Abstract
By collecting photons scattered out of the therapy beam, scatter imaging creates images of the treated volume. Two phantoms were used to assess the possible application of scatter imaging for markerless tracking of lung tumors during stereotactic body radiation therapy (SBRT) treatment. A scatter-imaging camera was assembled with a CsI flat-panel detector and a 5 mm diameter pinhole collimator. Scatter images were collected during the irradiation of phantoms with megavoltage photons. To assess scatter image quality, spherical phantom lung tumors of 2.1–2.8 cm diameters were placed inside a static, anthropomorphic phantom. To show the efficacy of the technique with a moving target (3 cm diameter), the position of a simulated tumor was tracked in scatter images during sinusoidal motion (15 mm amplitude, 0.25 Hz frequency) in a dynamic lung phantom in open-field, dynamic conformal arc (DCA), and volumetric modulated arc therapy (VMAT) deliveries. Anatomical features are identifiable on static phantom scatter images collected with 10 MU of delivered dose (2.1 cm diameter lung tumor contrast-to-noise ratio of 4.4). The contrast-to-noise ratio increases with tumor size and delivered dose. During dynamic motion, the position of the 3.0 cm diameter lung tumor was identified with a root-mean-square error of 0.8, 1.2, and 2.9 mm for open field (0.3 s frame integration), DCA (0.5 s), and VMAT (0.5 s), respectively. Based on phantom studies, scatter imaging is a potential technique for markerless lung tumor tracking during SBRT without additional imaging dose. Quality scatter images may be collected at low, clinically relevant doses (10 MU). Scatter images are capable of sub-millimeter tracking precision, but modulation decreases accuracy.
Keywords: Compton scatter, scatter imaging, image guidance, real-time, lung SBRT, tumor tracking, markerless
1. Introduction
Lung stereotactic body radiation therapy (SBRT) is an effective and efficient treatment technique for early-stage non-small cell lung cancer (Timmerman et al 2010, Shirvani et al 2012, Menten et al 2017, Ball et al 2019). In SBRT, large doses per fraction (10–34 Gy) are delivered to the tumor in one to five fractions (Bezjak et al 2019; Videtic et al 2015). Compared to treatments with conventional fractionation, SBRT treatments delivering similar physical doses in fewer fractions result in higher biologically effective doses, thereby increasing tumor control probability. However, delivering such large fractional doses while avoiding normal tissue complications requires smaller target margins and, therefore, methods to reduce treatment uncertainties such as prospective motion mitigation and image guidance. Compared to standard fractionation target delineation, where 1–1.5 cm is added to the clinical target volume (CTV) to give the planning target volume (PTV) (Hansen and Roach III 2010), reduced SBRT margins are facilitated by accurate internal target volume (ITV) demarcation on 4DCT and the minimization of setup margin through (i) more aggressive patient immobilization, (ii) pre-treatment CBCT for accurate patient positioning, and (iii) sub-millimeter technological accuracy in radiation delivery. Currently, a typical SBRT PTV is defined by adding a 5 mm set-up margin to the internal target volume (ITV) identified on the 4DCT (Bezjak et al 2019).
Although not widely adopted in the clinic, intrafraction imaging during delivery provides both verification of safe treatment and the possibility of further reduction in margins (Caillet et al 2017, Bertholet et al 2019). As a monitoring tool, intrafraction imaging ensures that the dose delivered to the tumor and normal tissues matches the planned dose. This is particularly important given observed deficiencies in 4DCT imaging, which has been shown to under-predict breathing-induced motion (Shi et al 2013). If identified during real-time monitoring, motion unaccounted-for by planning margins can trigger beam stoppage and/or off-line adaptation in the form of a replan. If gating is used, tracking the target directly via imaging will reduce errors from relying on external surrogates (Berbeco et al 2005b). If beam- (Schweikard et al 2000), multileaf collimator (MLC)- (Hansen et al 2016, Booth et al 2016), or couch-tracking (D’Souza et al 2005, Wilbert et al 2008, 2013) is possible, intrafraction imaging provides the guidance for real-time adaptation that may reduce the margins to the point that PTV = CTV + δ. In the theoretical limit δ approaches 0, but, realistically, the delay (latency) between image collection, analysis, and application of corrective motion requires the addition of a margin (δ) unless a sufficiently accurate predictive model can be used to estimate the future tumor position based on previous behavior. Reductions in tumor margins of up to 50% have been demonstrated (Keall et al 2019). Consequently, with tracking, only the tumor + δ is treated, not the full potential volume of motion. This possible reduction in PTV volume will allow for further dose escalation, improved normal tissue sparing, and concomitant better outcomes with less complications (Caillet et al 2017).
With the notable exception of the commercially available Cyberknife Synchrony (Sayeh et al 2007) (fiducial markers) and Xsight (Bahig et al 2013) (markerless), lung SBRT intra-fraction imaging techniques (electromagnetic transponder (Booth et al 2016), kV (Shirato et al 2000, Kim et al 2017), MV (Richter et al 2010), MR (Menten et al 2017)) have not been widely adopted due to toxicity associated with fiducial marker implantation (Calypso, marker-based kV and MV), additional imaging dose (kV), poor contrast (MV), cost (MR, room-mounted kV), obstruction from the gantry head (room-mounted kV), and possibly long latency times (MR) (Green et al 2018).
