Abstract
Difficulty processing sensory information may impede progress in school for students with autism spectrum disorder (ASD). We explore the relationship between sensory processing and school performance in 26 high-functioning youths with ASD and 26 controls (age 8–14) using measures of sensory, social, cognitive, and academic functioning. In the ASD group, bivariate Pearson correlations indicated a significant positive relationship between intelligence quotient (IQ) and the School Competence Scale (SCS) of the Child Behavior Checklist (CBCL), and a significant negative relationship between Dunn’s Sensory Processing Framework and SCS scores. Final hierarchical multiple linear regression model accounting for SCS scores in ASD included IQ, ADHD symptoms, and sensory features. An interaction between increased sensory sensitivity with reduced sensory avoidance behaviors explained the greatest amount of variance in SCS, meaning school performance is lowest for children with greater hypersensitivity and fewer avoidance behaviors. Results indicate a strong impact of sensory processing on school performance in ASD.
Autism spectrum disorder (ASD) is a condition defined by clinically significant impairments in social communication, social interaction, and restricted or repetitive interests and behaviors (American Psychiatric Association, 2013). Although social deficits are the core feature of ASD, sensory processing impairments are common in this population as well (Baum, Stevenson, & Wallace, 2015). Atypical sensory responses recently became part of the diagnostic criteria for ASD (American Psychiatric Association, 2013). While increasing numbers of children with ASD are educated in inclusive classroom settings (Snyder & Dillow, 2012), difficulty in social, cognitive, and sensory domains can interfere with student progress (Ashburner, Ziviani, & Rodger, 2008; Keen, Webster, & Ridley, 2016; Roley, Julie Bissell, & Clark, 2009; Whitby & Mancil, 2009). However, school-based interventions for ASD generally have low effectiveness (Bellini, Peters, Benner, & Hopf, 2007). Despite participation in mainstream classrooms, and having intelligence quotient (IQ) within normal range, some children with ASD still do not perform at the same level as their peers. In contrast, other children with ASD excel in mainstream schools (Keen et al., 2016; Whitby & Mancil, 2009). Understanding the factors that contribute to the successful inclusion of ASD students in general education is important to improving integration outcomes. Given the sensory demands in school settings (i.e., fluorescent lighting, loud classrooms, cafeteria smells), we focus on assessing the influence of sensory atypicalities on school performance in a sample of youths in mainstream education who are either typically developing (TD) or have ASD.
Background: Sensory Atypicalities in ASD
Sensory differences are reported by a majority of individuals with ASD (45%–95%; Ben-Sasson, Gal, Fluss, Katz-Zetler, & Cermak, 2019; Marco, Hinkley, Hill, & Nagarajan, 2011; Tomchek & Dunn, 2007) and occur at perceptual, neural, and behavioral levels (see Baum et al., 2015 and Harrison, Kats, Williams, & Aziz-Zadeh, 2019 for review). Sensory alterations have been observed in gustatory, olfactory, auditory, visual, tactile, somatosensory and proprioceptive domains, and persist across the lifespan (Baranek, David, Poe, Stone, & Watson, 2006; Dawson & Watling, 2000; Kern et al., 2007; O’Neill & Jones, 1997; Rogers, Hepburn, & Wehner, 2003; Schauder & Bennetto, 2016; Watling, Deitz, & White, 2001). Even when unisensory functioning is intact in individuals with ASD, impairments in multisensory integration are observed (Foxe et al., 2015; Stevenson et al., 2014, 2014; Stevenson, Segers, Ferber, Barense, & Wallace, 2014).
Sensory abnormalities are categorized as hyper- and hyporesponsivity, resulting from difficulty modulating sensory information (Baranek, 2002; O’Neill & Jones, 1997). Hyper-responsivity is an exaggerated experience of sensory information, which may manifest as avoidance of certain lights, sounds, and touch. Hyporesponsivity refers to a lack of, or attenuated experience of sensory stimuli, which may result in diminished behavioral responses to sensations such as sound, pain, or touch (Baranek, 2002; Little, Dean, Tomchek, & Dunn, 2017). Numerous models have attempted to define subcategories of sensory processing patterns (e.g., Ausderau et al., 2014; Dunn, 2014; Lane, Molloy, & Bishop, 2014; Tomchek, Little, Myers, & Dunn, 2018).
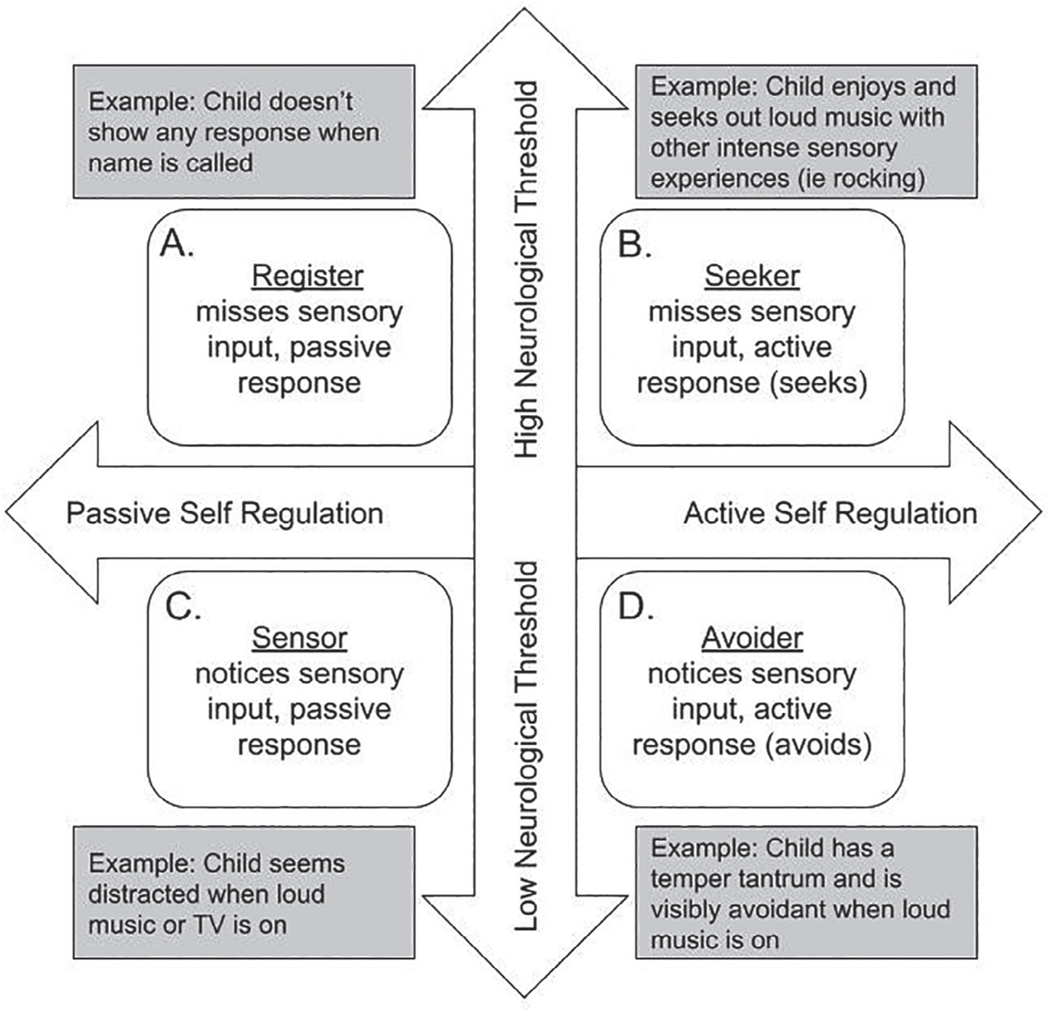
Dunn’s Sensory Processing Framework (Dunn, 2014) divides sensory processing along two continua and describes four unique subtypes of sensory processing. The first continuum—“neurological threshold”—describes an individual’s ability to detect sensory information, and the second continuum—“self-regulation”—refers to the way an individual responds to that stimuli. The threshold ranges from low (quick) to high (slow) detection of sensory information, where a low-threshold individual would be considered hypersensitive, and a high-threshold individual would be considered hyposensitive. The second continuum, self-regulation, ranges from passive to active responses in regulating sensory information; a passive self-regulation strategy would allow sensory stimuli to happen without interference, whereas an active self-regulation strategy would include engaging in behaviors to manage sensory input (e.g., covering one’s ears to prevent noise, or performing stimming behaviors for sensory input). The interactions of these continua result in four patterns of sensory processing labeled as: register, seeker, sensor, and avoider (Figure 1). The register pattern individual misses sensory input, the seeker pattern individual seeks sensory input, the sensor pattern individual intensely notices sensory input, and the avoider pattern individual moves away from sensory input (Figure 1).
