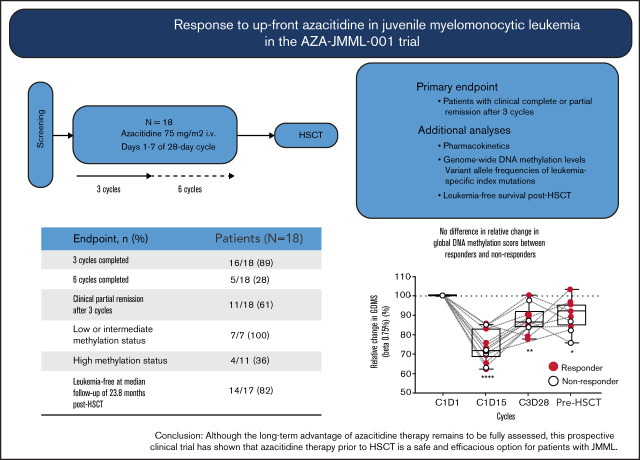
Key Points
Azacitidine monotherapy is well tolerated in a pediatric patient population, with a safety profile consistent with that seen in adults.
Azacitidine monotherapy is an effective agent in patients with JMML prior to stem cell transplantation
Visual Abstract
Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative therapy for most children with juvenile myelomonocytic leukemia (JMML). Novel therapies controlling the disorder prior to HSCT are needed. We conducted a phase 2, multicenter, open-label study to evaluate the safety and antileukemic activity of azacitidine monotherapy prior to HSCT in newly diagnosed JMML patients. Eighteen patients enrolled from September 2015 to November 2017 were treated with azacitidine (75 mg/m2) administered IV once daily on days 1 to 7 of a 28-day cycle. The primary end point was the number of patients with clinical complete remission (cCR) or clinical partial remission (cPR) after 3 cycles of therapy. Pharmacokinetics, genome-wide DNA-methylation levels, and variant allele frequencies of leukemia-specific index mutations were also analyzed. Sixteen patients completed 3 cycles and 5 patients completed 6 cycles. After 3 cycles, 11 patients (61%) were in cPR and 7 (39%) had progressive disease. Six of 16 patients (38%) who needed platelet transfusions were transfusion-free after 3 cycles. All 7 patients with intermediate- or low-methylation signatures in genome-wide DNA-methylation studies achieved cPR. Seventeen patients received HSCT; 14 (82%) were leukemia-free at a median follow-up of 23.8 months (range, 7.0-39.3 months) after HSCT. Azacitidine was well tolerated and plasma concentration-–time profiles were similar to observed profiles in adults. In conclusion, azacitidine monotherapy is a suitable option for children with newly diagnosed JMML. Although long-term safety and efficacy remain to be fully elucidated in this population, these data demonstrate that azacitidine provides valuable clinical benefit to JMML patients prior to HSCT. This trial was registered at www.clinicaltrials.gov as #NCT02447666.
Introduction
Juvenile myelomonocytic leukemia (JMML) is a rare, unique myeloproliferative/myelodysplastic neoplasia of early childhood driven by canonical Ras-pathway mutations in PTPN11, NRAS, KRAS, NF1, or CBL.1 The clinical course of the disease varies widely, but the majority of patients require early allogeneic hematopoietic stem cell transplantation (HSCT) for long-term survival.2,3 Age ≥2 years, severe thrombocytopenia, and a high fetal hemoglobin (HbF) level indicate aggressive disease with high risk for early death or relapse after HSCT.2-5 Recently, genome-wide DNA-methylation profiling identified distinct signatures that correlated with clinical and genetic features and seemed highly predictive of outcome.6-8 The objective of this prospective, open-label, phase 2 study was to evaluate the pharmacokinetics and pharmacodynamics, safety, and activity of the DNA-hypomethylating agent azacitidine prior to HSCT in patients with newly diagnosed JMML.
