Abstract
Background:
To assess the prognostic capability of the maximum standardized uptake values (SUVmax) measured in the primary tumor and axillary lymph nodes (ALNs) by pretreatment fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography and analyze outcomes according to the molecular breast cancer subtypes.
Methods:
The databases were systematically searched using keywords for breast cancer, positron emission tomography/computed tomography, and SUVmax; the extracted studies reported at least 1 form of survival data, event-free survival (EFS) and overall survival. Comparative analyses of the pooled hazard ratios (HRs) for EFS and overall survival were performed to assess their correlations with SUVmax. The pooled HR was estimated using random-effects model according to the results of heterogeneity.
Results:
Thirteen eligible studies comprising 3040 patients with breast cancer were included. The pooled HRs of high SUVmax in the primary tumor and ALN were 3.01 (95% CI 1.83–4.97, P < .00001; I2 = 82%) and 3.72 (95% CI 1.15–12.01; I2 = 92%; P = .03), respectively. Patients with higher SUVmax demonstrated a poorer survival prognosis. Furthermore, comparative analyses according to the molecular subtypes demonstrated that the SUVmax in the primary tumor or ALN can be a predictive parameter in patients with the luminal subtype disease. Subtype analysis results indicated a significant association of the luminal group, with a HR of 2.65 (95% CI 1.31–5.37; I2 = 27%; P = .007).
Conclusions:
SUVmax from pretreatment is a significant prognostic factor for EFS in patients with breast cancer. Despite several limitations, correlation with molecular subtype (luminal type) was demonstrated. Further large-scale studies are required to investigate the precise prognostic capability of SUVmax.
Keywords: breast cancer, meta-analysis, prognostic value, SUVmax
1. Introduction
Breast cancer, the second leading cause of cancer-related death and accounting for the highest number of solid cancers in women, is a heterogeneous malignancy that exhibits various patterns of progression, outcomes, and treatment responses.[1]
Although early diagnosis and effective treatment improved the survival rate, approximately 10% to 15% of locoregional recurrence was still reported after treatment.[2] As breast cancer is a very heterogeneous disease, accurate prediction of its prognosis is especially important in light of the variability of the disease characteristics before developing a treatment plan. It is affected by variable factors including tumor size, nuclear grade, axillary lymph node (ALN) involvement, and hormone receptor status. Fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), among various imaging modalities, has also been reported to be valuable in the initial staging, restaging, evaluating treatment response, and predicting the prognosis of breast cancer.[3,4]
Among the various values determined using 18F-FDG PET/CT, the most widely used parameter is the maximum standardized uptake value (SUVmax) that quantifies the rate of metabolic uptake of glucose by the tumor cells. Several recent systematic reviews and meta-analyses have found that the SUVmax of 18F-FDG could serve as a prognostic factor in various malignant solid tumors.[5–8] Like other one else, several studies have reported the correlation of higher SUVmax of the primary tumor with poorer prognostic behavior even in breast cancer.[9,10]
Tumor size and the number of involved lymph nodes are well-established prognostic factors in breast cancers;[11,12] similarly, the molecular subtypes also defined according to the estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) statuses are precise and useful prognostic indicators.[13,14]
However, the prognostic value of SUVmax according to each molecular subtype of breast cancer is controversial, and no consensus exists of its predictive capability.[15,16]
Therefore, we conducted a meta-analysis to assess the prognostic value of SUVmax and its correlation with molecular cancer subtype in patients with breast cancer.
2. Materials and methods
2.1. Study selection and data extraction
PubMed, EMBASE, and Medline databases were searched for original articles (until March 2021). The search strategy involved using the following terms “breast cancer,” “carcinoma,” “positron emission tomography,” “PET/CT,” “fluorine-18-fluorodeoxyglucose,” “18F-FDG,” “standardized uptake value,” or “SUVmax.” All searches were limited to human studies and English-language publications. The inclusion criteria for the studies were pretreatment, including surgery; use of 18F-FDG PET/CT as an initial imaging modality; measurement of SUVmax of the metabolic level of the primary lesions or ALN; and studies with at least one form of survival data, such as overall survival (OS), disease-free survival, event-free survival (EFS), progression-free survival, or metastasis-free survival. Reviews, abstracts, and editorial materials were excluded, and duplicate data were also removed. Authors independently performed the initial screening by reviewing the titles and abstracts according to the inclusion and exclusion criteria. Additionally, the following data were extracted from the publications: first author, year of publication, country of origin, study period, follow-up duration, age of patients, number of patients, and study design. Discrepancies were resolved by consensus.