Scatter imaging is a potential markerless technique for identifying the real-time position of lung tumors during SBRT (Redler et al 2018, Jones et al 2018). When a patient is treated with a megavoltage (MV) therapy beam, photons are scattered in all directions due to Compton scattering. These scattered photons may be collected to create an image of the treated volume. Scatter imaging is advantageous because there is no additional imaging dose, both the beam path and tumor are imaged, there is high contrast (only the irradiated volume scatters), simultaneous images may be collected from many vantage points if multiple cameras are employed, and no fiducials are required (Redler et al 2018). The technique is also conceptually and technologically simple—it only requires a collimator and a detector. Tracking breathing-induced tumor motion during lung SBRT is the ideal application for scatter imaging because: (1) tracking may provide clinical benefit (Caillet et al 2017), (2) the high dose rates used in SBRT maximize the number of photons collected at clinically-relevant time frames (<0.5 s) (Keall et al 2006), and (3) the large (electron) density differences between tumor (ρ ~ 1 g cm−3) and healthy lung (ρ ~ 0.25 g cm−3) translate into large intensity differences in the scatter images because the Compton scatter cross section is proportional to electron density.
To assess the possibility of scatter-image tumor tracking during SBRT treatments, planar scatter images of lung phantoms were collected with a single, pinhole-collimator camera during irradiation. For comparison, MV transmission images were simultaneously recorded with the electronic portal-imaging device (EPID). Two phantoms were used. An anthropomorphic torso phantom was used to assess scatter image quality at variable deposited dose for three different sized tumors. A dynamic phantom was used to assess scatter-image tracking accuracy. First, to provide a best-case benchmark, the dynamic phantom was used to place a tumor at fixed, stepwise positions, and its location was measured on high-dose, low-noise scatter images. Second, to characterize accuracy with motion, scatter images were collected with the dynamically moving phantom at variable frame rates during the delivery of three plan types: fixed open-field, dynamic conformal arc (DCA), and volumetric modulated arc therapy (VMAT).
2. Methods
2.1. Experimental setup
The experimental setup is depicted in figure 1. A Perkin Elmer XRD 0822 AP3 amorphous silicon detector (1024 × 1024 pixels, 200 μm pitch, 16 bit, 550 μm CsI:Tl, up to 30 Hz frame collection) was mounted on a conical 7 mm thick lead pinhole collimator outfitted with a knife-edge 5 mm diameter pinhole (Redler et al 2018). To reduce unwanted penetration through the pinhole collimator, additional shielding in the form of Wood’s metal blocks (thickness 1.8 ± 0.1 cm; 50% Bi, 26.7% Pb, 13.3% W, 10% Cd) was added between the gantry head and pinhole collimator, between the isocenter and pinhole collimator, and on the distal side of the pinhole collimator (to attenuate photons back-scattered from walls), as shown in figure 1(a). The camera was pointed at isocenter and positioned for 1:1 magnification: the isocenter-to-pinhole distance was equal to the detector-to-pinhole distance (18 cm). 6 MV radiation was delivered at 1200 MU min−1 in flattening filter free (FFF) mode by a TrueBeam linear accelerator (Varian Medical Systems (Palo Alto, CA, USA); HD120 MLC; 1 MU = 1 cGy at 100 cm SAD, depth = 1.5 cm for a 10 × 10 cm2 field). Transmission images were collected with the gantry-mounted aS1000 EPID. To avoid collision with the camera frame, the gantry rotation was limited to 290–211°.
Figure 1.
(a) A schematic of the experimental apparatus. Diagram is not to scale. Photons passing through the pinhole (solid lines) create a scatter image with inverted coordinates. Additional Wood’s metal shielding blocks were added to reduce collimator penetration. For example, head leakage (dotted line) and phantom scatter photons (dashed line) may penetrate the collimator wall. (b) A photo of the experimental setup with LUNGMAN phantom.
2.2. Phantoms
To characterize scatter image quality with an anthropomorphic phantom, spherical tumors of diameters 21, 25, and 28 mm were hand crafted using Adapt-It thermoplastic beads (RPD Inc. Albertville, MN, ρ = 1.1 g cm3). Each phantom tumor was consecutively placed inside the middle of the right lung of the N1 LUNGMAN multi-purpose chest phantom (Kyoto Kagaku Co., Ltd, Kyoto, Japan). The LUNGMAN phantom (figures 2(a) and (b)) is an accurate anatomical phantom with radiological properties that match human tissues. Although the branching of the bronchi is re-created with intricate phantom structures, much of the lung space is filled with air. Therefore, the scatter images collected with LUNGMAN are expected to exhibit more contrast and less attenuation than a human lung, where the healthy lung tissue has a density of ~0.25 g cm3 (Van Dyk et al 1982).
Figure 2.

An axial (a) and coronal (b) CT slice of the static LUNGMAN anthropomorphic phantom are shown with inserted 2.1 cm diameter lung tumor. An axial (c) and coronal (d) CT slice of the dynamic QUASAR phantom with lung tumor insert.
To characterize tracking accuracy in a dynamically moving phantom, a spherical phantom tumor (Adapt-It thermoplastic beads) of 30 mm diameter was embedded in a polyurethane foam cylinder of density 0.23 g cm−3. This cylinder served as the moving lung in a QUASAR Respiratory Motion Phantom (Modus Medical Devices Inc. London, ON, Canada; figures 2(c) and (d)), with a sinusoidal inferior-superior (IS) motion of amplitude 15 mm and frequency of 15 breaths per minute (BPM). Due to mixing imperfections, there was a low-density section of foam inferior to the tumor that had a mass density of 0.18 g cm−3.
2.2.1. Treatment planning
The phantoms were irradiated at 1200 MU min−1 with three beam types: fixed open-field, DCA, and VMAT. Although both DCA and VMAT plans are delivered in arcs, the techniques use different apertures to irradiate the PTV. In DCA, the aperture is adjusted to match the outer-edge of the PTV—as projected in the beam’s-eye-view—to irradiate the full PTV at every gantry angle. In VMAT, parts of the PTV are blocked by MLC leaves at some gantry positions such that the cumulative dose over the full delivery provides a distribution that is typically more conformal than that achieved by DCA delivery.