Fig. 1.
Overview of Dunn’s sensory processing framework. Note. The figure, modified (from Dunn, 2014) describes four subtypes of sensory functioning (Register, Seeker, Sensor, and Avoider) which fall at a unique point between two spectrums of neurological threshold and self-regulation.
Differences in sensory processing in ASD have been related to negative mental health outcomes, social and cognitive symptoms, maladaptive behavior (Ausderau et al., 2014; Cascio, Woynaroski, Baranek, & Wallace, 2016; Foss-Feig, Heacock, & Cascio, 2012; Reynolds, Bendixen, Lawrence, & Lane, 2011; Suarez, 2012), participation in family routines (Hochhauser & Engel-Yeger, 2010; Jasmin et al., 2009; Marquenie, Rodger, Mangohig, & Cronin, 2011; Nadon, Feldman, Dunn, & Gisel, 2011; Reynolds et al., 2011), and engagement in community activities (Bagby, Dickie, & Baranek, 2012). Adolescents with ASD experience challenges in processing verbal and visual information simultaneously, reorienting attention from one stimulus to another (Ashburner et al., 2008; Fleury et al., 2014), discrimination of motion, and with complex visual and auditory processing (Ashburner et al., 2008; Baum et al., 2015; van der Kruk et al., 2017). A range of stimuli processing, including noisy and visually complex environments found in the classroom, may negatively influence academic performance.
Academic Performance and Classroom Interventions
Since the passing of the Individuals with Disabilities Education Act (IDEA) in 1975, and the amendment in 2004, children with ASD frequently are “mainstreamed” into general education classrooms (McFarland et al., 2018). As of 2016, 40% of students with ASD receiving special education services spent a majority of their time in regular education classrooms (McFarland et al., 2018). While children with ASD do participate in inclusive classrooms, their academic performance often is not measured with standardized assessments in the same way TD children are, leaving gaps in what is known about their academic achievement (Fleury et al., 2014; Keen et al., 2016). Both students with ASD and their parents report negative academic experiences related to the achievement level and the quality of education programs, suggesting that additional support within the classroom is needed (Keen et al., 2016).
Many of the current interventions for adolescents with ASD focus on social skills, behavior, and only recently academic performance (Ashburner et al., 2008; Cotugno, 2009; Machalicek et al., 2008; van der Kruk et al., 2017; Wong et al., 2015). Evidence-based practices for children with ASD demonstrate some efficacy for improving academic performance including use of social narratives, video modeling, visual supports, priming, prompting, and reinforcement (Delano, 2007; Fleury et al., 2014; Koegel, Koegel, Frea, & Green-Hopkins, 2003; Schaefer Whitby, 2013; Wong et al., 2015). While there are some interventions that may marginally improve academic performance in students with ASD, studies have varying quality in experimental protocols and limited generalizability (Machalicek et al., 2008). Given the lack of investigation on school performance in ASD, mixed results of classroom intervention, and the profound influence of sensory issues on functioning, understanding the relationship between sensory processing and academic performance are a vital next step for the development of efficacious interventions.
Despite the prevalence of sensory differences in ASD and the potential impact on school outcomes, to our knowledge, only one study to date has assessed the impact of sensory processing patterns on school performance in ASD (Ashburner et al., 2008). In an Australian sample of children with ASD ages 6–10 years who participated in regular education classrooms, academic performance (as measured by the ASEBA Teacher Report Form subscale; Achenbach & Rescorla, 2001) was reduced in children with sensory processing impairments, while intelligence (measured by the K-BIT; Kaufman & Kaufman, 1990) did not significantly predict academic performance (Ashburner et al., 2008). The purpose of the present study is to examine the relationship between sensory differences and school performance with a sample of American children.
METHODS
Participants
Participants were age 8–14 years old, and included 26 TD individuals (mean age 11.24 years, five female) and 26 high-functioning participants with ASD (mean age 11.65 years, five female). Participants were recruited through public and private schools, social media advertisements, parent groups, community centers, word of mouth, therapy clinics, and public flyer postings Participants and their legal guardians were evaluated for their capacity to give informed consent in accordance with the study protocols approved by the University of Southern California Institutional Review Board. Inclusion criteria included: age 8–14 years old, English speaking, right-handed, parent-reported classroom placement at grade level, born after 36 weeks of gestation, and magnetic resonance imaging safety compatibility, as this was part of a larger motor focused brain imaging study. In order to investigate a population without an intellectual disability placed in general education classrooms, eligibility included a full-scale IQ (FSIQ-4) score greater than 80 on the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II; Wechsler, 2011). All ASD participants had a previous diagnosis through a clinical ASD diagnostic interview, an ASD diagnostic assessment, or both. Current clinical symptoms were confirmed using the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord, Rutter, & Bishop, 2012) and/or the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994). Participants with ASD were fully verbal and participating in classrooms for their age appropriate grade level. TD participants were excluded if they had any psychological diagnosis or neurological disorder, or if they scored below the 25th percentile on the Movement Assessment Battery for Children Second Edition (MABC-2; Schulz, Henderson, Sugden, & Barnett, 2011). Additional exclusion criteria included having an immediate family member with a diagnosis of ASD, or a Social Responsiveness Scale-2 (SRS-2; Constantino & Gruber, 2012) score that indicated a risk of ASD. ASD participants were excluded if they had an additional diagnosis of neurological or psychological disorder, except for attention deficit disorder (ADHD), Developmental Coordination Disorder (DCD), or generalized anxiety disorder—which are highly comorbid with ASD (Mazzone, Ruta, & Reale, 2012).
Assessments
IQ: Wechsler Abbreviated Scale of Intelligence (WASI-II)
The WASI-II (Wechsler, 2011) was administered by a trained research staff member. The WASI-II is a measure of intelligence normed for ages 6–90 and contains four subtests including Block Design, Vocabulary, Matrix Reasoning, and Similarities. Verbal Comprehension Index (VCI) consists of Vocabulary and Similarities subtests and Perceptual Reasoning Index (PRI) consists of Block Design and Matrix reasoning subtests.
