Abstract
The terms biliary sludge and cholesterol microlithiasis (hereafter referred to as microlithiasis) were originated from different diagnostic techniques and may represent different stages of cholesterol gallstone disease. Although the pathogenesis of biliary sludge and microlithiasis may be similar, microlithiasis could be preceded by biliary sludge, followed by persistent precipitation and aggregation of solid cholesterol crystals, and eventually, gallstone formation. Many clinical conditions are clearly associated with the formation of biliary sludge and microlithiasis, including total parenteral nutrition, rapid weight loss, pregnancy, organ transplantation, administration of certain medications, and a variety of acute and chronic illnesses. Numerous studies have demonstrated complete resolution of biliary sludge in approximately 40% of patients, a cyclic pattern of disappearing and reappearing in about 40%, and progression to gallstones in nearly 20%. Although only a minority of patients with ultrasonographic demonstration of biliary sludge develop gallstones, it is still a matter of controversy whether microlithiasis could eventually evolve to cholesterol gallstones. Biliary sludge and microlithiasis are asymptomatic in the vast majority of patients; however, they can cause biliary colic, acute cholecystitis, and acute pancreatitis. Biliary sludge and microlithiasis are most often diagnosed ultrasonographically and bile microscopy is considered the gold standard for their diagnosis. Specific measures to prevent the development of biliary sludge are not practical or cost-effective in the general population. Laparoscopic cholecystectomy offers the most definitive therapy on biliary sludge. Endoscopic sphincterotomy or surgical intervention is effective for microlithiasis-induced pancreatitis. Ursodeoxycholic acid can effectively prevent the recurrence of solid cholesterol crystals and significantly reduce the risk of recurrent pancreatitis.
Keywords: Biliary sludge, Cholesterol microlithiasis, Acute cholecystitis, Acute pancreatitis, Biliary colic, Cholesterol monohydrate crystals, Lithogenic bile
1. Introduction
Many clinical studies have found that biliary sludge and cholesterol microlithiasis (hereafter referred to as microlithiasis) could cause either an uncomplicated symptomatic gallstone-like disease (biliary colic), or a complicated gallstone-like disease (acute cholecystitis and acute pancreatitis).1–12 Furthermore, both biliary sludge and microlithiasis could represent the early stage of cholesterol gallstone disease because approximately 20% of patients with biliary sludge or microlithiasis can eventually develop cholesterol gallstones.3 However, in the vast majority (~60%) of subjects, biliary sludge and microlithiasis can spontaneously disappear after pathogenic conditions are cured, and under these circumstances, they are completely dissolved.13 If the gallbladder displays a well-regulated motility function, biliary sludge and microlithiasis can be totally eliminated into the intestine. Moreover, they can be completely dissolved in almost all of the individuals who are treated with ursodeoxycholic acid (UDCA) and/or cholecystokinin. This indicates that biliary sludge and microlithiasis are a reversible morbid condition during the development of cholesterol gallstone disease.2 Although the terms biliary sludge and microlithiasis are often used interchangeably in the literature, the former is frequently described in pregnant women and in patients on total parenteral nutrition (TPN), while the latter is usually considered as a pathogenic factor in patients with idiopathic acute pancreatitis.14–21 In clinical practice, physicians and surgeons have used many different definitions of biliary sludge and microlithiasis, which have made it difficult to compare various studies, including therapeutic strategies and effects.22 The diagnosis of biliary sludge is almost always based on ultrasonographic findings, while the exact diagnosis of microlithiasis requires the direct microscopic examination of bile. On transabdominal ultrasonography, biliary sludge has been defined as an amorphous mixture of particulate matter and bile, which occurs when various solutes in bile precipitate.23 Biliary sludge is echogenic and does not cast an acoustic shadow (Fig. 1). Although sludge is dependent on gravity, it slowly moves to the dependent portion of the gallbladder. Bile is usually collected for microscopy in patients without gallstones on ultrasonography, and is often aspirated from the duodenum, common bile duct, or gallbladder via an endoscope channel or a plastic cannula during the procedure of endoscopic retrograde cholangiopancreatography (ERCP).24–27 Bile collected in this context frequently contains classical birefringent solid cholesterol monohydrate crystals (Fig. 2).
Fig. 1. Longitudinal (top) and transversal (bottom) ultrasonographic scans of the gallbladder from a 56-year-old woman with drinking habits and a recent history of right hypochondrium pain.

The finding is suggestive of chronic cholecystitis, as indicated by a thickened gallbladder wall (up to 5 mm). Biliary sludge in the gallbladder is visible as an amorphous echogenic image that is distinctly different from the anechoic bile, and it can slowly move to the most dependent part of the gallbladder, as indicated by a red asterisk. A clear acoustic shadow is missing at the level of the distal gallbladder wall. Intra- and extra-hepatic bile ducts are not visible and therefore not dilated. Abbreviation: L, liver. We sincerely thank Drs. Francesco Minerva and Marica Noviello for providing us with these pictures.
Fig. 2. Fresh human gallbladder bile is examined by phase contrast and polarizing light microscopy, showing biliary sludge that is composed mainly of classical solid plate-like cholesterol monohydrate crystals, as well as varying sizes of calcium bilirubinate granules in golden color, and mucin gel.

Original magnification is × 800 by polarizing light microscopy.
During clinical practice, the criteria for differentiating between biliary sludge and microlithiasis are not entirely clear because they are very tiny and are not easily detected by current imaging techniques. It has been suggested that a gallstone be defined as a particle with a diameter greater than 3 mm that cannot be crushed by digital compression.28–30 Moreover, microlithiasis is a hard, ball-like object, and light yellow in color with smooth curved surface. Because of scattered and absorbed light, microlithiasis is opaque, and black in color under the microscope. In contrast, biliary sludge is soft, non-birefringent amorphous strands under phase contrast and polarizing light microscopy.31–33 Furthermore, it is unclear whether there are differences in the natural history, fate, the resulting complications, the responses to medical treatment, and prognosis between biliary sludge and microlithiasis. It has been questioned whether there is a difference in chemical composition of biliary lipids as well as pro-nucleating and anti-nucleating proteins between biliary sludge and microlithiasis. It is still unclear whether biliary sludge or microlithiasis is more predisposed to evolve into macroscopic stones and to cause complications. Nevertheless, biliary sludge and microlithiasis could have the same meaning and could result in the same complications. Thus, they could display the same fate and natural history. In this review article, we discuss similarities and differences between biliary sludge and microlithiasis and summarize their pathophysiological and clinical significances.
2. Etymology of biliary sludge and microlithiasis
Although a white crystalline substance, i.e., solid cholesterol monohydrate crystals, has been found to be the major component of cholesterol gallstones during the second half of the eighteenth century, it was until the early twentieth century that biliary cholesterol monohydrate crystals were identified by light microscopy to be colorless, transparent, thin plate-like solid crystals with parallel edges, as well as with 79.2° and 100.8° angles, and often a notched corner (Fig. 2).34–40 This microscopic finding provided an imaging basis for clinical diagnosis of gallbladder cholesterol gallstone disease before modern gallbladder imaging techniques such as ultrasonography, x-ray film, and computed tomography (CT) were available.28,29,41–48 Classical plate-like birefringent rhomboids were often detected in duodenal aspirates of patients with cholesterol gallstone disease although investigators also found some other forms of solid cholesterol crystals, i.e., in the form of needle-like or arc-like crystals, thin plates with irregular margins, or agglomerates.49–52
With the advent of abdominal ultrasonography in the early 1970s, the term biliary sludge was first used for the diagnosis of gallbladder disease.23,53,54 Ultrasonographic examination found that biliary sludge appears as low-level echoes that layer in the dependent portion of the gallbladder without acoustic shadowing and it generally shifts slowly with positioning (Fig. 1). Because abdominal ultrasonography is a noninvasive and inexpensive technique, it has been extensively used for clinical diagnosis of biliary diseases. In contrast, microlithiasis-induced acute pancreatitis is difficultly diagnosed by abdominal ultrasonography because the pancreas is located in the abdominal cavity behind the stomach. After alcohol is excluded as a causative factor for acute pancreatitis, ERCP should be performed and bile is aspirated from the duodenum. After being centrifuged, bile samples are examined by phase contrast and polarizing light microscopy.38,55–57 If solid cholesterol crystals are found, the diagnosis of microlithiasis-induced acute pancreatitis is established.58–62 Bile is now collected for microscopy in patients without gallstones on ultrasonography, and is usually aspirated from the duodenum or common bile duct via an endoscope channel or a 5-Franch plastic catheter during ERCP. Because the diameter of tubing is too small, large size (>5 mm) of gallstones, if existed, cannot be aspirated during the ERCP procedure for microscopic examination. Although it is possible that a tiny gallstone could be found in the duodenal lumen and/or the common bile duct during the examination directly by ERCP, the probability of finding such a small stone is very low.
Obviously, the terms biliary sludge and microlithiasis were originated from the clinical applications of different diagnostic techniques.
3. Definition and chemical composition of biliary sludge and microlithiasis
Biliary sludge is most commonly composed of classical solid plate-like cholesterol monohydrate crystals, as well as varying sizes of aggregated liquid crystals, calcium bilirubinate granules, and other calcium salts, most of which are embedded in strands of mucin gel produced by the gallbladder.31–33 Of note is that the chemical composition of biliary sludge varies with the clinical condition. For example, biliary sludge consists primarily of cholesterol monohydrate crystals in pregnant women,63–67 whereas calcium bilirubinate predominates in patients receiving TPN, a predisposing condition to gallbladder stasis and precipitation of solid cholesterol crystals from bile. Moreover, biliary sludge is composed mainly of calcium-ceftriaxone complexes (i.e., so-called pseudocholelithiasis) in patients receiving high-dose ceftriaxone therapy.68
Biliary sludge also contains a large proportion of undefined residues, protein-lipid complexes, soluble mucin proteins, and some minerals.69 In addition, xenobiotics such as ceftriaxone and biliary proteins are found to be important components. Calcium bilirubinate granules are almost invariably present, and bilirubin is usually found in its unconjugated, least soluble form. The source of the unconjugated bilirubin is controversial. Bilirubin is excreted by the liver mainly in its diglucuronide form, but small amounts of the monoglucuronide and unconjugated forms are also found. β-glucuronidase may be important for increasing unconjugated bilirubin because this enzyme can deconjugate bilirubin. Some pathogenic conditions, such as chronic low-grade biliary infection, are associated with pigment gallstones, in which the activity of this enzyme is increased. β-glucuronidase has also been identified in uninfected bile, probably having originated in the biliary epithelium. Non-enzymatic hydrolysis of bilirubin may occur.
On microscopy, microlithiasis is often defined as a tiny stone with a diameter <3 mm, which cannot be crushed by digital compression. Under polarizing light microscopy, these tiny stones always display rounded contours and black centers from light scattering/absorption. To date, such petty stones are often missing or undetected by conventional ultrasonography or current imaging techniques (Fig. 3).
Fig. 3. On microscopy, microlithiasis is often defined as a tiny stone with a diameter < 3 mm, which cannot be crushed by digital compression.