To provide best-case scatter images, phantoms were irradiated with jaw-defined (rectangular) open-field beams at 270° fixed gantry position. Jaw-defined, rectangular fields are not used clinically for SBRT treatments, but they provide unobstructed irradiation of the tumor and surrounding anatomy, and their simple geometry makes image interpretation easier. The anthropomorphic phantom was irradiated with a 4 × 4 cm2 beam (anterior-posterior (AP) x IS) to assess scatter image quality. A 4 × 30 cm2 was also used for characterization purposes to irradiate the entire SI extent of the phantom. 4 × 8 cm2 rectangular fields were used to capture the full dynamic motion of the tumor in the QUASAR phantom.
DCA and VMAT arcs were planned using the 4DCT images collected of the moving QUASAR phantom. Consistent with clinical practice at our institution and Bezjak et al (2019), the embedded spherical tumor was contoured on the 10 phase images of the 4DCT to create the ITV. The PTV was defined as a 5 mm expansion of the ITV. The DCA and VMAT arcs were designed to deliver 18 Gy to the PTV using two partial arcs from 30–181°. In both plans, the counter-clockwise arc was split into three sub-arcs (30–290, 290–211, 211–181°). For this study, only the 290–211° portion was delivered because the other sub-arcs would have caused a collision between the gantry head and the frame holding the scatter camera. The DCA arc was forward-planned using apertures that conformed to the PTV. The VMAT arc was inversely optimized with modulation, and it delivered 1.7 times more MU than the DCA plan over the 290–211° arc segment. The mean dose to the PTV from these DCA and VMAT 290–211° arc segments was 4.0 Gy and 4.4 Gy, respectively.
2.3. Scatter image collection and analysis
The 200 μm pixel pitch of the CsI detector is much smaller than the effective scatter image spatial resolution, which is limited by the 5 mm diameter pinhole. To reduce statistical noise, images were downsampled to 2 mm effective pitch in post-processing. Unless noted otherwise, the presented images were flipped vertically and horizontally (inverted axes) in post-processing to account for the pinhole so that the axes are consistent with viewing from a position anterior to the phantom. In the dynamic QUASAR studies, images were collected at frame rates of 1.67–4 Hz.
In an attempt to remove collimator-penetrating photons, scatter images were collected under identical conditions with and without the phantoms. The final presented scatter image is formed by subtracting the scatter image with the phantom from the background image without the phantom.
The position of the tumor in the QUASAR phantom was calculated with cross-correlation-based template tracking. Although common (e.g. Berbeco et al 2005a, Block et al 2016, Hazelaar et al 2018), the specific template tracking used here is described below. For the MV EPID images, a single frame image of the tumor was used as a template, and the tumor position was determined based on the maximum intensity in the cross correlation (convolution) of the template with the analyzed image frame. For a static tumor, analysis of 20 back-to-back MV images identified the same tumor position, indicating the accuracy of the cross correlation method is <0.26 mm (the projected pixel pitch) when applied to the MV EPID images. For scatter images, a 2.2–2.5 cm full-width-half-maximum Gaussian was used as the template, as shown in figure 3. After calculating the cross correlation (figure 3(c)) and identifying the point of maximum intensity, a Gaussian peak was then fit to the cross-correlation peak to provide sub-pixel resolution. For the scatter images collected during DCA and VMAT deliveries, the Laplacian (∇ ·∇) of the cross correlation was calculated and used for determining the tumor position (figure 3(d)). To improve the accuracy of tumor identification during the VMAT delivery, where portions of the tumor are not irradiated due to modulation, the final fitting step was modified. Rather than fitting the Laplacian peak to a Gaussian, the peak was fit to a Gaussian multiplied with the approximated beam fluence as measured on the chest wall (figure 3(f)). This final step is based on the simple relationship between the fluence reaching each voxel (F(r)), the electron density of the voxel (ρe(r)), and the number of photons Compton-scattered from that voxel (C(r)): C(r) ∝ F(r)ρe(r). The goal is to correct for the non-uniform fluence in VMAT deliveries to reveal the underlying electron density (assumed to be a Gaussian, figure 3(g)) so that the algorithm does not simply track features of the fluence distribution inside the patient instead of the tumor. Aside from limiting the search area to a subset of the image (box marked on figure 3(d)), no prior knowledge of the expected tumor position is used to modify the template used for tracking.
Figure 3.
(a–g) show the data analysis steps whereby the QUASAR phantom tumor position is identified on the scatter image during VMAT irradiation. Each dynamic scatter image (a) was convolved with a Gaussian template (b) to calculate a cross correlation image (c). For the dynamic studies irradiated with a fixed, open field, a Gaussian was fit to the cross-correlation image (c) to determine the position of the tumor. For dynamic studies irradiated with DCA or VMAT, the Laplacian (d) was calculated from the cross-correlation images. For DCA irradiation a Gaussian (g) was directly fit to the Laplacian image. For VMAT-formed images, the entrance fluence (f) was approximated by projecting the scatter intensity from the entrance chest wall, and the product (e) of this fluence (f) and the fitting Gaussian (g) was fit to the Laplacian image (d). The red circles on (a) and (g) indicate the position of the tumor identified through this process. Based on prior knowledge of the phantom anatomy, potential tumor positions were limited to a subset of the image (box marked in (d)).
To calibrate the tumor position in the QUASAR phantom measurements, transmission MV images were collected during the stepwise and open-field dynamic measurements. The amplitude of the tumor motion was determined by fitting a sinusoidal function to the tumor position identified in the MV image data (considered the ‘gold standard’ in this study). This MV data was collected with a fixed open-field irradiation either simultaneously (for the open-field studies) or immediately before (for the arc studies) scatter image collection. To register the MV and scatter datasets in time, the scatter tumor position data was then shifted in time to best fit this sinusoidal function. The offset of the sinusoidal function was also allowed to change to self-calibrate the registration between the camera and treatment room inferior-superior (IS) coordinates. Therefore, the reported root-mean-square errors represent a measure of precision.