School Performance: Child Behavior Checklist (CBCL)
The SCS score from the CBCL is a normalized summative score of mean performance based on 10–13 items (depending on responses) probing attendance, performance in classroom subjects, receipt of classroom services, grade repetition, and academic and behavioral problems (Achenbach & Rescorla, 2001). This measure was used as the primary outcome measure for school performance. The CBCL is standardized for ages 6–18 years and can be completed by the caregiver. On the SCS subscale T-scores between 65 and 69 are considered borderline, and T-scores over 70 fall in the clinical range. In two samples of children (n = 30 and n = 78) with high functioning ASD, the mean total scores were 69.5 (SD = 8.11) and 66.85 (SD = 7.36) while the mean total score for TD children (n = 67) was 42.33 (SD = 10.20) (Mazefsky, Anderson, Conner, & Minshew, 2011).
Social Functioning: Social Responsiveness Scale, 2nd Edition (SRS-2)
The Social Responsiveness Scale, 2nd Edition (SRS-2; Constantino & Gruber, 2012) is a rating scale that has been designed to be used as both a screener and as an aid to clinical diagnosis, for ASD. This study used the School-Aged form for ages 4–18 and was completed by the parent. The content of the School-Aged form probes core diagnostic symptoms of ASD including social communication, restrictive interests, and repetitive behaviors and deficits in reciprocal social interactions. SRS-2 total T-scores between 60 and 65 are considered mild, scores between 66 and 75 are considered moderate, and scores over 75 are considered severe.
ADHD Screener: Conners 3rd Edition (Conners 3AI)
The Conners 3 ADHD Index (Conners 3AI; Conners, 2008), is a parent measure for children ages 6 through 18 years and consists of 10 items from the larger Conners scale that best differentiate children with and without ADHD. It is age normed and used to identify if an evaluation of ADHD might be necessary. A T-score below 60 is considered an average score and clinically elevated scores range from 65–90.
Sensory Processing: Short Sensory Profile-2 (SSP-2)
The Short Sensory Profile 2 (SSP-2; Dunn, 2014) is a questionnaire designed to assess a child’s sensory processing patterns including Seeking, Sensitivity, Registering, and Avoiding. The measure is for caregivers of children ages 3–14 years and includes 34 items demonstrating the highest discriminative power of abnormal sensory processing from the items on the Sensory Profile-2. The measure is not scored for individual age categories. A raw score falling one standard deviation outside of the mean typical range indicates potential clinical relevance. The number of items and for each quadrant is as follows: Sensor (10 items); Seeker (7 items), Avoider (9 items), and Register (8 items).
Analysis
Group differences between TD and ASD groups were assessed using two-tailed independent sample t-tests. Pearson correlation coefficients were computed to assess relationships between all assessments in the TD and ASD groups. Due to lack of variance in TD sensory scores, and a specific interest in the impact of sensory functioning on school performance in ASD, further regression analysis was carried out in the ASD group only. For regression analysis, interaction terms were computed for all behavioral predictors with a Pearson correlation coefficient (r) of .3 or greater. In order to probe the impact of sensory data on school outcome scores, nuisance regressors of age, IQ, and ADHD symptoms were entered into the model before the sensory quadrant scores. Gender was not included as a nuisance regressor due to no significant differences between gender on any variables in the model. Data were centered and behavioral variables were entered into a hierarchical multiple linear regression model in the following order: Age, WASI-II: VCI, Conners 3AI, SSP-2 seeker, SSP-2 sensor, SSP-2 avoider, SSP-2 register, seeker*sensor, seeker*register, seeker*avoider, sensor*avoider, sensor*register, register*avoider, conners*seeker, and conners*sensor. After running this model, interaction terms that did not reach a significance level of p < .1, and did not change the explained variance (R2) of the model by >.01, were removed. In our choice of inclusion, we were more concerned about Type II error over Type I. The final model included: Age, WASI-II: VCI, Conners 3AI, SSP-2 seeker, SSP-2 sensor, SSP-2 avoider, SSP-2 register, seeker*sensor, seeker*register, seeker*avoider, and sensor*avoider. Homoscedasticity and normality of residuals were assessed, and the variance inflation (VIF) and tolerance statistics were used to assess independent variables for multicollinearity.
RESULTS
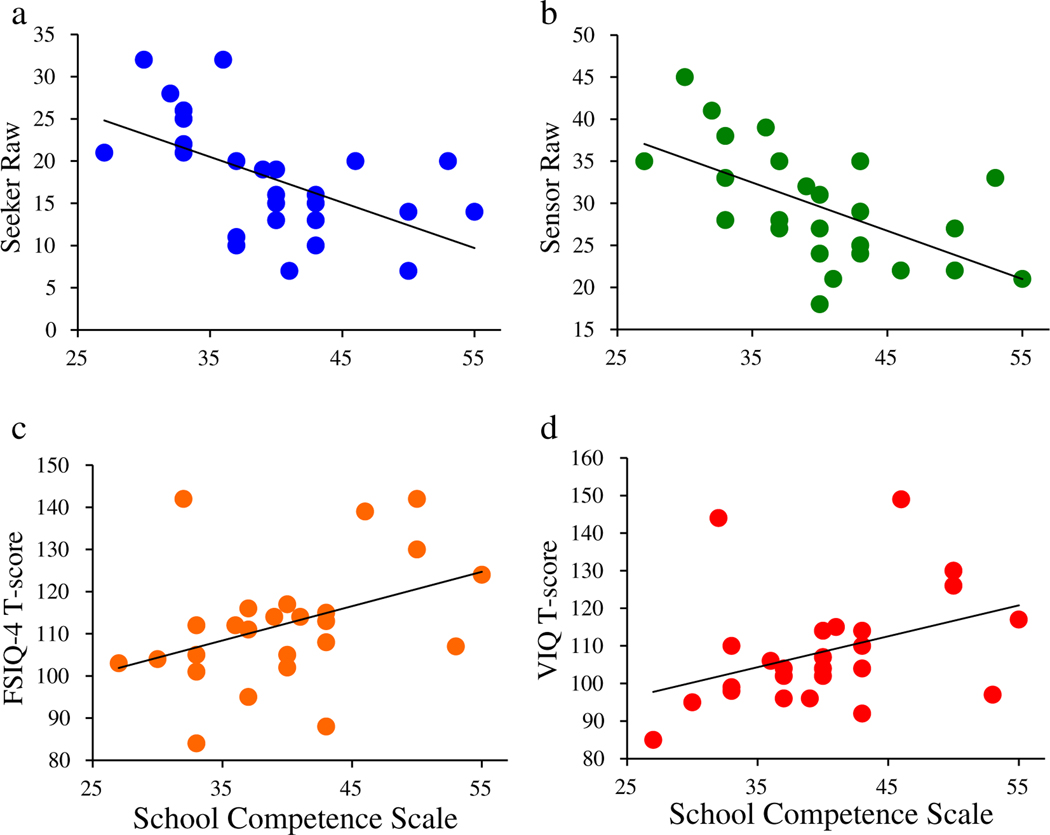
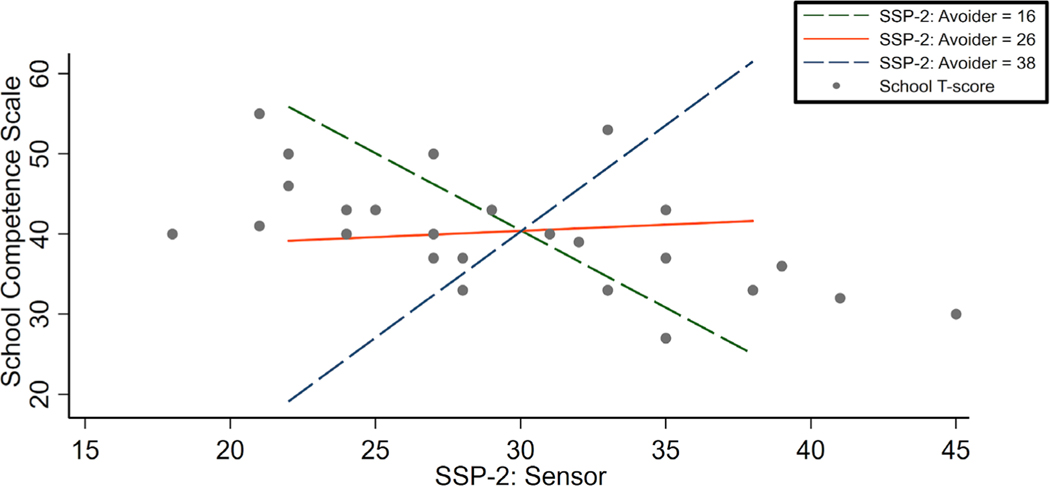