Methods
Study design
The Study With Azacitidine in Pediatric Subjects With Newly Diagnosed Advanced Myelodysplastic Syndrome (MDS) and Juvenile Myelomonocytic Leukemia (JMML) (AZA-JMML-001) trial was designed as a prospective, open-label, phase 2 study for children with newly diagnosed JMML. The trial was open at 39 centers in 13 European countries (supplemental Table 1) and enrolled from September 2015 to November 2017. Inclusion criteria were: age 1 month to <18 years; somatic mutation in PTPN11, KRAS, NRAS (with percentage HbF >5× the upper limit of normal [ULN] for age) or a clinical diagnosis of neurofibromatosis type 1 (NF1); peripheral blood (PB) monocyte count of ≥1.0 × 109/L; blast percentage in PB and bone marrow (BM) <20%; Lansky play score or Karnofsky performance status ≥60; normal renal function (National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 [NCI CTCAE v4.0] grade 1, maximum 1.5× the ULN) and normal liver function (NCI CTCAE v4.0 grade 1, maximum 2.5× ULN for transaminases and bilirubin); and SO2 >92% without additional supply of O2. Exclusion criteria included germline molecular aberrations in CBL, PTPN11, NRAS, or KRAS; prior treatment with another anticancer therapy or DNA-hypomethylating agent; treatment with any investigational agent within 4 weeks of signing informed consent/informed assent; central nervous system involvement; and isolated extramedullary disease. This study was conducted in accordance with the guidelines of Good Clinical Practice (defined by the International Council for Harmonization E6 guidelines) and the principles of the Declaration of Helsinki. The investigators gained approval from the local Human Investigations Committee at all 39 centers in 13 countries and obtained informed consent from each participant or each participant’s legal guardian where applicable.
Treatment
Following a screening period of ≤14 days, eligible patients were treated with azacitidine 75 mg/m2 IV over 10 to 40 minutes on days 1 to 7 of a 28-day cycle for a minimum of 3 and a maximum of 6 cycles. Patients <10 kg body weight or <1 year of age were treated with 2.5 mg/kg IV on days 1 to 7 of a 28-day cycle for a minimum of 3 and a maximum of 6 cycles. If, by cycle 3 day 28, a patient was in clinical complete remission (cCR) or clinical partial remission (cPR), the patient could receive cycles 4 to 6 of azacitidine at the investigator’s discretion. Patients were discontinued from treatment if they had disease progression at any point, were ready for HSCT between cycles 4 and 6, or had a neutrophil count <0.5 × 109/L on cycle 3 day 28 or on day 1 of cycles 5 or 6. The follow-up period was ≥1 year after the last dose, regardless of whether a patient had started a new anticancer treatment or had undergone HSCT.
Study end points
The primary end point was the proportion of patients with cCR or cPR at cycle 3 day 28. Response evaluation was based on international JMML response criteria,9 modified to allow for splenic response assessment in patients with a splenic longitudinal diameter greater than or equal to the ULN on ultrasound, and for initiation of preparative therapy for HSCT within 4 weeks from day 28 of cycle 3 or 6 (supplemental Table 2). Secondary end points included leukemia-free survival (LFS) after HSCT, overall survival (OS), percentage of patients undergoing HSCT, time to first HSCT, and DNA-methylation status in BM granulocytes.
Pharmacokinetics
Samples for the determination of plasma azacitidine levels were collected predose on cycle 1 days 5 to 7 and postdose (5 minutes, 30 minutes, and 1, 2, 4, and 6 hours) on cycle 1 day 7. For patients weighing ≤20 kg, day 7 predose and 2-hour postdose samplings were omitted. Samples were frozen and later analyzed using validated high-performance liquid chromatography/tandem mass spectrometry. Parameters were calculated using noncompartmental methods, including maximum observed plasma concentration, time of maximum observed plasma concentration, area under the plasma concentration–time curve (AUC∞), apparent total clearance, and apparent volume of distribution.
Pharmacodynamics
Genome-wide DNA-methylation analysis was performed using Infinium EPIC BeadChip arrays (Illumina, San Diego, CA) in DNA isolated from BM granulocytes collected at baseline and posttreatment. Overall, 61 samples from 18 patients that met quality control criteria were available for raw data processing.10 After filtering, 738 776 probes were retained for calculation of global DNA-methylation score (GDMS; mean β value of all cytosine guanine dinucleotide [CpG] probes retained with a β-value ≥0.75 predose on cycle 1 day 1), and JMML-specific DNA-methylation score (JDMS; defined as the mean β value of all JMML-specific CpG probes).6
Variant allele frequency of leukemia-specific index mutations
DNA from BM granulocytes was used for amplicon-based, next-generation deep sequencing to determine the variant allele frequencies (VAFs) of leukemia-specific index mutations. Targets (KRAS [NM_004985.4], NRAS [NM_002524.4], and PTPN11 [NM_002834.3]) were enriched using a custom in-house panel (AmpliSeq JMML panel v2, IAA11909_192; Thermo Fisher Scientific, Waltham, MA) and processed with the NEBNext Ultra II DNA library preparation kit (New England Biolabs, Ipswich, MA). Deep sequencing was performed using MiSeq (Illumina). The sequencing depth was at least 30 independent reads for each base position. A minimum of 30 reads carrying the mutation was required to call a variant (“Q30” score) and a minimum of 10% mutated reads was applied for variant detection.