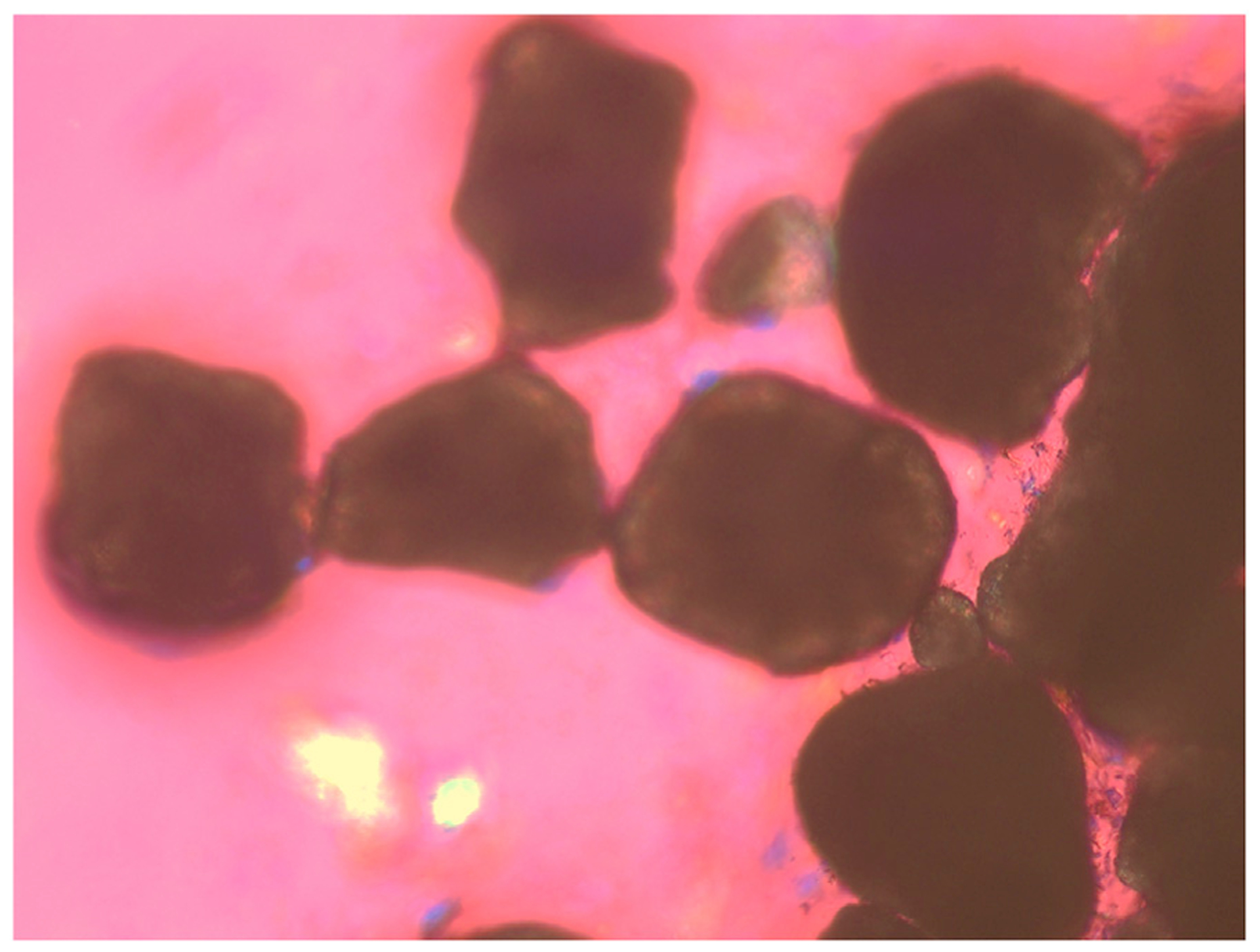
Under phase contrast and polarizing light microscopy, microlithiasis always display rounded contours and black centers from light scattering/absorption. Original magnification is × 100 by polarizing light microscopy.
4. Pathogenesis
Human bile is a golden yellow, dark green, or brownish complex solution that is composed mainly of water, organic solutes (i.e., lipids), inorganic salts, and some proteins. In bile, cholesterol, phospholipids, and bile acids are three major lipid components, which account for approximate 99% of total lipids by weight. Bilirubin is a minor solute and represents less than 1% of biliary lipids in bile. Approximately 95% of the cholesterol molecule in bile is in the unesterified form and <5% of the sterols are cholesterol precursors and dietary sterols.70–72 In contrast, the concentrations of cholesteryl esters are negligible in human bile. Because bile is an aqueous solution and cholesterol is basically insoluble in water, the physical-chemical mechanisms for cholesterol solubilization in bile are complex. Bile acids, which are synthesized in the liver through the conversion of cholesterol, can form simple and mixed micelles in bile. These micelles can aid in solubilizing cholesterol in bile.73–78 In addition, the vesicles that are composed primarily of phospholipids also greatly enhance the solubilization of cholesterol in bile.39,79–84 The precipitation of solid cholesterol monohydrate crystals represents a failure of biliary cholesterol homeostasis in which the physical-chemical balance of cholesterol solubility in bile is disturbed, thereby leading to the formation of cholesterol monohydrate crystals and gallstones. Over the past five decades, a lot of accumulated evidence from human and animal studies has greatly increased our understanding of the molecular, cellular and genetic mechanisms underlying the pathogenesis of cholesterol gallstone disease.
Based on clinical and epidemiological studies and animal experiments, we have proposed a novel concept that interactions of five primary defects play a critical role in the pathogenesis of cholesterol gallstone disease.85 These defects include: (i) genetic factors and Lith genes; (ii) hepatic hypersecretion of biliary cholesterol, leading to supersaturated bile; (iii) rapid phase transitions of cholesterol in bile; (iv) impaired gallbladder motility accompanied with hypersecretion of mucins and accumulation of mucin gel in the gallbladder lumen, as well as immune-mediated gallbladder inflammation; and (v) increased amounts of cholesterol of intestinal origin due to high efficiency of cholesterol absorption and/or slow intestinal motility, which aids “hydrophobe” absorption and augments synthesis of secondary bile acids by the anaerobic intestinal microflora.70,85,86 On the basis of numerous human and animal studies, hepatic cholesterol hypersecretion has been recognized to be the primary pathophysiologic defect, leading to the formation of cholesterol-supersaturated bile and solid cholesterol crystals, as well as the aggregation and growth of these solid crystals into microscopic stones first and then macroscopic gallstones. These abnormalities are caused by multiple Lith genes, with insulin resistance as part of the metabolic syndrome working with cholelithogenic environmental factors to induce the phenotype.70,87,88 The growth of solid cholesterol crystals starts as soon as cholesterol nucleation and crystallization occurs and this process is greatly accelerated by mucin gel, a potent pro-nucleating agent. Rapid growth and agglomeration of solid plate-like cholesterol monohydrate crystals into microlithiasis and eventually gallstones is a consequence of persistent hepatic hypersecretion of biliary cholesterol together with both gallbladder mucin hypersecretion and incomplete evacuation by the gallbladder due to its impaired motility dependent on defective smooth muscle in response to neuro-hormonal stimuli.89 Over the past decades, new progress has been made in the genetic analysis of Lith genes and the pathophysiology of gallstone disease. Many excellent review articles on these topics have been extensively published and interested readers can further read these papers.71,85,89–96
The extent to which patients with no identifiable risk factors develop biliary sludge and microlithiasis remains controversial. However, there are many clinical conditions that are clearly associated with the formation of biliary sludge and microlithiasis.71 These include parenteral supplementation, conditions associated with weight loss, pregnancy, a variety of acute and chronic illnesses, organ transplantation, and administration of certain medications. Numerous studies have found that physical and chemical interactions of gallbladder contents together with altered gallbladder motility and mucosal function serve as a nidus for the formation of biliary sludge and microlithiasis.2 It is likely that microlithiasis is preceded by the formation of biliary sludge, followed by microprecipitate aggregation and eventual stone formation.97–100 This hypothesis is supported by the typical finding of biliary sludge before stone recurrence in chemical dissolution studies. Others repudiate this hypothesis because of the infrequent development of gallstones in persons with biliary sludge and the spontaneous resolution of biliary sludge in most individuals. Of note, a genetic defect in the ATP-binding cassette (ABC) subfamily B member 4 (ABCB4) gene is the cause of a subtype of progressive familial intrahepatic cholestasis called progressive familial intrahepatic cholestasis type 3 (PFIC3), in which the phenotypic spectrum of PFIC3 ranges from neonatal cholestasis to cirrhosis in young adults.101–103 In addition, the ABCB4 defect is involved in two other human liver diseases such as intrahepatic cholestasis of pregnancy and cholesterol gallstone disease, i.e., low phospholipid-associated cholelithiasis (LPAC).104–110 It is still unclear why these three human liver diseases are induced by a single gene (ABCB4) deficiency, and its pathogenic mechanism is under an extensive investigation.111–113
The formation of biliary sludge greatly depends on the physical-chemical interactions of the three major lipid constituents, abnormalities of gallbladder mucosal function, and gallbladder dysmotility.85,89,90,93,114 It is most likely that cholesterol gallstones are formed initially from biliary sludge by persistent precipitation and aggregation of solid cholesterol crystals, and it is generally thought that biliary sludge is a necessary precursor to gallstones. For example, after chemical dissolution of gallstones, biliary sludge in the gallbladder lumen is usually seen on ultrasonography before gallstone recurrence. Thus, the pathogenesis of biliary sludge could be similar to that of cholesterol gallstones. However, controversial results have also been found from clinical studies that biliary sludge can spontaneously resolve in most persons and that gallstones form in only a small minority of persons with biliary sludge. This suggests that biliary sludge could not be an essential precursor to gallstones and that the pathogenesis of the two entities could differ. Nevertheless, it is impossible that gallstones could form from clear bile without the intermediate formation of microprecipitates, including classical cholesterol monohydrate crystals, anhydrous cholesterol crystals and sandy stones. It is still imperative to investigate how these microprecipitates aggregate and grow to form well-ordered, structured gallstones.
It is likely that microlithiasis may form through a different mechanism compared to the formation of cholesterol gallstones although both could start from gallbladder bile supersaturated with cholesterol. The natural history of microlithiasis is not completely defined yet. Although clinical studies have found that only a minority of patients with ultrasonographic demonstration of biliary sludge develop gallstones, it is still a matter of controversy whether microlithiasis could evolves to cholesterol gallstone disease.
Although the pathogenesis of microlithiasis could be the same to that of cholesterol gallstones, there are distinct differences. Rapid precipitation of solid cholesterol crystals from cholesterol-supersaturated bile is the key factor in the formation of microlithiasis.115 In bile aspirated from the duodenum after intravenous infusion of the cholecystokinin analogue ceruletide, patients with gallbladder microlithiasis have significantly lower concentrations of phospholipids compared to that in patients with macroscopic gallbladder stones.29 Relative phospholipid deficiency is caused mostly by missense mutations in the ABCB4 gene, also known as the multidrug resistance protein 3 (MDR3) gene. The ABCB4 gene encodes for an energy-dependent phospholipid efflux translocator at the canalicular membrane of the hepatocytes, which facilitates the transport of phospholipids from the inner to the outer canalicular membrane of hepatocytes for hepatic secretion into canalicular bile.116–119 Interestingly, some cases of acute pancreatitis caused by microlithiasis are associated with point mutations in the ABCB4 gene.120 Obviously, relative phospholipid deficiency in bile is related to rapid cholesterol crystallization and microlithiasis, as well as a high risk of developing acute pancreatitis. These findings can be explained by the physical-chemical properties of biliary cholesterol, phospholipids and bile acids in bile. Because cholesterol is poorly soluble in an aqueous environment, it is solubilized in bile in mixed micelles by bile acids and phospholipids. In case of cholesterol supersaturation, excess cholesterol is also solubilized in vesicles that are composited mainly of phospholipids. If excess cholesterol cannot be dissolved by micelles or vesicles, it could precipitate as solid cholesterol crystals.121 The importance of the relative amounts of bile acids vs. phospholipids in the model bile system has found for crystallization behavior.37 In contrast to usual cholesterol gallstone patients, biliary lipid composition of patients with microlithiasis plots in the left two-phase zone, in which besides classical plate-like cholesterol monohydrate crystals, various intermediate cholesterol crystals such as arc-like and needle-like crystals and tubular crystals can be detected, probably related to a faster crystallization process.37,51,122 In addition, these patients with microlithiasis have an increased cholesterol saturation index similarly to those with cholesterol gallstones. However, bile supersaturation of patients with microlithiasis is due to a decrease in biliary phospholipid concentrations and not to an increase in biliary cholesterol concentrations.