2.2. Statistical analysis
The primary outcome was EFS, defined as the time from initiation of therapy until recurrence or progression. Data regarding disease-free survival, relapse-free survival, and recurrence or progression-free survival were obtained as the primary outcomes and were redefined as EFS.[6,7] If available, the secondary endpoint was OS. The OS was defined as the time from therapy initiation until death irrespective of the cause.[5,7]. To reconstruct the estimated HR on the survival data, survival data were extracted using the methodology recommended by Parmar et al.[17] The effects of SUVmax on survival outcomes were estimated by pooling the HR effect size and 95% CI data. The pooled HR was estimated using random-effects model according to the results of heterogeneity. An HR >1 indicated worse prognosis in patients with high SUVmax, and an HR <1 was indicative of better prognosis. P values of the log-rank test, 95% CI, number of events, and number at risk provided by the authors were extracted to estimate the HR indirectly using ReviewManager (RevMan, version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration). The level of heterogeneity across individual studies was assessed using χ2 test and I2 statistics as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (http://handbook.cochrane.org). P values <.05 were considered statistically significant except for heterogeneity. The publication bias was evaluated by funnel plots.
2.3. Ethics approval
All analyses were based on previously published studies; therefore, no ethical approval and patient consent were required.
3. Results
3.1. Study characteristics
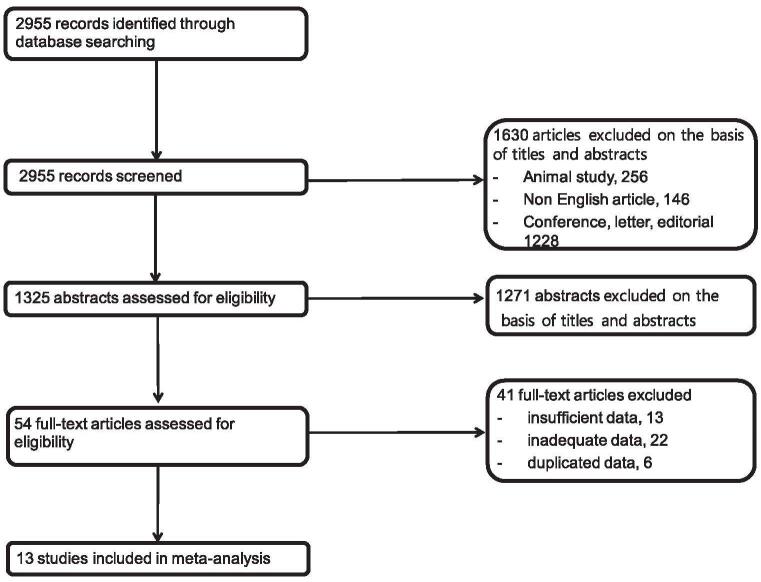
The searching of the electronic databases initially led to the identification of 2955 articles. After the exclusion of animal studies (n = 256), non-English articles (n = 146), conference abstracts, letter, editorial (n = 1228), and 1271 studies that did not meet the inclusion criteria based on the title and abstract, the full text of 54 articles was reviewed; finally, 13 eligible studies with 3040 patients were included in our meta-analysis[18–30] (Fig. 1). Visual inspection of the funnel plot did not identify substantial asymmetry.
Figure 1.
Flowchart for the identification of eligible studies.