The contrast-to-noise-ratio (CNR) was calculated on phantom scatter images as [mean(tumor)—mean(lung tissue)]/std dev(tumor).
3. Results
3.1. Scatter image processing
A 100 MU scatter image collected during irradiation of the QUASAR phantom while fixed at a static position is shown in figure 4. The phantom was irradiated from the phantom’s right (270° gantry angle) with a 4 × 8 cm2 (AP x IS) jaw-defined open field. The raw image (not flipped, collected with phantom: figure 4(a)), background (not flipped, no phantom: figure 4(b)), and final image (raw minus background, flipped to account for pinhole effect: figure 4(c)) are shown along with horizontal profiles through each image (all are flipped) at 0 cm inferior-superior (IS). Scatter images are 2D projections of the photons that pass through the collimator after being scattered out of the irradiated volume. Ideally, only the photons that pass through the pinhole aperture are collected. Here, the IS extent of the field was smaller than the field-of-view of the camera, and only the irradiated volume appears. The usable image was circular due to the conical shape of the pinhole collimator, and the bright corners correspond to saturated areas outside of the shielded collimator. The background, which was due to collimator penetration from head leakage and scatter from sources other than the phantom(jaws/gantry head, back room wall), decreased with distance from the gantry head. Because the background photons penetrate the collimator wall, they were not inverted by the pinhole like the signal photons. The QUASAR phantom right chest wall (entrance), tumor, and mediastinum (exit) are visible in both the raw and raw-minus-background images in figures 4(a)-(c).
Figure 4.
(a, Raw) 100 MU scatter image of the QUASAR phantom. (b, Bkg) 100 MU background scatter image collected under the same conditions as Raw, but without the QUASAR phantom. (c, Raw-Bkg) The difference image between Raw and Bkg. Raw and Bkg are not flipped, while Raw-Bkg has been flipped around (0,0) to correct for the pinhole coordinate inversion. Chest wall (i), tumor (ii), and mediastinum (iii) are labelled. (d, Profile) Scatter image profiles are displayed. Dashed lines in (a–c) indicate the locations of the profiles all flipped along IS = 0 cm. IS = Inferior—Superior. RL = Right—Left. Brighter pixel values correspond to higher photon counts. Colorbars display counts (arb. units).
3.2. Image quality: static anthropomorphic scatter images
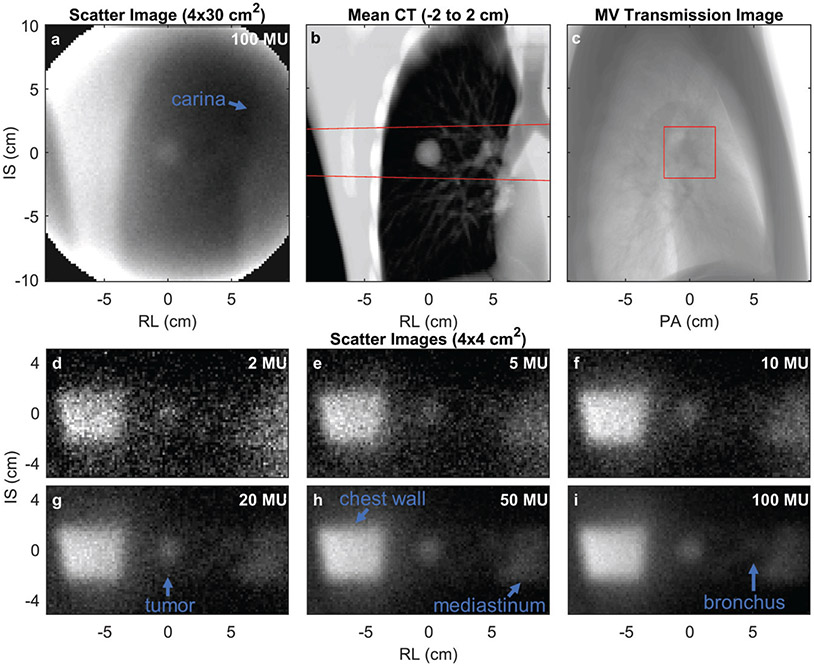
To assess scatter image quality, scatter images were collected during irradiation of the LUNGMAN phantom. The scatter image collected during a wide field irradiation (4 × 30 cm2) is shown in figure 5(a). For comparison, the irradiated volume is shown as an average of the irradiated coronal CT slices in figure 5(b). Anatomic features observed in the scatter image include the right chest wall, tumor, increased intensity associated with the primary bronchus, and the mediastinum, which displays decreased intensity from the air in the carina. Similar to scatter imaging, MV transmission imaging is a potential tracking technique whose images may be collected during treatment without additional imaging dose. A beam’s-eye-view MV transmission image (3 MU) of the LUNGMAN phantom was collected in the same geometry as the presented scatter images, and is shown in figure 5(c). This MV transmission image was collected with a large field-of-view to orient the viewer, and the actual 4 × 4 cm2 field size used for the scatter image experiments is marked with a box on the image. The tumor is not identifiable on the lateral MV transmission image (figure 5(c)).
Figure 5.
(a) A LUNGMAN scatter image (lung tumor φ = 2.1 cm) collected during irradiation from gantry = 270° with a 4 × 30 cm2 treatment field. (b) The corresponding coronal CT image averaged over the irradiated depth (−2 to 2 cm) shown in (a). (c) The MV transmission image collected for a 20 × 20 cm2 irradiation from LUNGMAN’s right (gantry = 270°). (d–i) The LUNGMAN scatter images collected with various indicated doses during irradiation with a 4 – 4 cm2 beam. The red lines in (b + c) indicate the beam path irradiated for collection of (d–i). PA = posterior—anterior. Anatomic structures are labelled on (a, g-i).