Hypersecretion of gallbladder mucin, including release of excess mucin gel, is also strongly associated with microlithiasis.98 Mucin gel can promote rapid cholesterol crystallization possibly by triggering cholesterol nucleation and offering low affinity binding sites for phospholipids and cholesterol.123 On the other hand, mucin gel may increase bile viscosity, thus leading to the formation of a gel matrix which can entrap solid cholesterol crystals and enhance aggregation and growth of solid cholesterol crystals into microlithiasis in the gallbladder.40,124–128
As discussed above, it is highly likely that biliary sludge and microlithiasis could represent two different steps during the early stage of cholesterol gallstone formation. Further studies on both the natural history and the pathogenesis of biliary sludge and microlithiasis are strongly needed to address these questions.
5. Epidemiology, natural history and clinical course
The prevalence of biliary sludge varies widely as reported in the literature. Most studies have examined patients in a specific clinical situation such as pregnancy or critical illness.3 Moreover, clinical investigations on the natural history of biliary sludge are usually based on abdominal ultrasonographic diagnosis, without microscopic examination or chemical composition analysis. Many of these studies are also limited by inadequate follow-up. In addition, it is not known whether the natural history of cholesterol monohydrate crystal-predominant sludge differs from that of calcium bilirubinate-predominant sludge.
Only one large study has been done with a well-defined follow-up protocol. In that study, three clinical outcomes are found: (i) complete resolution, (ii) a waxing and waning course, and (iii) gallstone formation.65 More importantly, biliary sludge found in patients with abdominal pain can spontaneously disappear in about 50% of cases and persist asymptomatically in about 20% of cases over a 3-year period. Over the same period, symptoms develop in 10%–15% of patients and gallstones are formed in 5%–15% of patients. If a specific precipitating cause for the formation of biliary sludge exists, biliary sludge usually resolves upon the removal of that cause. If the precipitating event recurs or persists, gallstones can form eventually. For instance, biliary sludge and gallstones often coexist after multiple pregnancies or prolonged TPN administration.
Similar to gallstones, biliary sludge is often asymptomatic in most patients. However, in addition to predisposing to gallstone formation, biliary sludge can lead to complications such as biliary colic.16 Approximately 31% of patients with non-alcoholic pancreatitis have biliary sludge, and up to 74% of patients with “idiopathic” pancreatitis have been found to be associated with biliary sludge, in which excess alcohol use, gallstones, metabolic abnormalities and drug-related causes have been excluded. Other complications of biliary sludge include acute cholangitis and acute acalculous cholecystitis.
If biliary sludge causes symptoms, a more provocative hypothesis is that the biliary pain and inflammation found in gallstone disease are mediated by the presence of biliary sludge. For example, symptomatic patients with gallstones who receive UDCA treatment have a striking alleviation of the symptoms in three months, although the number and size of their gallstones do not change. It is likely that solid cholesterol crystals in bile are dissolved before the gallstones do. Asymptomatic patients with gallstones who are receiving shock-wave lithotripsy can develop biliary colic, acute cholecystitis, or acute pancreatitis. In these patients, biliary sludge may have been created iatrogenically.
Our understanding of the clinical significance of biliary sludge has been limited by short-term studies involving patients with known risk factors, many of whom have coexisting gallstones and gallbladder pathologic condition. However, many clinical studies have demonstrated complete resolution of biliary sludge in approximately 40% of patients, a cyclic pattern of disappearing and reappearing in about 40%, and progression to gallstones in approximately 20%.
Potential outcomes of microlithiasis include remaining asymptomatic, developing biliary colic, or experiencing biliary and/or pancreatic complications such as acute cholecystitis, acute cholangitis, papillary stenosis, and acute pancreatitis. Although the course cannot be reliably predicted, removal of the precipitating event correlates with resolution of microlithiasis. Persistence of an underlying risk factor often leads to gallstone formation and complications such as acute pancreatitis.
Microlithiasis may lead to acute pancreatitis through several mechanisms. Small gallstones may transiently impact at the papilla, leading to pancreatic duct obstruction and eventual pancreatitis. Repeated exposure to microlithiasis may result in papillary stenosis and sphincter of Oddi dysfunction, both of which are often associated with acute pancreatitis. The smaller size of stones comprising microlithiasis (vs. macrolithiasis) does not imply a lower likelihood of acute pancreatitis. In contrast, there are some data suggesting that smaller stones may correlate with a greater tendency for the development of acute pancreatitis. However, there is also some uncertainty regarding the causal relationship between microlithiasis and acute pancreatitis. Some investigations found that microlithiasis does not cause acute pancreatitis, but instead indicates the prior presence of larger gallstones that leads to the pancreatitis.129 Pancreatitis itself may induce the formation of microlithiasis as a result of edema of the head of the pancreas that leads to biliary compression and gallbladder hypomotility. Gallbladder stasis also results from fasting, parenteral nutrition, and weight loss, with all these conditions being common in patients with pancreatitis. Also, gallbladder content is modified and may become more lithogenic as a result of pancreatitis.
6. Clinical manifestations and diagnosis
When patients have abdominal pain, true biliary colic should be distinguished from nonspecific abdominal discomfort.130 A cholecystectomy done for true biliary colic is usually curative, but symptoms often persist if the procedure is done in patients with nonspecific abdominal pain such as dyspepsia, gastric ulcer, duodenal ulcer, irritable bowel syndrome, causalgia, dysmenorrhea, psychosomatic pain, constipation, viral gastroenteritis, mesenteric adenitis, or choledocholelithiasis.131–134 Nevertheless, despite the availability of many imaging techniques that can be used to uncover biliary sludge and gallstones, the diagnosis of biliary colic is ultimately based on clinical judgment.
When biliary sludge is suspected, the choice of diagnostic method must be based on the clinical setting and the sensitivity, specificity, and cost of the diagnostic tests available (Fig. 4). In general, given its relatively low cost and noninvasiveness, transabdominal ultrasonography should be the initial test. However, because the sensitivity of this test is only approximately 60%,3,135 further imaging exams should be considered if the ultrasonographic result is negative and clinical suspicion remains high. For example, in a patient with recurring attacks of idiopathic pancreatitis.17 If the diagnosis is to be pursued, either endoscopic ultrasonography (EUS) or bile microscopy should be chosen.
Fig. 4. Suggested algorithm for the management of patients with biliary sludge.

Abbreviations: CT, computed tomography; ERC, endoscopic cholangiography; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasonography; MRCP, magnetic resonance cholangiography; UDCA, ursodeoxycholic acid; US, ultrasonography.
Clinically, biliary sludge is most often diagnosed ultrasonographically, according to the criteria defined above. The ultrasonographic pattern can be produced by calcium bilirubinate granules or aggregated cholesterol monohydrate crystals as small as 0.5–1 mm in diameter mixed with bile and mucin gel, independent of bile viscosity.1 Other entities, including blood, necrotic debris, multiple small gallstones, or pus, can also have an appearance similar to that of biliary sludge. However, the sensitivity of transabdominal ultrasonography for biliary sludge is only about 60%, and this procedure cannot define the chemical composition of biliary sludge.136 The appearance of biliary sludge on EUS is similar to that on transabdominal ultrasonography, and the sensitivity of EUS is approximately 96%.19,137–140 On CT, biliary sludge has greater attenuation than normal bile and layers within the gallbladder. Its appearance on magnetic resonance imaging (MRI) has not been well characterized.141–144
The evaluation usually begins with transabdominal ultrasonography because it is noninvasive and relatively inexpensive.145–147 The combination of mucin gel and solid cholesterol crystals greater than 50 μm in size produce low-amplitude echoes without a postacoustic shadow that layer in the dependent portion of the gallbladder and shift with positional changes. Larger gallstones produce high-amplitude echoes and a postacoustic shadow (Fig. 5). The use of transabdominal ultrasonography is limited by its low sensitivity (~50%–60%) and inability to determine the chemical composition of bile or its microprecipitates. The low sensitivity may be due to the operator-dependent nature of this procedure, the tendency for classical plate-like cholesterol monohydrate crystals, but not calcium bilirubinate crystals, to be imaged, and the intermittent presence of biliary sludge or microlithiasis. Repeat examination could improve the diagnostic rate. Notably, compared to fundamental ultrasonography, harmonic ultrasonography shows better conspicuity of gallbladder microlithiasis.148
Fig. 5. Transversal (left) and longitudinal (right) ultrasonographic scans of the gallbladder from a 64-year-old man with occasional discovery of a solitary stone.

The thickness of gallbladder wall is normal (<3 mm) and the gallstone is visible as a round-shaped hyperechoic image (8 mm in diameter, as pointed out by a red asterisk), which is dependent with gravity and is producing a distal echoic shadow, as indicated by a red arrow. Abbreviation: L, liver.
Generally, bile microscopy is considered the gold standard for diagnosis. Bile may be collected from the duodenum (during endoscopy), bile duct (during ERCP), or the gallbladder itself (during ERCP or by percutaneous aspiration). The sensitivity of this test for biliary sludge or gallstones varies from 67% to 86%, and the specificity ranges from 88% to 100%.52,149 The sensitivity is 83% when bile is obtained directly from the common bile duct during ERCP.61 The sensitivity of bile microscopy would be even higher if bile is obtained through duodenal intubation than through endoscopy. If gallbladder bile is collected, the sensitivity of bile microscopy is probably the highest among all sampling methods. The diagnostic rate of bile microscopy is the highest when bile is collected from the gallbladder, followed by from the bile duct and then from the duodenum. Although the sensitivity of bile microscopy varies according to the site of bile aspiration, there is likely minimal difference between sampling techniques when gallbladder bile is collected. To enhance gallbladder contractility, improve the collection of gallbladder bile, and increase the diagnostic rate, cholecystokinin is often administered before bile aspiration.
Direct microscopic examination of the content of the gallbladder is more sensitive than ultrasonography in the detection of biliary sludge. Thus, even though it is less clinically applicable than ultrasonography, the microscopic examination of bile is considered the diagnostic gold standard. In addition, bile microscopy allows the chemical composition of biliary sludge to be defined by precipitate structure.
Although many different protocols for bile microscopy have been described, some measures can maximize the sensitivity of this test. It should be recognized that gallbladder bile is preferred for bile microscopy. Although hepatic bile is supersaturated with cholesterol, rapid transit through the biliary ductal system rarely allows enough time for hepatic bile to form a solid cholesterol crystal large enough to be detectable on polarizing light microscopy. Hepatic bile is invariably yellow and free of precipitate, even when the gallbladder contains biliary sludge, microlithiasis or gallstones. Thus, using ductal bile to look for biliary sludge in patients with intact gallbladders is inappropriate.