Among the 13 studies, 12 studies evaluated the prognostic value of SUVmax measured in the primary tumor,[18,20–30] 4 studies evaluated the SUVmax for ALN,[20,22,24,30] and 2 articles included values for both primary lesion and ALN.[24,26]
In each study, patients were divided into 2 groups based on the SUVmax threshold (<optimal cutoff value and >optimal cutoff value). Different studies had different optimal cutoff values determined using different methodologies. In all the 13 studies, the optimal cutoff values were determined using the area under the receiver operating characteristic curve. The cutoff SUVmax in the primary tumor ranged between 2.9 and 11.1, and those of SUVmax in ALN ranged from 1.7 to 2.8 (Table 1).
Table 1.
Studies included in meta-analysis.
| Author | Year | Country | Study design | No. of patients | Histology | Staging (AJCC 7th) | Endpoints | Scanner | Lesion | FDG uptake time(min) | Image reconstruction method | Cutoff value | Determination of cutoff values |
| Jung et al | 2017 | Korea | R | 131 | IDC+ILC+other | I, II, III | EFS | PET/CT | Primary tumor | 60 | Maximization algorithm | 5.5 | AUC |
| Higuchi et al | 2017 | Japan | R | 387 | IDC+ILC+other | I, II, III | EFS | PET/CT | Primary tumor | 60 | 3.585 | AUC | |
| Kitajima et al | 2016 | Japan | R | 196 | IDC+ILC+other | I, II, III | EFS | PET/CT | Primary tumor/node | 60 | 2.9/1.7 | AUC | |
| Jo et al | 2015 | Korea | R | 508 | IDC | I, II, III | EFS | PET/CT | Primary tumor | 60 | Iterative reconstruction | 5.95 | AUC |
| Vicente et al | 2015 | Spain | R | 198 | IDC+ILC | II,III | EFS/OS | PET/CT | Primary tumor/node | 60 | Iterative reconstruction | 6.05/2.25 | AUC |
| Yue et al | 2015 | USA | R | 79 | IDC+ILC | I–IV | EFS | PET/CT | Primary tumor | 60 | Iterative reconstruction | 3.5 | AUC |
| Kim et al | 2015 | Korea | R | 153 | IDC | II, III | EFS | PET/CT | Primary tumor/node | 60 | Iterative reconstruction algorithm | 11.1/2.2 | AUC |
| Aogi et al | 2015 | Japan | R | 262 | IDC+ILC | I,II,III | EFS/OS | PET/CT | Primary tumor | 60–90 | Iterative reconstruction | 6.0 | AUC |
| Baba et al | 2014 | Japan | R | 79 | IDC+ILC+other | I,II,III | EFS/OS | PET | Primary tumor | 60 | 4.16 | AUC | |
| Cochet et al | 2014 | France | R | 142 | IDC+ILC | II,III,IV | EFS | PET/CT | Primary tumor | 60 | 5.7 | AUC | |
| Ahn et al | 2013 | Korea | R | 496 | IDC+ILC+other | I,II,III | EFS | PET | Primary tumor | 60 | Iterative transmission algorithm | 4 | AUC |
| Kadoya et al | 2013 | Japan | R | 344 | IDC+ILC+other | I,II,III | EFS | PET/CT | Primary tumor | 60–90 | Iterative algorithm | 3 | AUC |
| Song et al | 2012 | Korea | R | 65 | IDC | II,III | EFS | PET/CT | Primary tumor/node | 60 | Maximum iterative reconstruction algorithm | 6.9/2.8 | AUC |
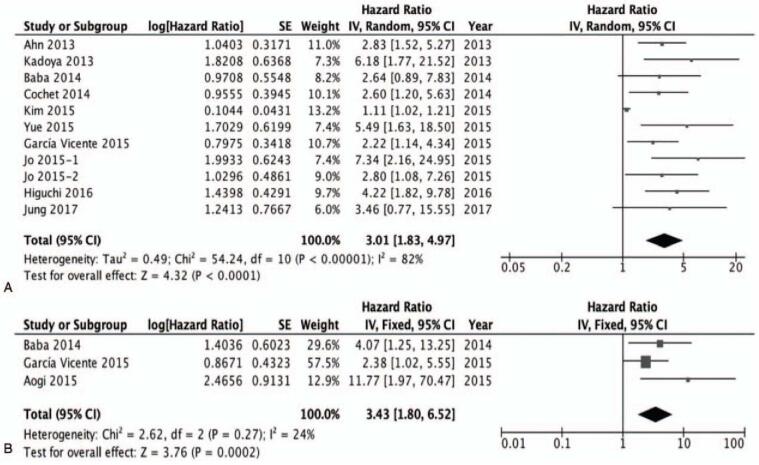
The EFS was analyzed based on 11 studies with SUVmax from the primary tumor.[18,20–24,26–30] The pooled HR for adverse events was 3.01 (95% CI 1.83–4.97, P < .00001; I2 = 82%) (Fig. 2A).
