Abstract
Prominent theories suggest that disruptions in amygdala reactivity and connectivity when processing emotional cues are key to the etiology of youth antisocial behavior (AB) and that these associations may be dependent on co-occurring levels of callous-unemotional (CU) traits. We examined the associations among AB, CU traits, and amygdala reactivity and functional connectivity while viewing emotional faces (fearful, angry, sad, happy) in 165 adolescents (46% male; 73.3% African American) from a representative, predominantly low-income community sample. AB was associated with increased amygdala activation in response to all emotions and was associated with greater amygdala reactivity to emotion only at low levels of CU traits. AB and CU traits were also associated with distinct patterns of amygdala connectivity. These findings demonstrate that AB-related deficits in amygdala functioning may extend across all emotions and highlight the need for further research on amygdala connectivity during emotion processing in relation to AB and CU traits within community populations.
Keywords: antisocial behavior, callous-unemotional traits, amygdala, generalized psychophysiological interactions, gPPI, emotion
Youth antisocial behavior (AB), including violence, aggression, and rule breaking, is a significant public health concern because of its negative impact on individuals, families, and society (Rivenbark et al., 2018). To better inform intervention efforts, researchers have focused on identifying specific behavioral impairments that may lead to AB, such as increased impulsivity and reward sensitivity, as well as difficulty with socioemotional processing, fear conditioning, and emotion regulation. Given this behavioral research, researchers have increasingly sought to identify the biological correlates of these impairments, with a recent focus on the neural substrates of these deficits (Blair, Leibenluft, & Pine, 2014; Hyde, Shaw, & Hariri, 2013).
The Amygdala and Emotion Processing in AB
The amygdala is involved in socioemotional processes key to the development of AB, including emotion processing, threat response, and fear conditioning (LeDoux, 2000). The amygdala is activated by emotional faces, particularly fearful and angry facial expressions (Adolphs, 2010), which can serve as important cues in stimulus-reinforcement learning processes that are thought to underlie the development of morality and prosocial behavior (Blair, 2017). Thus, studies of youth AB have focused on the role of amygdala activation in response to fearful or angry facial expressions. However, findings have been mixed, given that youth AB has been associated with both amygdala hyperactivation (e.g., Dotterer, Hyde, Swartz, Hariri, & Williamson, 2017; Herpertz et al., 2008; Sebastian et al., 2012; Sebastian et al., 2014; Viding et al., 2012) and amygdala hypoactivation in response to these cues (Ewbank et al., 2018; Jones, Laurens, Herba, Barker, & Viding, 2009; e.g., Lozier, Cardinale, VanMeter, & Marsh, 2014; Marsh et al., 2008; Passamonti et al., 2010).
AB With Co-Occurring Callous-Unemotional Traits
Notably, recent theories suggest that children and adolescents with AB show different patterns of amygdala functioning dependent on the presence of callous-unemotional (CU) traits (Blair, 2017; Hyde et al., 2013; Viding & McCrory, 2018). CU traits, including low empathy and remorselessness, are developmental precursors to some affective features of adult psychopathy and identify a subgroup of children and adolescents with severe and persistent AB (Frick, Ray, Thornton, & Kahn, 2014). These dual pathways have been supported in clinical samples (e.g., Jones et al., 2009; Marsh et al., 2008; Viding et al., 2012) such that CU traits moderate the association with AB and amygdala reactivity. AB with co-occurring CU traits is related to amygdala hypoactivation in response to fearful faces (potentially reflecting distress, or uncertain threat; Davis, Walker, Miles, & Grillon, 2010), whereas AB without co-occurring CU traits is related to amygdala hyperactivation in response to angry faces (potentially reflecting certain threat). This dual-pathway model has become key to neural-focused theories of AB to explain why children and adolescents with AB and CU traits appear to exhibit low empathic concern and more proactive aggression and why children and adolescents with AB display heightened threat sensitivity, emotion dysregulation, and reactive aggression (Blair, 2017; Hyde et al., 2013; Viding & McCrory, 2018). Although this model and findings are compelling, several larger studies of community samples have not been able to replicate these findings (e.g., Dotterer et al., 2017; for a clinical example, see Passamonti et al., 2010). Thus, it is unclear whether associations are present dimensionally across the range of AB and CU traits or are found only at the most severe levels of AB and CU traits, which highlights the need for dimensional studies of AB and CU traits (e.g., Hyde, Byrd, Votruba-Drzal, Hariri, & Manuck, 2014; Hyde et al., 2016).
Emotion-Processing Impairments in AB and CU Traits
Although the dual-pathway model posits that altered neural and behavioral sensitivity is specific to facial expression of fear and/or anger, few studies have confirmed the specificity of this association or have tested whether AB and CU traits are related to a more general neural deficit in processing interpersonal emotions. This gap in the literature is particularly striking given that the amygdala is sensitive to a range of emotions, including other negative emotions (sadness) and positive emotions (happiness; Adolphs, 2010). Indeed, a meta-analysis reported broad emotional-processing impairments associated with CU traits, including deficits in recognizing sadness and happiness (Dawel, O’Kearney, McKone, & Palermo, 2012). However, fewer neuroimaging studies have tested whether youth AB or CU traits are related to differences in amygdala reactivity to other emotions beyond fear and anger (e.g., happiness and sadness). In one exception, Passamonti et al. (2010) found that boys with early-onset conduct disorder demonstrated reduced amygdala reactivity to sad faces compared with control participants (CU traits were unrelated to amygdala reactivity to sad faces). In a sample of female adolescents with and without conduct disorder, Fairchild et al. (2014) found no differences in amygdala reactivity to sad faces among the participants, which suggests potential gender differences in these associations. Finally, beyond these two studies examining sad facial expressions, no studies of youth AB have examined amygdala reactivity to positive emotions (i.e., happy faces), a striking gap in the literature given research suggesting that AB is also associated with impaired processing of positive emotions (e.g., Tamamiya & Hiraki, 2013). Thus, studies are needed to test whether youth AB is characterized by a specific impairment in the neural processing of fear and anger or altered amygdala functioning when processing emotions broadly (e.g., sadness or happiness). Moreover, given that few past studies have examined neural reactivity to sad emotions, it is important to examine whether any differences in reactivity are specific to AB as opposed to CU traits and whether these associations differ by gender.
Amygdala Connectivity During Emotion Processing
Emotion processing relies on the functioning of and communication among multiple regions within the brain (Menon, 2011). Thus, impairments in amygdala functional connectivity may also contribute to socioemotional deficits in AB among children and adolescents. The amygdala is considered to be a hub region and is highly connected to numerous networks. For example, the amygdala functions as part of the salience network (SN), which adjusts arousal and attention on the basis of external cues and internal states and serves as a switching mechanism between itself other networks (Seeley et al., 2007). Recent theories have suggested that differences in connectivity between the amygdala and the SN and the default mode network (DMN) impede the processing of complex sensory information in a way that may lead to severe AB (Hamilton, Hiatt Racer, & Newman, 2015; Menon, 2011). That is, network functioning may affect emotion-based learning processes, including the processing of emotional cues, crucial to the development of prosocial behavior (Blair, 2017). Impaired connectivity within the SN and DMN has been linked to AB and CU traits in resting-state connectivity studies of children and adolescents and to psychopathic traits in adults (e.g., Aghajani et al., 2017; Broulidakis et al., 2016; Motzkin, Newman, Kiehl, & Koenigs, 2011; Philippi et al., 2015; Pu et al., 2017; Thijssen & Kiehl, 2017) and during socioemotional tasks in adults (e.g., Decety, Chen, Harenski, & Kiehl, 2013; Waller et al., 2018). Moreover, the few existing studies relating AB to task-based connectivity in children and adolescents have found impaired connectivity between the amygdala and key nodes of the SN (i.e., anterior insula [AI], anterior cingulate cortex [ACC]) and the DMN (i.e., ventromedial prefrontal cortex [vmPFC], posterior cingulate cortex [PCC], precuneus; e.g., Aghajani et al., 2018; Cardinale et al., 2018; Ewbank et al., 2018; Finger et al., 2012; Hwang et al., 2016; Marsh et al., 2008; Marsh et al., 2011).
Although findings from these initial studies provide support for the notion that disrupted network communication may be key to understanding youth AB, all but one of the previous studies were conducted in clinical samples of children and adolescents or forensic samples of adults (Aghajani et al., 2018; Cardinale et al., 2018; Ewbank et al., 2018; Finger et al., 2012; Hwang et al., 2016; Marsh et al., 2008; Marsh et al., 2011). Thus, it is unclear whether results generalize dimensionally to AB and CU traits in children and adolescents in the community. In addition, studies have varied substantially in task characteristics and connectivity analyses. For example, three studies examined connectivity across the entire task irrespective of task effects (Finger et al., 2012; Marsh & Blair, 2008; Marsh et al., 2011). With this method, it is unclear whether impaired connectivity is explicitly related to the specific requirements of the task or reflects differences in intrinsic connectivity. Generalized psychophysiological interactions (gPPI) is one approach to directly assess whether connectivity is disrupted during emotion processing within youth AB by examining how correlated activity among the amygdala and other brain regions is modulated by task characteristics (O’Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, 2012). Examining how task characteristics influence patterns of amygdala connectivity is particularly important given previous research in typically developing adolescents demonstrating that amygdala connectivity differs according to emotion type (Diano et al., 2017). Taken together, research is needed to determine whether AB is dimensionally associated with network-wide differences in amygdala activation and connectivity during basic emotion processing and whether these associations are modulated by the type of emotion that is being processed.
