or metabolic disorders such as obesity or diabetes mellitus, psychiatric disorders such as psychosis, schizophrenia, and bipolar disorder. For classification of endometriosis, the revised American Society for reproductive Medicine score and ENZIAN classification were used. Endometriosis was diagnosed by histological examination. The histological diagnosis of endometriosis was based on the presence of both endometriotic glands and stroma.
Twenty-five of the 65 patients received hormonal therapy at the time of the study; 15 patients took progestin-containing medication for more than 5 years; and 4 patients took gonadotropin releasing hormone analogue for approximately 2 years. Of these 25 patients, hormonal treatment improved the quality of life for 20 of them. In particular, there has been a significant reduction in painful symptoms, including dyspareunia and dyschezia. Dysmenorrhea persisted in 5 patients.
Samples were taken to assess the levels of CA 125, CA 19-9, and the hormone estradiol (E2) from all female patients on days 2 to 4 of the menstrual cycle. Furthermore, the patients were asked to fill in three questionnaires. The first one, the Revised Female Sexual Distress Scale (FSDS-R), is designed to assess sexual dysfunction. The second one, Trauma Symptoms Checklist (TSC-40), is designed to assess trauma/stress-related symptoms.
The third one, the Somatoform Dissociation Questionnaire (SDQ-20), is to detect dissociative symptoms.
All participants provided written informed consent and the study was approved by the ethics committee of Charles University Hospital and all methods were performed in accordance with the relevant instructions and regulations.
2.2. Measurement of female sexual dysfunction
The FSDS-R is used to assess sexually induced distress. This questionnaire contains 13 questions focusing on women's sexual dysfunction. Patients’ responses included their sexual feelings over the last 4 weeks and were recorded on a 5-point Likert scale; the total possible score is 52. A score of ≥11 will evaluate a woman as having a case of sexual dysfunction.[12] The average value of the FSDS-R questionnaire in the examined women was 29.2 (SD = 6.27). In our study, 61 patients had values above 11 in FSDS-R.
2.3. Psychometric measures of trauma/stress-related symptoms and dissociative symptoms response
2.3.1. Trauma Symptoms Checklist
Trauma and stress-related symptoms were evaluated using the TSC-40 questionnaire. This is a questionnaire with 40 questions. These are listed on a 4-point scale according to Likert. The score range is from 0 to 120. Questions include subgroups for sexual problems, depression, sleep disorders, anxiety, dissociation, and sexual abuse trauma (Sexual Abuse Trauma Index [SATI]). The average value of the TSC-40 questionnaire in the examined women was 23.48 (SD = 12.84).
2.3.1.1. Subscale composition and scoring for the Trauma Symptoms Checklist
The score for each subscale is the sum of the relevant items listed below. The TSC-40 total score contains several items not included in subscales. Dissociation: 7, 14, 16, 25, 31, 38. Anxiety: 1, 4, 10, 16, 21, 27, 32, 34, 39. Depression: 2, 3, 9, 15, 19, 20, 26, 33, 37. SATI: 5, 7, 13, 21, 25, 29, 31. Sleep disturbance: 2, 8, 13, 19, 22, 28. Sexual problems: 5, 9, 11, 17, 23, 29, 35, 40. A threshold is not determined.[13]
2.3.2. Somatoform dissociation questionnaire
Somatoform dissociative symptoms were measured using the SDQ-20 questionnaire. It contains 20 questions that record the feeling of pain, changes in perception, loss of control, gastrointestinal symptoms, etc. The subjects record their feelings on a 5-point scale according to Likert. A significant incidence of somatoform dissociative symptoms is recognized when a score is greater than 30.[14] The average score of the SDQ-20 questionnaire in the examined women was 25.53 (SD = 5.2). In our study 57 patients had a score above 30 in SDQ-20.
2.4. Neuroendocrine measures
For biochemical evaluations, 2 mL of blood serum samples were collected into a vacuette with a separating gel while the patients were under resting conditions according to routine procedures at the Institute of Sexology. The samples were taken from all participants between 7:00 am and 9:00 am and between the 2nd and 4th day of the menstrual cycle. The blood samples were transferred to the Central Laboratory of the Institute of Medical Biochemistry and Laboratory Diagnostics in a refrigerator at 4°C, (transport time was 20 minutes) where CA 125,CA 19-9, and E2 levels were measured by chemiluminescent microparticle immunoassays methods. For the study participants, the average values for CA 125, CA 19-9, and E2 were 16.47 U/mL (SD = 7.19), 15.81 U/mL (SD = 7.18) and 336.69 pg/mL (SD = 174.03), respectively.
