Abstract
Excess adipose tissue predisposes to an enhanced inflammatory state and can contribute to the pathogenesis and severity of asthma. Vitamin D has anti-inflammatory properties and low-serum levels are seen in children with asthma and in children with obesity. Here we review the intersection of asthma, obesity, and hypovitaminosis D in children. Supplementation with vitamin D has been proposed as a simple, safe, and inexpensive adjunctive therapy in a number of disease states. However, little research has examined the pharmacokinetics of vitamin D and its therapeutic potential in children who suffer from obesity-related asthma.
Keywords: Asthma, Obesity, Vitamin D, inflammation, pediatric, children
Introduction
Asthma in pediatric patients has multiple phenotypes and many factors contribute to its genesis. Both obesity and vitamin D deficiency have been associated with increased asthma symptoms in children and adults.1,2 This has led to interest in the intersection of asthma, obesity, and hypovitaminosis D, which may be of particular importance in children. [Figure 1] Low levels of serum vitamin D (25(OH)D) have been reported in children who have asthma and in children who are obese, making children who have both asthma and obesity particularly at risk for low vitamin D levels.3,4 It is hypothesized that supplementation with vitamin D could be beneficial in this patient population. A Cochrane review5 as noted that the dose of vitamin D to use in supplementation for asthma (and therefore obesity-related asthma) is still uncertain. This review highlights the association between asthma and obesity in children, the anti-inflammatory effects of vitamin D, and the therapeutic potential of vitamin D in children with obesity-related asthma.
Figure 1:
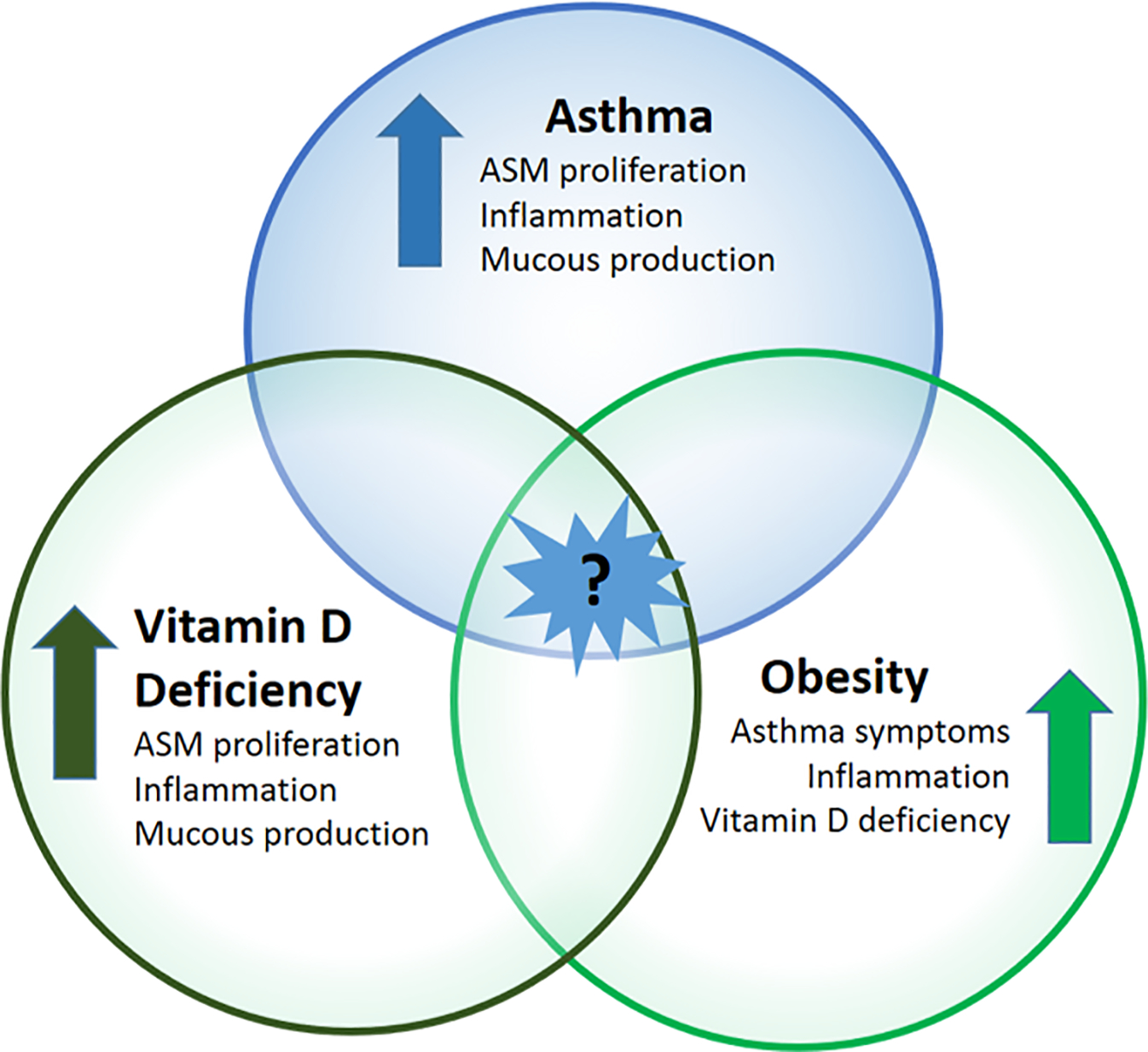
The intersection of asthma, obesity, and vitamin D deficiency in pediatric populations is not well known.