TD and ASD groups did not differ in their WASI-II: FSIQ-4 (p = .204) or WASI-II: PRI (Perceptual Reasoning Index) (p = .769). As Table 1 shows, the TD and ASD groups were significantly different in all other behavioral variables: WASI-II: VCI, CBCL: SCS, SRS-2, the Conners 3AI (Conners, 2008), and the SSP-2 seeker, avoider, sensor, and register subscales. In the TD group, bivariate Pearson correlations indicated no significant relationships between any behavioral variables and the SCS. In the ASD group, bivariate Pearson correlations indicated a positive relationship between FSIQ-4 scores and SCS scores (r = .396, p = .045, Figure 2). More specifically, the VCI approached a significant relationship with SCS (r = .382, p = .054, Figure 2). As for the sensory subtypes, bivariate Pearson correlations revealed significantly negative relationships between SCS scores and two of the four quadrants of sensory functioning; sensory seeker (r = −.553, p = .003), and sensory sensor (r = −.592, p = .001) in the ASD group (Figure 2), such that the greater the severity of sensory sensitivity and seeking, the lower the school performance (Table 2). Hierarchical linear regression models were run to assess explained variance in SCS (Figure 3) in the ASD group only. Model 1 included only nuisance regressors of Age, VCI, and Conners 3AI and accounted for 25% of the variance in mean SCS. In Model 2, sensory features and interaction terms were added to initial nuisance regressors in the following order: Age, VCI, Conners 3AI, SSP-2 seeker, SSP-2 sensor, SSP-2 avoider, SSP-2 register, seeker*sensor, seeker*register, seeker*avoider, sensor*avoider, sensor*register, register*avoider, conners*seeker, and conners*sensor. The addition of sensory quadrants and interactions to initial nuisance regressors to the model accounted for an R2 change of .590. The final hierarchical linear regression model including: Age, VCI, Conners 3AI, SSP-2 seeker, SSP-2 sensor, SSP-2 avoider, SSP-2 register, seeker*sensor, seeker*register, seeker*avoider, and sensor*avoider accounted for 83% of the variance in the mean SCS. The addition of the final interaction term (sensor*avoider) increased the R2 of the model by .286 when accounting for all other variables, indicating an important influence of this term (Table 3). In Figure 3, based on model parameters, we estimated points-to-plot at different levels of SSP-2 Avoider score, allowing us to visualize the changing relationship between the SSP-2 Sensor score and the SCS as SSP-2 Avoider score changed. Figure 3 demonstrates how low neurological threshold for sensory stimuli, and severity of avoidant behaviors interacts with school performance.
Table 1.
Descriptive Statistics and Group Comparisons of Behavioral Data
|
TH |
ASD |
|||||
|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | t | p |
| Age | 11.24 | 1.70 | 11.65 | 1.80 | −.842 | .404 |
| WASI-II: FSIQ-4 | 117.04 | 11.88 | 112.31 | 14.50 | 1.288 | .204 |
| WASI-II: VCI | 117.73 | 10.28 | 108.27 | 15.16 | 2.634 | 012 |
| WASI-II: PRI | 112.00 | 15.41 | 113.31 | 16.51 | −.295 | .769 |
| CBCL: SCS | 53.12 | 2.69 | 39.77 | 7.04 | 9.057 | .000 |
| SRS-2 | 44.23 | 6.46 | 74.08 | 9.12 | −13.617 | .000 |
| Conners 3AI | 46.81 | 6.91 | 84.19 | 9.17 | −16.612 | .000 |
| SSP-2: Seeker | 7.85 | 2.80 | 17.92 | 6.87 | −6.928 | .000 |
| SSP-2: Avoider | 11.31 | 4.19 | 25.73 | 6.69 | −9.314 | .000 |
| SSP-2: Sensor | 12.73 | 4.01 | 29.73 | 6.82 | −10.959 | .000 |
| SSP-2: Register | 9.54 | 3.10 | 18.50 | 5.83 | −6.924 | .000 |
Note. All measures are significantly different between typically developing (TD) and autism spectrum disorder (ASD) groups with the exception of age and full scale IQ; WASI-II: FSIQ-4 = Wechsler Abbreviated Scale of Intelligence, Second Edition: Full Scale IQ; WASI-II: VCI = Wechsler Abbreviated Scale of Intelligence, Second Edition: Verbal Comprehension Index; WASI-II: PRI = Wechsler Abbreviated Scale of Intelligence, Second Edition: Perceptual Reasoning Index; CBCL: SCS = Child Behavior Checklist, School Competence Scale; SRS-2 = Social Responsiveness Scale, Second Edition; Conners 3AI = Conners 3 ADHD Index; SSP-2 = Short Sensory Profile, Second Edition.
Fig. 2.
Behavioral Correlations with School Competence Scale in the autism spectrum disorder (ASD) group. Note. In the ASD group, three measures significantly correlated with the School Competence Scale, and one measure approached significance. (a) the Short Sensory Profile, Seeker Subscale; r = −.553, p = .003; (b) the the Short Sensory Profile, Sensor Subscale; r = −.592, p = .001; (c) the Wechsler Abbreviated Scale of Intelligence, Second Edition, Full Scale IQ score; r = .396, p = .045; (d) the Wechsler Abbreviated Scale of Intelligence, Second Edition, Verbal IQ score; r = .382, p = .054.
Table 2.