Figure 2.
A, The prognostic value of SUVmax (primary tumor) for EFS. B, The prognostic value of SUVmax (primary tumor) for OS. EFS = event-free survival, OS = overall survival, SUVmax = maximum standardized uptake values.
In subgroup analysis, in 9 studies using PET/CT, the pooled HR for adverse events was 3.11 (95% CI 1.74–5.58). In 2 studies using PET, the pooled HR for adverse events was 2.78 (95% CI 1.62–4.77).
Among the 13 studies, 3 studies additionally included the result of the OS rate, such that the 3 studies with SUVmax from the primary tumor were included in the second analysis of OS.[26,29,30] The pooled HR was 3.43 (1.80–6.52; I2 = 24%; P = .0002) (Fig. 2B).
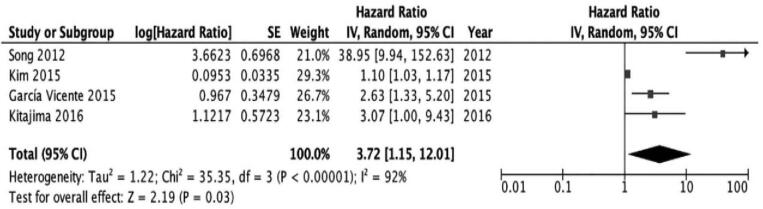
In an analysis of the prognostic value of high SUVmax from the primary tumor, primary tumors with a high SUVmax were found to be associated with progression and recurrence and had poor OS rates. Similarly, the prognostic value of high SUVmax from ALN was also analyzed based on 4 studies.[20,22,23,24] In an analysis of the prognostic value of high SUVmax from ALN, a similar predictive value of SUVmax as that of the primary tumor was observed. Additionally, ALN with a high SUVmax was associated with progression and recurrence. The pooled HR for adverse events, including death, was 3.72 (95% CI 1.15–12.01; I2 = 92%; P = .03), which was also significant (Fig. 3).
Figure 3.
The prognostic value of SUVmax (axillary lymph node) for EFS. EFS = event-free survival, SUVmax = maximum standardized uptake values.
3.2. Molecular biological subtype comparative analyses
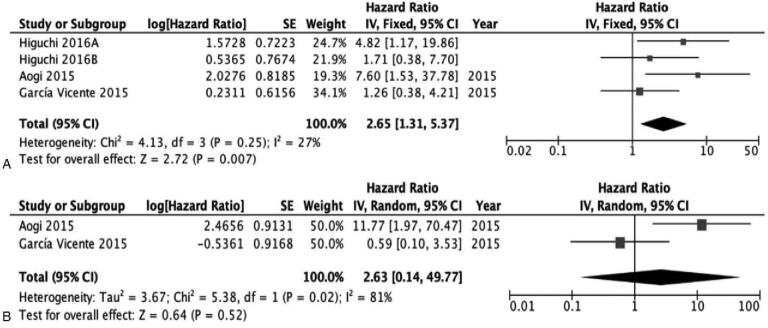
Of the 13 eligible studies, the results of 4 were extracted according to each molecular subtype. Using the same method, the subgroup comparative analyses according to molecular subtype were obtained. Three studies[19,22,25] were included for analyzing the prognostic value of SUVmax in patients with luminal subtype (ER+/HER−) and 2 studies each were used in the assessments of triple-negative (ER−/HER−) and[23,25] HER2(+) subtype (ER−/HER+) tumors.[19,25] SUVmax could serve as a prognostic factor for EFS in patients with the luminal subtype of breast cancer. The results of subtype comparative analyses indicated a significant association of the luminal subgroup of tumors, with a HR of 2.65 (95% CI 1.31–5.37; I2 = 27%; P = .007) (Fig. 4A).