Current Study
In the current study, we examined associations between AB and CU traits and amygdala activation and connectivity during socioemotional processing within a relatively large, mixed-gender sample of adolescents drawn from a population-based sample of births in large U.S. cities. Associations were examined between neural reactivity and connectivity dimensionally across a wide range of AB and CU traits, including normative and clinical levels of AB. Furthermore, by using a mixed-gender sample with large representation of low-income and African American adolescents, the current study addresses gaps in the current literature, which has been informed primarily from studies of boys of European ancestry (or clinical samples that may confound race and clinical status). Moreover, we examined amygdala reactivity to traditional emotional cues implicated in AB (angry and fearful faces) as well as reactivity to other emotions (sadness, happiness) to test the specificity of potential neural-reactivity differences to emotion. We also extend previous research by examining both amygdala reactivity and connectivity to regions with the SN and DMN within the same study.
Our primary aim was to determine whether AB was associated with individual differences in amygdala activation and functional connectivity during socioemotional processing. Guided by existing neural theories of youth AB (Blair, 2013; Viding & McCrory, 2018), we hypothesized that AB would be uniquely associated with increased amygdala activation in response to angry faces and that CU traits would be uniquely associated with reduced amygdala activation in response to fearful faces (with no association between AB and CU traits to amygdala reactivity to sad or happy faces). In addition, we conducted exploratory analyses examining associations among AB, CU traits, and amygdala connectivity to the SN and DMN while viewing emotional reactivity relative to neutral faces given recent network theories that suggest disrupted connectivity between the SN and DMN may underlie AB and CU traits (Hamilton et al., 2015). Finally, we explored gender and race as potential moderators given that prior research in this area has focused on boys of European descent (e.g., Jones et al., 2009; Marsh et al., 2008; Viding et al., 2012).
Method
Participants
The study sample was drawn from 237 adolescents from Detroit, Toledo, or Chicago who were part of the Study of Adolescent Neural Development (SAND; Goetschius et al., 2019; Hein et al., 2018), a substudy of the Fragile Families and Child Wellbeing Study (FFCWS; Reichman, Teitler, Garfinkel, & McLanahan, 2001), which contains multiple measures of context, psychopathology, brain function, and biology. The FFCWS is a longitudinal cohort of 4,898 (52.4% boys) children born in 20 large U.S. cities from 1998 to 2000 (Reichman et al., 2001) that was oversampled for nonmarital births (~3:1). This sample contains substantial representation of African American children and adolescents as well as adolescents from families living in low-income contexts. Families living in Detroit, Toledo, and Chicago were invited to take part in additional data collection at the University of Michigan as part of the SAND study when the focal child was 15 years old (see the Supplemental Material available online). The complete list of measures and data for this project is publicly available from the National Institute of Mental Health data archive (https://nda.nih.gov/). The University of Michigan Medical School Institutional Review Board approved this study (UM IRBMED: HUM00074392). All adolescent participants provided written informed assent, and their primary caregivers provided written consent for both themselves and their adolescent children after the study was explained and questions were answered. Within the SAND study, the MRI component introduced sources of data loss (see Table S1 in the Supplemental Material), which resulted in 167 adolescents with available and high-quality functional MRI (fMRI) data. Two participants were excluded because of missing data on behavioral variables of interest (i.e., AB and CU traits). Of the 165 adolescents who were included in the present analyses, 53.9% were female; 76.4% were Black/African American and 12.1% were White/European American; and 46.7% of families reported annual income below $25,000. Participants included in the current sample did not significantly differ in demographics and behavioral variables of interest from participants without neuroimaging data or participants with neuroimaging data excluded after quality checks (see Table S2 in the Supplemental Material).
Procedures
Amygdala-reactivity paradigm.
Participants completed an event-related emotional-faces task during fMRI acquisition (Hein et al., 2018). In this task, participants were asked to identify the gender of the actor by pressing their thumb for male or their index finger for female on a button box. Faces from the NimStim set (Tottenham et al., 2009) were used and were counterbalanced for gender and race (European American and African American). There were 100 pseudo-randomized trials comprising 20 trials of each of the following type of emotional faces: fearful, happy, sad, neutral, and angry. Each trial consisted of a fixation cross (500 ms), followed by a face (250 ms), then a black screen (1,500 ms) during which participants responded to the face, and finally a second black screen (jittered intertrial interval: 2 s, 4 s, or 6 s). The task is particularly well suited for studying basic, automatic emotion processing because the quick presentation time of the face stimuli does not provide opportunity for participants to saccade away from the stimuli. Accuracy and response times were recorded.
BOLD fMRI acquisition parameters.
The fMRI data were collected using a 3T MRI scanner (Discovery MR750; GE Healthcare, Chicago, IL) with an eight-channel head coil. We collected functional T2*-weighted blood-oxygen-level-dependent (BOLD) images with a gradient echo spiral sequence (repetition time = 2,000 ms, echo time = 30 ms, contiguous 3-mm axial slices, flip angle = 90°, field of view = 22 cm, voxel size = 3.44 mm × 3.44 mm × 3 mm) aligned with the anterior commissure–posterior commissure (AC–PC) plane.
Preprocessing and quality-control procedures.
Anatomical images were homogeneity corrected using SPM (Version 12; https://www.fil.ion.ucl.ac.uk/spm/), then skull-stripped using the Brain Extraction Tool in FSL (Version 5.0.7; Jenkinson, Pechaud, & Smith, 2005; Smith, 2002). The following preprocessing steps were applied to the functional imaging data: removal of large temporal spikes in k-space data (> 2 SD), field-map correction and image reconstruction using custom code in MATLAB (Version 9.3; The MathWorks, Natick, MA), and slice-timing correction using SPM. In addition, the remainder of preprocessing was done in SPM and included gray-matter segmenting anatomical images, realigning segmented anatomical and functional images to the AC–PC plane, coregistering anatomical and functional images, spatially normalizing functional images into Montreal Neurological Institute (MNI) space, and smoothing functional images with a Gaussian filter set to 8 mm (full width at half maximum). After preprocessing, Artifact Detection Tools (ART) software (Release art-2015-10; http://www.nitrc.org/projects/artifact_detect) identified motion outliers (> 2 mm movement or 3.5° rotation). Outliers were censored from individual participant models using a single regressor for each outlier volume. Given the susceptibility of the amygdala to signal loss, only those participants with a minimum of 70% coverage in the right and left amygdala, as defined by the amygdala region of interest (ROI) in the Automated Anatomical Labeling (AAL) atlas regions of interest (ROIs; Maldjian, Laurienti, & Burdette, 2004), were included in group-level analyses. To ensure that participants were engaged in the task, only those participants with accuracy of 75% or greater were included in group analyses (Table S1 in the Supplemental Material). Condition effects were modeled at the individual level; incorrect trials were modeled as a separate condition and excluded from subsequent analyses.
Group-level analyses: activation.
The general linear model of SPM was used for first- and second-level analyses. Linear contrasts employing canonical hemodynamic response functions were used to estimate condition-specific (i.e., fearful > neutral) BOLD activation for each individual and scan. These individual contrast images (i.e., weighted sum of the beta images) were then used in second-level random effects models that account for both scan-to-scan and participant-to-participant variability to determine mean expression-specific reactivity using one-sample t tests. The main goal of this study was to examine amygdala reactivity to emotional faces relative to neutral facial expressions, consistent with previous research that has primarily used conditions involving neutral or calm faces as the contrast to other emotions (e.g., Jones et al., 2009; Sebastian et al., 2014; Viding et al., 2012; White et al., 2012). Thus, we present results for the contrasts of fearful > neutral (i.e., activity during fearful blocks minus activity during neutral blocks), angry > neutral, sad > neutral, and happy > neutral.
Given that our focus was on amygdala reactivity to emotional facial expressions, we created an ROI mask of the AAL bilateral amygdala using the WFU PickAtlas Tool (Version 1.04; https://www.nitrc.org/projects/wfu_pickatlas/). We conducted all amygdala analyses using a small volume correction via the 3DClustSim program (December 2015; https://afni.nimh.nih.gov/pub/dist/doc/htmldoc/programs/3dClustSim_sphx.html), which uses a Monte Carlo simulation to provide thresholds that will achieve a family-wise error correction for multiple comparisons of p < .05 within each ROI (Cox, Chen, Glen, Reynolds, & Taylor, 2017). We used the latest version of 3DClustSim and the recommended method for estimating and using noise smoothness values in 3dFWHMx (https://afni.nimh.nih.gov/pub/dist/doc/htmldoc/programs/3dFWHMx_sphx.html), the -acf option (spatial AutoCorrelation Function estimated by calculating moments of differences out to a larger radius than before; Cox et al., 2017). The -acf option computes the spatial autocorrelation of the data as a function of radius (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). We used a voxel-wise threshold of p < .01 at an α of .05, which resulted in a cluster threshold of 25 contiguous voxels for small-volume correction in the amygdala ROI. We focus on reporting results of the most stringent models (associations among amygdala reactivity and AB or CU traits controlling for covariates and the overlap of AB and CU traits; 3dClustSim uncorrected p [punc] < .01, α < .05). The pattern of results was similar in models that did not control for the overlap of AB and CU traits (i.e., no evidence of suppression; results available on request). Given the number of separate analyses we conducted and the more exploratory nature of sad and happy facial expression contrasts (sad > neutral; sad < neutral; happy > neutral; happy < neutral), we also examined and report whether findings specific to these contrasts remained significant at a stricter threshold, accounting for the four models (punc < .01; α < .05 / 4 = .0125; k = 55 contiguous voxels for small volume correction in the amygdala ROI).