Vitamin D and Inflammation
Vitamin D is more accurately classified as a lipophilic hormone that has profound effects on a multitude of bodily functions beyond bone health than as a vitamin, per se.6 However, Vitamin D insufficiency and deficiency are generally defined in terms of calcium metabolism and bone health. The Endocrine Society defines vitamin D deficiency as 25(OH)D levels less than 20 ng/ml and vitamin D insufficiency as levels of 21 to 29 ng/ml.7 The optimal 25(OH)D levels to maximize Vitamin D’s other properties, such as regulating inflammation, are still being studied. Vitamin D is thought to reduce inflammation via multiple mechanisms, including inhibiting the activation of NF-κB signaling, and inhibiting p38 MAP kinase phosphorylation and activities of macrophages, B cells, T cells, neutrophils, dendritic cells, and mast cells (Figure 2).8,9 Vitamin D’s pluripotent effects on immune cells may explain the association between vitamin D deficiency and poor asthma control. Notably, low-25(OH)D levels have been associated with increased markers of oxidative stress and inflammation in children.10
Figure 2:
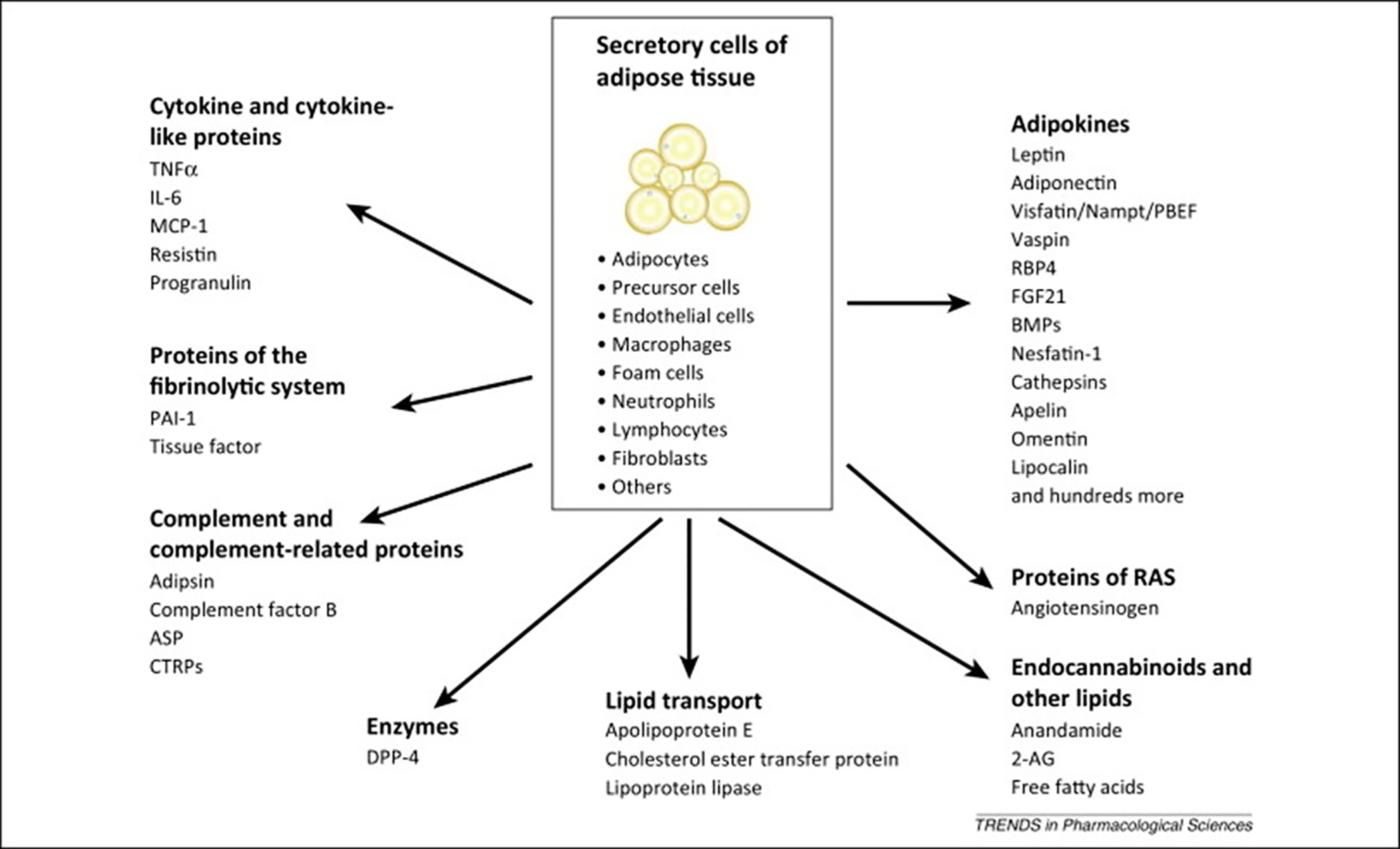
Factors released or secreted by adipose tissue.54
Vitamin D and Asthma
Epidemiologic studies have reported low levels of serum 25(OH)D in children with difficult to treat asthma irrespective of body weight. A nationwide study using NHANES data reported a positive correlation between vitamin D insufficiency defined as serum 25(OH)D level less than 30 ng/ml and current asthma or current wheeze in children.4 In a study looking at vitamin D levels in 235 children with asthma, only 76 of whom were considered to have obesity, the mean serum 25(OH)D level was 20.6 ng/ml (interquartile range, 13.5–26.0).11 The Childhood Asthma Management Program (CAMP), the largest prospective clinical trial in pediatric asthma, found that 35% of children ages 5–12 years with mild or moderate asthma were vitamin D insufficient (level less than 30 ng/ml).12 Hollams and colleagues13 studied plasma vitamin 25(OH)D levels at birth and 0.5, 1, 2, 3, 4, 5, and 10 years of age in an at-risk cohort and noted that the number of times a child was found to be deficient in vitamin D (defined as a level of less than 20 ng/ml) was positively associated with the risk for asthma and wheezing at 10 years of age. This suggests that low vitamin D is present at the inception of asthma. Szentpetery and colleagues14 examined vitamin D levels and Th2 markers in children with asthma in Puerto Rico. Vitamin D insufficiency was significantly associated with severe exacerbation frequency in children with non-atopic asthma, lending credence to vitamin D’s role in the inflammatory milieu not directly related to allergy. However, a link between serum IgE and vitamin D levels has been seen by others. In a study of 100 children with asthma, it was found that exacerbation severity and serum IgE levels were inversely correlated with vitamin D levels.15