Figure 4.
In luminal subtype. A, The prognostic value of SUVmax (primary tumor) for EFS. B, The prognostic value of SUVmax (primary tumor) for OS. EFS = event-free survival, SUVmax = maximum standardized uptake values.
Given the availability of OS data from 2 studies,[22,25] we were able to analyze the prognostic value of SUVmax for OS. However, the pooled HR for OS was 2.63 (95% CI 0.14–49.77; I2 = 81%; P = .52), which was not significant (Fig. 4B).
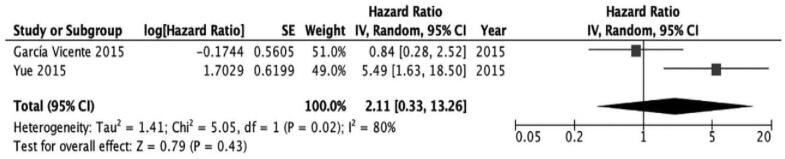
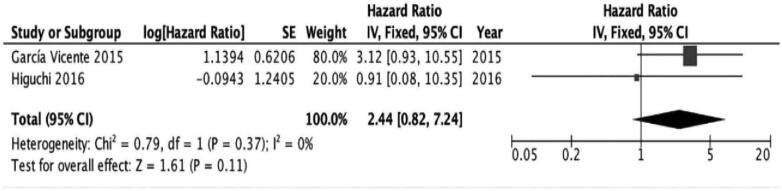
No significant association was found in the assessment of patients with triple-negative subtype and patients with HER2(+) subtype (triple negative; HR [95% CI 0.33–13.26; I2 = 80%; P = 0.43], HER2(+); HR 2.44 [95% CI 0.82–7.24; I2 = 0%; P = .11]) (Figs. 5 and 6).
Figure 5.
The prognostic value of SUVmax (primary tumor) for EFS in triple negative subtype.
Figure 6.
The prognostic value of SUVmax (primary tumor) for EFS in HER2(+) subtype. EFS = event-free survival, HER2 = human epidermal growth factor receptor 2, SUVmax = maximum standardized uptake values.
4. Discussion
The Precision Medicine Initiative, launched in January 2015, requires more accurate diagnoses and prognoses, utilizing optimal treatment patterns, reducing the risk of treatments and side effects, and ensuring less cost.[32] To improve treatment outcome, effective personalized therapeutic strategies are required for more aggressive treatments, especially in patients with more aggressive diseases.[31] Therefore, before operation or for adjuvant treatment, stratifying the risk of patients for recurrence or progression is very essential. 18F-FDG PET/CT is a clinically useful noninvasive imaging modality for the diagnosis of metastases or preoperative initial staging of breast cancers.[33,34] Recently, as a prognostic factor in breast cancer, the use of the SUVmax value as the most widely used parameter in the clinical settings has increased.[35] The SUVmax of primary lesion has significant prognostic value for EFS or OS in different cancers, such as cervical, lung, and esophageal cancers.[5–8]
Additionally, several reports have suggested that tumors with high SUVmax are associated with poor prognosis in patients with breast cancer.[10,21,29] Increasing SUVmax was related to the aggressive behavior of the cancer, and patients with high SUVmax might have a higher risk of recurrence or progression.[36] Therefore, in patients with high SUVmax, more aggressive treatment is considerably effective and benefits EFS or OS. In different cancers, tumor size and the number of involved lymph nodes are well-established significant prognostic factors as these are closely associated with the progression and the development of distant metastases.[12] Especially, tumor size and ALN involvement in breast cancer are also significant predictors of relapse and in determining cancer staging.[37,38] Furthermore, defining molecular subtypes according to ER and HER2 statuses should be considered to stratify the risks of recurrence or death in patients with breast cancer, following which adjuvant treatments are determined based on each subtype.[13,35]
To investigate not only the prognostic value of SUVmax in patients with breast cancer but also conduct a comparative assessment according to each molecular subtype, this meta-analysis reanalyzed approximately 3040 patients from 13 studies by calculating the pooled HR for EFS and/or OS in patients with high SUVmax compared with those with low SUVmax. Patients with a high SUVmax of the primary tumor or ALN demonstrated a higher risk of adverse events than those with a low SUVmax. Hence, in terms of biological subtypes, only the luminal subtype group with a high SUVmax demonstrated a higher risk of adverse events. The luminal subtype group with high SUVmax might, thus, have a higher risk of recurrence or progression than the low SUVmax group.