Using a 4 × 4 cm2 jaw-defined field (see field edge shown in figures 5(b)-(c)), scatter images were collected during the delivery of 2, 5, 10, 20, 50, and 100 MU (frame integration times of 0.1, 0.25, 0.5, 1, 2.5, and 5 s at 1200 MU min−1) from the phantom’s right side (gantry at 270°, figures 5(d)-(i)). The entrance chest wall (left side of image), tumor, and mediastinum are identifiable in the images. For the images collected with ≥20 MU, the primary bronchus is also visible. The CNR calculated on these images is plotted as a function of delivered MU in figure 6. The CNR increases with delivered dose and with lung tumor phantom diameter.
Figure 6.
The LUNGMAN tumor contrast-to-noise-ratio for scatter images collected with various doses during irradiation with a 4 × 4 cm2 beam. Different tumor diameters (φ) are indicated. The images for φ = 2.1 cm are shown in figures 5(d)-(i). Each data point represents the CNR measured on a scatter image collected with the specified MU.
3.3. Tracking precision
3.3.1. Stepwise scatter images
To characterize how precisely the position of lung tumors can be identified on scatter images, a lung tumor phantom was placed at fixed positions using the QUASAR phantom. The position was measured with both an MV transmission image and a 100 MU scatter image. The difference between the MV-measured and scatter-measured positions are shown in figure 7. The displayed data has been shifted (registered) to minimize the difference between the MV and scatter measured positions. Therefore, the results do not reflect any global systematic errors that might arise from misregistration between the scatter-image camera coordinates and the room coordinates.
Figure 7.
The difference between the static QUASAR phantom tumor position (IS direction, z) identified in corresponding MV transmission images and scatter images (see figure 4 Raw-Bkg for an example image). The standard deviation of the differences is 0.2 mm. The characterized zMV uncertainty (standard deviation) is ≤0.26 mm.
3.3.2. Dynamic scatter images
During dynamic tumor motion (15 mm amplitude, 4 s period), the QUASAR phantom lung tumor position was identified during static open field, DCA, and VMAT irradiations. Example scatter images and measured position traces are plotted in figure 8.
Figure 8.
From dynamic QUASAR phantom studies, the MV transmission images (a, d, g), corresponding scatter images (b, e, h), and measured tumor positions (c, f, i) are shown for a fixed open 4 × 8 cm2 field (300 ms frames, gantry = 270°, a–c), a dynamic conformal arc delivery (500 ms frames, d–f), and a VMAT delivery (500 ms frames, g–i). The positions identified in the images shown in (a–b, d–e, g–h) are indicated with arrows in (c, f, i). u is the horizontal MV EPID image coordinate. In the shown VMAT-irradiation case (g and h), most of the bottom portion of the tumor is shadowed due to blocking from multi-leaf collimator leaves.
For the QUASAR studies, the root-mean-square-errors between the scatter- and MV- identified tumor positions are tabulated in table 1. As the scatter image frame integration time is increased from 0.3 to 0.6 s or 0.25 to 0.5 s, the accuracy of identifying the tumor position improves. As the plan complexity is increased from a fixed open field to a dynamic conformal arc to a VMAT plan, the scatter imaging tumor tracking precision degrades.
Table 1.
Compared to MV-identified positions, the root-mean-squared error (RMSE) is shown for QUASAR tumor positions identified in scatter images collected with variable frame integration times and plan types. The dynamic motion, 4 × 8 cm2 delivery field data RMSE are averaged over five trials, and the standard deviation is provided.
| Motion | Plan type | Integration time (ms) | MU / frame | RMSE (mm) |
|---|---|---|---|---|
| Stepwise | 4 x 8 cm2 | 5000 | 100 | 0.2 |
| Dynamic | 4 x 8 cm2 | 300 | 6 | 0.8 ± 0.08 |
| 4 x 8 cm2 | 600 | 12 | 0.6 ± 0.02 | |
| DCA | 250 | 5 | 1.4 | |
| DCA | 500 | 10 | 1.2 | |
| VMAT | 250 | 5 | 3.9 | |
| VMAT | 500 | 10 | 2.9 |
4. Discussion:
As shown in previous experimental and simulation studies (Yan et al 2016, Redler et al 2018, Jones et al 2018, Garnica-Garza 2018), scatter imaging is a potentially useful technique for image guidance during radiation therapy. Using a simple pinhole camera (figure 1), photons scattered during radiation therapy delivery can be collected to form scatter images. As shown by comparing figures 5(a)-(i), only the irradiated volume appears in the scattered image. This provides high contrast, but portions of the tumor that are not irradiated during the scatter image collection time will appear dark in the image. As an indication of the high contrast relative to transmission imaging, the tumor and other anatomical features are easily identified on scatter images (figure 5(i)), but difficult to discern on the corresponding MV portal image (figure 5(c)).
Although a 7-mm-thick pinhole collimator was used, additional Wood’s metal shielding was added to reduce collimator wall penetration from gantry head leakage, scatter off of the jaws, and scatter from the phantom. As observed in the background image (figure 4 Bkg), there is increased intensity near the gantry head, which is attributed to high-energy collimator penetration from head leakage photons and photons scattered off of the primary gantry jaws. Note that when the image is flipped to correct for pinhole coordinate inversion (which only applies physically to photons passing through the pinhole aperture, not penetrating the collimator), this penetration intensity appears to be on the opposite side from the gantry head (figure 4 Profiles). When the phantom is placed in the beam, there is additional intensity that appears in the image (figure 4 Raw). In addition to the desired pinhole-imaged features, there is also intensity in areas where none is expected, such as appearing to originate from air in front of the phantom (compare Raw and Bkg profiles at RL = −9 cm in figure 4) and from outside the treated volume (inferior and superior to the horizontal beam path imaged in figure 4). This additional, uniform intensity is attributed to photons scattered from the phantom that penetrate through the pinhole collimator walls (for example, see dashed line in figure 1(a)).