Children with asthma and low serum 25(OH)D levels are predisposed to worse asthma outcomes. Gupta and collaborators16 found lower vitamin D levels in children who had difficult-to-treat asthma or poor asthma control; further, vitamin D deficiency has been associated with lower pulmonary function in children with obesity.4 Lautenbacher and colleagues17 found that vitamin D deficiency was associated with pulmonary function decline among obese children of Hispanic and African-American descent but not their healthy weight controls.17 In another study, Bose et al18 found a relationship between indoor air quality, asthma, and vitamin D levels in obese children in an urban environment. They observed that higher serum 25(OH)D levels appeared to mitigate the effects of increase indoor air pollution. Vitamin D works through binding to the vitamin D receptor (VDR), which is expressed on nearly all cells and tissues.9,19 Adipose tissue can upregulate VDR expression and expression of pro-inflammatory cytokines.20 Vitamin D and its actions through VDR may have a profound influence on airways inflammation and asthma particularly in overweight individuals. Collectively, these studies have contributed to a growing interest in the potential role of vitamin D deficiency in the genesis of asthma and a potential role for supplementation in its treatment, particularly in children who are overweight.
Intersection between asthma and obesity
Both asthma and obesity have become major health concerns in pediatric populations. Asthma is one of the leading causes of hospitalization in children and is a common cause of outpatient healthcare utilization.21 Obesity is also common and increasing in the U.S. Using National Health and Nutrition Examination Survey (NHANES) data between the years 2013 and 2016, Ogden et al reported a 17.8% prevalence of obesity (BMI ≥95th percentile for age and sex) in children ages 2–19 years and a 5.8% prevalence of severe obesity (BMI ≥ 99% for age and sex). A review of health data from over 500,000 children estimated that 23% to 27% of new asthma cases in children with obesity could be directly attributed to obesity and that in the absence of obesity, 10% of all cases of asthma could be avoided.22 Thus, obesity may be contributing substantially to the morbidity and healthcare costs associated with childhood asthma.
There are various phenotypes associated with obesity-related asthma.1 While the effect of obesity on asthma in adults is in large part mechanical in nature, the immunomodulatory effects of adipose tissue appear to play a major role in childhood obesity-related asthma. Adipose tissue can act as an endocrine organ releasing soluble factors that have effects on distant targets (Figure 3). Studies have shown that obesity is associated with elevated levels of circulating pro-inflammatory adipokines and cytokines including leptin, tumor necrosis factor alpha (TNF-α), and numerous interleukins (ILs).23 Excess leptin levels may be especially relevant as it affects both innate and adaptive immunity and can influence predisposition to asthma by increasing proliferation and action of monocytes, delaying eosinophil apoptosis, activating T-cell proliferation, and stimulating B-cell cytokine production (Figure 4).24 Higher leptin levels were associated with lower FEV1, in a population of 138 eight-year-old children.25 Metabolic factors such as insulin resistance and dyslipidemia also contribute to the inflammatory milieu that may predispose children with obesity to asthma.26 Conversely, having asthma may contribute to obesity since an inability to exercise without developing shortness of breath may limit a person’s ability to burn off calories and increase time inside consuming excess calories. Thus, there is a strong and interconnected pathophysiology between asthma and obesity.27