However, a significant predictive value for EFS in patients with other biological subtypes was not identified. This may have been due to the insufficient statistical power, as there were only 2 or 3 studies available for the comparative study of each subtype, comprising a relatively small number of patients. Furthermore, the limited sample size and significant heterogeneity could also result in low statistical efficiency. Further research is required to investigate whether the correlation between each biological subtype and SUVmax of the primary tumor or ALN can be of effective prognostic value in patients with breast cancer. However, there were certain advantages to our meta-analysis. HR was used to calculate the prognostic value in this meta-analysis. HR is the most appropriate measure for prognosis because the odds ratio is measured at a single point in time; therefore, it is not recommended as a surrogate method for analyzing time-to-event outcomes.[39] However, this meta-analysis had several limitations also. First, the included articles were restricted to the English language only; thus, the potential effect of language bias should be considered. Second, a potential publication bias in the studies cannot be clearly excluded even though funnel plots showed no clear evidence. Lastly, all included studies were retrospective in nature, and so selection bias could not be excluded.
5. Conclusion
Our meta-analysis indicated that high SUVmax of the primary lesion or ALN could predict a higher risk of adverse events in the patients. In patients with the luminal subtype, there is a correlation between the prognostic value of SUVmax for EFS and molecular subtypes. The pretreatment SUVmax is a significant prognostic factor in patients with breast cancer because a high SUVmax can be considered a high risk for treatment failure; therefore, patients with high SUVmax may benefit from a more aggressive treatment. Therefore, pretreatment SUVmax in patients with breast cancer could serve as a prognostic factor for planning an effective treatment strategy. Given the limitations due to sample size and heterogeneity, further research, including large-scale prospective studies, is required to investigate more precise prognostic capabilities of SUVmax.
Acknowledgments
The authors thank Editage (www.editage.co.kr) for English language editing
Author contributions
The first author, Moon il Lee planed and wrote this manuscript
Other co-authors played a role in the data organization and modulation of this article
Corresponding author planed, revised, edited and submitted this article
Conceptualization: Kyoungjune Pak.
Data curation: Hyun Yul Kim.
Investigation: Youn Joo Jung, Dong Il Kim, Seok Kyung Kang.
Methodology: Kyoungjune Pak.
Resources: Hyun Yul Kim.
Supervision: Kyoungjune Pak, Seong Jang Kim, Hyun Yul Kim.
Validation: Seungju Lee, Chang Shin Jung.
Writing – original draft: Moon il Lee.
Writing – review & editing: Hyun Yul Kim.
Footnotes
Abbreviations: 18F-FDG PET/CT = fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography, ALN = axillary lymph node, EFS = event-free survival, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, HR = hazard ratios, MFS = metastasis-free survival, OS = overall survival, SUVmax = maximum standardized uptake values.
How to cite this article: Lee Mi, Jung YJ, Kim DI, Lee S, Jung CS, Kang SK, Pak K, Kim SJ, Kim HY. Prognostic value of SUVmax in breast cancer and comparative analyses of molecular subtypes: A systematic review and meta-analysis. Medicine. 2021;100:31(e26745).
All analyses were based on previously published studies; therefore, no ethical approval and patient consent were required.
There are currently no funding sources.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
IDC = invasive ductal carcinoma, ILC = invasive lobular carcinoma, R = retrospective.