As described in the Methods, the presented scatter images are difference images in which a background (collected without the phantom) has been subtracted. Clinically, this requires collecting scatter images during delivery to an empty couch prior to treatment so that these background images may be subtracted from the patient treatment images in real-time. This is not ideal, as it involves extra effort and subtraction adds noise to the image. Ideally, the collimator thickness would be increased to reduce penetration to levels far below signal levels. Two potential sources of photons, head leakage (up to 6 MeV) and scattered photons (≤ 511 keV at 90° scatter angle), are both expected to be higher energy than diagnostic keV photons. As a result, attenuation of background photons to negligible levels may not be logistically possible. For example, the lead half-value layer for 6 and 0.511 MeV photons is 1.4 and 0.4 cm, respectively.
As shown in figures 5 and 6, at clinical dose rates, quality scatter images are achievable with sufficiently fast acquisition for real-time image guidance. For example, for a 2.1 cm diameter tumor inserted into the anthropomorphic LUNGMAN phantom, a tumor CNR of 4.4 is measured for 10 MU (0.5 s at 1200 MU min−1) of beam-on time, which substantially drops with shorter exposures. As the tumor size is increased, the CNR increases due to the larger number of photons scattered out of the larger tumor volume.
To characterize the tracking precision under ideal, low-noise conditions, scatter images of the QUASAR phantom in static positions were collected during a 100 MU delivery with a static open 4 × 8 cm2 field (figure 4 Raw-Bkg, figure 7). Under these low-noise situations, the tumor was tracked with a RMSE of 0.2 mm. These measurements provide a best-case scenario test of the ability of scatter imaging to identify tumor positions. Although the detector pixels were binned to provide a resolution of 2 mm, by fitting the scatter images to Gaussian templates, tumor locations with sub-2-mm resolution were identified.
The goal of real-time tracking is to identify the tumor position with a lag of <0.5 s (Keall et al 2006). For monitoring to ensure the tumor does not stray from the treatment volume, 0.5 s of delay (a potential errant 10 MU at 1200 MU min−1 dose rate) is clinically acceptable. If real-time tumor tracking is used to guide corrective motion of the gantry head, couch, or MLCs, then filters will be used to predict the current lung tumor position based on the lagged image. Predictive filters have trouble predicting more than 0.5 s in the future (Keall et al 2006). In this study, a variety of integration times were tested. Despite the large range of potential motion during these integration times—the ± 15 mm, 0.25 Hz QUASAR tumor can move up to 5.9, 7.0, 11.5, and 13.6 mm during 0.25, 0.3, 0.5, and 0.6 s periods, respectively—the tumor tracking accuracy improved with frame integration time. This suggests that, for the tested conditions, the image quality benefits of longer integration outweigh the averaging effects of blurring.
Based on the presented phantom studies (figure 8, table 1), scatter imaging is capable of tracking moving lung tumors (30 mm diameter) with sub-millimeter precision (0.8 mm RMSE) at 0.3 s integration times when the tumor is irradiated with an open field from a fixed angle (4 x 8 cm2 field). When the field is conformed and delivered in an arc (dynamic arc therapy), the accuracy degrades to 1.2 mm RMSE (0.5 s frame integration). When modulation is introduced (VMAT), the accuracy is further degraded (0.5 s frame integration: 2.9 mm RMSE) because blocked portions of the tumor do not appear in the scatter image. For example, if the bottom half of the tumor is blocked during a frame, the tracking algorithm will identify the tumor as being erroneously high based on just the irradiated top half (compare figures 3(e)-(g)). Here, a simple procedure is used in the fitting algorithm to attempt to correct for modulation in the scatter images. Rather than identifying the position of the tumor based on fitting a Gaussian to the point of maximum intensity within each frame, an intensity mask (figure 3(f)) is measured in each scatter image by horizontally integrating the scatter intensity in the chest wall, where the beam enters the phantom. This profile approximately measures the fluence entering the phantom along the IS direction. Tumor position identification then proceeds by fitting the measured data to a Gaussian multiplied by the fluence intensity mask. The oval QUASAR phantom provides an idealized volume for the chest wall fluence measurement. Patients have less homogeneous chest walls, and more complex lung and body shapes. Instead of approximating the fluence by integrating the scatter image along the chest wall, the expected fluence for each control point may be calculated before treatment by analyzing the radiation plan MLC DICOM files.
As the tumor-tracking accuracy improves, the margins used to define the CTV to PTV expansion can also be reduced. Ideally, for SBRT, the tumor will be tracked with <1 mm accuracy. Here, for the 3 cm diameter QUASAR tumor, sub-millimeter RMSE tracking precision was observed for fixed-angle, open fields, which may be closely analogous to static conformal SBRT, but not representative of more advanced arc-based and/or modulated deliveries. Tracking of the phantom tumor during DCA therapy, which is occasionally used in our clinic for SBRT treatments, was also reasonably precise for potential clinical adoption. For the most common SBRT treatment plan type in our clinic, VMAT, modulation reduces the accuracy to a point that scatter imaging is not feasible for clinical use under the current conditions. Potential strategies for improving accuracy in the presence of modulation include: (i) usage of multiple scatter imaging cameras to gather more information on the tumor position, (ii) assessment of the MLC control point files prior to treatment so that scatter images may be ignored or less-heavily weighted if they are collected when modulation is expected to result in poor scatter images, (iii) usage of MLC-specific simulated images to create libraries of templates that include modulation, and (iv) improvement of the scatter imaging camera so that fine tumor features may be used to identify the tumor in the presence of partial obfuscation from modulation.