2.5. Statistical methods
The statistical evaluation of all psychometric measurements included descriptive statistics and Spearman correlation coefficients. Statistical methods were evaluated using Statistica software, version 12 (StatSoft CR s.r.o., Prague, Czech Republic).
3. Results
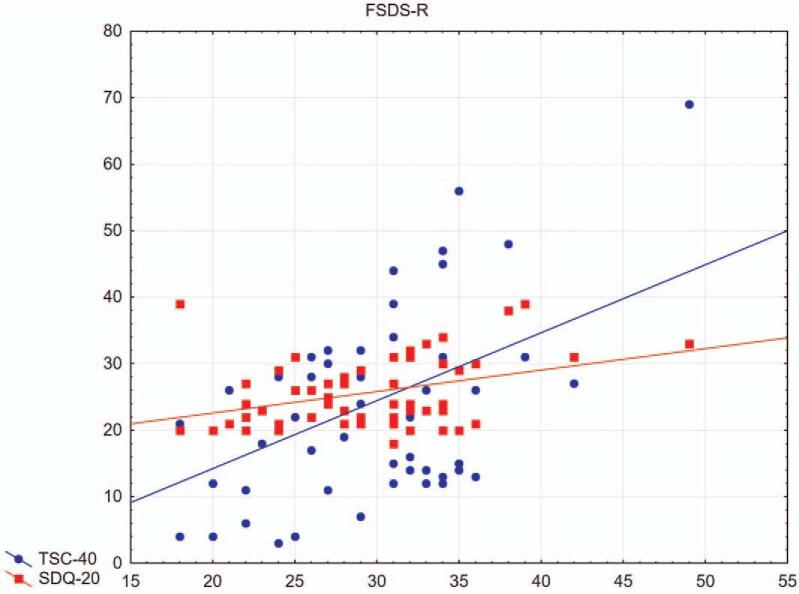
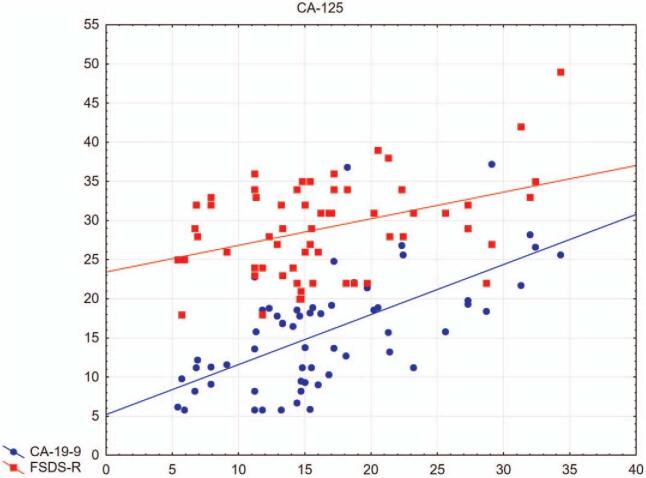
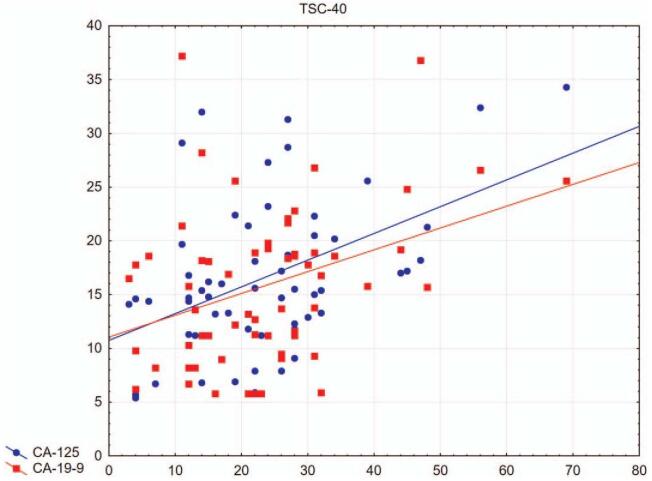
The results showed significant Spearman correlations between the values of the revised range of sexual difficulties for sexual dysfunction (FSDS-R), trauma/stress-related symptoms (TSC-40) (R = 0.31), and dissociative symptoms (SDQ-20) (R = 0.33) (Fig. 1). A positive correlation was also found between CA 125 and CA 19-9 (R = 0.63) and between CA 125 and the results of the values of the revised scale of sexual difficulties for sexual dysfunction (FSDS-R) (R = 0.29) (Fig. 2). Also significantly correlated were psychosocial trauma/stress symptoms (TSC-40) with CA 125 (R = 0.38) and CA 19-9 (R = 0.33) (Fig. 3). No relationship between these values and E2 levels was found.