Behavioral Data Correlations with School Competence Scale Scores
|
TH |
ASD |
|||
|---|---|---|---|---|
| Variable | r | p | r | p |
| WASI-II: FSIQ-4 | −.192 | .347 | .396 | .045 |
| WASI-II: VCI | .052 | .803 | .382 | .054 |
| WASI-II: PRI | −.336 | .093 | .301 | .136 |
| SRS-2 | −.155 | .450 | −.029 | .888 |
| Conners 3AI | −.312 | .121 | −.340 | .089 |
| SSP-2: Seeker | −.074 | .720 | −.553 | .003 |
| SSP-2: Avoider | −.076 | .712 | −.235 | .248 |
| SSP-2: Sensor | −.088 | .669 | −.592 | .001 |
| SSP-2: Register | .031 | .879 | −.248 | .222 |
Note. Three sensory domains and IQ are significantly correlated with School Competence Scale scores; WASI-II: FSIQ-4 = Wechsler Abbreviated Scale of Intelligence, Second Edition; WASI-II: VCI = Wechsler Abbreviated Scale of Intelligence, Second Edition: Verbal Comprehension Index; WASI-II: PRI = Wechsler Abbreviated Scale of Intelligence, Second Edition: Perceptual Reasoning Index; SRS-2 = Social Responsiveness Scale, Second Edition; Conners 3AI = Conners 3 ADHD Index; SSP-2 = Short Sensory Profile, Second Edition. ASD = autism spectrum disorder; TD = typically developing.
Fig. 3.
Model-based estimates at Selected Points of Sensor and Avoider on School Competence Scale. Note. Based on model parameters, we estimated points-to-plot at several different levels of Short Sensory Profile (SSP): Avoider to visualize the relationship between the Short Sensory Profile-2 (SSP-2) Sensor score and the School Competence Scale as SSP-2 Avoider score changes in the autism spectrum disorder (ASD) group. The green dashed line starts at the minimum score of the avoider subscale, the orange solid line represents the median of the avoider subscale, and the blue dashed line represents the maximum score of the avoider subscale. These lines demonstrate how low neurological threshold for sensory stimuli, and severity of avoidant behaviors interact with school performance. Actual data points are plotted over the prediction lines as gray scatter dots.
Table 3.
Summary of Hierarchical Regression Analysis for School Competence Scale in autism spectrum disorder (ASD)
|
Model 1 |
Model 2 |
Model 3 |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | B | SEB | β | B | SEB | β | B | SEB |
| Age | −0.289 | 0.723 | −0.074 | −1.346 | 0.605 | −0.345 | −1.279 | 0.517 |
| WASI-II: VCI | 0.174 | 0.085 | 0.374 | 0.001 | 0.078 | 0.001 | −0.011 | 0.068 |
| Conners 3AI | −0.244 | 0.142 | −0.318 | −0.264 | 0.202 | −0.343 | −0.280 | 0.136 |
| SSP-2: Seeker | −0.606 | 0.272 | −0.591 | −0.614 | 0.200 | |||
| SSP-2: Sensor | 0.088 | 0.303 | 0.086 | 0.097 | 0.248 | |||
| SSP-2: Avoider | −0.093 | 0.194 | −0.089 | −0.062 | 0.164 | |||
| SSP-2: Register | 0.072 | 0.228 | 0.060 | 0.004 | 0.165 | |||
| Seeker × Sensor | −0.087 | 0.052 | −0.652 | −0.083 | 0.028 | |||
| Seeker × Register | 0.072 | 0.050 | 0.384 | 0.108 | 0.030 | |||
| Seeker × Avoider | −0.083 | 0.059 | −0.613 | −0.116 | 0.037 | |||
| Sensor × Avoider | 0.193 | 0.057 | 1.286 | 0.208 | 0.043 | |||
| Sensor × Register | 0.048 | 0.056 | 0.213 | |||||
| Register × Avoider | −0.015 | 0.054 | −0.077 | |||||
| Conners × Seeker | −0.028 | 0.044 | −0.192 | |||||
| Conners × Sensor | 0.006 | 0.022 | 0.069 | |||||
| R2 | 0.258 | 0.848 | 0.829 | |||||
| Change in R2 | 0.590 | −0.019 | ||||||
Note. WASI-II: VCI = Wechsler Abbreviated Scale of Intelligence, Second Edition: Verbal Comprehension Index; Conners 3AI = Conners 3 ADHD Index; SSP-2 = Short Sensory Profile, Second Edition; Seeker × Sensor = Short Sensory Profile, Second Edition Seeker and Sensor subscale interaction; Seeker × Register = Short Sensory Profile, Second Edition, Seeker and Register subscale interaction; Seeker × Avoider = Short Sensory Profile, Second Edition, Seeker and Avoider subscale interaction; Sensor × Avoider = Short Sensory Profile, Second Edition, Sensor and Avoider subscale interaction; Sensor × Register = Short Sensory Profile, Second Edition, Sensor and Register subscale interaction; Register × Avoider = Short Sensory Profile, Second Edition, Register and Avoider subscale interaction; Conners × Seeker = Conners 2 ADHS Index and Short Sensory Profile, Second Edition Seeker subscale interaction; Conners × Sensor = Conners 2 ADHS Index and Short Sensory Profile, Second Edition Sensor subscale interaction.
DISCUSSION
Here we aimed to better understand how processing of sensory information may impact classroom outcomes in children with ASD. The strongest model to predict school performance scores included IQ, ADHD symptom severity, and interacting sensory sensitivity (neurological threshold) and sensory responsiveness (passive-active self-regulation) features. Of these variables, an interaction between increased sensory sensitivity with reduced sensory avoidance behaviors explained the greatest amount of variance in SCS scores in children and adolescents with ASD suggesting that school performance scores are lowest for children with greater hypersensitivity and fewer self-regulation avoidance behaviors. This interaction of sensory sensitivity and self-regulation avoidance behavior had a strong influence on the model even after controlling for other variables such as age, IQ, ADHD symptoms, and other sensory features of sensitivity and behaviors. These results differ from Ashburner et al. (2008) who found that under responsiveness/seeks sensation and auditory filtering sections of the SSP predicted 47% of the variance in academic performance, while IQ and results of parent-reported ASD severity were not predictors of academic performance. The SSP (McIntosh, Miller, & Shyu, 1999) is longer and provides total scores on specific sensory domain sensitivities (i.e., tactile, taste/smell, visual/auditory) whereas the SSP-2 provides a broader overview of sensory thresholds and regulation across a number of sensory domains. Age range, country of educational system, and ASD criteria for inclusion also differed between the studies. The current study required clinical diagnosis of ASD and the ADOS-2 and ADI-R observational assessments completed by research certified study personnel, whereas Ashburner et al. (2008) used pediatrician diagnoses and caregiver questionnaires. Finally, the current study’s statistical research methods and data analysis differed from Ashburner’s group.
Children in the classroom are constantly exposed to complex auditory and visual stimuli. For a child with ASD and sensory hypersensitivity, having avoidant self-regulation patterns can leave a child in distress, leading to limited engagement or opportunity to process information during classroom activities (Ashburner et al., 2008; Baum et al., 2015; van der Kruk et al., 2017). Our results pose potential opportunities for targeted sensory interventions and classroom adaptation for children with high-functioning ASD and hypersensitivity with passive self-regulation. Previous literature indicates small effect sizes of positive changes in classroom behavior using sensory-based adaptations in school settings (Case-Smith, Weaver, & Fristad, 2015). Taken together, these results suggest a need for designing and testing interventions to address hypersensitivity with passive self-regulation in the classroom. Interventions currently used that have a small effect size could be further investigated for effectiveness based on specific sensory subtypes.