Group-level analyses: connectivity.
To examine amygdala connectivity with regions within the SN and default DMN during the emotional-faces-matching paradigm, we defined the right and left amygdala (i.e., ROI masks of the AAL left and right amygdala using WFU PickAtlas) as the seed regions. We examined the following target regions previously characterized as key nodes of the SN (AAL definitions): bilateral ACC and bilateral AI. In addition, we examined the following target regions previously characterized as key nodes of the DMN (AAL definitions): vmPFC (an ROI mask comprising BA 10, BA 14, and BA 25; Marsh et al., 2008; Motzkin, Philippi, Wolf, Baskaya, & Koenigs, 2015), bilateral PCC, and bilateral precuneus (Menon, 2011; Seeley et al., 2007). Psychophysiological interaction (PPI) analyses from the gPPI toolbox (McLaren, Ries, Xu, & Johnson, 2012) in SPM were used to assess functional connectivity. Two general linear models at the individual level were constructed (i.e., right and left amygdala seeds). Using the gPPI toolbox, the time series of the left or right amygdala seed was entered as the physiological variable in the design matrix, the onset times for conditions in our task were entered as psychological variables (i.e., fearful, angry, sad, happy, and neutral faces), and all product terms among the amygdala seed and conditions were entered as the interaction terms. Because we were interested in whether functional coupling of the left or right amygdala and our ROIs from the SN and DMN varied as a function of condition, we specified four primary contrasts at the individual level: fearful-faces interaction term > neutral interaction, angry-faces interaction term > neutral interaction, sad-faces interaction term > neutral interaction, and happy-faces interaction term > neutral interaction.
Group-level models were constructed to examine contrasts across all participants. In our analysis, a significant interaction of emotional face (i.e., fearful, angry, sad, happy) > neutral face at the group level would suggest that activity between the left or right amygdala and the target ROI was more strongly positively correlated while participants were looking at emotional facial expressions than at neutral facial expressions. As with the activation analyses, we conducted all ROI analyses using a small volume correction via the 3DClustSim program (voxel-wise threshold punc < .01, α < .05; cluster thresholds range = 69–167 contiguous voxels based on the target ROI mask and amygdala seed; Cox et al., 2017). We focus reporting results of the most stringent models (associations between amygdala connectivity and AB or CU traits controlling for covariates and the overlap of AB and CU Traits; 3dClustSim punc < .01, α < .05). The pattern of results was similar in models that did not control for the overlap of AB and CU traits (results available on request). Given that connectivity analyses were exploratory without specific hypotheses, we also examined and report whether findings remained significant at a stricter threshold, accounting for the eight models (punc < .01; α < .05 / 8 = .00625; cluster thresholds range = 120–376 contiguous voxels based on the target ROI mask and amygdala seed).
Behavioral measures
The SAND is an archival study designed to flexibly test multiple scientific questions having to do with poverty effects on the brain and brain–psychopathology associations. We selected the measures used in this report by identifying all existing measures of AB and CU traits. We used all existing measures of CU traits. We selected measures of various forms of AB (i.e., both aggression and rule breaking). We submitted measures of AB and CU traits to confirmatory factor analyses (CFAs; i.e., two CFAs: AB and CU traits). Because of the poor model fit of the AB CFA, we removed one measure of AB on the basis of modification indices. After this step, the model fit of the AB factor was good, and all other measures significantly loaded on the factor. We decided on two final factors before examining associations with brain activity (i.e., these measures were selected theoretically and via factor analysis and were not chosen or trimmed on the basis of associations with brain activity).
Antisocial behavior.
We assessed AB at age 15 using a multi-informant, multimethod approach combining multiple indicators from multiple different measures: (a) parent-reported rule breaking and (b) aggression from the Child Behavior Checklist (Achenbach, 1991), (c) total score (excluding substance use items) of the youth-reported Self-Report of Delinquency (Elliott, Huizinga, & Ageton, 1985), and (d) combined lifetime symptom count (i.e., past and present subclinical and clinical threshold symptoms) of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) conduct disorder and oppositional defiant disorder (American Psychiatric Association, 2013) on the basis of clinician-ratings assessed via a modified version of the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 1997). “Present” symptom counts included symptoms that the participant endorsed in the 6 months immediately preceding the study visit. “Past” symptom counts included symptoms that the participant endorsed as occurring any time before the 6 months immediately preceding the study visit. To combine these four measures into a multi-informant, multimethod score for AB, we used CFA in Mplus (Version 7.3; Muthén & Muthén, 2014) with maximum likelihood estimation with robust standard errors (to account for skew and zero-inflation; see the Supplemental Material). Although we focused on dimensional measures of AB in this study, participants reported a range of AB from normative to clinical; several participants met diagnostic criteria for conduct disorder (past diagnosis: n = 13, 7.9%; current diagnosis: n = 5, 3%) and oppositional defiant disorder (past diagnosis: n = 10, 6.1%; current diagnosis: n = 6, 3.6%).
CU traits.
We also assessed CU traits at age 15 using a multimethod, multi-informant approach combining three different measures: total scores for (a) parent-reported and (b) youth-reported Inventory of Callous-Unemotional Traits (Frick, Bodin, & Barry, 2000) and (c) clinician ratings of total lifetime symptom counts (i.e., past and present subclinical and clinical threshold symptoms) using the Michigan Addendum to the K-SADS, which consists of items that are meant to overlap with the recently developed DSM–5 “limited prosocial emotions” specifier (American Psychiatric Association, 2013) derived from the Clinical Assessment of Prosocial Emotions (Frick, 2016) and embedded into the K-SADS interview (Kaufman et al., 1997). “Present” symptom counts included symptoms that the participant endorsed in the 6 months immediately preceding the study visit. “Past” symptom counts included symptoms that the participant endorsed as occurring any time before the 6 months immediately preceding the study visit. We created a sum score of present and past symptom counts to create the lifetime symptom count. We created a latent construct of CU traits specifying scales from these measures to load onto a CU traits factor using CFA with full information maximum likelihood estimation with robust standard errors (MLR) in Mplus; see the Supplemental Material). MLR is able to accommodate variables with significant skew (as expected in measures of AB) and can provide unbiased estimates without violation of assumptions under these conditions (Muthén & Muthén, 2017; Yuan & Bentler, 2000). Although we focused on dimensional measures of CU traits, a small percentage of participants did meet diagnostic criteria for the “with limited prosocial emotion” specifier (past diagnosis: n = 4, 2.4%; current diagnosis: n = 4, 2.4%).
Covariates and moderators.
To rule out any impact of demographic factors on the associations among neural correlates and AB and CU traits, we also accounted for the effects of the following covariates, all assessed at age 15: (a) self-reported gender, (b) self-reported race (two dichotomous codes: African American vs. Other; European American vs. Other), (c) parent-reported family monthly income, and (d) self-reported pubertal development assessed using the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988); total scores of the scale range from 1 to 4 (note that youth reports were missing for four participants, and we used parent-reported scores in these cases). In addition, we examined gender and race as moderators.
Results
For all analyses, we focus on reporting results of the most stringent models (associations controlling for covariates and the overlap of AB and CU traits; 3dClust Sim punc < .01, α < .05); however, the pattern of findings was similar for less stringent models.
Are AB and CU traits associated with amygdala reactivity to emotional faces?
Across all types of emotional faces (i.e., fearful > neutral, angry > neutral, sad > neutral, and happy > neutral), we found a strikingly similar pattern of findings in which AB was positively correlated with right and left amygdala activation, controlling for CU traits and other demographic factors (Table 1), which indicates a more generalized difference in amygdala reactivity to emotion. When we used a more stringent threshold (punc < .01, α < .0125) for exploratory analyses of sad and happy contrasts, the positive association between AB and amygdala activation in response to sad > neutral faces remained significant, but the association with happy > neutral faces did not. In contrast to associations with AB, CU traits were not associated with amygdala reactivity to any of the emotional facial expressions.
Table 1.