As discussed above, cholesterol monohydrate crystals demonstrate birefringence under polarizing light microscopy and are classically rhomboidal-shaped with a notched corner (Fig. 2). Calcium bilirubinate granules are non-birefringent and appear as brown or reddish-brown minute participates (Fig. 2). The number of solid cholesterol crystals required to establish a positive study varies widely among clinical investigations. Bile microscopy is a semiquantitative study with the number of solid cholesterol crystals being influenced by the method of sampling. Grading systems have been developed to quantify the number of solid cholesterol crystals, but this is mostly for research purposes and of little clinical utility. It has never been shown that the quantity of solid cholesterol crystals correlates with clinical outcome. Most investigators regard the presence of even a small number of solid cholesterol crystals as a positive result.
EUS has been less widely studied than bile microscopy.150 The sensitivity of EUS is 96% compared with 67% for duodenal drainage. However, the specificities of these two methods are similar from 86% to 91%. The sensitivity of EUS and bile microscopy combined is approximately 92%. More importantly, EUS operates at a higher frequency than transabdominal ultrasonography and minimizes the influence of bowel gas or subcutaneous tissue on image quality.151–153 EUS produces higher image resolution and greater diagnostic sensitivity (~95%). Stimulated biliary drainage can be performed at the time of EUS, which has been shown to further enhance diagnostic capability.
If patients with suspected biliary sludge have a negative result of transabdominal ultrasonography, further testing largely depends on the clinical situation. We recommend bile sampling for patients in whom the clinical suspicion of biliary sludge is high and in whom further treatment, such as cholecystectomy, would be considered. The choice of a method of bile sampling depends on the clinical situation.154 For example, if upper gastrointestinal tract disorders remain in the differential diagnosis, an upper gastrointestinal endoscopy should be performed and bile should be aspirated for bile microscopy during the procedure. If patients have no indication for upper gastrointestinal endoscopy, a bile sample could be obtained through duodenal intubation. Patients with recurrent episodes of idiopathic acute pancreatitis generally undergo ERCP, and bile can be sampled from the duodenum or the common bile duct during the procedure.155–157 If patients have an indication for EUS, such as evaluation of abnormalities found from previous imaging exams, they should undergo this procedure first. If biliary sludge is not identified on imaging exams, bile can be collected for microscopy. If patients have no indication for EUS, the test is not recommended because it has relatively low availability and high cost. Notably, ERCP is very helpful in finding gallstones in the common bile duct, as illustrated in Fig. 6.
Fig. 6. ERCP shows a filling defect image of a gallstone (as pointed out by a red arrow) of ~15 mm in diameter within the proximal CBD.

Abbreviations: ERCP, endoscopic retrograde cholangiopancreatography; CBD, common bile duct. We sincerely thank Dr. Giuseppe Scaccianoce for providing us with this picture.
As shown in Fig. 7, the optimal methods for detecting microlithiasis and the extent to which a diagnosis is sought largely depend on the clinical setting and prior evaluation.158–161 The two most widely used techniques are transabdominal ultrasonography and bile microscopy. Although CT and MRI may detect microlithiasis, their cost and unproven utility should preclude their use solely for this purpose unless otherwise indicated.17,162–164 As shown in Fig. 8, a gallstone is found by CT scans in the papilla of Vater in a patient with acute pancreatitis.
Fig. 7. Suggested algorithm for treatment in patients with microlithiasis-induced pancreatitis.

Abbreviations: CT, computed tomography; ERC, endoscopic cholangiography; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasonography; MRI, magnetic resonance imaging; US, ultrasonography.
Fig. 8. CT scans were performed in a patient with acute abdominal pain and elevated pancreatic enzymes.

Because this patient was allergic, the CT scans were performed in emergency without contrast medium injection. The final diagnosis was biliary pancreatitis. (A) and (B): A radiopaque gallstone of ~10 mm in diameter (arrow) is visible in the distal part of the common bile duct (i.e., the papilla of Vater). (A) and (C): The PA is enlarged due to edema. (B): The CBD is dilated above the stone to a diameter of 12.2 mm (D): The GB wall shows a normal thickness. Abbreviations: CT, computed tomography; PA, pancreas; CBD, common bile duct; GB, gallbladder. We sincerely thank Dr. Cristina Jablonska for providing us with these pictures.
Bile microscopy is the gold standard for the diagnosis of microlithiasis with an overall sensitivity of about 65%–90%. Bile sampling should be performed only if less invasive studies are negative, the clinical suspicion of microlithiasis is high, and the results could be used to guide management. Techniques vary with respect to the site of bile aspiration, use of cholecystokinin, processing of samples, and criteria for a positive test.
7. Treatment
Specific measures to prevent the development of biliary sludge are not practical or cost-effective in the general population. Although many studies have been performed concerning two potential preventive strategies, including use of the hydrophilic tertiary bile acid UDCA and an intestinal hormone cholecystokinin, none of these methods can be recommended for routine clinical use, even in high-risk populations.165
UDCA is an orally administered bile acid that has been extensively studied for the dissolution of cholesterol gallstones and the treatment of primary biliary cirrhosis.166–169 It reduces hepatic cholesterol secretion into bile and prolongs the time of cholesterol nucleation and crystallization. Some studies have investigated the effects of UDCA on the treatment of biliary sludge. In patients who are rapidly losing weight, UDCA reduces the incidence of gallstones by 50%–100%.170,171 In patients with biliary sludge and idiopathic acute pancreatitis, after the initial treatment with UDCA to dissolve solid cholesterol crystals, sustaining maintenance therapy successfully prevents the recurrence of biliary sludge and pancreatitis. Notably, UDCA effectively prevents the recurrence of solid cholesterol crystals and significantly reduces the risk of recurrent pancreatitis.30 The UDCA therapy may be a reasonable alternative in poor operative candidates or the very elderly.
Cholecystokinin or the cholecystokinin analogues could be used for prophylaxis against the development of biliary sludge in patients in whom gallbladder stasis is an underlying cause of biliary sludge, particularly in patients receiving prolonged total parenteral nutrition.172,173 Clinical studies have found that after patients initially free of biliary sludge or gallstones are randomly assigned to be treated with a daily intravenous infusion of cholecystokinin or placebo, no patients treated with cholecystokinin develops biliary sludge or gallstones.172 In contrast, 62% of the patients receiving placebo suffer from either biliary sludge or gallstones. Moreover, few side effects of cholecystokinin or cholecystokinin analogues are found in these patients. However, the safety and efficacy of cholecystokinin and the cholecystokinin analogues in other clinical settings have not been investigated yet.
In general, patients should be evaluated specifically for biliary sludge or gallstones only after they develop symptoms. Regularly monitoring asymptomatic patients for the development of biliary sludge or gallstones has not been generally done, even in high-risk patients. However, biliary sludge is often found incidentally on imaging exams performed for other reasons, and its management in these circumstances has been disputed. If a specific precipitating cause of biliary sludge is present, attempts should be made to eliminate it. For management purposes, biliary sludge and gallstones should be considered similar in almost all respects. Thus, asymptomatic patients with biliary sludge should be observed and managed expectantly (Fig. 4).
When symptoms or complications of biliary sludge occur, therapy should be considered immediately. Patients with uncomplicated biliary colic are at moderate risk for future pain and more serious complications, but up to 30% of patients have no further symptoms. Thus, clinical judgment must be used to decide whether to proceed with therapy in these patients.115 If more serious complications such as acute pancreatitis have occurred, therapy should be considered more strongly. The definitive therapy for biliary sludge is cholecystectomy, using either the laparoscopic or the open route.115 However, if patients are under poor surgical conditions, nonsurgical interventions, such as oral UDCA dissolution or percutaneous cholecystostomy for drainage, should be considered (Fig. 4).115 The long-term efficacy of these treatments has not been proven. Therefore, these treatments should be used only in patients who require therapy but are not under good surgical conditions. The recurrence rate of biliary sludge after oral UDCA dissolution is not known, but gallstones recur in approximately 50% of patients.167 The efficacy of percutaneous cholecystostomy and drainage in the treatment of biliary sludge has not been well established. Other therapeutic options include surgical intervention, endoscopic sphincterotomy, and chemical dissolution. Notably, the benefits of these therapies have been demonstrated by a significant decrease in recurrent episodes of pancreatitis after therapy (<10%) versus a recurrence rate of approximately 66%–75% in untreated patients.62,170,174–176
Laparoscopic cholecystectomy offers the most definitive therapy and is generally considered the treatment of choice (Fig. 4).122,177–179 Laparoscopic cholecystectomy is indicated in good operative candidates of almost any age because the risks of future gallbladder-related symptoms and complications outweigh the operative risks.90 Endoscopic sphincterotomy is an effective alternative and may obviate the need for cholecystectomy in the very elderly or those with significant comorbid illness.180–184 The benefits of sphincterotomy include enhanced gallbladder motility and reduced gallbladder stasis on the evidence given by a significant reduction in gallbladder fasting volume and residual volume and by an increase in gallbladder ejection fraction. This improvement in gallbladder motility after endoscopic sphincterotomy may persist for at least 5 years. Endoscopic sphincterotomy may reduce bile lithogenicity by modifying biliary lipid composition, leading to a significant prolongation in time of cholesterol nucleation and crystallization.
There is no demonstrable difference in the clinical benefit of cholecystectomy or endoscopic sphincterotomy for microlithiasis-induced pancreatitis.185 However, the use of endoscopic therapy is favored only in patients with poor operative conditions or the very elderly, unless their clinical course justifies more aggressive intervention.186 The continued risk of gallbladder-related symptoms or complications such as biliary colic, cholecystitis, and cholangitis after endoscopic sphincterotomy could need more definitive therapy in patients with good surgical conditions and with a reasonable life expectancy. The safety of laparoscopic cholecystectomy and the risk of immediate and late complications of endoscopic sphincterotomy further argue for the roles of these surgeries.
For the treatment of microlithiasis, endoscopic sphincterotomy is performed in 25 patients and cholecystectomy is carried out in two patients. Notably, this is partially explained by the coexistence of sphincter of Oddi dysfunction in many of these patients, which served as an indication for endoscopic sphincterotomy. However, in another studies on 12 patients with microlithiasis alone, only 2 are offered cholecystectomy. Interestingly, although endoscopic sphincterotomy is performed in all 5 patients with common bile duct stones, 4 of the 5 patients also undergo elective cholecystectomy. Biliary sphincter manometry has found that in 60 patients with biliary-type pain after cholecystectomy, 25 of whom ultimately prove to have elevated sphincter pressures. In addition, solid cholesterol monohydrated crystals are found in 3 patients, 2 without and 1 with elevated sphincter pressure. Of special note, in this selected group of patients, the vast majority of them have symptoms caused by sphincter of Oddi dysfunction.175,187 Taken together, the treatments of these patients may help us understand the choice of surgical intervention for microlithiasis.