References
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- [2].Freedman GM, Fowble BL. Local recurrence after mastectomy or breast-conserving surgery and radiation. Oncology (Williston Park) 2000;14:1561–81. [PubMed] [Google Scholar]
- [3].Bernsdorf M, Berthelsen AK, Wielenga VT, et al. Preoperative PET/CT in early-stage breast cancer. Ann Oncol 2012;23:2277–82. [DOI] [PubMed] [Google Scholar]
- [4].Chen L, Yang Q, Bao J, Liu D, Huang X, Wang J. Direct comparison of PET/CT and MRI to predict the pathological response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. Sci Rep 2017;7:8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sarker A, Im HJ, Cheon GJ, et al. Prognostic implications of the SUVmax of primary tumors and metastatic lymph node measured by 18F-FDG PET in patients with uterine cervical cancer: a meta-analysis. Clin Nucl Med 2016;41:34–40. [DOI] [PubMed] [Google Scholar]
- [6].Im HJ, Pak K, Cheon GJ, et al. Prognostic value o volumetric parameters of 18F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging 2015;42:241–51. [DOI] [PubMed] [Google Scholar]
- [7].Pak K, Cheon GJ, Nam HY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med 2014;55:884–90. [DOI] [PubMed] [Google Scholar]
- [8].Xie M, Wu K, Liu Y, Jiang Q, Xie Y. Predictive value of F-18 FDG PET/CT quantization parameters in diffuse large B cell lymphoma: a meta-analysis with 702 participants. Med Oncol 2015;32:446. [DOI] [PubMed] [Google Scholar]
- [9].Nieweg OE, Kim EE, Wong WH, et al. Positron emission tomography with fluorine-18-deoxyglucose in the detection and staging of breast cancer. Cancer 1993;71:3920–5. [DOI] [PubMed] [Google Scholar]
- [10].Buck A, Schirrmeister H, Kuhn T, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging 2002;29:1317–23. [DOI] [PubMed] [Google Scholar]
- [11].Diao W, Tian F, Jia Z. The prognostic value of SUVmax measuring on primary lesion and ALN by 18F-FDG PET or PET/CT in patients with breast cancer. Eur J Radiol 2018;105:01–7. [DOI] [PubMed] [Google Scholar]
- [12].Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989;63:181e7. [DOI] [PubMed] [Google Scholar]
- [13].Park YH, Lee SJ, Cho EY, et al. Clinical relevance of TNM staging system according to breast cancer subtypes. Ann Oncol 2011;22:1554e60. [DOI] [PubMed] [Google Scholar]
- [14].Groheux D, Martineau A, Teixeira L, et al. 18FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res 2017;19:03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Evangelista L, Cervino AR, Ghiotto C, et al. Could semiquantitative FDG analysis add information to the prognosis in patients with stage II/III breast cancer undergoing neoadjuvant treatment? Eur J Nucl Med Mol Imaging 2015;42:1648–55. [DOI] [PubMed] [Google Scholar]
- [16].Kitajima K, Miyoshi Y, Yamano T, Odawara S, Higuchi T, Yamakado K. Prognostic value of FDG-PET and DWI in breast cancer. Ann Nucl Med 2018;32:44–53. [DOI] [PubMed] [Google Scholar]
- [17].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform metaanalyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [18].Jung JH, Son SH, Kim DH, et al. CONSORT-independent prognostic value of asphericity of pretherapeutic F-18 FDG uptake by primary tumors in patients with breast cancer. Med (Baltimore) 2017;96:e8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Higuchi T, Nishimukai A, Ozawa H, et al. Prognostic significance of preoperative 18F-FDG PET/CT for breast cancer subtypes. Breast 2016;30:05–12. [DOI] [PubMed] [Google Scholar]
- [20].Kitajima K, Fukushima K, Miyoshi Y, et al. Diagnostic and prognostic value of 18F-FDG PET/CT for axillary lymph node staging in patients with breast cancer. Jpn J Radiol 2015;33: [DOI] [PubMed] [Google Scholar]
- [21].Jo JE, Kim JY, Lee SH, Kim S, Kang T. Preoperative 18F-FDG PET/CT predicts disease-free survival in patients with primary invasive ductal breast cancer. Acta Radiol 2015;56:1463–70. [DOI] [PubMed] [Google Scholar]