Figure 3:
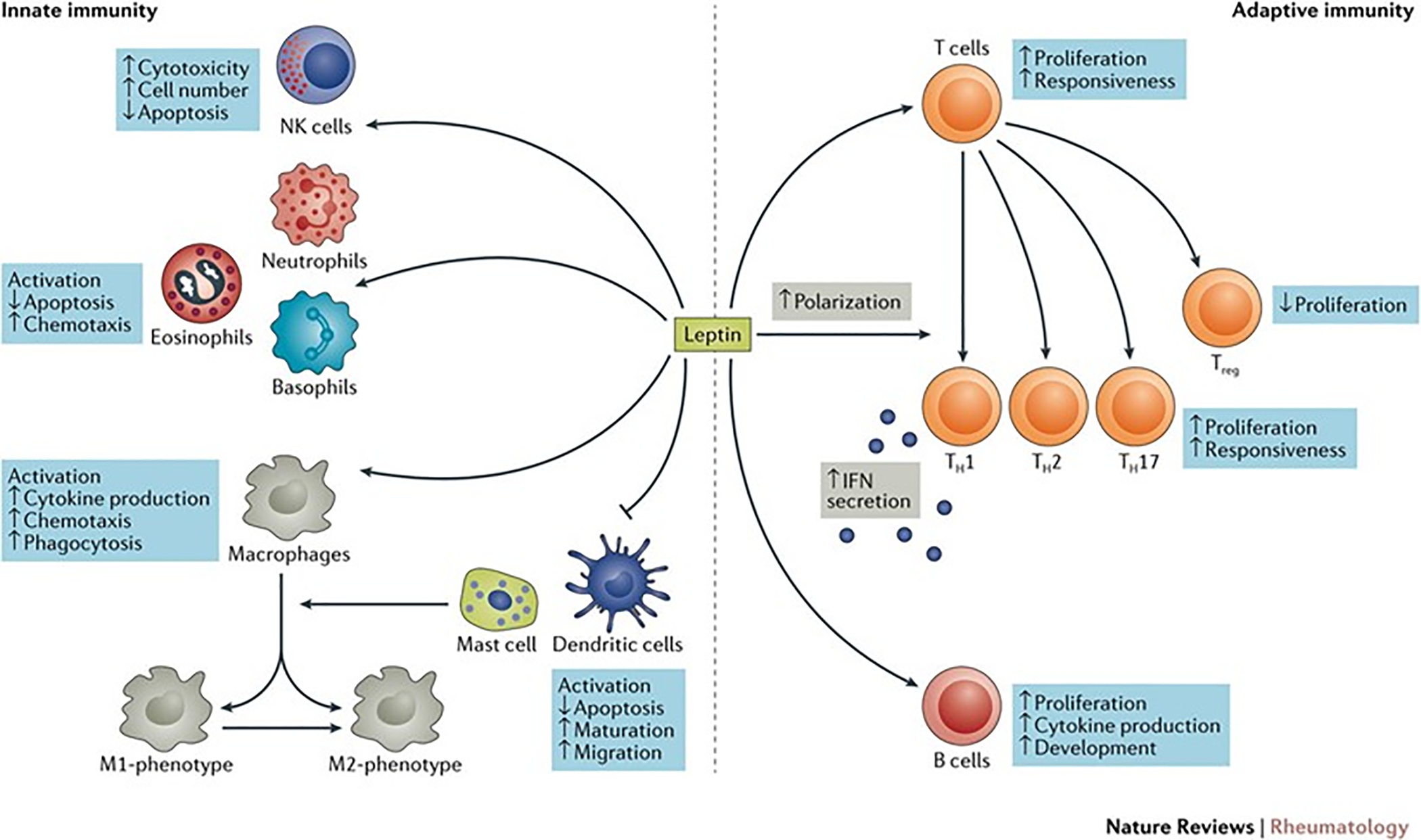
The effects of leptin on innate and adaptive immunity.24
Figure 4:
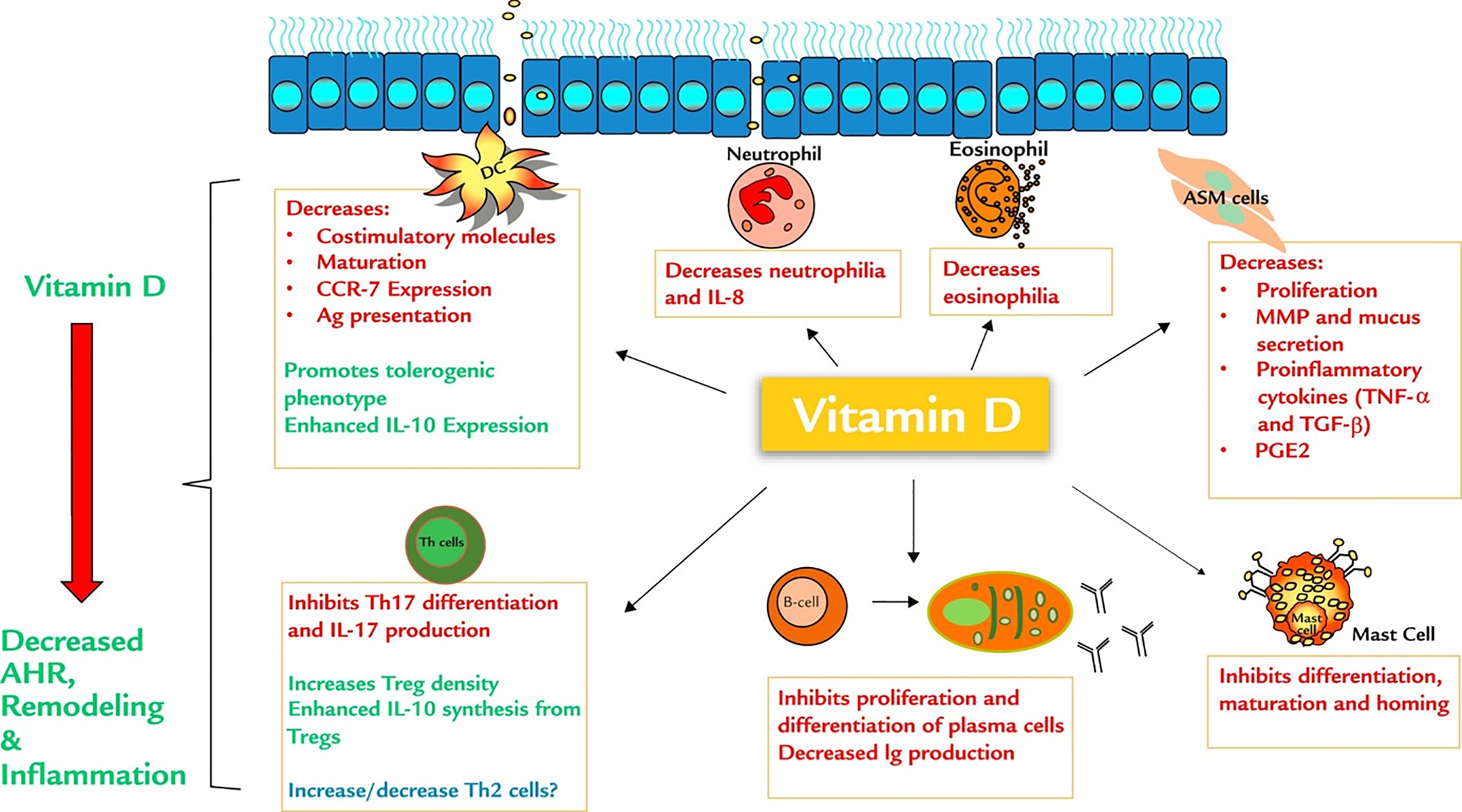
Immunomodulatory effects of vitamin D on inflammatory cells in allergic asthma.35
Vitamin D and Obesity
The correlation between low vitamin D and obesity has been studied extensively and was described in an excellent recent review.28 In this review, the authors examined the cause and effect nature of low vitamin D levels in obesity, noting both the potential role of obesity impairing vitamin D uptake and utilization and the role of vitamin D deficiency in leading to obesity. Turer et al.3 observed that 79% of children who were overweight (body mass index [BMI] 85th-95th percentile for age and gender) and 86% of children with obesity (BMI ≥95th percentile) met the criteria for vitamin D insufficiency (serum vitamin D measured as 25(OH)D <30 ng/ml). This is likely related to the large volume of distribution in these children and to the adipose tissue reservoir that can sequester large amounts of this fat-soluble vitamin.29,30 A study comparing vitamin D bioavailability in adults of normal weight versus those with obesity attributed decreased vitamin D levels to deposition of vitamin D in body fat compartments.29 Data on vitamin D pharmacokinetics in children with obesity is lacking. Addressing this critical gap in our understanding of vitamin D pharmacokinetics is an essential first step in investigating the role of vitamin D as a treatment for pediatric obesity-related asthma.
It should be noted that there is a potential interaction between obesity, asthma, vitamin D deficiency and socioeconomic status. These factors intermingle since the quality of food available can affect both obesity and vitamin D status. Natural food sources are generally low in vitamin D (see next section) and those that do have higher levels of vitamin D tend to be more expensive, making them less accessible to lower income populations. At the same time, readily available foods tend to be high in starches and sugars and contribute to obesity. A study in adults concluded “a low socioeconomic status is a major independent risk factor for vitamin D deficiency in obese individuals. These new findings highlight the need to pay particular attention to the health impact of vitamin D deficiency in socioeconomically disadvantaged populations.”31 Indoor and outdoor air pollution are also more common in low socioeconomic communities and are independent factors for development of asthma, making the intersection of asthma, obesity and vitamin D status complex.32
Vitamin D Supplements for Asthma