In patients with acute recurrent pancreatitis, should the search for microlithiasis be abandoned or should such efforts be intensified? It is highly likely that such a decision is largely made based on the conditions including the clinical manifestation, approach to diagnosis, and indications for therapeutic intervention (Fig. 7).188–190 The presence of biliary sludge or microlithiasis in patients with acute recurrent pancreatitis usually leads to therapeutic intervention because of the tendency for pancreatitis to recur and the associated morbidity and mortality. Because of the prevalence of microlithiasis in this group of patients and the limited sensitivity of diagnostic studies, it is reasonable to proceed with laparoscopic cholecystectomy.191–195 Endoscopic sphincterotomy and UDCA administration are options for patients with poor operative conditions or the very elderly. If this approach is followed, there is no need to search for microlithiasis, because a negative evaluation would not alter your management plan.
However, for the patients who refrain from recommending therapeutic intervention without clear evidence of microlithiasis, transabdominal ultrasonography or laboratory evidence of microlithiasis may suffice. If stronger evidence is desired, bile microscopy during ERCP should be then considered. If the imaging exams continue to show no signs of microlithiasis, surveillance should be carried out by EUS with bile microscopy or repeating transabdominal ultrasonography in several months. If microlithiasis cannot be found despite all efforts and patients still experience recurrent pancreatitis, elective cholecystectomy should be reconsidered.191–195
8. Conclusions and future research directions
Biliary sludge is often found in diverse clinical situations, but has not yet been well understood. Its clinical course varies in which evolution to gallstones, a waxing and waning course, and complete resolution are all possible outcomes. In addition, biliary sludge could lead to biliary and/or pancreatic complications, including biliary colic, acute pancreatitis, and acute cholecystitis. It is most often diagnosed on transabdominal ultrasonography, although bile microscopy is considered the gold standard for diagnosis. If patients with biliary sludge are asymptomatic, they can be managed expectantly. If patients develop symptoms or complications, cholecystectomy should be considered as the definitive therapy.
Much is still to be learned about biliary sludge and microlithiasis. Continuing controversies involve the natural history of biliary sludge vs. microlithiasis, risk factors for their formation, and their true importance in the spectrum of biliary tract disease. Future prospective clinical studies may help clarify these issues and further increase our understanding of the pathogenesis of these biliary tract abnormalities.
Long-term and larger prospective studies are strongly needed to provide insights into the natural history, pathogenesis, clinical significance, ideal means of diagnosis, and optimum form of therapy on patients with biliary sludge or microlithiasis. Only then can our efforts be focused on the management of these patients.
Acknowledgements
This work was supported in part by research grants DK101793, DK114516 and DK106249, and AA025737 (to D. Q.-H. Wang), as well as P30 DK020541 (to Marion Bessin Liver Research Center), all from the National Institutes of Health (United States Public Health Service).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Wang DQ, Portincasa P. Recent advance in epidemiology, pathogenesis, diagnosis and management. New York: Nova Biomedical; 2017. [Google Scholar]
- 2.Wang DQ, Afdhal NH. Gallstone disease. In: Feldman M, Friedman LS, Brandt L, eds. Sleisenger and Fordtran’s gastrointestinal and liver disease. 10th ed. Philadelphia: Elsevier Saunders; 2014:1100–1133. [Google Scholar]
- 3.Ko CW, Sekijima JH, Lee SP. Biliary sludge. Ann Intern Med. 1999;130:301–311. [DOI] [PubMed] [Google Scholar]
- 4.Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326:589–593. [DOI] [PubMed] [Google Scholar]
- 5.Venneman NG, van Brummelen SE, van Berge-Henegouwen GP, van Erpecum KJ. Microlithiasis: An important cause of “idiopathic” acute pancreatitis? Ann Hepatol. 2003;2:30–35. [PubMed] [Google Scholar]
- 6.Gerke H, Baillie J. Biliary microlithiasis: A neglected cause of recurrent pancreatitis and biliary colic? J Gastroenterol Hepatol. 2005;20:499–501. [DOI] [PubMed] [Google Scholar]
- 7.Shaffer EA. Gallbladder sludge: What is its clinical significance? Curr Gastroenterol Rep. 2001;3:166–173. [DOI] [PubMed] [Google Scholar]
- 8.Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149:1731–1741 (e3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell RM, Byrne MF. Biliary emergencies: Pancreatitis, cholangitis, and more. Semin Gastrointest Dis. 2003;14:77–86. [PubMed] [Google Scholar]
- 10.Mitchell RM, Byrne MF, Baillie J. Pancreatitis. Lancet. 2003;361:1447–1455. [DOI] [PubMed] [Google Scholar]
- 11.Vidarsdottir H, Moller PH, Vidarsdottir H, Thorarinsdottir H, Björnsson ES. Acute pancreatitis: A prospective study on incidence, etiology, and outcome. Eur J Gastroenterol Hepatol. 2013;25:1068–1075. [DOI] [PubMed] [Google Scholar]
- 12.McNabb-Baltar J, Ravi P, Isabwe GA, et al. A population-based assessment of the burden of acute pancreatitis in the United States. Pancreas. 2014;43:687–691. [DOI] [PubMed] [Google Scholar]
- 13.Jüngst C, Kullak-Ublick GA, Jüngst D. Gallstone disease: Microlithiasis and sludge. Best Pract Res Clin Gastroenterol. 2006;20:1053–1062. [DOI] [PubMed] [Google Scholar]
- 14.Lee SP, Hayashi A, Kim YS. Biliary sludge: Curiosity or culprit? Hepatology. 1994;20:523–525. [PubMed] [Google Scholar]
- 15.Angelico M, Della Guardia P. Review article: Hepatobiliary complications associated with total parenteral nutrition. Aliment Pharmacol Ther. 2000;14(Suppl 2):54–57. [DOI] [PubMed] [Google Scholar]
- 16.Janowitz P, Kratzer W, Zemmler T, Tudyka J, Wechsler JG. Gallbladder sludge: Spontaneous course and incidence of complications in patients without stones. Hepatology. 1994;20:291–294. [PubMed] [Google Scholar]
- 17.Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol. 2001;96:2540–2555. [DOI] [PubMed] [Google Scholar]
- 18.Messing B, Bories C, Kunstlinger F, Bernier JJ. Does total parenteral nutrition induce gallbladder sludge formation and lithiasis? Gastroenterology. 1983;84:1012–1019. [PubMed] [Google Scholar]
- 19.Bolondi L, Gaiani S, Testa S, Labò G. Gall bladder sludge formation during prolonged fasting after gastrointestinal tract surgery. Gut. 1985;26:734–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houssin D, Castaing D, Lemoine J, Bismuth H. Microlithiasis of the gallbladder. Surg Gynecol Obstet. 1983;157:20–24. [PubMed] [Google Scholar]
- 21.Goodman AJ, Neoptolemos JP, Carr-Locke DL, Finlay DB, Fossard DP. Detection of gall stones after acute pancreatitis. Gut. 1985;26:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cucher D, Kulvatunyou N, Green DJ, Jie T, Ong ES. Gallstone pancreatitis: A review. Surg Clin North Am. 2014;94:257–280. [DOI] [PubMed] [Google Scholar]
- 23.Conrad MR, Janes JO, Dietchy J. Significance of low level echoes within the gallbladder. AJR Am J Roentgenol. 1979;132:967–972. [DOI] [PubMed] [Google Scholar]
- 24.Kaw M, Brodmerkel GJ Jr. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic recurrent pancreatitis. Gastrointest Endosc. 2002;55:157–162. [DOI] [PubMed] [Google Scholar]
- 25.Cooperman M, Ferrara JJ, Carey LC, Thomas FB, Martin EW Jr, Fromkes JJ. Idiopathic acute pancreatitis: The value of endoscopic retrograde cholangiopancreatography. Surgery. 1981;90:666–670. [PubMed] [Google Scholar]
- 26.Feller ER. Endoscopic retrograde cholangiopancreatography in the diagnosis of unexplained pancreatitis. Arch Intern Med. 1984;144:1797–1799. [PubMed] [Google Scholar]
- 27.Geenen JE, Nash JA. The role of sphincter of Oddi manometry and biliary microscopy in evaluating idiopathic recurrent pancreatitis. Endoscopy. 1998;30:A237–A241. [DOI] [PubMed] [Google Scholar]
- 28.Sharma BC, Agarwal DK, Dhiman RK, Baijal SS, Choudhuri G, Saraswat VA. Bile lithogenicity and gallbladder emptying in patients with microlithiasis: Effect of bile acid therapy. Gastroenterology. 1998;115:124–128. [DOI] [PubMed] [Google Scholar]
- 29.Fracchia M, Pellegrino S, Secreto P, et al. Biliary lipid composition in cholesterol microlithiasis. Gut. 2001;48:702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ros E, Navarro S, Bru C, Garcia-Pugés A, Valderrama R. Occult microlithiasis in ‘idiopathic’ acute pancreatitis: Prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology. 1991;101:1701–1709. [DOI] [PubMed] [Google Scholar]
- 31.Lee SP, Nicholls JF. Nature and composition of biliary sludge. Gastroenterology. 1986;90:677–686. [DOI] [PubMed] [Google Scholar]
- 32.Lee SP, Maher K, Nicholls JF. Origin and fate of biliary sludge. Gastroenterology. 1988;94:170–176. [DOI] [PubMed] [Google Scholar]
- 33.Angelico M, De Santis A, Capocaccia L. Biliary sludge: A critical update. J Clin Gastroenterol. 1990;12:656–662. [DOI] [PubMed] [Google Scholar]
- 34.Chevreul ME. Examen des graisses d’homme, de mouton, de boeuf, de jaguar et d’oie. Ann Chim Phys. 1816;2:339–372. [Google Scholar]
- 35.Berthelot M Sur plusiers alcools nouveaux. Ann Chim Phys. 1859;56:51e98. [Google Scholar]
- 36.Reinitzer F Beitrage zur Kenntniss des Cholesterins. Sitzber Akad Wiss Wien Mathnaturw K1 Abt I. 1888;97:167–187. [Google Scholar]
- 37.Wang DQ, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: Influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res. 1996;37:606–630. [PubMed] [Google Scholar]
- 38.Wang DQ, Carey MC. Characterization of crystallization pathways during cholesterol precipitation from human gallbladder biles: Identical pathways to corresponding model biles with three predominating sequences. J Lipid Res. 1996;37:2539–2549. [PubMed] [Google Scholar]