Antisocial Behavior Is Associated With Amygdala Activation in Response to Emotional Compared With Neutral Faces, Controlling for Demographic Factors and Callous-Unemotional Traits
| Contrast | Cluster size | t(l64) | z | Peak coordinates |
|---|---|---|---|---|
| Fearful faces > neutral faces | ||||
| Left amygdala | 78 | 4.45 | 4.32 | −18, 0, −16 |
| Right amygdalaa | 120 | 4.81 | 4.64 | 18, 4, −16 |
| 4.12 | 4.01 | 26, −6, −12 | ||
| Angry faces > neutral faces | ||||
| Left amygdala | 41 | 4.17 | 4.05 | −22, −8, −14 |
| Right amygdalaa | 85 | 3.81 | 3.72 | 28, −8, −14 |
| 3.57 | 3.49 | 22, 6, −16 | ||
| Sad faces > neutral faces | ||||
| Left amygdalaa | 79b | 5.12 | 4.91 | −20, −6, −16 |
| 3.05 | 3.00 | −30, 4, −18 | ||
| Right amygdala | 92b | 3.88 | 3.79 | 18, 4, −16 |
| 3.48 | 3.41 | 28, −8, −14 | ||
| Happy faces > neutral faces | ||||
| Left amygdala | 41 | 4.18 | 4.06 | −20, −6, −12 |
| Right amygdala | 54 | 3.12 | 3.07 | 18, 2, −16 |
Note: All models included gender, puberty, family monthly income, race (two dichotomous codes), and callous-unemotional traits as covariates. There were no associations between callous-unemotional traits and amygdala activation in response to emotional compared with neutral faces when we controlled for demographic factors and antisocial behavior.
This cluster had multiple significant peaks.
These exploratory findings were significant at a more stringent threshold controlling for four models (sad > neutral; sad < neutral; happy > neutral; happy < neutral; p < .01, α < .0125, cluster threshold k = 55).
Given that our results were not consistent with dominant theories in the literature (i.e., CU traits were not related to amygdala reactivity to any emotional facial expression), we also examined whether CU traits moderated associations between AB and amygdala activation in response to emotional faces (Viding et al., 2012) using a continuous interaction term, controlling for the main effects of AB and CU traits (see Table S5 in the Supplemental Material). That is, because most clinical studies have examined children and adolescents with high levels of AB and CU traits (e.g., Jones et al., 2009; Marsh et al., 2008; Sebastian et al., 2014; Viding et al., 2012; White et al., 2012), we hypothesized that associations between CU traits and amygdala reactivity to fearful facial expressions might emerge only at higher levels of AB.
In testing this interaction, we found that there were significant interactions between AB and CU traits in relation to bilateral amygdala activation for three of the contrasts (i.e., angry faces > neutral faces, sad faces > neutral faces, happy faces > neutral faces; see the Supplemental Material). Consistent with the main effect models, AB was associated with increased activation in response to angry, sad, and happy faces compared with neutral faces. However, the associations between AB and increased amygdala activation in response to angry, sad, and happy faces compared with neutral faces were present only at mean or low levels of CU traits (Fig. 1; also see Table S6 in the Supplemental Material). All but one of the associations (i.e., angry > neutral) remained when correcting for multiple comparisons across facial emotion type (Bonferroni; p < .0125). CU traits were not a significant moderator of associations between AB and amygdala reactivity to fearful facial expressions.
Fig. 1.
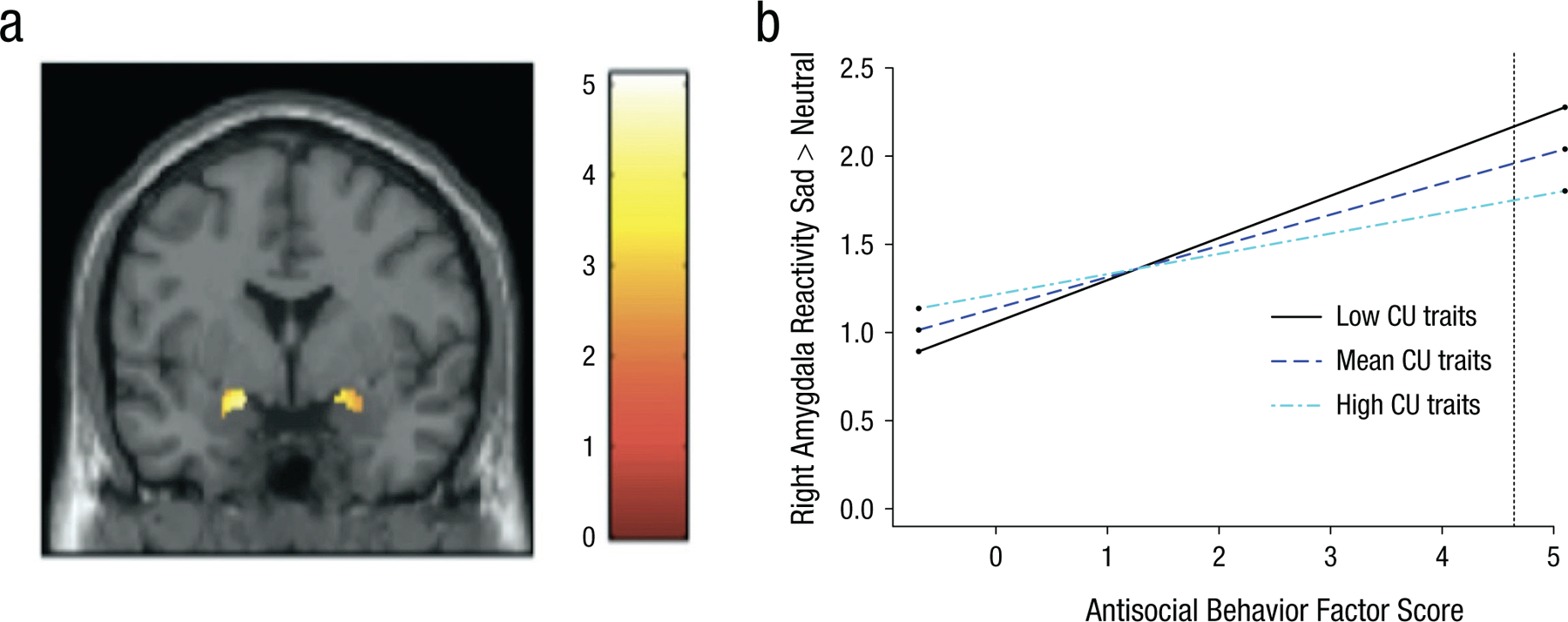
Relation of increased antisocial behavior (AB) to increased amygdala reactivity while viewing sad facial expressions. In (a), the functional cluster for bilateral amygdala reactivity to sad > neutral facial expressions. The voxel-wise threshold is set at p < .01, which resulted in a cluster threshold of 25 contiguous voxels for small volume correction in the amygdala region of interest (ROI). The color bar shows t values. Details about the significant clusters are reported in Table 1. In (b), callous-unemotional traits (CU) moderate the association between AB and right amygdala reactivity to sad > neutral facial expressions. Simple slopes plotted at mean levels and ±1 SD above and below the mean for CU traits, as recommended by Aiken, West, and Reno (1991) and using an online computational tool (Preacher, Curran, & Bauer, 2006). The stars next to the lines indicate significant slopes. At low and mean levels of CU traits but not at high levels of CU traits, increased right amygdala reactivity to sad > neutral facial expressions was significantly related to higher AB. The dashed line indicates the level of AB at which the association is significant (AB factor score > 4.65; 0.6% of the sample).
In addition, we examined whether gender and race further moderated these findings and found that gender but not race moderated these associations (see Table S7 in the Supplemental Material). Specifically, for boys, as in the whole sample, AB was associated to greater amygdala reactivity to angry and sad faces at low or mean levels of CU traits. In contrast, the association was reversed in girls such that AB was related to increased amygdala reactivity to fearful, angry, and sad faces only at high levels of CU traits (see Table S8 in the Supplemental Material). That is, the results in the entire sample were due mostly to the same pattern in boys, whereas for girls, the pattern was the opposite. For the most part, these associations survived Bonferroni correction for multiple comparisons across analyses (see the Supplemental Material).
Are AB and CU traits associated with amygdala connectivity while viewing emotional faces?
The overall patterns of connectivity are summarized in Table 2. We found that AB was generally associated with weaker positive amygdala connectivity to regions within the SN (i.e., insula, ACC) while viewing fearful, sad, and happy faces but stronger positive amygdala connectivity to the ACC while viewing angry faces (Fig. 2). Moreover, while viewing fearful and sad faces, AB was associated with stronger positive amygdala connectivity to the PCC and precuneus (within the DMN). In contrast, while viewing happy faces, AB was associated with weaker positive amygdala connectivity to the PCC and precuneus (within the DMN). Moreover, AB was associated with weaker positive amygdala–vmPFC connectivity across all emotional faces (see Table S9 in the Supplemental Material). At a more stringent threshold (punc < .01, α < .0125), AB was still associated with stronger positive amygdala connectivity to the precuneus while viewing both fearful and sad faces as well as weaker positive amygdala connectivity to the vmPFC and insula while viewing sad and happy faces.
Table 2.