Safety
Adverse events (AEs) were graded according to the NCI CTCAE v4.0 and recorded until 28 days after the last dose of azacitidine. Thereafter, only serious AEs suspected of being related to azacitidine were recorded.
Statistical methods
The data cutoff for this analysis was 24 May 2019. The primary end point response rate was assessed using the Simon optimal 2-stage design. Using a lower boundary of interest of 10% and an upper boundary of 30% in the response rate, with a significance level of 5% and 90% power, it was determined that 35 patients were required (18 in stage 1 and 17 in stage 2). Stage 2 of the study was not executed because the threshold for confirmed response for the whole study (7 of 35 responders in stages 1 and 2 combined) was met in stage 1, with 9 patients achieving a confirmed clinical response. In patients receiving HSCT, LFS was defined as time from the date of HSCT until leukemia progression, relapse, or death, whichever occurred first, and was evaluated using the Kaplan-Meier method. OS was defined as the time from first dose of azacitidine until death due to any cause, and was evaluated using the Kaplan-Meier method. DNA-methylation levels were evaluated using a repeated measures analysis-of-variance test.
Results
Patient characteristics
From September 2015 to November 2017, 18 children with newly diagnosed JMML were enrolled; the median age was 2.1 years (range, 0.3-6.9 years) (Table 1; supplemental Table 3). JMML genetic subtype was defined by a somatic mutation in PTPN11 (13 of 18 [72%]), NRAS (3 of 18 [17%]), or KRAS (1 of 18 [6%]), or by the clinical diagnosis of NF1 (1 of 18 [6%]). Methylation class was high, intermediate, and low in 11 (61%), 5 (28%), and 2 (11%) patients, respectively. The spleen tip was palpable at a median of 4 cm (range, 2-14 cm) below the costal margin and HbF was elevated for age in 15 patients (83%). Median time from diagnosis to azacitidine initiation was 25 days (range, 6-80 days). Twelve patients (67%) were platelet transfusion dependent at cycle 1 day 1 and 16 (90%) at cycle 2 day 1.
Table 1.
Baseline patient and disease characteristics
| Characteristic | N = 18 |
|---|---|
| Age, median (range), y | 2.1 (0.3-6.9) |
| Time from initial diagnosis to C1D1, median (range), d | 25 (6-80) |
| Sex, no. (%) | |
| Male | 11 (61) |
| Female | 7 (39) |
| Somatic mutation/genetic subtype, no. (%) | |
| PTPN11 | 13 (72) |
| NRAS | 3 (17) |
| KRAS | 1 (6) |
| NF1 | 1 (6) |
| Methylation class, no. (%) | |
| High | 11 (61) |
| Intermediate | 5 (28) |
| Low | 2 (11) |
| Karyotype, no. (%) | |
| Normal | 12 (67) |
| −7 | 3 (17) |
| −Y | 1 (6) |
| del(9) | 1 (6) |
| +21c | 1 (6) |
| WBC, median (range), ×109/L | 19.7 (4.3-59.0) |
| HbF, median (range) | 18.4 (0.7-59.1) |
| Elevated, no. (%) | 15 (83) |
| Normal for age, no. (%) | 3 (17) |
| Platelet count, median (range), ×109/L | 28 (7-85) |
| Patients receiving platelet transfusion, no. (%) | 12* (67) |
| Blasts in BM, median (range), % | 6 (0-19) |
| Spleen tip palpable below costal margin, median (range), cm | 4 (2-14) |
C, cycle; D, day; WBC, white blood cell.
At C2D1, 16 patients (90%) were platelet transfusion dependent.
Therapy delivered and clinical response
Sixteen patients (90%) completed 3 cycles of azacitidine, and 5 of these 16 completed 6 cycles. Two patients (11%) discontinued therapy before completing 3 cycles due to disease progression (supplemental Table 3). At cycle 3 day 28, 11 of 18 patients (61%; 95% confidence interval, 26.0-74.0) achieved cPR (Table 2). Overall, all patients of the low-methylation (LM; n = 2) and intermediate-methylation (IM; n = 5) classes, as well as 4 of the 11 patients of the high-methylation (HM) class, achieved cPR. At cycle 3 day 28, 7 patients (39%), all belonging to the HM class, had clinical progressive disease (cPD).
Table 2.