Sensory Interventions
Sensory integration involves the neurological processing of sensory information, including tactile, auditory, visual, vestibular and proprioceptive, and has been described as the foundation of higher-level learning (Ayres, 1972; Baranek, 2002; Lane, 2020; Parham, 1998). A variety of interventions have been utilized to address children’s sensory needs (Baranek, 2002; Case-Smith et al., 2015; Lang et al., 2012; May-Benson & Koomar, 2010; Roley et al., 2009; Watling & Hauer, 2015). Approaches to intervention include (1) “sensory based interventions (SBI),” adapting the sensory environment (e.g., ball seating or changing lighting; Case-Smith et al., 2015 ; Watling & Hauer, 2015); (2) Sensory integration, a child-centered approach that provides appropriate sensory experiences coupled with an adaptive response (e.g., combination of swinging, deep pressure, or riding a scooter board; Ayres, 1972, 1979; Baranek, 2002, Lang et al., 2012; Schoen et al., 2019); (3) “Sensory integration-based,” interventions including elements of sensory integration but do not require an adaptive response, and may be more cognitively focused (e.g., sensory diets or weighted vests; Baranek, 2002; Watling & Hauer, 2015). These interventions may have short-term improvements (Weitlauf, Sathe, McPheeters, & Warren, 2017), however, most have not looked at their effects on academic performance.
The literature on sensory interventions in the classroom is sparse and has a number of limitations. SBI interventions such as wearing weighted vests or bouncing on a therapy ball have been associated with improvement in classroom behaviors in children with ASD (Case-Smith et al., 2015). However, in other studies, SBI either had mixed results in academic tasks, or was found to be less effective than behavioral interventions at decreasing challenging behaviors (Case-Smith et al., 2015; Watling & Hauer, 2015). One study that modified the sensory environment with sound absorbing walls and halogen lighting found an increase in academic performance in students with ASD (Watling & Hauer, 2015). Van der Kruk et al. (2017) reviewed auditory processing interventions in ASD and found improvements in listening, speech recognition, and on task-behaviors in the classroom when a personal microphone amplifying the voice of the teacher (frequency modulation) was used. However, all of the reviewed studies mentioned above had considerable limitations, including small sample sizes (ranged from three to 13 participants), lack of randomization, and varying criteria for ASD diagnosis. Despite these limitations, other examples of sensory interventions outside an ASD sample may provide a basis for effective classroom-based sensory intervention models (Worthen, 2010). With the knowledge that sensory and social impairments are core deficits in ASD, and that sensory impairments may precede deficits in social functioning (Thye, Bednarz, Herringshaw, Sartin, & Kana, 2018), interventions addressing both sensory and social symptoms may improve overall outcomes (Hilton, Graver, & LaVesser, 2007). In fact, improvement has been reported in both social functioning and sensory processing in an occupation-based horseback riding intervention (Pfeiffer, Clark, & Arbesman, 2018). Additionally, therapeutic robots targeting improved social interaction have also been suggested to decrease sensory sensitivity in children with ASD (Sartorato, Przybylowski, & Sarko, 2017). Exploring the integration of sensory interventions with established social interventions may be useful to address ASD symptoms.
Limitations
There are limitations which should be considered when interpreting this study’s findings. First, it is a relatively small sample for a population that is incredibly heterogeneous. Unfortunately, due to a limited sample size, and disproportionate representation of males, we cannot analyze differences in sensory and school performance patterns based on demographic information such as gender or age. Future research is needed with greater female representation as there is literature supporting the supposition that gender differences may exist (Mandy et al., 2012). This study only included participants with ASD who were high-functioning, verbal, and had IQ above 80, therefore, the results can only be generalized to a very specific subset of individuals with ASD. In addition, the main outcome used to assess sensory functioning was a brief parent report, which did not capture teacher or therapist evaluation. It is also important to note that while sensory sensitivities were correlated with school outcomes and the implications of this relationship are discussed, we cannot extend causality of school performance to sensory functioning, and therefore, we can only speculate that sensory intervention may improve performance in the classroom. Future research in larger samples is needed to clarify the nature of these relationships.
CONCLUSIONS
A growing number of children and adolescents with ASD are now participating in general education classrooms. While IQ is considered to be a major factor in academic performance, our findings suggest that sensory processing is also critical to the academic performance of youths with ASD. While some sensory interventions do have positive effects, sensory interventions implemented in the classroom have mixed results. The increase of neurodiversity in inclusive classrooms calls for a better understanding of sensory needs in order to improve academic performance of youths with ASD. While others find that sensory impairments inhibit meaningful engagement in social and employment domains (Keen et al., 2016), our study indicates sensory impairments also negatively influence academic outcomes in children and adolescents with ASD. This research suggests that sensory needs should be considered as factors influencing academic performance. More research is needed to extend these findings in order to examine the best way to address these issues in school settings.
ACKNOWLEDGMENTS—
We would like to express deep gratitude to the families who participated in this research study. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD079432. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflicts of interest to report.
REFERENCES
- Achenbach TM, & Rescorla LA (2001) Manual for the ASEBA school-age forms & profiles. Burlington: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. (5th ed.). Arlington, VA: Author. [Google Scholar]
- Ashburner J, Ziviani J, & Rodger S. (2008). Sensory processing and classroom emotional, behavioral, and educational outcomes in children with autism spectrum disorder. American Journal of Occupational Therapy, 62(5), 564–573. 10.5014/ajot.62.5.564 [DOI] [PubMed] [Google Scholar]
- Ausderau K, Sideris J, Furlong M, Little LM, Bulluck J, & Baranek GT (2014). National survey of sensory features in children with ASD: Factor structure of the sensory experience questionnaire (3.0). Journal of Autism and Developmental Disorders, 44(4), 915–925. 10.1007/s10803-013-1945-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres AJ (1972). Improving academic scores through sensory integration. Journal of Learning Disabilities, 5(6), 338–343. 10.1177/002221947200500605 [DOI] [Google Scholar]
- Ayres AJ (1979). Sensory Integration and the Child. Los Angeles: Western Psychological Services. [Google Scholar]
- Bagby MS, Dickie VA, & Baranek GT (2012). How sensory experiences of children with and without autism affect family occupations. American Journal of Occupational Therapy, 66(1), 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT (2002). Efficacy of sensory and motor interventions for children with autism. Journal of Autism and Developmental Disorders, 32(5), 397–422. 10.1023/A:1020541906063 [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, & Watson LR (2006). Sensory experiences questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, 47(6), 591–601. 10.1111/j.1469-7610.2005.01546.x [DOI] [PubMed] [Google Scholar]