The recognition of the association of low-vitamin D levels and asthma incidence has led to a growing interest in evaluating the effect of vitamin D supplementation on asthma outcomes in children and adults.33–36 Supplementation might be even more important in children with obesity-related asthma, given that both conditions are associated with inflammation and decreased serum 25(OH)D levels. Studies of supplementation have been hampered by the fact that the level of 25(OH)D that might ameliorate asthma symptoms is not known. Intra-cellular levels of 1,25 dihydroxyvitamin D3 may need to be higher to achieve anti-inflammatory effects than those required for bone health. What serum level best achieves extra-skeletal effects is unknown. Hollis, et al.37 showed that optimal conversion of vitamin D3 to 25(OH)D occurs when serum levels of 25(OH)D are ≥40 ng/ml. An evaluation of NHANES data involving 6,000 children 6 to 19 years of age suggested that optimal vitamin D status defined by estimated maximum parathyroid hormone (PTH) suppression also occurs at 25(OH)D levels ≥ 40 ng/ml.38 Data in adults suggests that serum 25(OH)D level above 38 ng/ml reduce the risk of acute viral respiratory tract infections.39 These studies imply that serum levels of 25(OH)D greater than the Endocrine Society’s recommended 30 ng/ml are necessary to achieve the desired extra-skeletal (and intracellular) effects. For this reason, Litunjua and Weiss have suggested that a level of approximately 40 ng/ml may be needed to down-regulate inflammation and protect against infection.40
There are studies that suggest that asthma control indeed is optimized at a serum 25(OH)D ≥ level 40 ng/ml. In the CAMP study, which included more than 1,000 children, the risk of asthma exacerbations was inversely associated with serum 25(OH)D levels; the lowest risk occurred in patients with levels ≥40 ng/ml.12 The Vitamin D Add-on Therapy Enhances Corticosteroid Responsiveness in Asthma (VIDA) study enrolled more than 400 adults with asthma and decreased serum 25(OH)D levels at entry (mean 18.8 in the treated group and 19.9 in the placebo group). Mean BMI was more than 30 in both groups. Although vitamin D supplementation (100,000 IU loading dose followed by 4,000 IU daily) did not alter the rate of exacerbation in the first 28 weeks compared to placebo, a trend toward improved asthma control was seen in adults who achieved a mean 25(OH)D level of 41.9 ng/ml at 12 weeks of treatment.34
Unfortunately, pediatric studies of vitamin D supplementation for asthma have been few, with none specifically targeted to children with obesity-related asthma and none trying to achieve a level of ≥40 ng/ml. A meta-analysis of vitamin D supplementation trials in children found that supplementation is likely to reduce the risk of asthma exacerbations.41 None of the trials were designed to achieve a specific target level of 25(OH)D and few children achieved a serum 25(OH)D level of ≥40 ng/ml. Despite this, the analysis suggested that children receiving vitamin D had reduced odds of exacerbation compared to placebo treated children. An individual patient data meta-analysis of seven vitamin D supplementation trials including children and adults demonstrated a significant reduction in asthma exacerbations requiring treatment with systemic corticosteroids in those receiving supplements.33 Again, serum levels ≥40 ng/ml were not consistently reached.