Achievement of the primary end point
| Response at C3D28 | ||
|---|---|---|
| cPR responders, n = 11 |
cPD nonresponders, n = 7 |
|
| Total, no./Total no. (%) | 11/18 (61) | 7/18 (39) |
| Age, median (range), y | 1.2 (0.3-4.7) | 3.0 (1.8-6.9) |
| Genetic subtype, no. (%)* | ||
| PTPN11 | 8 (73) | 5 (71) |
| KRAS | 1 (9) | 0 |
| NRAS | 2 (18) | 1 (14) |
| NF1 | 0 | 1 (14) |
| HbF, median (range)* | 16.5 (0.7-56.8) | 38 (10.0-59.1) |
| Elevated, no. (%) | 8 (73) | 7 (100) |
| Normal for age, no. (%) | 3 (27) | 0 |
| Karyotype, no. (%)* | ||
| Normal | 7 (64) | 5 (71) |
| −7 | 3 (27) | 0 |
| −Y | 0 | 1 (14) |
| +21c | 1 (9) | 0 |
| del(9) | 0 | 1 (14) |
| Methylation, no. (%)* | ||
| Low | 2 (18) | 0 |
| Intermediate | 5 (46) | 0 |
| High | 4 (36) | 7 (100) |
C, cycle; D, day.
Percentage based on 11 responders with cPR and 7 nonresponders with cPD.
Patients who achieved cPR tended to be younger than patients without response (Table 2), with 7 of the 8 children (88%) <2 years achieving cPR (supplemental Table 3). Importantly, 6 of the 16 patients (38%) who needed platelet transfusions at or shortly after therapy initiation were transfusion-free after 3 cycles of azacitidine. Five of these 6 patients (all responders) had platelet counts >100 ×109/L at the end of treatment (supplemental Tables 3 and 4). The palpable spleen size in the 11 responders decreased by a median of 3.5 cm after 3 cycles and ranged from 0 to 2 cm below the costal margin after 6 cycles. Spleen diameter by ultrasound (a variable for response assessment) decreased, but remained above the ULN for age in all but 1 responder; hence, this patient was assessed in cCR at the secondary end point after 6 cycles of azacitidine (supplemental Table 3).
OS and HSCT
With a median follow-up time from cycle 1 day 1 of 26.9 months (range, 18.2-44.4 months), 16 of 18 patients (90%) were alive (Table 3; supplemental Table 3; supplemental Figure 1). Seventeen of the 18 patients (94%) underwent allogeneic HSCT from a matched sibling donor, unrelated donor (9 of 10 or 10 of 10 allelic match), or a haploidentical parent in 2, 14, and 1 children, respectively (supplemental Table 5). The median time from diagnosis and last dose of azacitidine to HSCT was 5.5 months (range, 3.4-20.2 months) and 2.3 months (range, 1.2-17.0 months), respectively. Thirteen patients (77%) and 4 patients (24%) were grafted with BM or PB cells, respectively. Preparative regimens consisted of busulfan, cyclophosphamide, and melphalan in 13 patients (77%); 2 patients (12%) received busulfan, fludarabine, and melphalan; and 2 patients (12%) received treosulfan, thiotepa, and fludarabine. There were 2 primary graft failures; grade II-IV acute graft-versus-host disease was diagnosed in 4 patients (24%). One patient experienced mild chronic graft-versus-host disease. With a median follow-up time from HSCT of 23.0 months (7.0-39.3 months) for patients still alive at time of analysis, 1 patient had succumbed to nonrelapse mortality and 2 patients (12%) experienced relapse post-HSCT, 1 of whom was successfully regrafted. Of the 17 patients who underwent HSCT, 14 (82%) were leukemia-free at a median follow-up of 23.8 months (range, 7.0-39.3 months) (Table 3; supplemental Figure 1).
Table 3.
LFS after HSCT and OS
| no. (%) or median (range) | |
|---|---|
| LFS after HSCT, n = 17* | |
| Patients who were leukemia-free | 14 (82) |
| Post-HSCT relapse | 2 (12) |
| HSCT-related deaths | 1 (6) |
| Follow-up time from HSCT, mo | 23.8 (7.0-39.3) |
| OS, n = 18 | |
| Patients alive | 16 (90) |
| Follow-up time from C1D1, mo | 26.9 (18.2-44.4) |
C, cycle; D, day.
17 patients who received HSCT; 1 nonresponding patient died prior to HSCT.