Summary of Associations Between Amygdala Connectivity While Viewing Emotional Faces and Antisocial Behavior Compared With Callous-Unemotional Traits
| Emotion, Network, and Node | Antisocial behavior | Callous-unemotional traits |
|---|---|---|
| Fearful | ||
| Salience | ||
| ACC | Weaker positive connectivity | — |
| Insula | Weaker positive connectivity | Stronger positive connectivity |
| Default mode | ||
| vmPFC | Weaker positive connectivity | Stronger positive connectivity |
| PCC | Stronger positive connectivity | — |
| Precuneus | Stronger positive connectivitya | — |
| Sad | ||
| Salience | ||
| ACC | — | — |
| Insula | Weaker positive connectivitya | — |
| Default mode | ||
| vmPFC | Weaker positive connectivitya | Stronger positive connectivity |
| PCC | Stronger positive connectivity | — |
| Precuneus | Stronger positive connectivitya | — |
| Angry | ||
| Salience | ||
| ACC | Stronger positive connectivity | — |
| Insula | — | — |
| Default mode | ||
| vmPFC | Weaker positive connectivity | — |
| PCC | — | — |
| Precuneus | — | — |
| Happy | ||
| Salience | ||
| ACC | — | — |
| Insula | Weaker positive connectivitya | Stronger positive connectivity |
| Default mode | ||
| vmPFC | Weaker positive connectivitya | — |
| PCC | Weaker positive connectivity | — |
| Precuneus | Weaker positive connectivity | — |
Note: Results from models that included gender, puberty, family monthly income, and race (two dichotomous codes). All models also controlled for the overlap between antisocial behavior and callous-unemotional traits. ACC = anterior cingulate cortex; vmPFC = ventromedial prefrontal cortex; PCC = posterior cingulate cortex; — = association was not significant.
These are exploratory findings that were significant at a more stringent threshold controlling for eight models (fearful > neutral; fearful < neutral; angry > neutral; angry < neutral; sad > neutral; sad < neutral; happy > neutral; happy < neutral; p < .01, α < .00625).
Fig. 2.
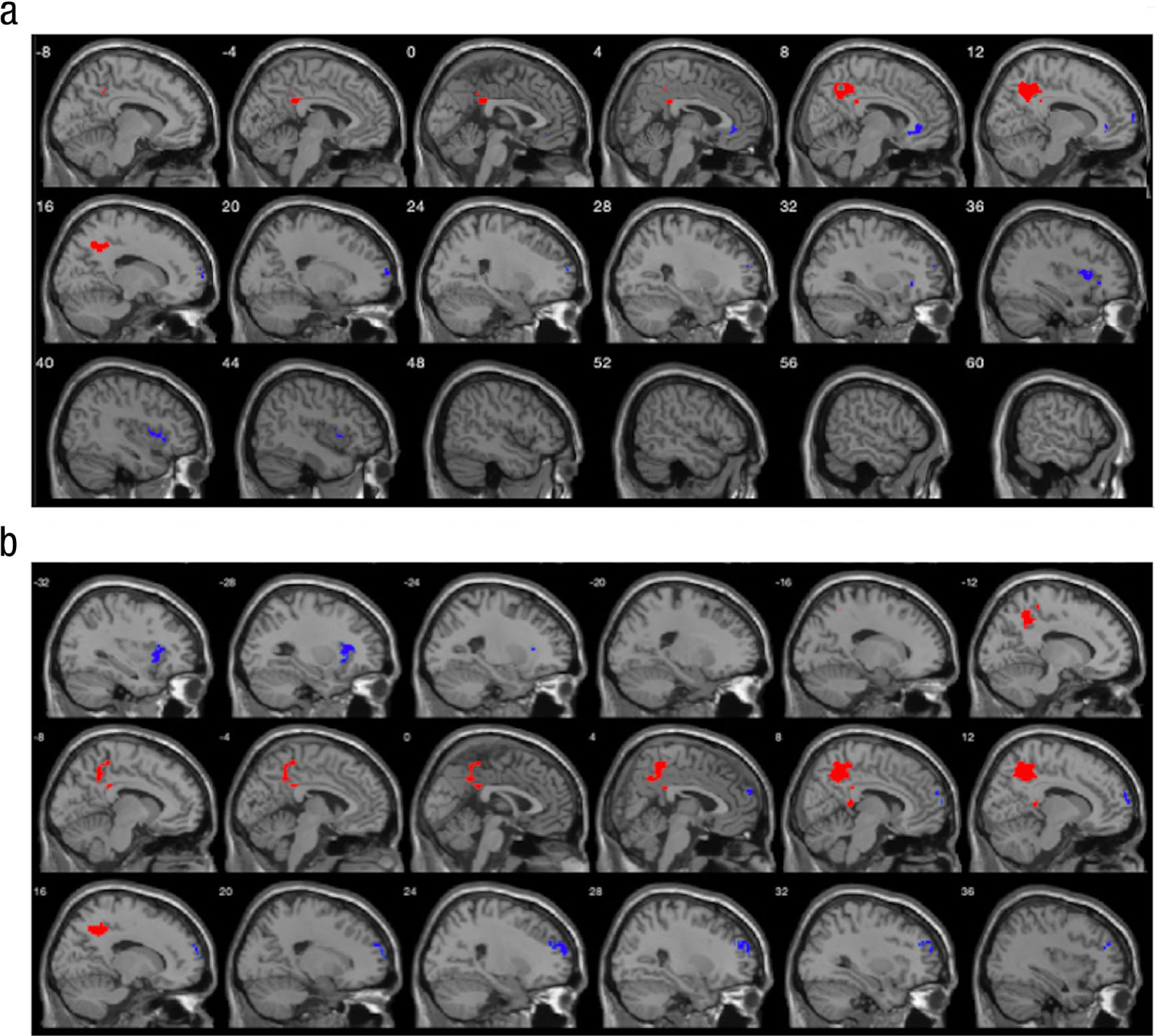
Association of group-level psychophysiological interactions (PPI) effects of left and right amygdala connectivity with antisocial behavior when processing fearful and sad facial expressions. In (a), group-level PPI effects of left and right amygdala connectivity are associated with antisocial behavior while viewing fearful compared with neutral facial expressions. N = 165. For group-level PPI effects of the amygdala, stronger positive connectivity is shown in red and weaker positive connectivity is shown in blue. Details about the significant clusters are reported in Table S9 in the Supplemental Material available online. (b) Group-level PPI effects of left and right amygdala connectivity are associated with antisocial behavior while viewing sad compared with neutral facial expressions.
In contrast, CU traits were associated with stronger positive connectivity to the insula (within the SN) while viewing happy and fearful faces and stronger positive connectivity to the vmPFC (within the DMN) viewing fearful and sad faces (see Table S10 in the Supplemental Material). Note that none of these results related to CU traits survived a more stringent threshold (punc < .01, α < .0125).
Discussion
In the current study, we found that AB was associated with individual differences in both amygdala functioning and amygdala connectivity in response to a range of emotions (i.e., fear, anger, sadness, and happiness) in a large, mixed-gender, community sample of under-represented minority adolescents. AB was associated with increased bilateral amygdala activation in response to each emotion. In contrast to our hypothesis and prior research (e.g., Jones et al., 2009; Marsh et al., 2008; Viding et al., 2012), CU traits were not associated with amygdala activation. A set of exploratory analyses indicated that CU traits were instead a significant moderator of associations between AB and amygdala reactivity such that AB was related only to amygdala reactivity to angry, sad, and happy faces when CU traits were low. Finally, AB was associated with stronger positive amygdala connectivity to regions of the DMN (i.e., PCC, precuneus) when processing fearful and sad faces but weaker positive amygdala connectivity to the ACC and insula (within the SN). Patterns of connectivity differed, however, while viewing angry faces (i.e., stronger positive amygdala–ACC connectivity) and happy faces (i.e., weaker positive amygdala connectivity to PCC, precuneus, and insula). Across all emotions, AB was associated with weaker positive connectivity vmPFC, whereas CU traits were associated with stronger positive amygdala–insula connectivity (happy and fearful) and stronger positive amygdala–vmPFC connectivity (fearful and sad).
These findings represent a challenge to existing theory in the field regarding the role of amygdala reactivity in AB and CU traits because CU traits were not directly related to lower amygdala reactivity, nor was AB related to lower amygdala reactivity at high levels of CU traits. Moreover, in contrast to an emphasis in the field on the specificity of amygdala dysfunction related to anger or fear, the positive association between AB and amygdala reactivity was general to all types of emotion explored in this study (although the specific association with happy > neutral did not survive a more stringent threshold for exploratory multiple corrections). In addition, the findings support and build on existing network theories of AB by demonstrating unique patterns of amygdala connectivity during emotion process that differed for AB as opposed to CU traits. Specifically, AB was associated with stronger positive connectivity to regions within the DMN but weaker positive amygdala connectivity to the vmPFC and regions within the SN. Conversely, CU traits were associated with stronger positive amygdala connectivity to the vmPFC and regions within the SN (e.g., insula). These findings are particularly important given the use of a relatively large, well-sampled cohort of adolescents with a substantial range of AB and CU traits (from normative to clinical) and dimensional analyses.
Increased amygdala reactivity was related to AB for all emotional faces
As hypothesized and consistent with theory (Blair et al., 2014), AB was associated with increased amygdala reactivity to angry faces. Moreover, AB was more broadly associated with amygdala response to fearful, sad, and happy facial expressions. Although youth studies have previously connected AB to greater amygdala reactivity to fearful faces during basic emotion processing (e.g., Lozier et al., 2014) and sad faces (e.g., Passamonti et al., 2010), no work has extended this effect to happy (although happy faces have been used as stimuli in an empathy paradigm; Seara-Cardoso, Sebastian, Viding, & Roiser, 2016). However, the association between AB and amygdala activation in response to happy faces did not survive a stricter threshold, which highlights the need for replication of this finding. Moreover, the results challenge the prevailing notion in the field (e.g., Blair et al., 2014) that AB is related specifically to amygdala reactivity to anger or fear but may be broadly associated with increased reactivity to others’ emotions. Consistent with recent meta-analyses examining recognition of emotion in others (Dawel et al., 2012), individual differences in amygdala reactivity related to AB may be related nonspecifically to processing others’ emotions.