There are limitations of the presented study. As a feasibility study, phantoms were used. Although anthropomorphic and radiographically accurate for kV x-rays, the LUNGMAN phantom represents one body type and accentuates CNR due to lower density surrounding tumors than true human lung tissue. Photons scattered out of patients with thicker chest walls will undergo more attenuation. The QUASAR phantom approximates a human thorax with simple geometric shapes. Rigid, spherical, and relatively large phantom tumors were used, and motion was only applied along the IS direction. Although the predominate breathing-induced motion is IS, realistic tumors may translate and rotate along all 6 degrees of freedom in addition to deforming, which will complicate template matching approaches. To determine the precision of phantom-lung tumor tracking, the scatter-identified traces were fit to the MV-measured, gold-standard traces. Offsets in both time and position were allowed. Therefore, the latency (time offset) and the overall positional accuracy (positional offset) were not characterized. Independent registration between the scatter camera and treatment coordinates was attempted by matching scatter and kV images collected of a complex pattern, and an error of 1.1 mm was measured. This offset, however, can be removed through calibration. Any systematic offset between scatter image and room coordinates will be dependent on the engineering of the future scatter imaging camera. Although the large amplitude (15 mm) applied to the QUASAR dynamic tumor is at the extreme of realistic motion, which will accentuate errors due to blurring, the applied sinusoidal motion is simpler than real breathing patterns. All the images were processed after collection. Clinical implementation will require synchronous processing with limited latency. The scatter imaging camera was placed 18 cm from isocenter to create a 1:1 magnification, in a position that would result in collision with the gantry head during a full arc delivery. As a result, only a portion of the arc plan (290–211°) was delivered, and this simple mounting system cannot be employed clinically. Collision may be avoided, however, by mounting the camera to a robotic arm (e.g. from the ceiling) or with a gantry-mounted scatter imaging system, as is already implemented for modern on-board imaging systems. Although only one camera was used, scatter image cameras may be placed at many angles surrounding the patient to track tumor motion in all directions (Jones et al 2018). In theory, 3D images of the irradiated volume may be reconstructed. Interestingly, for a fixed camera, the angle between the treatment beam and pinhole collimator will change during an arc delivery. Because the efficiency and resulting photon energy changes with scattering angle, the number of scattered photons and detector efficiency will change during the arc delivery (Jones et al 2018). The presented studies were only performed with 6 MV FFF beam energies because this is the only energy used in our clinic for lung SBRT treatments. 10 MV FFF energies may be advantageous, however, because they can be delivered with dose rates up to 2400 MU min−1 (on the linear accelerator used here), which would increase the scatter imaging intensity collected for a set camera integration time. 10 MV FFF energies will generate a higher relative proportion of forward-scattered photons, however, and result in a greater intensity background because the higher energy photons will be less attenuated by the gantry head shielding and camera collimator.
5. Conclusion
Scatter images of the static anthropomorphic phantom demonstrate that quality scatter images (CNR > 4) may be collected for clinically relevant doses (10 MU = 0.5 s at 1200 MU min−1). Scatter images are also capable of sub-millimeter tumor tracking, but modulation decreases accuracy. Future implementation of scatter image-based markerless lung tumor tracking will require advancements that improve tracking accuracy during VMAT delivery.
Acknowledgments
Research reported here was supported by the National Cancer Institute under award number R21CA213407. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. KCJ thanks the Cohn Fellowship for support.
References
- Bahig H, Campeau M-P, Vu T, Doucet R, Béliveau Nadeau D, Fortin B, Roberge D, Lambert L, Carrier J-F and Filion E 2013. Predictive parameters of cyberknife fiducial-less (xsight lung) applicability for treatment of early non-small cell lung cancer: a single-center experience Int. J. Radiat. Oncol. Biol. Phys 87 583–9 [DOI] [PubMed] [Google Scholar]
- Ball D et al. 2019. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial Lancet Oncol. 20 494–503 [DOI] [PubMed] [Google Scholar]
- Bezjak A et al. 2019. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG Oncology/RTOG 0813 Trial J Clin. Oncol 37 1316–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbeco RI, Mostafavi H, Sharp GC and Jiang SB 2005a. Towards fluoroscopic respiratory gating for lung tumours without radiopaque markers Phys. Med. Biol 50 4481–90 [DOI] [PubMed] [Google Scholar]
- Berbeco RI, Nishioka S, Shirato H, Chen GTY and Jiang SB 2005b. Residual motion of lung tumours in gated radiotherapy with external respiratory surrogates Phys. Med. Biol 50 3655–67 [DOI] [PubMed] [Google Scholar]
- Bertholet J. et al. Real-time intrafraction motion monitoring in external beam radiotherapy. Phys. Med. Biol. 2019;64:15TR01. doi: 10.1088/1361-6560/ab2ba8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AM, Patel R, Surucu M, Harkenrider MM and Roeske JC 2016. Evaluation of a template-based algorithm for markerless lung tumour localization on single- and dual-energy kilovoltage images Br. J. Radiol 89 20160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JT, Caillet V, Hardcastle N, O’Brien R, Szymura K, Crasta C, Harris B, Haddad C, Eade T and Keall PJ 2016. The first patient treatment of electromagnetic-guided real time adaptive radiotherapy using MLC tracking for lung SABR Radiother. Oncol 121 19–25 [DOI] [PubMed] [Google Scholar]