- 39.Gantz DL, Wang DQ, Carey MC, Small DM. Cryoelectron microscopy of a nucleating model bile in vitreous ice: Formation of primordial vesicles. Biophys J. 1999;76:1436–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HH, Portincasa P, Liu M, Tso P, Samuelson LC, Wang DQ. Effect of gallbladder hypomotility on cholesterol crystallization and growth in CCK-deficient mice. Biochim Biophys Acta. 2010;1801:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.JUNIPER K Jr, BURSON EN Jr. Biliary tract studies. II. The significance of biliary crystals. Gastroenterology. 1957;32:175–208; discussion, 208–211. [PubMed] [Google Scholar]
- 42.Ramond MJ, Dumont M, Belghiti J, Erlinger S. Sensitivity and specificity of microscopic examination of gallbladder bile for gallstone recognition and identification. Gastroenterology. 1988;95:1339–1343. [DOI] [PubMed] [Google Scholar]
- 43.Burnstein MJ, Vassal KP, Strasberg SM. Results of combined biliary drainage and cholecystokinin cholecystography in 81 patients with normal oral cholecystograms. Ann Surg. 1982;196:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porterfield G, Cheung LY, Berenson M. Detection of occult gallbladder disease by duodenal drainage. Am J Surg. 1977;134:702–704. [DOI] [PubMed] [Google Scholar]
- 45.Foss DC, Laing RR. Detection of gallbladder disease in patients with normal oral cholecystograms. Results using a simplified biliary drainage technique. Am J Dig Dis. 1977;22:685–689. [DOI] [PubMed] [Google Scholar]
- 46.Sigman HH, Goldberg N, Niloff PH, Lachance C. Use of biliary drainage in diagnosis of biliary tract disease. Am J Gastroenterol. 1977;67:439–443. [PubMed] [Google Scholar]
- 47.Reisberg IR, Mabee GW. Endoscopic biliary drainage for detection of gallbladder disease. Gastrointest Endosc. 1979;25:6–9. [DOI] [PubMed] [Google Scholar]
- 48.Susann PW, Sheppard F, Baloga AJ. Detection of occult gallbladder disease by duodenal drainage collected endoscopically. A clinical and pathologic correlation. Am Surg. 1985;51:162–165. [PubMed] [Google Scholar]
- 49.Abeysuriya V, Deen KI, Navarathne NM. Biliary microlithiasis, sludge, crystals, microcrystallization, and usefulness of assessment of nucleation time. Hepatobiliary Pancreat Dis Int. 2010;9:248–253. [PubMed] [Google Scholar]
- 50.Landi K, Sinard J, Crawford JM, Topazian M. Cholesterol crystal morphology in acalculous gallbladder disease. J Clin Gastroenterol. 2003;36:364–366. [DOI] [PubMed] [Google Scholar]
- 51.Neoptolemos JP, Davidson BR, Winder AF, Vallance D. Role of duodenal bile crystal analysis in the investigation of ‘idiopathic’ pancreatitis. Br J Surg. 1988;75:450–453. [DOI] [PubMed] [Google Scholar]
- 52.Buscail L, Escourrou J, Delvaux M, et al. Microscopic examination of bile directly collected during endoscopic cannulation of the papilla. Utility in patients with suspected microlithiasis. Dig Dis Sci. 1992;37:116–120. [DOI] [PubMed] [Google Scholar]
- 53.Filly RA, Allen B, Minton MJ, Bernhoft R, Way LW. In vitro investigation of the origin of echoes with biliary sludge. J Clin Ultrasound. 1980;8:193–200. [DOI] [PubMed] [Google Scholar]
- 54.Lee SP. Pathogenesis of biliary sludge. Hepatology. 1990;12:200S–203S; discussion 203S–205S. [PubMed] [Google Scholar]
- 55.Sedaghat A, Grundy SM. Cholesterol crystals and the formation of cholesterol gallstones. N Engl J Med. 1980;302:1274–1277. [DOI] [PubMed] [Google Scholar]
- 56.Mouiel J, Chauvin P, Borelli JP, Bus JJ, Giaume F, Bourgeon R. Role of biliary microlithiasis in acute pancreatitis. Apropos of a series of 31 cases. Chirurgie. 1975;101:258–265. [PubMed] [Google Scholar]
- 57.Marks JW, Bonorris G. Intermittency of cholesterol crystals in duodenal bile from gallstone patients. Gastroenterology. 1984;87:622–627. [PubMed] [Google Scholar]
- 58.Block MA, Priest RJ. Acute pancreatitis related to grossly minute stones in a radiographically normal gallbladder. Am J Dig Dis. 1967;12:934–938. [DOI] [PubMed] [Google Scholar]
- 59.Negro P, Flati G, Flati D, Porowska B, Tuscano D, Carboni M. Occult gallbladder microlithiasis causing acute recurrent pancreatitis. A report of three cases. Acta Chir Scand. 1984;150:503–506. [PubMed] [Google Scholar]
- 60.Goldstein F, Kucer FT, Thornton JJ 3rd, Abramson J. Acute and relapsing pancreatitis caused by bile pigment aggregates and diagnosed by biliary drainage. Am J Gastroenterol. 1980;74:225–230. [PubMed] [Google Scholar]
- 61.Delchier JC, Benfredj P, Preaux AM, Metreau JM, Dhumeaux D. The usefulness of microscopic bile examination in patients with suspected microlithiasis: A prospective evaluation. Hepatology. 1986;6:118–122. [DOI] [PubMed] [Google Scholar]
- 62.Saraswat VA, Sharma BC, Agarwal DK, Kumar R, Negi TS, Tandon RK. Biliary microlithiasis in patients with idiopathic acute pancreatitis and unexplained biliary pain: Response to therapy. J Gastroenterol Hepatol. 2004;19:1206–1211. [DOI] [PubMed] [Google Scholar]
- 63.Maringhini A, Marcenò MP, Lanzarone F, et al. Sludge and stones in gallbladder after pregnancy. Prevalence and risk factors. J Hepatol. 1987;5:218–223. [DOI] [PubMed] [Google Scholar]
- 64.Maringhini A, Ciambra M, Baccelliere P, et al. Biliary sludge and gallstones in pregnancy: Incidence, risk factors, and natural history. Ann Intern Med. 1993;119:116–120. [DOI] [PubMed] [Google Scholar]
- 65.Ko CW, Beresford SA, Schulte SJ, Matsumoto AM, Lee SP. Incidence, natural history, and risk factors for biliary sludge and stones during pregnancy. Hepatology. 2005;41:359–365. [DOI] [PubMed] [Google Scholar]
- 66.Everson GT. Pregnancy and gallstones. Hepatology. 1993;17:159–161. [PubMed] [Google Scholar]
- 67.Valdivieso V, Covarrubias C, Siegel F, Cruz F. Pregnancy and cholelithiasis: Pathogenesis and natural course of gallstones diagnosed in early puerperium. Hepatology. 1993;17:1–4. [PubMed] [Google Scholar]
- 68.Park HZ, Lee SP, Schy AL. Ceftriaxone-associated gallbladder sludge. Identification of calcium-ceftriaxone salt as a major component of gallbladder precipitate. Gastroenterology. 1991;100:1665–1670. [DOI] [PubMed] [Google Scholar]
- 69.Ko CW, Schulte SJ, Lee SP. Biliary sludge is formed by modification of hepatic bile by the gallbladder mucosa. Clin Gastroenterol Hepatol. 2005;3:672–678. [DOI] [PubMed] [Google Scholar]
- 70.Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res. 2009;50(Suppl):S406–S411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang HH, Portincasa P, Wang DQ. Molecular pathophysiology and physical chemistry of cholesterol gallstones. Front Biosci. 2008;13:401–423. [DOI] [PubMed] [Google Scholar]
- 72.Carey MC, Lamont JT. Cholesterol gallstone formation. 1. Physical-chemistry of bile and biliary lipid secretion. Prog Liver Dis. 1992;10:139–163. [PubMed] [Google Scholar]
- 73.Carey MC, Cohen DE. Update on physical state of bile. Ital J Gastroenterol. 1995;27:92–100. [PubMed] [Google Scholar]
- 74.Freeman JB, Meyer PD, Printen KJ, Mason EE, DenBesten L. Analysis of gallbladder bile in morbid obesity. Am J Surg. 1975;129:163–166. [DOI] [PubMed] [Google Scholar]
- 75.Shaffer EA, Braasch JW, Small DM. Bile composition at and after surgery in normal persons and patients with gallstones. Influence of cholecystectomy. N Engl J Med. 1972;287:1317–1322. [DOI] [PubMed] [Google Scholar]
- 76.Shaffer EA, Small DM. Biliary lipid secretion in cholesterol gallstone disease. The effect of cholecystectomy and obesity. J Clin Invest. 1977;59:828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaffer EA, Small DM. Gallstone disease: Pathogenesis and management. Curr Probl Surg. 1976;13:3–72. [DOI] [PubMed] [Google Scholar]
- 78.Donovan JM, Carey MC. Separation and quantitation of cholesterol “carriers” in bile. Hepatology. 1990;12:94S–104S; discussion 104S–105S. [PubMed] [Google Scholar]
- 79.Small DM. Cholesterol nucleation and growth in gallstone formation. N Engl J Med. 1980;302:1305–1307. [DOI] [PubMed] [Google Scholar]
- 80.Bourgès M, Small DM, Dervichian DG. Biophysics of lipid associations. 3. The quaternary systems lecithin-bile salt-cholesterol-water. Biochim Biophys Acta. 1967;144:189–201. [PubMed] [Google Scholar]
- 81.Bourgès M, Small DM, Dervichian DG. Biophysics of lipidic associations. II. The ternary systems: Cholesterol-lecithin-water. Biochim Biophys Acta. 1967;137:157–167. [DOI] [PubMed] [Google Scholar]
- 82.Brecher P, Chobanian J, Small DM, Chobanian AV. The use of phospholipid vesicles for in vitro studies on cholesteryl ester hydrolysis. J Lipid Res. 1976;17:239–247. [PubMed] [Google Scholar]
- 83.Carey MC. Aqueous bile salt-lecithin-cholesterol systems: Equilibrium aspects. Hepatology. 1984;4:151S–154S. [DOI] [PubMed] [Google Scholar]
- 84.Hay DW, Cahalane MJ, Timofeyeva N, Carey MC. Molecular species of lecithins in human gallbladder bile. J Lipid Res. 1993;34:759–768. [PubMed] [Google Scholar]
- 85.Wang HH, Portincasa P, Afdhal NH, Wang DQ. Lith genes and genetic analysis of cholesterol gallstone formation. Gastroenterol Clin North Am. 2010;39:185–207, vii–viii. [DOI] [PubMed] [Google Scholar]
- 86.Wang DQ, Afdhal NH. Gallstone disease. In: Feldman M, Friedman LS, Brandt L, eds. Sleisenger and Fordtran’s gastrointestinal and liver disease. 9th ed. Philadelphia: Elsevier Saunders; 2010:1089–1120. [Google Scholar]
- 87.Méndez-Sánchez N, Chavez-Tapia NC, Motola-Kuba D, et al. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol. 2005;11:1653–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nervi F, Miquel JF, Alvarez M, et al. Gallbladder disease is associated with insulin resistance in a high risk Hispanic population. J Hepatol. 2006;45:299–305. [DOI] [PubMed] [Google Scholar]
- 89.Portincasa P, Di Ciaula A, Wang HH, et al. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology. 2008;47:2112–2126. [DOI] [PubMed] [Google Scholar]
- 90.Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers. 2016;2:16024. [DOI] [PubMed] [Google Scholar]
- 91.Krawczyk M, Wang DQ, Portincasa P, Lammert F. Dissecting the genetic heterogeneity of gallbladder stone formation. Semin Liver Dis. 2011;31:157–172. [DOI] [PubMed] [Google Scholar]
- 92.de Bari O, Wang TY, Liu M, Paik CN, Portincasa P, Wang DQ. Cholesterol cholelithiasis in pregnant women: pathogenesis, prevention and treatment. Ann Hepatol. 2014;13:728–745. [PubMed] [Google Scholar]
- 93.Portincasa P, Moschetta A, Ciaula AD, et al. Pathophysiology and cholesterol gallstone disease. In: Borzellino G, Cordiano C, eds. Biliary lithiasis: Basic science, current diagnosis and management. 1st ed. Biliary Lithiasis. Springer Milan; 2008:19–49. [Google Scholar]