Due to these inconclusive findings, a Cochrane review concluded that the optimum circulating level is as yet undetermined.5 Despite promising clues, it is still unknown if attaining a 25(OH)D level of ≥40 ng/ml provides added benefit. Several large non-asthma studies in adults have shown no benefit of supplementation even when mean levels were well above 40 ng/ml.42–44 Thus, the appropriate serum 25(OH)D level that should be targeted for optimal health outcomes remains unknown.
Prevention of deficiency or insufficiency is preferable to the need for treatment. Sunlight is the best natural source of vitamin D for humans given the lack of foods with significant amounts of vitamin D. A light-skinned person wearing minimal clothing (e.g., a bath suit) when exposed to enough sunlight to generate mild erythema produces endogenous vitamin D equivalent to ingesting 10,000 to 25,000 IU.45 Those with more darkly pigmented skin will produce much less vitamin D with the same exposure. Milk has been fortified with vitamin D in the U.S.A. since the 1930’s at a time rickets was a major problem. Additional fortification of wheat has been suggested as a cost effective way to prevent vitamin D deficiency in higher latitudes.46 A list of foods containing natural occurring vitamin D is available at https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/#h3. For those children found to be deficient, weekly boluses of 50,000 IU of vitamin D2 × 6 weeks followed by daily supplements of 1,000 IU of vitamin D3 has been suggested by the Endocrine Society, but likely do not provide sufficient amounts of vitamin D to raise serum vitamin 25(OH)D to anti-inflammatory levels.7
Vitamin D Pharmacokinetics in Children with Obesity
It is vital to understand the pharmacokinetics of vitamin D in children with asthma and obesity prior to performing an intervention trial. It is likely that children with obesity will require substantially greater than normally recommended doses to achieve concentrations of 25(OH)D ≥40 ng/ml due to their adipose sink and large volume of distribution. A small study of vitamin D administered at a dose of 5000 IU daily over two months increased mean 25(OH)D levels from 15 to 40 ng/ml in 14 children with obesity.47 Another study involving 35 adolescents with obesity and diabetes mellitus found that vitamin D3 supplementation of 4000 IU daily for 6 months increased mean 25(OH)D levels by 19.5 ng/ml in the supplemented group compared to 2.8 ng/ml in the placebo group.48 93% of the treated group reached serum levels consistent with vitamin D sufficiency, but the mean level was still <40 ng/ml. Vitamin D levels increased from baseline to 3 months and again from 3 to 6 months of therapy without reaching a plateau, implying that levels could continue to rise with prolonged supplementation.
Using a loading dose strategy should allow individuals to achieve a more rapid therapeutic level, particularly in the setting of adiposity. In the VIDA trial, adult participants who had a mean BMI of 30 kg/m2 received a single oral vitamin D3 loading dose of 100,000 IU followed by 4000 IU daily.34 Loading doses have also been used in children. Alansari, et al.49 examined the effects of 300,000 or 600,000 IU of vitamin D2 by intramuscular injection as a loading dose followed by 400 IU of oral vitamin D3 daily versus oral therapy only in 231 children ages 6 to 14 years. The loading dose achieved a 7 ng/ml greater increase in 25(OH)D, compared to the standard treatment approach of 400 IU daily.
Safety of Vitamin D Supplementation
Vitamin D supplementation is extremely safe. The upper bound of normal serum 25(OH)D in most clinical laboratories is 100 ng/ml; however, the Endocrine Society’s practice guidelines on vitamin D state that vitamin D intoxication is usually not observed until serum 25(OH)D levels are >150 ng/ml.15 Symptoms of vitamin D intoxication may include nausea, vomiting, loss of appetite, constipation, dehydration, fatigue, irritability, confusion, weakness, and/or weight loss. Recent published dosing regimens, including 4000 IU daily for 6 months and repeated 600,000 IU intramuscular loading doses every 3 months over a 6-month period in children, were well tolerated with no untoward effects.48,49 Young adults with vitamin D deficiency have been treated with 50,000 IU weekly for 8 weeks and then continued on 50,000 IU every other week for up to 6 years without serious side effects or changes in serum calcium levels.50 Vitamin D intoxication associated with hypercalcemia, hyperphosphatemia, and suppressed parathyroid hormone level may occur, but is typically only seen in patients who are receiving massive doses of vitamin D (50,000 to 1 million IU per day for several months to years).51,52 Indeed, case reports of vitamin D toxicity in association with mislabeling, manufacturing errors, or consumer misuse demonstrate the uncommon nature of this problem and the tolerability of even very high levels. In one report in which intoxicated subjects became symptomatic, it was noted that symptoms abated once 25(OH)D levels fell below 400 ng/ml.53 Thus, there is a very large safety window for administration of vitamin D supplements.