Figure 1.
Relationship of FSDS-R with TSC-40 and SDQ-20. Significant Spearman correlations between sexual dysfunction, stress-related symptoms, and dissociative symptoms (R = 0.31 and R = 0.33, respectively) observed in patients in the study group. FSDS-R = Revised Female Sexual Distress Scale, SDQ-20 = Somatoform Dissociation Questionnaire, TSC-40 = Trauma Symptoms Checklist.
Figure 2.
Relationship of CA 125 with CA 19-9 and FSDS-R. Significant Spearman correlations between neuroendocrine marker CA 125 with CA 19-9 and sexual dysfunction (R = 0.63 and R = 0.29, respectively) observed in patients in the study group. CA 125 = cancer antigen 125, CA 19-9 = cancer antigen 19-9, FSDS-R = Revised Female Sexual Distress Scale.
Figure 3.
Relationship of TSC-40 with CA 125 and CA 19-9. Significant Spearman correlations between stress-related symptoms and neuroendocrine markers (R = 0.38 and R = 0.33, respectively) observed in patients in the study group. CA 125 = cancer antigen 125, CA 19-9 = cancer antigen 19-9, TSC-40 = Trauma Symptoms Checklist.
4. Discussion
E2, which is produced in the yellow body of the ovaries from the testosterone, is the predominant estrogen in women of childbearing age and plays an important role in endometriosis.[15]
The results of the present study are consistent with the hypothesis of possible relationships between trauma- and stress-related symptoms, dissociative symptoms, and neuroendocrine markers in patients with endometriosis that, in approximately 50% of cases, is accompanied by sexual dysfunction adversely affecting the patients’ health, sexual partner, and social life. In the group of patients examined at the Institute of Sexology for sexual dysfunction, we found significant relationships, confirmed by Spearman correlation, between the results of the TSC-40 questionnaire which mainly describes psychosocial trauma and stress, questionnaire SDQ-20 that focuses on dissociative symptoms and questionnaire FSDS -R that focuses on the quality of sexual life and sexual function, and between neuroendocrine markers CA 125 and CA 19-9 that are considered one of the possible markers of endometriosis.[16–18] These results represent the first findings in the literature regarding the relationship of neuroendocrine markers CA 125 and CA 19-9 and sexual dysfunction with psychosocial trauma/stress symptoms and dissociative symptoms in women with endometriosis.
In conclusion, these findings suggest that CA 125 and CA 19-9 evaluations and their relationship to chronic trauma/stress symptoms and dissociative symptoms in women with endometriosis may have significant clinical implications and may play a role in the earlier diagnosis of endometriosis, which is usually delayed due to misinterpretation of symptoms, especially in young women.[8,19] In this context, these results may initiate new psychosomatic research on endometriosis and a discussion of the relationship between neuroendocrine changes in endometriosis and psychosocial stress/trauma symptoms. Further research in this area is needed.
Author contributions
Conceptualization: Ludek Fiala.
Data curation: Ludek Fiala.
Formal analysis: Ludek Fiala, Jiri Lenz.
Investigation: Ludek Fiala.
Software: Petr Bob.
Supervision: Petr Bob.
Writing – original draft: Ludek Fiala.
Writing – review & editing: Jiri Lenz.