- Baum SH, Stevenson RA, & Wallace MT (2015). Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Progress in Neurobiology, 134, 140–160. 10.1016/j.pneurobio.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini S, Peters JK, Benner L, & Hopf A. (2007). A meta-analysis of school-based social skills interventions for children with autism spectrum disorders. Remedial and Special Education, 28(3), 153–162. 10.1177/07419325070280030401 [DOI] [Google Scholar]
- Ben-Sasson A, Gal E, Fluss R, Katz-Zetler N, & Cermak SA (2019). Update of a meta-analysis of sensory symptoms in ASD: A new decade of research. Journal of Autism and Developmental Disorders, 49(12), 4974–4996. 10.1007/s10803-019-04180-0 [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Woynaroski T, Baranek GT, & Wallace MT (2016). Toward an interdisciplinary approach to understanding sensory function in autism spectrum disorder. Autism Research, 9(9), 920–925. 10.1002/aur.1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case-Smith J, Weaver LL, & Fristad MA (2015). A systematic review of sensory processing interventions for children with autism spectrum disorders. Autism, 19(2), 133–148. 10.1177/1362361313517762 [DOI] [PubMed] [Google Scholar]
- Conners KC (2008) Conners 3rd edition manual. New York, NY: Multi-Health Systems. [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social responsiveness scale: SRS-2. Torrance, CA: Western Psychological Services. [Google Scholar]
- Cotugno AJ (2009). Social competence and social skills training and intervention for children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(9), 1268–1277. 10.1007/s10803-009-0741-4 [DOI] [PubMed] [Google Scholar]
- Dawson G, & Watling R. (2000). Interventions to facilitate auditory, visual, and motor integration in autism: A review of the evidence. Journal of Autism and Developmental Disorders, 30(5), 415–421. 10.1023/A:1005547422749 [DOI] [PubMed] [Google Scholar]
- Delano ME (2007). Video modeling interventions for individuals with autism. Remedial and Special Education, 28(1), 33–42. 10.1177/07419325070280010401 [DOI] [Google Scholar]
- Dunn W. (2014) Sensory profile 2. Bloomington, MN: Pearson Psychcorp. [Google Scholar]
- Fleury VP, Hedges S, Hume K, Browder DM, Thompson JL, Fallin K, … Vaughn S. (2014). Addressing the academic needs of adolescents with autism spectrum disorder in secondary education. Remedial and Special Education, 35(2), 68–79. 10.1177/0741932513518823 [DOI] [Google Scholar]
- Foss-Feig JH, Heacock JL, & Cascio CJ (2012). Tactile responsiveness patterns and their association with core features in autism spectrum disorders. Research in Autism Spectrum Disorders, 6(1), 337–344. 10.1016/j.rasd.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Molholm S, Del Bene VA, Frey H-P, Russo NN, Blanco D, … Ross LA (2015). Severe multisensory speech integration deficits in high-functioning school-aged children with autism spectrum disorder (ASD) and their resolution during early adolescence. Cerebral Cortex, 25(2), 298–312. 10.1093/cercor/bht213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LA, Kats A, Williams ME, & Aziz-Zadeh L. (2019). The importance of sensory processing in mental health: A proposed addition to the research domain criteria (RDoC) and suggestions for RDoC 2.0. Frontiers in Psychology, 10, 103. 10.3389/fpsyg.2019.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton C, Graver K, & LaVesser P. (2007). Relationship between social competence and sensory processing in children with high functioning autism spectrum disorders. Research in Autism Spectrum Disorders, 1(2), 164–173. 10.1016/j.rasd.2006.10.002 [DOI] [Google Scholar]
- Hochhauser M, & Engel-Yeger B. (2010). Sensory processing abilities and their relation to participation in leisure activities among children with high-functioning autism spectrum disorder (HFASD). Research in Autism Spectrum Disorders, 4(4), 746–754. 10.1016/j.rasd.2010.01.015 [DOI] [Google Scholar]
- Jasmin E, Couture M, McKinley P, Reid G, Fombonne E, & Gisel E. (2009). Sensori-motor and daily living skills of preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(2), 231–241. 10.1007/s10803-008-0617-z [DOI] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (1990) K–BIT: Kaufman brief intelligence test manual. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Keen D, Webster A, & Ridley G. (2016). How well are children with autism spectrum disorder doing academically at school? An overview of the literature. Autism, 20(3), 276–294. 10.1177/1362361315580962 [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Grannemann BD, Garver CR, Johnson DG, Andrews AA, … Schroeder JL (2007). Sensory correlations in autism. Autism, 11(2), 123–134. 10.1177/1362361307075702 [DOI] [PubMed] [Google Scholar]
- Koegel LK, Koegel RL, Frea W, & Green-Hopkins I. (2003). Priming as a method of coordinating educational services for students with autism. Language, Speech & Hearing Services in Schools, 34(3), 228. 10.1044/0161-1461(2003/019) [DOI] [PubMed] [Google Scholar]
- Lane AE (2020). Practitioner Review: Effective management of functional difficulties associated with sensory symptoms in children and adolescents. [DOI] [PubMed] [Google Scholar]
- Lane AE, Molloy CA, & Bishop SL (2014). Classification of children with autism spectrum disorder by sensory subtype: A case for sensory-based phenotypes. Autism Research, 7(3), 322–333. 10.1002/aur.1368 [DOI] [PubMed] [Google Scholar]
- Lang R, O’Reilly M, Healy O, Rispoli M, Lydon H, Streusand W, … Giesbers S. (2012). Sensory integration therapy for autism spectrum disorders: A systematic review. Research in Autism Spectrum Disorders, 6(3), 1004–1018. 10.1016/j.rasd.2012.01.006 [DOI] [Google Scholar]
- Little LM, Dean E, Tomchek SD, & Dunn W. (2017). Classifying sensory profiles of children in the general population. Child: Care, Health and Development, 43(1), 81–88. 10.1111/cch.12391 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Bishop SL (2012) Autism diagnostic observation schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A. (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- Machalicek W, O’Reilly MF, Beretvas N, Sigafoos J, Lancioni G, Sorrells A, … Rispoli M. (2008). A review of school-based instructional interventions for students with autism spectrum disorders. Research in Autism Spectrum Disorders, 2(3), 395–416. 10.1016/j.rasd.2007.07.001 [DOI] [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, & Skuse D. (2012). Sex differences in autism spectrum disorder: Evidence from a large sample of children and adolescents. Journal of Autism and Developmental Disorders, 42(7), 1304–1313. 10.1007/s10803-011-1356-0 [DOI] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LBN, Hill SS, & Nagarajan SS (2011). Sensory processing in autism: A review of neurophysiologic findings. Pediatric Research, 69, 48R–54R. 10.1203/PDR.0b013e3182130c54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquenie K, Rodger S, Mangohig K, & Cronin A. (2011). Dinnertime and bedtime routines and rituals in families with a young child with an autism spectrum disorder. Australian Occupational Therapy Journal, 58(3), 145–154. 10.1111/j.1440-1630.2010.00896.x [DOI] [PubMed] [Google Scholar]
- May-Benson TA, & Koomar JA (2010). Systematic review of the research evidence examining the effectiveness of interventions using a sensory integrative approach for children. American Journal of Occupational Therapy, 64(3), 403–414. 10.5014/ajot.2010.09071 [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, Anderson R, Conner CM, & Minshew N. (2011). Child behavior checklist scores for school-aged children with autism: Preliminary evidence of patterns suggesting the need for referral. Journal of Psychopathology and Behavioral Assessment, 33(1), 31–37. 10.1007/s10862-010-9198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone L, Ruta L, & Reale L. (2012). Psychiatric comorbidities in asperger syndrome and high functioning autism: Diagnostic challenges. Annals of General Psychiatry, 11(1), 16. 10.1186/1744-859X-11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland J, Hussar B, Wang X, Zhang J, Wang K, Rathbun A, … & Mann FB (2018). The condition of education 2018. NCES 2018–144. National Center for Education Statistics. Retrieved from https://eric.ed.gov/?id=ED583502 [Google Scholar]