Pharmacokinetics
Following IV administration, azacitidine rapidly reached maximum plasma concentrations and then was rapidly eliminated with a terminal elimination half-life of 0.3 hours (supplemental Table 6). Maximum observed plasma concentration was 1066.3 ng/mL, and overall exposure (area under the plasma concentration–time curve, [AUC0-t]) was 240.2 h × ng/mL and 386.9 h × ng/mL for patients with JMML, respectively. High interpatient variability, typical of azacitidine pharmacokinetics, was observed (coefficient of variation range, 53.4% to 215.3%).11
Pharmacodynamics
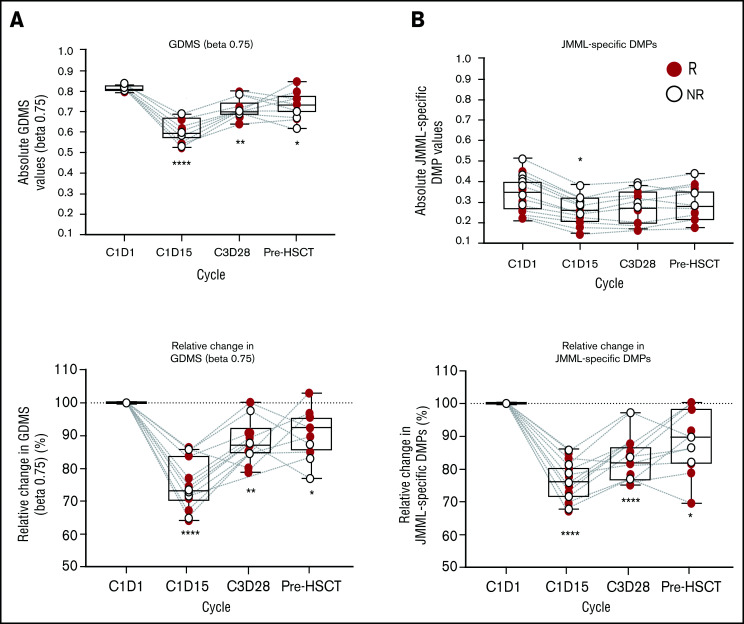
To characterize the pharmacodynamic activity of azacitidine in JMML cells, genome-wide DNA-methylation analysis was performed in BM granulocytes at cycle 1 day 1, cycle 1 day 15, cycle 3 day 28, and pre-HSCT. At baseline, patients exhibited uniform GDMS but variable DNA-methylation levels at JDMS CpG sites (Figure 1), reflecting the DNA-methylation classes in the study cohort (Table 1). The JDMS was higher in patients ≥2 years of age (supplemental Figure 2), consistent with their overrepresentation in the HM group (supplemental Table 3). In addition, male patients had higher DNA-methylation levels at baseline, possibly reflecting their older median age compared with female patients. Profound reductions in both GDMS and JDMS at cycle 1 day 15 were attained in all 18 patients (Figure 1), confirming the DNA-hypomethylating activity of azacitidine in JMML. Remethylation occurred at end-of-cycle time points (cycle 3 day 28 and pre-HSCT), but DNA methylation did not fully revert to baseline levels in all but 2 patients. Of note, there was no difference in the extent of DNA hypomethylation or completeness of remethylation between clinical responders and nonresponders. The maximum plasma concentration of azacitidine or area under the concentration-time curve did not correlate with the extent of DNA hypomethylation (supplemental Figure 3). A correlative analysis of DNA-methylation class with clinical response to azacitidine revealed a 16.0 odds ratio for IM/LM patients to respond (95% confidence interval, 1.3-194.6; P = .049) compared with HM patients.
Figure 1.
DNA-methylation levels in JMML BM granulocytes during treatment with azacitidine. (A) GDMS for all CpG probes with β ≥0.75 at predose on C1D1. (B) JDMS derived from differentially methylated probes (JMML DMPs) across all time points. Data illustrated as absolute values or relative change. Statistics performed via Kruskal-Wallis one-way analysis of variance/Dunn test. Significance vs C1D1: *P < .05; **P < .01; ***P < .001; ****P < .0001. C, cycle; D, day; NR, nonresponder; R, responder.
VF of leukemia-specific mutations
VAFs of individual heterozygous index point mutations were measured during azacitidine treatment in 15 of the 18 patients (83%) (not performed in 1 NF1- and 2 PTPN11-mutated patients; all HM) (supplemental Table 7). The mean VAF at baseline (cycle 1 day 1) was 45.19% ± 2.22%. Four patients achieved a reduction in VAF by at least 10%, attaining a mean VAF of 27.05% ± 6.08% (compared with 44.0% ± 3.27% in the remaining patients). Among these 4 patients, the clinical response was cPR in 3 and cPD in 1 patient.