Footnotes
Abbreviations: CA 125 = cancer antigen 125, CA 19-9 = cancer antigen 19-9, E2 = estradiol, FSDS-R = Revised Female Sexual Distress Scale, SATI = Sexual Abuse Trauma Index, SDQ-20 = Somatoform Dissociation Questionnaire, TSC-40 = Trauma Symptoms Checklist.
How to cite this article: Fiala L, Lenz J, Bob P. Effect of psychosocial trauma and stress on sexual dysfunction in women with endometriosis. Medicine. 2021;100:31(e26836).
This study was not supported by any project.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin Noirth Am 1997;24:235–58. [DOI] [PubMed] [Google Scholar]
- [2].Moradi M, Parker M, Sneddon A, Lopez V, Ellwood D. Impact of endometriosis on women's lives: a qualitative study. BMC Womens Health 2014;14:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Králíčková M, Fiala L, Losan P, Tomes P, Vetvicka V. Altered Immunity in Endometriosis: What Came First? Immunol Invest 2018;47:569–82. [DOI] [PubMed] [Google Scholar]
- [4].Cloke B, Christian M. The role of androgens and the androgen receptor in cycling endometrium. Mol Cell Endocrinol 2012;358:166–75. [DOI] [PubMed] [Google Scholar]
- [5].Simitsidellis I, Saunders PTK, Gibson DA. Androgens and endometrium: new insights and new targets. Mol Cell Endocrinol 2018;465:48–60. [DOI] [PubMed] [Google Scholar]
- [6].Kralickova M, Vetvicka V. Immunological aspects of endometriosis: a review. Ann Transl Med 2015;3:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cloke B, Christian M. The role of androgens and the androgen receptor in cycling endometrium. Mol Cell Endocrinol 2012;358:66–75. [DOI] [PubMed] [Google Scholar]
- [8].Fiala L, Bob P, Raboch J. Oncological markers CA 125, CA 19-9 and endometriosis. Medicine (Baltimore) 2018;97:e13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gibson DA, Foster PA, Simitsidellis I, et al. Sulfation pathways: a role for steroid sulfatase in intracrine regulation of endometrial decidualisation. J Mol Endocrinol 2018;61:M57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Human Reprod Update 2014;20:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ito TE, Abi Khalil ED, Taffel M, Moawad GN. Magnetic resonance imaging correlation to intraoperative findings of deeply infiltrative endometriosis. Fertil Steril 2017;107:e11–2. [DOI] [PubMed] [Google Scholar]
- [12].DeRogatis LR. Assessment of sexual function/dysfunction via patient reported outcomes. Int J Impot Res 2008;20:35–44. [DOI] [PubMed] [Google Scholar]
- [13].Briere J. Dissociative symptoms and trauma exposure: specificity, affect dysregulation, and posttraumatic stress. J Ner Ment Dis 2006;194:78–82. [DOI] [PubMed] [Google Scholar]
- [14].Nijenhuis ER, Spinhoven P, Van Dyck R, Van der Hart O, Vanderlinden J. The development and psychometric characteristics of the Somatoform Dissociation Questionnaire (SDQ-20), stress. J Ner Ment Dis 1996;184:688–94. [DOI] [PubMed] [Google Scholar]
- [15].Du Y, Zhang Z, Xiong W, et al. Estradiol promotes EMT in endometriosis via MALAT1/miR200 s sponge function. Reproduction 2019;157:179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nisenblat V, Bossuyt PM, Shaikh R, et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;2016:CD012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Socolov R, Socolov D, Sindilar A, Pavaleanu I. An update on the biological markers of endometriosis. Minerva Ginecol 2017;69:462–7. [DOI] [PubMed] [Google Scholar]
- [18].Hirsch M, Duffy JMN, Deguara CSS Davis##JD, Khan KS. Diagnostic accuracy of cancer antigen 125 (CA125) for endometriosis in symptomatic women: a multi-center study. Eur J Obstet Gynecol Reprod Biol 2017;210:102–7. [DOI] [PubMed] [Google Scholar]
- [19].Ahn SH, Singh V, Tayade C. Biomarkers in endometriosis: challenges and opportunities. Fertil Steril 2017;107:523–32. [DOI] [PubMed] [Google Scholar]