- McIntosh DN, Miller LJ, & Shyu V. (1999). Development and validation of the short sensory profile. In Dunn W (Ed.), Sensory profile: User’s manual. (pp. 59–73). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Nadon G, Feldman DE, Dunn W, & Gisel E. (2011). Mealtime problems in children with autism spectrum disorder and their typically developing siblings: A comparison study. Autism, 15(1), 98–113. 10.1177/1362361309348943 [DOI] [PubMed] [Google Scholar]
- O’Neill M, & Jones RSP (1997). Sensory-perceptual abnormalities in autism: A case for more research? Journal of Autism and Developmental Disorders, 27(3), 283–293. 10.1023/A:1025850431170 [DOI] [PubMed] [Google Scholar]
- Parham LD (1998). The relationship of sensory integrative development to achievement in elementary students: Four-year longitudinal patterns. The Occupational Therapy Journal of Research, 18(3), 105–127. 10.1177/153944929801800304 [DOI] [Google Scholar]
- Pfeiffer B, Clark GF, & Arbesman M. (2018). Effectiveness of cognitive and occupation-based interventions for children with challenges in sensory processing and integration: A systematic review. American Journal of Occupational Therapy, 72(1), 7201190020p1–7201190020p9. 10.5014/ajot.2018.028233 [DOI] [PubMed] [Google Scholar]
- Reynolds S, Bendixen RM, Lawrence T, & Lane SJ (2011). A pilot study examining activity participation, sensory responsiveness, and competence in children with high functioning autism spectrum disorder. Journal of Autism and Developmental Disorders, 41(11), 1496–1506. 10.1007/s10803-010-1173-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, & Wehner E. (2003). Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders, 33(6), 631–642. 10.1023/B:JADD.0000006000.38991.a7 [DOI] [PubMed] [Google Scholar]
- Roley SS, Julie Bissell MA, & Clark GF (2009). Providing occupational therapy using sensory integration theory and methods in school-based practice. American Journal of Occupational Therapy, 63(6), 823–842. 10.5014/ajot.63.6.823 [DOI] [PubMed] [Google Scholar]
- Sartorato F, Przybylowski L, & Sarko DK (2017). Improving therapeutic outcomes in autism spectrum disorders: Enhancing social communication and sensory processing through the use of interactive robots. Journal of Psychiatric Research, 90, 1–11. 10.1016/j.jpsychires.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Schaefer Whitby PJ (2013). The effects of solve it! On the mathematical word problem solving ability of adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities, 28(2), 78–88. 10.1177/1088357612468764 [DOI] [Google Scholar]
- Schauder KB, & Bennetto L. (2016). Toward an interdisciplinary understanding of sensory dysfunction in autism spectrum disorder: An integration of the neural and symptom literatures. Frontiers in Neuroscience, 10, 1–18. 10.3389/fnins.2016.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen SA, Lane SJ, Mailloux Z, May-Benson T, Parham LD, Smith Roley S, & Schaaf RC (2019). A systematic review of Ayres sensory integration intervention for children with autism. Autism Research, 12(1), 6–19. 10.1002/aur.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J, Henderson SE, Sugden DA, & Barnett AL (2011). Structural validity of the movement ABC-2 test: Factor structure comparisons across three age groups. Research in Developmental Disabilities, 32(4), 1361–1369. 10.1016/j.ridd.2011.01.032 [DOI] [PubMed] [Google Scholar]
- Snyder TD, & Dillow SA (2012). Digest of education statistics, 2011. NCES 2012–001. Retrieved from http://eric.ed.gov/?id=ED544580
- Stevenson RA, Segers M, Ferber S, Barense MD, & Wallace MT (2014). The impact of multisensory integration deficits on speech perception in children with autism spectrum disorders. Frontiers in Psychology, 5, 1–3. 10.3389/fpsyg.2014.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Schneider BC, Eberly HE, Woynaroski TG, Camarata SM, & Wallace MT (2014). Multisensory temporal integration in autism spectrum disorders. Journal of Neuroscience, 34(3), 691–697. 10.1523/JNEUROSCI.3615-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Woynaroski TG, Schneider BC, Eberly HE, Camarata SM, & Wallace MT (2014). Evidence for diminished multisensory integration in autism spectrum disorders. Journal of Autism and Developmental Disorders, 44(12), 3161–3167. 10.1007/s10803-0142179-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez MA (2012). Sensory processing in children with autism spectrum disorders and impact on functioning. Pediatric Clinics, 59(1), 203–214. 10.1016/j.pcl.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Thye MD, Bednarz HM, Herringshaw AJ, Sartin EB, & Kana RK (2018). The impact of atypical sensory processing on social impairments in autism spectrum disorder. Developmental Cognitive Neuroscience, 29, 151–167. 10.1016/j.dcn.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek SD, & Dunn W. (2007). Sensory processing in children with and without autism: A comparative study using the short sensory profile. American Journal of Occupational Therapy, 61(2), 190–200. 10.5014/ajot.61.2.190 [DOI] [PubMed] [Google Scholar]
- Tomchek SD, Little LM, Myers J, & Dunn W. (2018). Sensory subtypes in preschool aged children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 48(6), 2139–2147. 10.1007/s1080 [DOI] [PubMed] [Google Scholar]
- van der Kruk Y, Wilson WJ, Palghat K, Downing C, Harper-Hill K, & Ashburner J. (2017). Improved signal-to-noise ratio and classroom performance in children with autism spectrum disorder: A systematic review. Review Journal of Autism and Developmental Disorders, 4(3), 243–253. 10.1007/s40489-017-0111-7 [DOI] [Google Scholar]
- Watling R, & Hauer S. (2015). Effectiveness of Ayres sensory integration® and sensory-based interventions for people with autism spectrum disorder: A systematic review. American Journal of Occupational Therapy, 69(5), 6905180030p1–6905180030p12. 10.5014/ajot.2015.018051 [DOI] [PubMed] [Google Scholar]
- Watling RL, Deitz J, & White O. (2001). Comparison of sensory profile scores of young children with and without autism spectrum disorders. American Journal of Occupational Therapy, 55(4), 416–423. 10.5014/ajot.55.4.416 [DOI] [PubMed] [Google Scholar]
- Wechsler D. (2011) Wechsler abbreviated scale of intelligence-(WASI-II). San Antonio, TX: NCS Pearson. [Google Scholar]
- Weitlauf AS, Sathe N, McPheeters ML, & Warren ZE (2017). Interventions targeting sensory challenges in autism spectrum disorder: A systematic review. Pediatrics, 139(6), e20170347. 10.1542/peds.2017-0347 [DOI] [PubMed] [Google Scholar]
- Whitby PJS, & Mancil GR (2009). Academic achievement profiles of children with high functioning autism and asperger syndrome: A review of the literature. Education and Training in Developmental Disabilities, 44(4), 551–560. Retrieved from JSTOR. https://www.jstor.org/stable/24234262?seq=1#page_scan_tab_contents [Google Scholar]
- Wong C, Odom SL, Hume KA, Cox AW, Fettig A, Kucharczyk S, … Schultz TR (2015). Evidence-based practices for children, youth, and young adults with autism spectrum disorder: A comprehensive review. Journal of Autism and Developmental Disorders, 45(7), 1951–1966. 10.1007/s10803-014-2351-z [DOI] [PubMed] [Google Scholar]
- Worthen E. (2010). Sensory-based interventions in the general education classroom: A critical appraisal of the topic. Journal of Occupational Therapy, Schools, & Early Intervention, 3(1), 76–94. 10.1080/19411241003684217 [DOI] [Google Scholar]