AB was related to amygdala reactivity only at low levels of CU traits
Although the associations found between amygdala reactivity and AB support half of the dual-pathway model (i.e., AB without co-occurring CU traits was associated with increased reactivity), we did not identify any significant associations between CU traits and amygdala reactivity during socioemotional processing. In a set of post hoc analyses, we found that CU traits were a significant moderator of associations between AB and amygdala reactivity to angry, sad, and happy faces, but again, not in the way expected by theory. AB was associated with increased amygdala reactivity only at low or mean levels of CU traits; there was no indication that AB was related to low amygdala reactivity at high levels of CU traits (or that CU traits were associated with low amygdala reactivity at high levels of AB). Moreover, region of significance analyses revealed that results were significant at most levels of CU traits and that these associations were diminished only at severe levels of CU traits (see Supplemental Results). However, given the exploratory nature of these analyses and lack of research on broad emotion processing in relation to AB and CU traits, further research will be needed to determine the robustness of these findings (however, for other community studies failing to replicate amygdala reactivity associations with CU traits, see Dotterer et al., 2017; Hyde et al., 2016; Szabó et al., 2017).
Note that the sample in the current study differs from most previous samples that have supported associations between CU traits and reduced amygdala reactivity, which have been clinical and predominantly European-origin male samples. We used exploratory analyses to check whether race or gender may explain the extent to which our findings diverge from those from clinical samples. These analyses indicated that race was not a factor and that the group pattern was due primarily to the pattern seen in the boys. These results may not be surprising given that much of the literature in youth studies has focused on samples of primarily boys. Our exploratory analyses revealed a different neuroetiologic pattern related to AB and CU traits in girls. Note that only one other study explicitly tested gender moderation in neural correlates of AB and CU traits (e.g., Dotterer et al., 2017), which emphasizes the need for more research to clarify how the etiology of AB may differ between girls and boys.
It is possible that associations between CU traits and reduced amygdala reactivity during emotion processing emerge only at the most severe levels of CU traits found in clinical samples. Although our sample did contain adolescents with diagnosable levels of AB and CU traits (i.e., 14 participants with conduct disorder and oppositional defiant disorder past or present diagnosis; 5 participants with diagnosable “with limited prosocial emotions” qualifier), we did not have enough participants to do a subgroup analysis and may have not had enough participants with very severe AB and CU traits to replicate previous clinical studies. Thus, lower levels of CU traits represented in community samples, even samples enriched for individuals at higher risk for AB, could explain the null findings of the current study and the few other existing studies in nonclinical samples (e.g., Dotterer et al., 2017; Hyde et al., 2016; Szabó et al., 2017). At the same time, given the well-sampled and larger nature of our sample and the presence of multiple nonreplications in community samples (e.g., Dotterer et al., 2017; Hyde et al., 2016; Szabó et al., 2017), we must also consider whether “well-established” findings based on clinical studies are as robust as we believe them to be (e.g., Finger et al., 2012; Jones et al., 2009; Lozier et al., 2014; Marsh et al., 2008; Sebastian et al., 2014; Viding et al., 2012; White et al., 2012).
AB was primarily associated with stronger positive amygdala connectivity with the default mode network but weaker positive amygdala connectivity with the salience network
PCC and precuneus.
AB was associated with stronger positive connectivity between the amygdala and two key regions of the DMN (i.e., PCC, precuneus) during distress processing but weaker positive connectivity while viewing happy faces. Associations between AB and amygdala–precuneus connectivity while viewing fearful and sad faces were particularly robust and remained significant at a stringent threshold (punc < .01, α < .00625). Previous studies of resting-state connectivity have also suggested that AB may be characterized by disrupted connectivity within the DMN (Chen et al., 2015; e.g., Dalwani et al., 2014; Pu et al., 2017; Zhou et al., 2016). The PCC and precuneus are strongly activated during tasks that involve working memory and autobiographical memory retrieval (Buckner, Andrews-Hanna, & Schacter, 2008) as well as emotion processing (e.g., Göttlich, Ye, Rodriguez-Fornells, Münte, & Krämer, 2017; Maddock, Garrett, & Buonocore, 2003) and empathy (Carrington & Bailey, 2009). These findings suggest that AB may be characterized by individual differences in amygdala connectivity to regions within the DMN, which could potentially affect social cognition and perspective-taking via atypical processing of both positive (i.e., happy) and negative (i.e., sad, fearful) emotional cues.
vmPFC.
Note that across all emotions, AB was also associated with weaker connectivity between the amygdala and the vmPFC (often characterized as a node in the DMN), a region that is central to existing theories of AB and psychopathic traits (Blair, 2017). Associations between AB and amygdala–vmPFC connectivity while viewing sad and happy faces were particularly robust and remained significant at a stringent threshold (punc < .01, α < .00625). In contrast to the PCC and precuneus, the vmPFC is recruited in more complex mental simulation, such as retrieving associative information to form mental representations that would then guide decision-making (Andrews-Hanna, Smallwood, & Spreng, 2014). Within existing theories of AB and CU traits, weaker amygdala–vmPFC connectivity is thought to interfere with the transfer of important reinforcement expectancy information (e.g., emotional cues) from the amygdala to the vmPFC, a process that is critical to the development of morality and prosocial behavior (Blair, 2017). In support of this notion, reduced amygdala–vmPFC task-based connectivity has been previously associated with youth AB during socioemotional tasks (e.g., Finger et al., 2012; Marsh et al., 2008; Marsh et al., 2011). Note that in a recent study of young adults, impulsive and antisocial components of psychopathy (“factor 2”) was specifically associated with reduced amygdala–vmPFC connectivity while viewing fearful but not angry faces (Waller et al., 2018). Thus, both studies suggested that weaker amygdala–vmPFC connectivity may be associated with AB but to different emotional probes across development. However, because we did not have strong a priori hypotheses, further research will be needed to replicate these findings.
Insula and ACC.
AB was associated with weaker positive amygdala connectivity to the ACC and insula (within the SN) while viewing fearful and sad faces as well as weaker positive amygdala–insula connectivity while viewing happy faces. However, AB was associated with stronger positive amygdala connectivity to the ACC while viewing angry faces. Associations between AB and amygdala–insula connectivity while viewing sad and happy faces were particularly robust and remained significant at a stringent threshold (punc < .01, α < .00625). The insula and ACC incorporate autonomic nervous signals with conscious thought processing to represent one’s emotional state and the emotional valence of external stimuli (Uddin, 2015). Previous research has similarly found reduced connectivity between the amygdala and ACC and insula during harm processing in children with conduct problems and CU traits (Yoder, Lahey, & Decety, 2016). However, the only previous study that has examined amygdala connectivity to the ACC while viewing angry faces instead found reduced amygdala–ACC connectivity. Note that this finding was specific to adolescents with childhood-onset conduct disorder compared with participants with adolescent-onset conduct disorder and control participants (Ewbank et al., 2018). Increased communication specifically within the SN (i.e., between the amygdala and ACC) while processing anger cues may indicate reduced switching and recruiting areas from other networks that would facilitate normative emotion processing. Thus, stronger positive amygdala–ACC connectivity could represent a neural pathway underlying increased sensitivity to certain threat, particularly in adolescents with subclinical AB and without co-occurring CU traits.
CU traits were associated with weaker positive amygdala–vmPFC connectivity and stronger positive amygdala–insula connectivity
vmPFC.
Surprisingly, in contrast to our findings with AB and to previous studies of CU traits in adolescents (Finger et al., 2012; Marsh & Blair, 2008; Marsh et al., 2011) and the interpersonal and affective components of psychopathy in adults (Waller et al., 2018), we found that CU traits were associated with stronger positive amygdala–vmPFC connectivity while viewing fearful and sad faces. It could be that weaker connectivity between the amygdala and vmPFC to distress cues emerges only at extreme levels of CU traits. Moreover, few of the previous studies in children and adolescents used gPPI and thus were unable to distinguish whether reduced amygdala–vmPFC connectivity was specific to viewing fearful or sad faces (Finger et al., 2012; Marsh & Blair, 2008; Marsh et al., 2011). Note that a recent study by our group (Waller et al., 2018) on a sample of young adults did not find significant associations among amygdala–vmPFC connectivity and affective-interpersonal features of psychopathy (i.e., “factor 1”; grandiosity, callousness) but did find that adolescent CU traits were associated with stronger positive amygdala–vmPFC connectivity. However, it may be difficult to compare findings given that adolescent CU traits and factor 1 psychopathy do not consist of the same features. Moreover, the findings in the current study did not remain significant after correcting for a strict threshold (punc < .01, α < .0125), and thus we draw caution in interpretation.
Insula.