Safety
All patients had ≥1 AE, with 10 patients (55.6%) experiencing treatment-related AEs. The most common treatment-related treatment-emergent AEs were anemia, thrombocytopenia, neutropenia, and constipation reported in 2 patients each (11%) (supplemental Table 8). Six patients (33%) experienced ≥1 grade 3 or 4 treatment-related AEs. One patient discontinued treatment due to a serious AE of abdominal pain that was not treatment-related. No AEs leading to dose reduction, dose interruption, or death were recorded during the study.
Discussion
In this study of upfront azacitidine monotherapy in children with newly diagnosed JMML, 11 of 18 patients achieved cPR after 3 cycles of therapy. Both the decrease in spleen size and the considerable platelet responses demonstrate that the drug was effective in JMML. Severe thrombocytopenia with platelet transfusion refractoriness is a major clinical challenge in high-risk JMML.2 Sixteen of the 18 patients in this study were transfusion dependent at initiation of therapy or shortly thereafter. Following 3 cycles of azacitidine treatment, 6 of the 16 patients were transfusion-free, and 5 of these 6 had platelet counts >100 × 109/L. Intriguingly, early platelet response is an independent positive predictor of OS in adult patients with MDSs or chronic myelomonocytic leukemia treated with DNA-hypomethylating agents.12,13
This is the first prospective study designed to apply the international response criteria on JMML therapy.9 Clinical variables for response assessment proved suitable, but had to be modified by reducing the required longitudinal spleen diameter by ultrasound for response assessment and by allowing preparative therapy of HSCT within 4 weeks from cycle 3 day 28. Spleen size decreased slowly in responders; after 3 or 6 cycles of azacitidine, the spleen was no longer palpable below the costal margin in 4 children, whereas spleen diameter by ultrasound remained above the ULN in all but 1 patient.
The study design excluded patients with JMML at low risk for early death, such as those with canonical CBL mutations or NRAS mutations with only moderately elevated HbF. Not surprisingly, 17 of the 18 patients enrolled presented with ≥1 of the established clinical4,5 or molecular14 high-risk factors including age ≥2 years (10 of 18), HbF ≥10% (13 of 18), platelet count <33 × 109/L (13 of 18), or presence of PTPN11 mutation (13 of 18) (supplemental Table 3).
Recently, using genome-wide DNA-methylation analysis, methylation subgroups of JMML have been described by 3 independent study groups.6-8 DNA methylation correlated with clinical and molecular risk factors and emerged as the strongest predictor of OS in multivariate analysis. Consistent with these observations, 11 of the 18 study patients belonged to the HM subgroup (Table 1).6
Pharmacodynamics analysis examined the course of DNA methylation in JMML BM granulocytes during azacitidine therapy. There was a drastic short-term (cycle 1 day 15) reduction in both global and JMML-specific CpG methylation, consistent with inhibition of cellular DNA methyltransferases. Remethylation occurred toward the end of treatment cycles, as previously observed in other diseases.15,16 However, DNA methylation did not return to baseline levels except in 2 patients. Although clinical responses did not correlate with the extent of DNA hypomethylation or occurrence of DNA methylation, the DNA-methylation analysis predicted the probability of response, as all 7 LM/IM patients and 4 of 11 HM children (36%) responded. Considering that a DNA-hypomethylating agent like azacitidine would be expected to have a more pronounced effect in hypermethylated phenotypes, this result may appear surprising. However, other mechanisms of action are known for azacitidine that do not depend directly on DNA hypomethylation (ie, induction of apoptosis through RNA incorporation by disrupting RNA synthesis, activation of DNA-replication stress, reactivation of endogenous retroviral elements, and immune-modulatory mechanisms).17-19 Likely, the better response in LM/IM patients confirms the concept that these methylation groups represent lower-risk variants of the disease.
Following IV administration of azacitidine, plasma concentration-time profiles in patients with JMML were similar to profiles observed in adults with MDS, demonstrating rapid time of maximum observed plasma concentration and elimination half-life.11 Arithmetic mean (standard deviation) of the area under the plasma concentration–time curve (AUC0-t) for adult and pediatric populations was 1025 (298.06) vs 744.2 (1152) (data not shown), respectively, demonstrating similar exposures with high variability for adult and pediatric patients. Azacitidine monotherapy was well tolerated in this patient population, with a safety profile consistent with that seen in adults.