- 94.Wang DQ, Afdhal NH. Genetic analysis of cholesterol gallstone formation: Searching for Lith (gallstone) genes. Curr Gastroenterol Rep. 2004;6:140–150. [DOI] [PubMed] [Google Scholar]
- 95.Lammert F, Miquel JF. Gallstone disease: From genes to evidence-based therapy. J Hepatol. 2008;48(Suppl 1):S124–S135. [DOI] [PubMed] [Google Scholar]
- 96.Lammert F, Matern S. The genetic background of cholesterol gallstone formation: an inventory of human lithogenic genes. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:163–170. [DOI] [PubMed] [Google Scholar]
- 97.Wang DQ, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: Physical-chemistry of gallbladder bile. J Lipid Res. 1997;38:1395–1411. [PubMed] [Google Scholar]
- 98.Lee SP, LaMont JT, Carey MC. Role of gallbladder mucus hypersecretion in the evolution of cholesterol gallstones. J Clin Invest. 1981;67:1712–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marks JW, Bonorris GG, Albers G, Schoenfield LJ. The sequence of biliary events preceding the formation of gallstones in humans. Gastroenterology. 1992;103:566–570. [DOI] [PubMed] [Google Scholar]
- 100.Shiffman ML, Sugerman HJ, Kellum JM, Brewer WH, Moore EW. Gallstone formation after rapid weight loss: A prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86:1000–1005. [PubMed] [Google Scholar]
- 101.de Vree JM, Jacquemin E, Sturm E, et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci U S A. 1998;95: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deleuze JF, Jacquemin E, Dubuisson C, et al. Defect of multidrug-resistance 3 gene expression in a subtype of progressive familial intrahepatic cholestasis. Hepatology. 1996;23:904–908. [DOI] [PubMed] [Google Scholar]
- 103.Jacquemin E, Hadchouel M. Genetic basis of progressive familial intrahepatic cholestasis. J Hepatol. 1999;31:377–381. [DOI] [PubMed] [Google Scholar]
- 104.Jacquemin E, Cresteil D, Manouvrier S, Boute O, Hadchouel M. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet. 1999;353:210–211. [DOI] [PubMed] [Google Scholar]
- 105.Lammert F, Marschall HU, Glantz A, Matern S. Intrahepatic cholestasis of pregnancy: Molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33:1012–1021. [DOI] [PubMed] [Google Scholar]
- 106.Dixon PH, Sambrotta M, Chambers J, et al. An expanded role for heterozygous mutations of ABCB4, ABCB11, ATP8B1, ABCC2 and TJP2 in intrahepatic cholestasis of pregnancy. Sci Rep. 2017;7:11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosmorduc O, Hermelin B, Poupon R. MDR3 gene defect in adults with symptomatic intrahepatic and gallbladder cholesterol cholelithiasis. Gastroenterology. 2001;120:1459–1467. [DOI] [PubMed] [Google Scholar]
- 108.Rosmorduc O, Hermelin B, Boelle PY, Parc R, Taboury J, Poupon R. ABCB4 gene mutation-associated cholelithiasis in adults. Gastroenterology. 2003;125: 452–459. [DOI] [PubMed] [Google Scholar]
- 109.Rosmorduc O, Poupon R. Low phospholipid associated cholelithiasis: association with mutation in the MDR3/ABCB4 gene. Orphanet J Rare Dis. 2007;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poupon R, Rosmorduc O, Boëlle PY, et al. Genotype-phenotype relationships in the low-phospholipid-associated cholelithiasis syndrome: A study of 156 consecutive patients. Hepatology. 2013;58:1105–1110. [DOI] [PubMed] [Google Scholar]
- 111.Jacquemin E Role of multidrug resistance 3 deficiency in pediatric and adult liver disease: One gene for three diseases. Semin Liver Dis. 2001;21:551–562. [DOI] [PubMed] [Google Scholar]
- 112.Sundaram SS, Sokol RJ. The multiple facets of ABCB4 (MDR3) deficiency. Curr Treat Options Gastroenterol. 2007;10:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lucena JF, Herrero JI, Quiroga J, et al. A multidrug resistance 3 gene mutation causing cholelithiasis, cholestasis of pregnancy, and adulthood biliary cirrhosis. Gastroenterology. 2003;124:1037–1042. [DOI] [PubMed] [Google Scholar]
- 114.Di Ciaula A, Wang DQ, Portincasa P. An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol. 2018;34:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Portincasa P, Wang DQ. Gallstones. In: Podolsky DK, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC, eds. Yamada’s textbook of gastroenterology. 6th ed. 2. Hoboken, New Jersey: Wiley-Blackwell; 2015:1808–1834. [Google Scholar]
- 116.Smit JJ, Schinkel AH, Oude Elferink RP, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. [DOI] [PubMed] [Google Scholar]
- 117.Oude Elferink RP, Ottenhoff R, van Wijland M, Smit JJ, Schinkel AH, Groen AK. Regulation of biliary lipid secretion by mdr2 P-glycoprotein in the mouse. J Clin Invest. 1995;95:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Groen AK, Van Wijland MJ, Frederiks WM, Smit JJ, Schinkel AH, Oude Elferink RP. Regulation of protein secretion into bile: studies in mice with a disrupted mdr2 p-glycoprotein gene. Gastroenterology. 1995;109:1997–2006. [DOI] [PubMed] [Google Scholar]
- 119.Oude Elferink RP, Ottenhoff R, van Wijland M, Frijters CM, van Nieuwkerk C, Groen AK. Uncoupling of biliary phospholipid and cholesterol secretion in mice with reduced expression of mdr2 P-glycoprotein. J Lipid Res. 1996;37:1065–1075. [PubMed] [Google Scholar]
- 120.Fein F, Hermelin B, Becker MC, Felix S, Carbonnel F. Acute recurrent biliary pancreatitis associated with the ABCB4 gene mutation. Gastroenterol Clin Biol. 2007;31:106–109. [DOI] [PubMed] [Google Scholar]
- 121.Lammert F, Wang DQ, Hillebrandt S, et al. Spontaneous cholecysto- and hepatolithiasis in Mdr2−/− mice: a model for low phospholipid-associated cholelithiasis. Hepatology. 2004;39:117–128. [DOI] [PubMed] [Google Scholar]
- 122.Alexakis N, Lombard M, Raraty M, et al. When is pancreatitis considered to be of biliary origin and what are the implications for management? Pancreatology. 2007;7:131–141. [DOI] [PubMed] [Google Scholar]
- 123.Wang DQ, Cohen DE, Lammert F, Carey MC. No pathophysiologic relationship of soluble biliary proteins to cholesterol crystallization in human bile. J Lipid Res. 1999;40:415–425. [PubMed] [Google Scholar]
- 124.van Erpecum KJ, Wang DQ, Moschetta A, et al. Gallbladder histopathology during murine gallstone formation: Relation to motility and concentrating function. J Lipid Res. 2006;47:32–41. [DOI] [PubMed] [Google Scholar]
- 125.Wang HH, Liu M, Portincasa P, Tso P, Wang DQ. Lack of endogenous cholecystokinin promotes cholelithogenesis in mice. Neurogastroenterol Motil. 2016;28:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang HH, Portincasa P, Wang DQ. The cholecystokinin-1 receptor antagonist devazepide increases cholesterol cholelithogenesis in mice. Eur J Clin Invest. 2016;46:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang TY, Portincasa P, Liu M, Tso P, Wang DQ. Mouse models of gallstone disease. Curr Opin Gastroenterol. 2018;34:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pazzi P, Gamberini S, Buldrini P, Gullini S. Biliary sludge: The sluggish gallbladder. Dig Liver Dis. 2003;35(Suppl 3):S39–S45. [DOI] [PubMed] [Google Scholar]
- 129.Diehl AK. Gallstone size and the risk of gallbladder cancer. JAMA. 1983;250:2323–2326. [PubMed] [Google Scholar]
- 130.Shaffer E Acalculous biliary pain: new concepts for an old entity. Dig Liver Dis. 2003;35(Suppl 3):S20–S25. [DOI] [PubMed] [Google Scholar]
- 131.Heaton KW. Diagnosis of acute non-specific abdominal pain. Lancet. 2000;355:1644. [DOI] [PubMed] [Google Scholar]
- 132.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–II47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hearing SD, Thomas LA, Heaton KW, Hunt L. Effect of cholecystectomy on bowel function: A prospective, controlled study. Gut. 1999;45:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Poulin EC, Schlachta CM, Mamazza J. Early laparoscopy to help diagnose acute non-specific abdominal pain. Lancet. 2000;355:861–863. [DOI] [PubMed] [Google Scholar]
- 135.Venu RP, Geenen JE, Toouli J, Stewart E, Hogan WJ. Endoscopic retrograde cholangiopancreatography. Diagnosis of cholelithiasis in patients with normal gallbladder x-ray and ultrasound studies. JAMA. 1983;249:758–761. [DOI] [PubMed] [Google Scholar]
- 136.Portincasa P, Wang DQ. Gallstones. In: Podolsky DK, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC, eds. Yamada’s atlas of gastroenterology. 5th ed. Hoboken, New Jersey: Wiley-Blackwell; 2016:335–353. [Google Scholar]
- 137.Dahan P, Andant C, Lévy P, et al. Prospective evaluation of endoscopic ultrasonography and microscopic examination of duodenal bile in the diagnosis of cholecystolithiasis in 45 patients with normal conventional ultrasonography. Gut. 1996;38:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cooperberg PL, Gibney RG. Imaging of the gallbladder. Radiology. 1987;163:605–613. [DOI] [PubMed] [Google Scholar]
- 139.Cano N, Cicero F, Ranieri F, Martin J, di Costanzo J. Ultrasonographic study of gallbladder motility during total parenteral nutrition. Gastroenterology. 1986;91:313–317. [DOI] [PubMed] [Google Scholar]
- 140.Gaf a M, Sarli L, Miselli A, Pietra N, Carreras F, Peracchia A. Sludge and microlithiasis of the biliary tract after total gastrectomy and postoperative total parenteral nutrition. Surg Gynecol Obstet. 1987;165:413–418. [PubMed] [Google Scholar]
- 141.Calvo MM, Bujanda L, Heras I, et al. Magnetic resonance cholangiography versus ultrasound in the evaluation of the gallbladder. J Clin Gastroenterol. 2002;34:233–236. [DOI] [PubMed] [Google Scholar]
- 142.Calvo MM, Bujanda L, Calderón A, et al. Comparison between magnetic resonance cholangiopancreatography and ERCP for evaluation of the pancreatic duct. Am J Gastroenterol. 2002;97:347–353. [DOI] [PubMed] [Google Scholar]
- 143.Calvo MM, Bujanda L, Calderón A, et al. Role of magnetic resonance cholangiopancreatography in patients with suspected choledocholithiasis. Mayo Clin Proc. 2002;77:422–428. [DOI] [PubMed] [Google Scholar]
- 144.Cavdar F, Yildar M, Tellioğlu G, Kara M, Tilki M, Titiz Mİ. Controversial issues in biliary pancreatitis: When should we perform MRCP and ERCP? Pancreatology. 2014;14:411–414. [DOI] [PubMed] [Google Scholar]