CU traits were also associated with stronger positive amygdala–insula connectivity with the insula while viewing fearful and happy faces, in contrast to the pattern associated with AB (i.e., weaker connectivity). To our knowledge, this is the first study to examine amygdala–insula connectivity while viewing happy facial expressions. Thus, future research is needed on the neural correlates of positive emotion processing associated with CU traits to determine the replicability of these associations, particularly given that this result was not robust enough to remain significant at a stricter statistical threshold.
Strengths and limitations
The current study benefitted from several strengths, including multi-informant, multimethod measures of both AB and CU traits and a large, diverse sample in terms of gender, socioeconomic status, and race. Moreover, we used an fMRI paradigm that included a variety of emotional faces, in contrast to previous studies that have solely focused on fearful or angry facial expressions. Despite these strengths, there are limitations worth noting. First, the current study was not preregistered and contains multiple hypothesis-driven and exploratory analyses. Although we have provided information on which exploratory analyses survived strict correction for multiple comparisons, many of these findings require replication in a second sample. Second, our study was also cross-sectional in nature, and thus the directionality of associations is unknown. Relatedly, AB often includes the use of substances and exposure to other neurotoxicants, and thus neural correlates may be the cause or consequence of AB; given that there was extremely low endorsement of substance use within the sample (12-item substance-use subscale of the Self Report of Delinquency; range = 0–24, M = .90, SD = 1.8), higher rates of substance use in other samples may also contribute to differential findings. Third, we used an implicit emotion-processing task that was relatively simple in nature and did not include more nuanced emotions (i.e., disgust, surprise). Previous work has suggested that associations between AB and CU or psychopathic traits and amygdala functioning may differ as a function of task difficulty or attentional load (Larson et al., 2013; White et al., 2012). Moreover, the presentation of emotional stimuli in the current task was very brief (250 ms) compared with previous studies (e.g., 2,000 ms, Marsh et al., 2008; 1,750 ms, Sebastian et al., 2014). Neural correlates of AB and CU traits may therefore differ for more automatic compared with prolonged emotion processing.
In addition, our task constrained the ability for eye movements to change attention and amygdala reactivity, which may also explain our divergent results (Han, Alders, Greening, Neufeld, & Mitchell, 2012). Because of the use of multiple types of facial emotions, we had fewer trials of each type of face than other studies (e.g., Marsh et al., 2008), which potentially lowers our power to detect nuanced effects, including our ability to look at all possible contrasts (e.g., fear > anger, which did not have main task effects). Moreover, recent studies have found that task-related activation and connectivity may be unreliable as an individual difference measure because of poor internal consistency (Infantolino, Luking, Sauder, Curtin, & Hajcak, 2018). Indeed, research suggests that neural activation (i.e., habituation; Thompson & Spencer, 1966) and connectivity can vary over time (i.e., dynamic connectivity, time-varying connectivity; Cohen, 2018). Thus, the current results as well as previous findings using similar approaches are likely limited by poor internal consistency of traditionally used fMRI paradigms. Future studies will benefit from using task designs that better account for variation in activation and connectivity over time. Furthermore, in using gPPI to examine connectivity, we were not able to examine directed connectivity (i.e., which node predicts activation in the other node), a limitation of gPPI and potentially fMRI generally. In addition, our connectivity analyses were exploratory, and we did not have a priori hypotheses. Thus, the connectivity results should be regarded as tentative and in need of replication. Finally, as previously noted, although we did have cases that met diagnosable levels of AB (n = 14) and CU traits (n = 5), most of the sample did not and varied dimensionally along the continuum seen in the community; thus, we did not have the power to examine the question of whether previous results exist only in extreme clinical cases.
Conclusions
In sum, in the current study, we found that AB was associated with increased bilateral amygdala activation in response to both negative and positive emotional faces, whereas CU traits were not associated with amygdala activation during socioemotional processing. In a set of exploratory analyses, we found that AB was associated with increased reactivity to angry, sad, and happy faces only at low or mean levels of co-occurring CU traits and that this pattern differed for boys compared with girls. Finally, in a set of exploratory gPPI analyses, we identified unique patterns of connectivity associated with AB for each emotion type. In particular, AB was associated with stronger positive amygdala connectivity with the precuneus; weaker positive connectivity of the insula during the processing of fearful, sad, and happy faces; and weaker positive amygdala–vmPFC connectivity across all emotions, with the strongest effects for happy and sad faces. Moreover, although CU traits were not associated with activation, CU traits were associated with stronger positive amygdala–insula connectivity while viewing happy and fearful faces and stronger positive amygdala–vmPFC connectivity while viewing fearful and sad faces, although these results were less robust and did not survive a more stringent threshold. Thus, examining patterns of connectivity provided additional information, beyond activation, in understanding potential neural mechanisms underlying emotion processing for children and adolescents with high levels of CU traits. Taken together, these findings emphasize the interactive nature of neural mechanisms of socioemotional processing and highlight the utility of network-level analyses in examining neural correlates of AB and CU traits, particularly in community samples that include a range of AB and CU traits.
Supplementary Material
Acknowledgments
We acknowledge the past work of the Fragile Families and Child Wellbeing Study, the families for sharing their experiences with us, and the project staff for making the study possible.
Funding
The research reported in this article was supported by National Institute of Mental Health Grant R01-MH103761 (to C. S. Monk). R. Waller was supported by National Institute on Alcohol and Alcoholism Grant T32-AA007477. H. L. Dotterer was supported by a National Science Foundation Graduate Research Fellowship. T. C. Hein was supported by a Doris Duke Fellowship for the Promotion of Child Well-Being. L. W. Hyde was supported by a NARSAD Young Investigator Award from the Brain and Behavior Foundation.
Footnotes
Declaration of Conflicting Interests
The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Supplemental Material
Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/2167702620922829
References
- Achenbach TM (1991). Integrative guide for the 1991 CBCL/4-18, YSR, and TRF profiles. Burlington: Department of Psychiatry, University of Vermont. [Google Scholar]
- Adolphs R (2010). What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences, 1191, 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajani M, Klapwijk ET, Colins OF, Ziegler C, Domschke K, Vermeiren RR, & van der Wee NJ (2018). Interactions between oxytocin receptor gene methylation and callous-unemotional traits impact socio-affective brain systems in conduct-disordered offenders. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 379–391. [DOI] [PubMed] [Google Scholar]
- Aghajani M, Klapwijk ET, van der Wee NJ, Veer IM, Rombouts SA, Boon AE, … Colins OF (2017). Disorganized amygdala networks in conduct-disordered juvenile offenders with callous-unemotional traits. Biological Psychiatry, 82, 283–293. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: SAGE. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR (2013). The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience, 14, 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR (2017). Emotion-based learning systems and the development of morality. Cognition, 167, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Leibenluft E, & Pine DS (2014). Conduct disorder and callous–unemotional traits in youth. New England Journal of Medicine, 371, 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broulidakis MJ, Fairchild G, Sully K, Blumensath T, Darekar A, & Sonuga-Barke EJ (2016). Reduced default mode connectivity in adolescents with conduct disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 55, 800–808.e1. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Cardinale EM, Breeden AL, Robertson EL, Lozier LM, VanMeter JW, & Marsh AA (2018). Externalizing behavior severity in youths with callous–unemotional traits corresponds to patterns of amygdala activity and connectivity during judgments of causing fear. Development and Psychopathology, 30, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington SJ, & Bailey AJ (2009). Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping, 30, 2313–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhou J, Liu C, Witt K, Zhang Y, Jing B, … Li L (2015). Regional homogeneity of resting-state brain abnormalities in violent juvenile offenders: A biomarker of brain immaturity? The Journal of Neuropsychiatry and Clinical Neurosciences, 27, 27–32. [DOI] [PubMed] [Google Scholar]
- Cohen JR (2018). The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. NeuroImage, 180, 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI clustering in AFNI: False-positive rates redux. Brain Connectivity, 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalwani MS, Tregellas JR, Andrews-Hanna JR, Mikulich-Gilbertson SK, Raymond KM, Banich MT, … Sakai JT (2014). Default mode network activity in male adolescents with conduct and substance use disorder. Drug and Alcohol Dependence, 134, 242–250. doi: 10.1016/j.drugalcdep.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35, 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawel A, O’Kearney R, McKone E, & Palermo R (2012). Not just fear and sadness: Meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neuroscience & Biobehavioral Reviews, 36, 2288–2304. [DOI] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski C, & Kiehl KA (2013). An fMRI study of affective perspective taking in individuals with psychopathy: Imagining another in pain does not evoke empathy. Frontiers in Human Neuroscience, 7, Article 489. doi: 10.3389/fnhum.2013.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano M, Tamietto M, Celeghin A, Weiskrantz L, Tatu MK, Bagnis A, … Costa T (2017). Dynamic changes in amygdala psychophysiological connectivity reveal distinct neural networks for facial expressions of basic emotions. Scientific Reports, 7, Article 45260. doi: 10.1038/srep45260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotterer HL, Hyde LW, Swartz JR, Hariri AR, & Williamson DE (2017). Amygdala reactivity predicts adolescent antisocial behavior but not callous-unemotional traits. Developmental Cognitive Neuroscience, 24, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DS, Huizinga D, & Ageton SS (1985). Explaining delinquency and drug use. Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- Ewbank MP, Passamonti L, Hagan CC, Goodyer IM, Calder AJ, & Fairchild G (2018). Psychopathic traits influence amygdala–anterior cingulate cortex connectivity during facial emotion processing. Social Cognitive and Affective Neuroscience, 13, 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Passamonti L, Walsh ND, Goodyer IM, & Calder AJ (2014). Atypical neural responses during face processing in female adolescents with conduct disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 53, 677–687.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, Majestic C, Evangelou I, Gupta K, … Fowler K (2012). Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Research: Neuroimaging, 202, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ (2016). Clinical assessment of prosocial emotions, version 1.1 (CAPE 1.1). Unpublished manuscript, Department of Psychology, Louisiana State University, Baton Rouge, Louisiana. [Google Scholar]