Following previous case reports, this is the first prospective study on azacitidine therapy in children with JMML.20-23 Limitations of the study are the small sample size and short follow-up time. Whether a response to azacitidine prior to HSCT will translate into improved OS with longer observation time remains to be determined. With a median time of 23.8 months from HSCT, 14 of the 17 patients with high-risk features who received transplants were in first cCR, 2 relapsed (1 was rescued with a second allograft), and 1 had nonrelapse mortality. In conclusion, although the long-term advantage of azacitidine therapy remains to be fully assessed, this prospective clinical trial has shown that azacitidine therapy prior to HSCT is a safe and efficacious option for patients with JMML. Whether response to upfront therapy like azacitidine or combination chemotherapy24 is simply a biomarker for favorable disease or can effectively reduce post-HSCT JMML recurrence will have to be addressed in future clinical trials.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all patients and their families, as well as investigators from all participating centers in Austria, Belgium, Czech Republic, Denmark, France, Germany, Ireland, Italy, Spain, Sweden, Switzerland, The Netherlands, and the United Kingdom. The authors acknowledge the support of Brigitte Strahm (Freiburg, Germany) for central response review, Berna Beverloo (Rotterdam, The Netherlands), Gudrun Göhring, and Brigitte Schlegelberger (Hanover, Germany) for central cytogenetic reference review, and European Working Group of Myelodysplastic Syndromes (EWOG-MDS) National Coordinators Michael Dworzak (Austria), Barbara de Moerloose (Belgium), Henrik Hasle (Denmark), Owen Smith (Ireland), Albert Catala (Spain), and Karin Belander Stralin (Sweden) for patient recruitment.
This work was supported by Bristol Myers Squibb. The authors received writing and editorial assistance from Emily Poulin, and Jacqueline Moy, of Excerpta Medica, funded by Bristol Myers Squibb.
Authorship
Contribution: C.M.N., F.L., M.M.v.d.H.-E., B.B., N.B., and J.P. were responsible for the study conception and design; J.S., C.R., A.B., T.K., C.M., G.M., K.N., S.R., M.S.L., M.Z., C.M.N., and F.L. were responsible for the provision of study material or patients; J.S., C.R., A.B., T.K., C.M., G.M., K.N., S.R., M.S.L., M.Z., C.M.N., F.L., M.P., D.M., C.F., and D.B.L. were responsible for the collection and assembly of data; C.F., D.B.L., M. Schönung, I.B., P.N., M. Simcock, M.P., D.M., A.G., and C.M.N. were responsible for data analysis and interpretation; C.M.N., C.F., and F.L. drafted the manuscript; and all authors provided approval of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: C.M.N. has served in a consultancy or advisory role for Bristol Myers Squibb (BMS) and Novartis. C.R. has served in a consultancy or advisory role for Amgen, BMS, Celgene, EUSA Pharma, Genentech, Pfizer, Novartis, and Roche. A.B. has received honoraria from AstraZeneca, BMS, Jazz Pharmaceuticals, Novartis, and Servier; has served in a consultancy or advisory role for BMS, Jazz Pharmaceuticals, Novartis, and Servier; and has received research funding from Servier. K.N. has received honoraria from Bayer, and has served in a consultancy or advisory role for Bayer and Y-mABs Therapeutics Inc. B.B. and N.B. were employed by BMS and have equity ownership in BMS. J.P., M.S., and A.G. are employed by, and have equity ownership in, BMS. D.M. is employed by BMS. M.M.v.d.H.-E. is employed by the Princess Máxima Center for Pediatric Oncology (Utrecht, The Netherlands). F.L. has served in a consultancy or advisory role for Amgen, Bellicum Pharmaceuticals, Neovii, and Novartis; and has served on a speakers’ bureau for Amgen, Bluebird bio, Jazz Pharmaceuticals, Medac, Miltenyi Biotec, Novartis, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Charlotte N. Niemeyer, Division of Pediatric Hematology and Oncology, Department of Pediatrics and Adolescent Medicine, University Medical Center Freiburg, Mathildenst 1, 79106 Freiburg, Germany; e-mail: charlotte.niemeyer@uniklinik-freiburg.de.