- 145.Şurlin V, Săftoiu A, Dumitrescu D. Imaging tests for accurate diagnosis of acute biliary pancreatitis. World J Gastroenterol. 2014;20:16544–16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fusaroli P, Kypraios D, Caletti G, Eloubeidi MA. Pancreatico-biliary endoscopic ultrasound: A systematic review of the levels of evidence, performance and outcomes. World J Gastroenterol. 2012;18:4243–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.De Lisi S, Leandro G, Buscarini E. Endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography in acute biliary pancreatitis: A systematic review. Eur J Gastroenterol Hepatol. 2011;23:367–374. [DOI] [PubMed] [Google Scholar]
- 148.Choi CS, Ku YJ, Yoon DY, et al. Harmonic ultrasonography for the detection of microlithiasis in the gallbladder. Ultrasonography. 2014;33:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moskovitz M, Min TC, Gavaler JS. The microscopic examination of bile in patients with biliary pain and negative imaging tests. Am J Gastroenterol. 1986;81:329–333. [PubMed] [Google Scholar]
- 150.Wilcox CM, Seay T, Kim H, Varadarajulu S. Prospective endoscopic ultrasound-based approach to the evaluation of idiopathic pancreatitis: Causes, response to therapy, and long-term outcome. Am J Gastroenterol. 2016;111:1339–1348. [DOI] [PubMed] [Google Scholar]
- 151.Anderloni A, Galeazzi M, Ballarè M, et al. Early endoscopic ultrasonography in acute biliary pancreatitis: A prospective pilot study. World J Gastroenterol. 2015;21:10427–10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Anderloni A, Repici A. Role and timing of endoscopy in acute biliary pancreatitis. World J Gastroenterol. 2015;21:11205–11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Anderloni A, Ballarè M, Pagliarulo M, et al. Prospective evaluation of early endoscopic ultrasonography for triage in suspected choledocholithiasis: Results from a large single centre series. Dig Liver Dis. 2014;46:335–339. [DOI] [PubMed] [Google Scholar]
- 154.Levy MJ. The hunt for microlithiasis in idiopathic acute recurrent pancreatitis: Should we abandon the search or intensify our efforts? Gastrointest Endosc. 2002;55:286–293. [DOI] [PubMed] [Google Scholar]
- 155.Dill JE. Endosonography/bile drainage combination for difficult-to-diagnose gallbladder disease. J Laparoendosc Adv Surg Tech A. 1998;8:361–365. [DOI] [PubMed] [Google Scholar]
- 156.Dill JE, Dill BP. EUS for idiopathic pancreatitis. Gastrointest Endosc. 2006;64:845; author reply 845–847. [DOI] [PubMed] [Google Scholar]
- 157.Dill JE, Hill S, Callis J, Berkhouse L, Evans P, Martin D. Combined endoscopic ultrasound and stimulated biliary drainage in the diagnosis of cholecystitis and microlithiasis. Endoscopy. 1995;27:218. [DOI] [PubMed] [Google Scholar]
- 158.Andersson R, Andersson B, Haraldsen P, Drewsen G, Eckerwall G. Incidence, management and recurrence rate of acute pancreatitis. Scand J Gastroenterol. 2004;39:891–894. [DOI] [PubMed] [Google Scholar]
- 159.Ardengh JC, Malheiros CA, Rahal F, Pereira V, Ganc AJ. Microlithiasis of the gallbladder: Role of endoscopic ultrasonography in patients with idiopathic acute pancreatitis. Rev Assoc Med Bras. 1992;56:27–31, 2010. [DOI] [PubMed] [Google Scholar]
- 160.Dill JE, Dill BP. Microlithiasis and pancreatitis. Gastrointest Endosc. 2002;56:784; author reply 784–785. [DOI] [PubMed] [Google Scholar]
- 161.Al-Haddad M, Wallace MB. Diagnostic approach to patients with acute idiopathic and recurrent pancreatitis, what should be done? World J Gastroenterol. 2008;14:1007–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thevenot A, Bournet B, Otal P, Canevet G, Moreau J, Buscail L. Endoscopic ultrasound and magnetic resonance cholangiopancreatography in patients with idiopathic acute pancreatitis. Dig Dis Sci. 2013;58:2361–2368. [DOI] [PubMed] [Google Scholar]
- 163.Venu RP, Geenen JE, Hogan W, Stone J, Johnson GK, Soergel K. Idiopathic recurrent pancreatitis. An approach to diagnosis and treatment. Dig Dis Sci. 1989;34:56–60. [DOI] [PubMed] [Google Scholar]
- 164.Sarva RP, Farivar S, Fromm H, Poller W. Study of the sensitivity and specificity of computerized tomography in the detection of calcified gallstones which appears radiolucent by conventional roentgenography. Gastrointest Radiol. 1981;6:165–167. [DOI] [PubMed] [Google Scholar]
- 165.Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–e15. [DOI] [PubMed] [Google Scholar]
- 166.Di Ciaula A, Wang DQ, Garruti G, et al. Therapeutic reflections in cholesterol homeostasis and gallstone disease: A review. Curr Med Chem. 2014;21:1435–1447. [DOI] [PubMed] [Google Scholar]
- 167.Di Ciaula A, Wang DQ, Wang HH, Bonfrate L, Portincasa P. Targets for current pharmacologic therapy in cholesterol gallstone disease. Gastroenterol Clin North Am. 2010;39:245–264. Viii–ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Portincasa P, Ciaula AD, Bonfrate L, Wang DQ. Therapy of gallstone disease: what it was, what it is, what it will be. World J Gastrointest Pharmacol Ther. 2012;3:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Portincasa P, Di Ciaula A, de Bari O, Garruti G, Palmieri VO, Wang DQ. Management of gallstones and its related complications. Expert Rev Gastroenterol Hepatol. 2016;10:93–112. [DOI] [PubMed] [Google Scholar]
- 170.Shiffman ML, Kaplan GD, Brinkman-Kaplan V, Vickers FF. Prophylaxis against gallstone formation with ursodeoxycholic acid in patients participating in a very-low-calorie diet program. Ann Intern Med. 1995;122:899–905. [DOI] [PubMed] [Google Scholar]
- 171.Broomfield PH, Chopra R, Sheinbaum RC, et al. Effects of ursodeoxycholic acid and aspirin on the formation of lithogenic bile and gallstones during loss of weight. N Engl J Med. 1988;319:1567–1572. [DOI] [PubMed] [Google Scholar]
- 172.Sitzmann JV, Pitt HA, Steinborn PA, Pasha ZR, Sanders RC. Cholecystokinin prevents parenteral nutrition induced biliary sludge in humans. Surg Gynecol Obstet. 1990;170:25–31. [PubMed] [Google Scholar]
- 173.Wu ZS, Yu L, Lin YJ, et al. Rapid intravenous administration of amino acids prevents biliary sludge induced by total parenteral nutrition in humans. J Hepatobiliary Pancreat Surg. 2000;7:504–509. [DOI] [PubMed] [Google Scholar]
- 174.Portincasa P, Moschetta A, Puglisi F, et al. Medical treatment of gallstone disease. In: Borzellino G, Cordiano C, eds. Biliary lithiasis: Basic science, current diagnosis and management. Springer Milan; 2008:149–157. [Google Scholar]
- 175.Testoni PA, Caporuscio S, Bagnolo F, Lella F. Idiopathic recurrent pancreatitis: Long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol. 2000;95:1702–1707. [DOI] [PubMed] [Google Scholar]
- 176.Barton P, Steininger R, Maier A, Mühlbacher F, Lechner G. Biliary sludge after liver transplantation: 2. Treatment with interventional techniques versus surgery and/or oral chemolysis. AJR Am J Roentgenol. 1995;164:865–869. [DOI] [PubMed] [Google Scholar]
- 177.Stevens CL, Abbas SM, Watters DA. How does cholecystectomy influence recurrence of idiopathic acute pancreatitis? J Gastrointest Surg. 2016;20:1997–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol. 2007;5:75–79. [DOI] [PubMed] [Google Scholar]
- 179.Räty S, Pulkkinen J, Nordback I, et al. Can laparoscopic cholecystectomy prevent recurrent idiopathic acute pancreatitis?: A prospective randomized multicenter trial. Ann Surg. 2015;262:736–741. [DOI] [PubMed] [Google Scholar]
- 180.Carr-Locke DL. Role of endoscopy in gallstone pancreatitis. Am J Surg. 1993;165:519–521. [DOI] [PubMed] [Google Scholar]
- 181.Carr-Locke DL. Acute gallstone pancreatitis and endoscopic therapy. Endoscopy. 1990;22:180–183. [DOI] [PubMed] [Google Scholar]
- 182.Neoptolemos JP, Carr-Locke DL, London N, Bailey I, Fossard DP. ERCP findings and the role of endoscopic sphincterotomy in acute gallstone pancreatitis. Br J Surg. 1988;75:954–960. [DOI] [PubMed] [Google Scholar]
- 183.Neoptolemos JP, London N, Slater ND, Carr-Locke DL, Fossard DP, Moosa AR. A prospective study of ERCP and endoscopic sphincterotomy in the diagnosis and treatment of gallstone acute pancreatitis. A rational and safe approach to management. Arch Surg. 1986;121:697–702. [DOI] [PubMed] [Google Scholar]
- 184.Soetikno RM, Carr-Locke DL. Endoscopic management of acute gallstone pancreatitis. Gastrointest Endosc Clin N Am. 1998;8:1–12. [PubMed] [Google Scholar]
- 185.Yaghoobi M ERCP for gallstone pancreatitis. N Engl J Med. 2014;370:1955–1956. [DOI] [PubMed] [Google Scholar]
- 186.Ricci F, Castaldini G, de Manzoni G, Borzellino G, Rodella L, Kind R. Minimally invasive treatment of acute biliary pancreatitis. Surg Endosc. 1997;11:1179–1182. [DOI] [PubMed] [Google Scholar]
- 187.Elton E, Howell DA, Parsons WG, Qaseem T, Hanson BL. Endoscopic pancreatic sphincterotomy: Indications, outcome, and a safe stentless technique. Gastrointest Endosc. 1998;47:240–249. [DOI] [PubMed] [Google Scholar]
- 188.Jain R Biliary sludge: When should it not be ignored? Curr Treat Options Gastroenterol. 2004;7:105–109. [DOI] [PubMed] [Google Scholar]
- 189.Das R, Yadav D, Papachristou GI. Endoscopic treatment of recurrent acute pancreatitis and smoldering acute pancreatitis. Gastrointest Endosc Clin N Am. 2015;25:737–748. [DOI] [PubMed] [Google Scholar]
- 190.Monkhouse SJ, Court EL, Dash I, Coombs NJ. Two-week target for laparoscopic cholecystectomy following gallstone pancreatitis is achievable and cost neutral. Br J Surg. 2009;96:751–755. [DOI] [PubMed] [Google Scholar]
- 191.Kamal A, Akhuemonkhan E, Akshintala VS, Singh VK, Kalloo AN, Hutfless SM. Effectiveness of guideline-recommended cholecystectomy to prevent recurrent pancreatitis. Am J Gastroenterol. 2017;112:503–510. [DOI] [PubMed] [Google Scholar]
- 192.Hernandez V, Pascual I, Almela P, et al. Recurrence of acute gallstone pancreatitis and relationship with cholecystectomy or endoscopic sphincterotomy. Am J Gastroenterol. 2004;99:2417–2423. [DOI] [PubMed] [Google Scholar]
- 193.Uhl W, Warshaw A, Imrie C, et al. IAP guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2:565–573. [DOI] [PubMed] [Google Scholar]
- 194.Toouli J, Brooke-Smith M, Bassi C, et al. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17(Suppl):S15–S39. [DOI] [PubMed] [Google Scholar]
- 195.Tenner S, Baillie J, DeWitt J, et al. American college of gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415, 1416. [DOI] [PubMed] [Google Scholar]