Summary
There is ample evidence that both asthma and obesity are inflammatory conditions, both are associated with decreased serum 25(OH)D levels and that there is the potential for intervention with vitamin D supplementation to decrease the inflammatory properties of both diseases. Determining the pharmacokinetics of vitamin D in the specific population of children and adolescents with overweight/obesity and asthma is critical if interventional trials are to be planned. A multicenter trial is currently underway on the pharmacokinetics of vitamin D supplementation in obese children with asthma (Clinicaltrials.gov # NCT03686150). Future studies will be needed in assessing the efficacy of vitamin supplementation as a treatment for obesity-related asthma.
Acknowledgements
Guarantor Statement: Brian O’Sullivan takes responsibility for the content of the manuscript.
Funding information:
Supported by the Office of the Director, National Institutes of Health. Grant numbers: U24OD024957, UG1OD024944, UG1OD024945, UG1OD024946, UG1OD024959, UG1OD024958, UG1OD024951, UG1OD024948, UG1OD024943, UG1OD024954, UG1OD024942, UG1OD024950, UG1OD024952, UG1OD024953, UG1OD024947, UG1OD024950, UG1OD024956, UG1OD024955, UG1OD024949
Role of the sponsor: The Office off the Director at the National Institutes of Health did not have any input in the development of this manuscript.
Abbreviation List
- BMI
Body Mass Index
- CAMP
Childhood Asthma Management Program
- IL
interleukins
- NHANES
National Health and Nutrition Examination Survey
- PTH
parathyroid hormone
- SOCS1
suppressor of cytokine signaling 1
- TNF-α
tumor necrosis factor alpha
- VDR
vitamin D receptor
- VIDA
Vitamin D Add-on Therapy Enhances Corticosteroid Responsiveness in Asthma
Footnotes
Summary Conflict of Interests: Authors do not have conflicts of interest
References
- 1.Bates J Physiological mechanisms of airway hyperresponsiveness in obese asthma. Am J Respir Cell Mol Biol. 2016;24(5):618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon AE, Poynter ME. Mechanisms of Asthma in Obesity. Pleiotropic Aspects of Obesity Produce Distinct Asthma Phenotypes. Am J Respir Cell Mol Biol. 2016;54(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics. 2013;131(1):e152–161. [DOI] [PubMed] [Google Scholar]
- 4.Han YY, Forno E, Celedón JC. Vitamin D Insufficiency and Asthma in a US Nationwide Study. J Allergy Clin Immunol Pract. 2017;5(3):790–796.e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martineau AR, Cates CJ, Urashima M, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9(9):Cd011511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deluac HF. Historical overview of vitamin D. In: Vitamin D 4 ed.: Academic Press; 2018:3–12. [Google Scholar]
- 7.Holick MF, Binkley NC, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96: 1911–1930. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. [DOI] [PubMed] [Google Scholar]
- 9.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Codoñer-Franch P, Tavárez-Alonso S, Simó-Jordá R, Laporta-Martín P, Carratalá-Calvo A, Alonso-Iglesias E. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J Pediatr. 2012;161(5):848–854. [DOI] [PubMed] [Google Scholar]
- 11.Reinehr T, Langrock C, Hamelmann E, et al. 25-Hydroxvitamin D concentrations are not lower in children with bronchial asthma, atopic dermatitis, obesity, or attention-deficient/hyperactivity disorder than in healthy children. Nutr Res. 2018;52:39–47. [DOI] [PubMed] [Google Scholar]
- 12.Brehm JM, Schuemann B, Fuhlbrigge AL, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126(1):52–58.e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollams EM, Teo SM, Kusel M, et al. Vitamin D over the first decade and susceptibility to childhood allergy and asthma. J Allergy Clin Immunol. 2017;139(2):472–481.e479. [DOI] [PubMed] [Google Scholar]
- 14.Szentpetery SE, Han YY, Brehm JM, et al. Vitamin D insufficiency, plasma cytokines, and severe asthma exacerbations in school-aged children. J Allergy Clin Immunol Pract. 2018;6(1):289–291.e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammadzadeh I, Darvish S, Qujeq D, Hajiahmadi M, Vaghari-Tabari M. Association of serum 25-OH vitamin D3 with serum IgE and the Pediatric Asthma Severity Score in patients with pediatric asthma. Allergy Asthma Proc. 2020;41:126–133. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Sjoukes A, Richards D, et al. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184(12):1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lautenbacher LA, Jariwala SP, Markowitz ME, Rastogi D. Vitamin D and pulmonary function in obese asthmatic children. Pediatr Pulmonol. 2016;51(12):1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bose S, Diette GB, Woo H, et al. Vitamin D Status Modifies the Response to Indoor Particulate Matter in Obese Urban Children with Asthma. J Allergy Clin Immunol Pract. 2019;7(6):1815–1822.e1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol Metab Clin North Am. 2010;39(2):255–269, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonas MI, Kurylowicz A, Bartoszewicz Z, et al. Vitamin D Receptor Gene Expression in Adipose Tissue of Obese Individuals is Regulated by miRNA and Correlates with the Pro-Inflammatory Cytokine Level. Int J Mol Sci. 2019;20(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akinbami L, Simon AE, Rossen LM. Changing Trends in asthma prevalence among children. Pediatrics. 2016. 137(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang JE, Bunnell HT, Hossain MJ, et al. Being Overweight or Obese and the Development of Asthma. Pediatrics. 2018;142(6). [DOI] [PubMed] [Google Scholar]