- Caillet V, Keall PJ, Colvill E, Hardcastle N, O’Brien R, Szymura K and Booth JT 2017. MLC tracking for lung SABR reduces planning target volumes and dose to organs at risk Radiother. Oncol 124 18–24 [DOI] [PubMed] [Google Scholar]
- D’Souza WD, Naqvi SA and Yu CX 2005. Real-time intra-fraction-motion tracking using the treatment couch: a feasibility study Phys. Med. Biol 50 4021. [DOI] [PubMed] [Google Scholar]
- Garnica-Garza HM 2018. Directional scatter imaging for the stereoscopic tracking of fiducial markers in a single kV exposure Med. Phys 45 703–13 [DOI] [PubMed] [Google Scholar]
- Green OL et al. 2018. First clinical implementation of real-time, real anatomy tracking and radiation beam control Med. Phys 45 3728–40 [DOI] [PubMed] [Google Scholar]
- Hansen EK and Roach M III 2010. Handbook of Evidence-Based Radiation Oncology (Berlin: Springer; ) [Google Scholar]
- Hansen R, Ravkilde T, Worm Esben S, Toftegaard J, Grau C, Macek K and Poulsen Per R 2016. Electromagnetic guided couch and multileaf collimator tracking on a TrueBeam accelerator Med. Phys 43 2387–98 [DOI] [PubMed] [Google Scholar]
- Hazelaar C, Dahele M, Mostafavi H, van der Weide L, Slotman B and Verbakel W 2018. Markerless positional verification using template matching and triangulation of kV images acquired during irradiation for lung tumors treated in breath-hold Phys. Med. Biol 63 115005. [DOI] [PubMed] [Google Scholar]
- Jones KC, Redler G, Templeton A, Bernard D, Turian JV and Chu JCH 2018. Characterization of Compton-scatter imaging with an analytical simulation method Phys. Med. Biol 63 025016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keall P, Poulsen P and Booth J T 2019. See, think, and act: real-time adaptive radiotherapy Semin. Radiat. Oncol 29 228–35 [DOI] [PubMed] [Google Scholar]
- Keall PJ et al. 2006. The management of respiratory motion in radiation oncology report of AAPM Task Group 76a Med. Phys 33 3874–900 [DOI] [PubMed] [Google Scholar]
- Kim JH, Nguyen DT, Huang CY, Fuangrod T, Caillet V, O’Brien R, Poulsen P, Booth J and Keall P 2017. Quantifying the accuracy and precision of a novel real-time 6 degree-of-freedom kilovoltage intrafraction monitoring (KIM) target tracking system Phys. Med. Biol 62 5744–59 [DOI] [PubMed] [Google Scholar]
- Menten MJ, Wetscherek A and Fast MF 2017. MRI-guided lung SBRT: present and future developments Physica Med. 44 139–49 [DOI] [PubMed] [Google Scholar]
- Redler G, Jones KC, Templeton A, Bernard D, Turian J and Chu JCH 2018. Compton scatter imaging: A promising modality for image guidance in lung stereotactic body radiation therapy Med. Phys 45 1233–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, Wilbert J, Baier K, Flentje M and Guckenberger M 2010. Feasibility study for markerless tracking of lung tumors in stereotactic body radiotherapy Int. J. Radiat. Oncol. Biol. Phys 78 618–27 [DOI] [PubMed] [Google Scholar]
- Sayeh S, Wang J, Main WT, Kilby W and Maurer CR 2007. Treating Tumors that Move with Respiration eds Urschel HC et al. (Berlin Heidelberg: Springer; ) 15–29 [Google Scholar]
- Schweikard A, Glosser G, Bodduluri M, Murphy MJ and Adler JR 2000. Robotic motion compensation for respiratory movement during radiosurgery Comput. Aided Surg 5 263–77 [DOI] [PubMed] [Google Scholar]
- Shi X, Chen S, D’Souza WD and Mistry NN 2013. Margins determined using 4dct often underestimate tumor motion in thoracic tumors Int. J. Radiat. Oncol. Biol. Phys 87 S67–S8 [Google Scholar]
- Shirato H et al. 2000. Physical aspects of a real-time tumor-tracking system for gated radiotherapy Int. J. Radiat. Oncol. Biol. Phys 48 1187–95 [DOI] [PubMed] [Google Scholar]
- Shirvani SM, Jiang J, Chang JY, Welsh JW, Gomez DR, Swisher S, Buchholz TA and Smith BD 2012. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly Int. J. Radiat. Oncol. Biol. Phys 84 1060–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman R et al. 2010. Stereotactic body radiation therapy for inoperable early stage lung cancer JAMA 303 1070–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyk J, Keane TJ and Rider WD 1982. Lung density as measured by computerized tomography: implications for radiotherapy Int. J. Radiat. Oncol. Biol. Phys 8 1363–72 [DOI] [PubMed] [Google Scholar]
- Videtic GMM, Hu C, Singh AK, Chang JY, Parker W, Olivier KR, Schild SE, Komaki R, Urbanic JJ and Choy H 2015. A randomized phase 2 study comparing two stereotactic body radiation therapy schedules for medically inoperable patients With stage I peripheral non-small cell lung cancer: NRG Oncology RTOG 0915 (NCCTG N0927) Int. J. Radiat. Oncol. Biol. Phys 93 757–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbert J, Baier K, Hermann C, Flentje M and Guckenberger M 2013. Accuracy of real-time couch tracking during 3-dimensional conformal radiation therapy, intensity modulated radiation therapy, and volumetric modulated arc therapy for prostate cancer Int. J. Radiat. Oncol. Biol. Phys 85 237–42 [DOI] [PubMed] [Google Scholar]
- Wilbert J et al. 2008. Tumor tracking and motion compensation with an adaptive tumor tracking system (ATTS): system description and prototype testing Med. Phys 35 3911–21 [DOI] [PubMed] [Google Scholar]
- Yan H, Tian Z, Shao Y, Jiang SB and Jia X 2016. A new scheme for real-time high-contrast imaging in lung cancer radiotherapy: a proof-of-concept study Phys. Med. Biol 61 2372–88 [DOI] [PMC free article] [PubMed] [Google Scholar]