- [22].Garcia Vicente AM, Soriano Castrejon A, Lopez-Fidalgo JF, et al. Basal 18F-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography as a prognostic biomarker in patients with locally advanced breast cancer. Eur J Nucl Med Mol Imaging 2015;42:1804–13. [DOI] [PubMed] [Google Scholar]
- [23].Yong Y, Cui X, Bose S, Audeh W, Zhang X, Fraass B. Stratifying triple-negative breast cancer prognosis using 18F-FDG-PET/CT imaging. Breast Cancer Res Treat 2015;153:607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim YH, Yoon HJ, Kim Y, Kim BS. Axillary lymph node-to-primary tumor standard uptake value ratio on preoperative 18F-FDG PET/CT: a prognostic factor for invasive ductal breast cancer. J Breast Cancer 2015;18:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aogi K, Kadoya T, Sugawara Y, et al. Utility of 18F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat 2015;150:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baba S, Isoda T, Maruoka Y, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med 2014;55:736–42. [DOI] [PubMed] [Google Scholar]
- [27].Cochet A, Cochet ID, Riedinger J, et al. 18F-FDG PET/CT provides powerful prognostic stratification in the primary staging of large breast cancer when compared with conventional explorations. Eur J Nucl Med Mol Imaging 2014;41:428–37. [DOI] [PubMed] [Google Scholar]
- [28].Ahn SG, Park JT, Lee HM, et al. Standardized uptake value of 18F-fluorodeoxyglucose positron emission tomography for prediction of tumor recurrence in breast cancer beyond tumor burden. Breast Cancer Res 2014;16:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kadoya T, Aogi K, Kiyoto S, Masumoto N, Sugawara Y, Okada M. Role of maximum standardized uptake value in fluorodeoxyglucose positron emission tomography/computed tomography predicts malignancy grade and prognosis of operable breast cancer: a multi-institute study. Breast Cancer Res Treat 2013;141:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Song BI, Lee SW, Jeong SY, et al. 18F-FDG uptake by metastatic axillary lymph nodes on pretreatment PET/CT as a prognostic factor for recurrence in patients with invasive ductal breast cancer. J Nucl Med 2012;53:1337–44. [DOI] [PubMed] [Google Scholar]
- [31].Bouchelouche K, Turkbey B, Choyke PL. PSMA PET and radionuclide therapy in prostate cancer. Semin Nucl Med 2016;46:522–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kuntz TM, Gilbert JA. Introducing the microbiome into precision medicine. Trends Pharmacol Sci 2017;38:81–91. [DOI] [PubMed] [Google Scholar]
- [33].Lee JH. Radionuclide methods for breast cancer staging. Semin Nucl Med 2013;43:294e8. [DOI] [PubMed] [Google Scholar]
- [34].Garami Z, Hascsi Z, Varga J, et al. The value of 18-FDG PET/CT in early-stage breast cancer compared to traditional diagnostic modalities with an emphasis on changes in disease stage designation and treatment plan. Eur J Surg Oncol 2012;38:31–7. [DOI] [PubMed] [Google Scholar]
- [35].Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol 2015;26:1533e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim J, Lee Y, Lee D, et al. The prognostic significance of the lymph node ratio in axillary lymph node positive breast cancer. J Breast Cancer 2011;78:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kim JY, Ryu MR, Choi BO, et al. The prognostic significance of the lymph node ratio in axillary lymph node positive breast cancer. J Breast Cancer 2011;14:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lale Atahan I, Yildiz F, Ozyigit G, et al. Percent positive axillary lymph node metastasis predicts survival in patients with non-metastatic breast cancer. Acta Oncol 2008;47:232–8. [DOI] [PubMed] [Google Scholar]
- [39].Michiels S, Piedbois P, Burdett S, Syz N, Stewart L, Pignon JP. Metaanalysis when only the median survival times are known: a comparison with individual patient data results. Int J Technol Assess Health Care 2005;21:119–25. [DOI] [PubMed] [Google Scholar]