- 23.Singer K, Lumeng CN. The initiation of metabolic inflammation in childhood obesity. J Clin Invest. 2017;127(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abella V, Scotece M, Conde J, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13(2):100–109. [DOI] [PubMed] [Google Scholar]
- 25.Eising JB, Uiterwaal CS, Evelein AM, Visseren FL, van der Ent CK. Relationship between leptin and lung function in young healthy children. Eur Respir J. 2014;43(4):1189–1192. [DOI] [PubMed] [Google Scholar]
- 26.Vijayakanthi N, Greally JM, Rastogi D. Pediatric Obesity-Related Asthma: The Role of Metabolic Dysregulation. Pediatrics. 2016;137(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baffi CW, Winnica DE, Holguin F. Asthma and obesity: mechanisms and clinical implications. Asthma Res Pract. 2015;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migliaccio S, Di Nisio A, Mele C, Scappaticcio L, Savastano S, Colao A. Obesity and hypovitaminosis D: causality or casualty? Int J Obes Suppl. 2019;9(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. [DOI] [PubMed] [Google Scholar]
- 30.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring). 2012;20(7):1444–1448. [DOI] [PubMed] [Google Scholar]
- 31.Leger-Guist’hau J, Domingues-Faria C, et al. Low socioeconomic status is a newly identified independent risk factor for poor vitamin D status in severely obese adults. J Hum Nutr Diet. 2017; 30:203–215. [DOI] [PubMed] [Google Scholar]
- 32.O’Lenick CR, Winquist A, et al. Assessment of neighbourhood-level socioeconomic status as a modifier of air pollution–asthma associations among children in Atlanta. J Epidemiol Community Health. 2017;71:129–36. [DOI] [PubMed] [Google Scholar]
- 33.Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5(11):881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro M, King TS, Kunselman SJ, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. Jama. 2014;311(20):2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall SC, Agrawal DK. Vitamin D and Bronchial Asthma: An Overview of Data From the Past 5 Years. Clin Ther. 2017;39(5):917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerley CP, Elnazir B, Faul J, Cormican L. Vitamin D as an adjunctive therapy in asthma. Part 2: A review of human studies. Pulm Pharmacol Ther. 2015;32:75–92. [DOI] [PubMed] [Google Scholar]
- 37.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007;103(3–5):631–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginde AA, Wolfe P, Camargo CA Jr., Schwartz RS. Defining vitamin D status by secondary hyperparathyroidism in the U.S. population. J Endocrinol Invest. 2012;35(1):42–48. [DOI] [PubMed] [Google Scholar]
- 39.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5(6):e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litonjua AA, Weiss ST. Vitamin D status through the first 10 years of life: A vital piece of the puzzle in asthma inception. J Allergy Clin Immunol. 2017;139(2):459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riverin BD, Maguire JL, Li P. Vitamin D Supplementation for Childhood Asthma: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(8):e0136841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manson JE, Cook NR, Lee IM, et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med. 2019;380(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. Jama. 2019;322(8):736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N Engl J Med. 2019;381(6):520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. [DOI] [PubMed] [Google Scholar]
- 46.Aguiar M, Andronis L, Pallan M, Högler W, Frew E. The economic case for prevention of population vitamin D deficiency: a modelling study using data from England and Wales. Euro J Clin Nutrit. 2020; 74:825–33. [DOI] [PubMed] [Google Scholar]
- 47.Elitsur Yoram PDL. Randomized vitamin D supplementation in vitamin D deficient obese children from West Virginia. Arch Clin Gastroenterol. 2016;2(1):065–068. [Google Scholar]
- 48.Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr. 2013;97(4):774–781. [DOI] [PubMed] [Google Scholar]
- 49.Alansari K, Davidson BL, Yousef KI, Mohamed ANH, Alattar I. Rapid vs Maintenance Vitamin D Supplementation in Deficient Children With Asthma to Prevent Exacerbations. Chest. 2017;152(3):527–536. [DOI] [PubMed] [Google Scholar]
- 50.Pietras SM, Obayan BK, Cai MH, Holick MF. Vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med. 2009;169(19):1806–1808. [DOI] [PubMed] [Google Scholar]
- 51.Vieth R Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69(5):842–856. [DOI] [PubMed] [Google Scholar]
- 52.Vogiatzi MG, Jacobson-Dickman E, DeBoer MD. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99(4):1132–1141. [DOI] [PubMed] [Google Scholar]
- 53.Araki T, Holick MF, Alfonso BD, et al. Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J Clin Endocrinol Metab. 2011;96(12):3603–3608. [DOI] [PubMed] [Google Scholar]
- 54.Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015; 36(7):461–70. [DOI] [PubMed] [Google Scholar]