- Frick PJ, Bodin SD, & Barry CT (2000). Psychopathic traits and conduct problems in community and clinic-referred samples of children: Further development of the psychopathy screening device. Psychological Assessment, 12, 382–393. [PubMed] [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, & Kahn RE (2014). Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychological Bulletin, 140, 1–57. [DOI] [PubMed] [Google Scholar]
- Goetschius LG, Hein TC, Mattson WI, Lopez-Duran N, Dotterer HL, Welsh RC, … Monk CS (2019). Amygdala-prefrontal cortex white matter tracts are widespread, variable and implicated in amygdala modulation in adolescents. NeuroImage, 191, 278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlich M, Ye Z, Rodriguez-Fornells A, Münte TF, & Krämer UM (2017). Viewing socio-affective stimuli increases connectivity within an extended default mode network. NeuroImage, 148, 8–19. [DOI] [PubMed] [Google Scholar]
- Hamilton RK, Hiatt Racer K, & Newman JP (2015). Impaired integration in psychopathy: A unified theory of psychopathic dysfunction. Psychological Review, 122, 770–791. [DOI] [PubMed] [Google Scholar]
- Han T, Alders GL, Greening SG, Neufeld RWJ, & Mitchell DGV (2012). Do fearful eyes activate empathy-related brain regions in individuals with callous traits? Social Cognitive and Affective Neuroscience, 7, 958–968. doi: 10.1093/scan/nsr068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, Mattson WI, Dotterer HL, Mitchell C, Lopez-Duran N, Thomason ME, … Monk CS (2018). Amygdala habituation and uncinate fasciculus connectivity in adolescence: A multi-modal approach. NeuroImage, 183, 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, … Herpertz-Dahlmann B (2008). Emotional processing in male adolescents with childhood-onset conduct disorder. Journal of Child Psychology and Psychiatry, 49, 781–791. [DOI] [PubMed] [Google Scholar]
- Hwang S, Nolan ZT, White SF, Williams WC, Sinclair S, & Blair RJR (2016). Dual neurocircuitry dysfunctions in disruptive behavior disorders: Emotional responding and response inhibition. Psychological Medicine, 46, 1485–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Byrd AL, Votruba-Drzal E, Hariri AR, & Manuck SB (2014). Amygdala reactivity and negative emotionality: Divergent correlates of antisocial personality and psychopathy traits in a community sample. Journal of Abnormal Psychology, 123, 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, & Hariri AR (2013). Understanding youth antisocial behavior using neuroscience through a developmental psychopathology lens: Review, integration, and directions for research. Developmental Review, 33, 168–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Murray L, Gard A, Hariri AR, & Forbes EE (2016). Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young, low-income, urban men. Clinical Psychological Science, 4, 527–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantolino ZP, Luking KR, Sauder CL, Curtin JJ, & Hajcak G (2018). Robust is not necessarily reliable: From within-subjects fMRI contrasts to between-subjects comparisons. NeuroImage, 173, 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Pechaud M, & Smith S (2005, June 12–16). BET2: MR-based estimation of brain, skull and scalp surfaces. Paper presented at the 11th annual meeting of the Organization for Human Brain Mapping. Toronto, Ontario, Canada. [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, & Viding E (2009). Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry, 166, 95–102. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Larson CL, Baskin-Sommers AR, Stout DM, Balderston NL, Curtin JJ, Schultz DH, … Newman JP (2013). The interplay of attention and emotion: Top-down attention modulates amygdala activation in psychopathy. Cognitive, Affective, & Behavioral Neuroscience, 13, 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184. [DOI] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, & Marsh AA (2014). Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry, 71, 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, & Buonocore MH (2003). Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping, 18, 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, & Burdette JH (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21, 450–455. [DOI] [PubMed] [Google Scholar]
- Marsh AA, & Blair RJR (2008). Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neuroscience & Biobehavioral Reviews, 32, 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Jurkowitz IT, Schechter JC, Henry HY, … Blair RJR (2011). Reduced amygdala–orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research: Neuroimaging, 194, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, … Blair RJR (2008). Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry, 165, 712–720. doi: 10.1176/appi.ajp.2007.07071145 [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61, 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, & Koenigs M (2011). Reduced prefrontal connectivity in psychopathy. The Journal of Neuroscience, 31, 17348–17357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, & Koenigs M (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry, 77, 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2014). Mplus Version 7.3 [Software]. Los Angeles, CA: Author. [Google Scholar]
- Muthén LK, & Muthén BO (2017). Mplus user’s guide (8th ed.). Los Angeles, CA: Author. [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, & Johansen-Berg H (2012). Tools of the trade: Psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience, 7, 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, & Calder AJ (2010). Neural abnormalities in early-onset and adolescence-onset conduct disorder. Archives of General Psychiatry, 67, 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17, 117–133. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Pujara MS, Motzkin JC, Newman J, Kiehl KA, & Koenigs M (2015). Altered resting-state functional connectivity in cortical networks in psychopathy. The Journal of Neuroscience, 35, 6068–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31, 437–448. [Google Scholar]
- Pu W, Luo Q, Jiang Y, Gao Y, Ming Q, & Yao S (2017). Alterations of brain functional architecture associated with psychopathic traits in male adolescents with conduct disorder. Scientific Reports, 7(1), 11349. doi: 10.1038/s41598-017-11775-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman NE, Teitler JO, Garfinkel I, & McLanahan SS (2001). Fragile families: Sample and design. Children and Youth Services Review, 23, 303–326. [Google Scholar]
- Rivenbark JG, Odgers CL, Caspi A, Harrington H, Hogan S, Houts RM, … Moffitt TE (2018). The high societal costs of childhood conduct problems: Evidence from administrative records up to age 38 in a longitudinal birth cohort. Journal of Child Psychology and Psychiatry, 59, 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seara-Cardoso A, Sebastian CL, Viding E, & Roiser JP (2016). Affective resonance in response to others’ emotional faces varies with affective ratings and psychopathic traits in amygdala and anterior insula. Social Neuroscience, 11, 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJ, Cecil CA, Lockwood PL, De Brito SA, Fontaine NM, & Viding E (2012). Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Archives of General Psychiatry, 69, 814–822. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJ, Dadds MR, Cecil CA, Lockwood PL, Hyde ZH, … Viding E (2014). Neural responses to fearful eyes in children with conduct problems and varying levels of callous-unemotional traits. Psychological Medicine, 44, 99–109. doi: 10.1017/S0033291713000482 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó E, Kocsel N, Edes A, Pap D, Galambos A, Zsombok T, … Juhász G (2017). Callous-unemotional traits and neural responses to emotional faces in a community sample of young adults. Personality and Individual Differences, 111, 312–317. [Google Scholar]
- Tamamiya Y, & Hiraki K (2013). Individual differences in the recognition of facial expressions: An event-related potentials study. PLOS ONE, 8(2), Article e57325. doi: 10.1371/journal.pone.0057325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S, & Kiehl KA (2017). Functional connectivity in incarcerated male adolescents with psychopathic traits. Psychiatry Research: Neuroimaging, 265, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, & Spencer WA (1966). Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review, 73(1), 16–43. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16, 55–61. [DOI] [PubMed] [Google Scholar]
- Viding E, & McCrory E (2018). Understanding the development of psychopathy: Progress and challenges. Psychological Medicine, 48, 566–577. [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, & McCrory EJ (2012). Amygdala response to preattentive masked fear in children with conduct problems: The role of callous-unemotional traits. American Journal of Psychiatry, 169, 1109–1116. [DOI] [PubMed] [Google Scholar]
- Waller R, Gard AM, Shaw DS, Forbes EE, Neumann CS, & Hyde LW (2018). Weakened functional connectivity between the amygdala and the ventromedial prefrontal cortex is longitudinally related to psychopathic traits in low-income males during early adulthood. Clinical Psychological Science, 7, 628–635. doi: 10.1177/2167702618810231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, … Blair RJR (2012). Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: Decreased emotional response versus increased top-down attention to non-emotional features. American Journal of Psychiatry, 169, 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KJ, Lahey BB, & Decety J (2016). Callous traits in children with and without conduct problems predict reduced connectivity when viewing harm to others. Scientific Reports, 6, Article 20216. doi: 10.1038/srep20216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K-H, & Bentler PM (2000). Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociological Methodology, 30, 165–200. [Google Scholar]
- Zhou J, Yao N, Fairchild G, Cao X, Zhang Y, Xiang Y-T, … Wang X (2016). Disrupted default mode network connectivity in male adolescents with conduct disorder. Brain Imaging and Behavior, 10, 995–1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