References
- 1.Niemeyer CM, Flotho C.. Juvenile myelomonocytic leukemia: who’s the driver at the wheel? Blood. 2019;133(10):1060-1070. [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F, Niemeyer CM.. How I treat juvenile myelomonocytic leukemia. Blood. 2015;125(7):1083-1090. [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Nöllke P, Zecca M, et al. ; European Blood and Marrow Transplantation Group . Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005;105(1):410-419. [DOI] [PubMed] [Google Scholar]
- 4.Passmore SJ, Hann IM, Stiller CA, et al. Pediatric myelodysplasia: a study of 68 children and a new prognostic scoring system. Blood. 1995;85(7):1742-1750. [PubMed] [Google Scholar]
- 5.Niemeyer CM, Arico M, Basso G, et al. ; European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS) . Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. Blood. 1997;89(10):3534-3543. [PubMed] [Google Scholar]
- 6.Lipka DB, Witte T, Toth R, et al. RAS-pathway mutation patterns define epigenetic subclasses in juvenile myelomonocytic leukemia. Nat Commun. 2017;8(1):2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stieglitz E, Mazor T, Olshen AB, et al. Genome-wide DNA methylation is predictive of outcome in juvenile myelomonocytic leukemia. Nat Commun. 2017;8(1):2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami N, Okuno Y, Yoshida K, et al. Integrated molecular profiling of juvenile myelomonocytic leukemia. Blood. 2018;131(14):1576-1586. [DOI] [PubMed] [Google Scholar]
- 9.Niemeyer CM, Loh ML, Cseh A, et al. Criteria for evaluating response and outcome in clinical trials for children with juvenile myelomonocytic leukemia. Haematologica. 2015;100(1):17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assenov Y, Müller F, Lutsik P, Walter J, Lengauer T, Bock C.. Comprehensive analysis of DNA methylation data with RnBeads. Nat Methods. 2014;11(11):1138-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcucci G, Silverman L, Eller M, Lintz L, Beach CL.. Bioavailability of azacitidine subcutaneous versus intravenous in patients with the myelodysplastic syndromes. J Clin Pharmacol. 2005;45(5):597-602. [DOI] [PubMed] [Google Scholar]
- 12.van der Helm LH, Alhan C, Wijermans PW, et al. Platelet doubling after the first azacitidine cycle is a promising predictor for response in myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) patients in the Dutch azacitidine compassionate named patient programme. Br J Haematol. 2011;155(5):599-606. [DOI] [PubMed] [Google Scholar]
- 13.Zeidan AM, Lee JW, Prebet T, et al. Platelet count doubling after the first cycle of azacitidine therapy predicts eventual response and survival in patients with myelodysplastic syndromes and oligoblastic acute myeloid leukaemia but does not add to prognostic utility of the revised IPSS. Br J Haematol. 2014;167(1):62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida N, Yagasaki H, Xu Y, et al. Correlation of clinical features with the mutational status of GM-CSF signaling pathway-related genes in juvenile myelomonocytic leukemia. Pediatr Res. 2009;65(3):334-340. [DOI] [PubMed] [Google Scholar]
- 15.Si J, Boumber YA, Shu J, et al. Chromatin remodeling is required for gene reactivation after decitabine-mediated DNA hypomethylation. Cancer Res. 2010;70(17):6968-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G.. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26(4):577-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flotho C, Claus R, Batz C, et al. The DNA methyltransferase inhibitors azacitidine, decitabine and zebularine exert differential effects on cancer gene expression in acute myeloid leukemia cells. Leukemia. 2009;23(6):1019-1028. [DOI] [PubMed] [Google Scholar]
- 18.Roulois D, Loo Yau H, Singhania R, et al. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell. 2015;162(5):961-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolff F, Leisch M, Greil R, Risch A, Pleyer L.. The double-edged sword of (re)expression of genes by hypomethylating agents: from viral mimicry to exploitation as priming agents for targeted immune checkpoint modulation. Cell Commun Signal. 2017;15(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furlan I, Batz C, Flotho C, et al. Intriguing response to azacitidine in a patient with juvenile myelomonocytic leukemia and monosomy 7. Blood. 2009;113(12):2867-2868. [DOI] [PubMed] [Google Scholar]
- 21.Cseh A, Niemeyer CM, Yoshimi A, et al. Bridging to transplant with azacitidine in juvenile myelomonocytic leukemia: a retrospective analysis of the EWOG-MDS study group. Blood. 2015;125(14):2311-2313. [DOI] [PubMed] [Google Scholar]
- 22.Hashmi SK, Punia JN, Marcogliese AN, et al. Sustained remission with azacitidine monotherapy and an aberrant precursor B-lymphoblast population in juvenile myelomonocytic leukemia. Pediatr Blood Cancer. 2019;66(10):e27905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcu A, Colita A, Radu LE, et al. Single-center experience with epigenetic treatment for juvenile myelomonocytic leukemia. Front Oncol. 2020;10:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hecht A, Meyer J, Chehab FF, et al. Molecular assessment of pretransplant chemotherapy in the treatment of juvenile myelomonocytic leukemia. Pediatr Blood Cancer. 2019;66(11):e27948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.