Abstract
Elimination of schistosomiasis as a public health problem among all disease-endemic countries in 2030 is an ambitious goal. Recent achievements resulting from mass drug administration (MDA) with praziquantel is promising but may need to be complemented with also other means. Schistosomiasis was highly prevalent in China before the initiation of the national schistosomiasis control program in the mid-1950s, and, at that time, the country bore the world’s highest burden of schistosomiasis. The concerted control efforts, upheld without interruption for more than a half century, have resulted in elimination of the disease as a public health problem in China as of 2015. Here, we describe the current status of schistosomiasis in China, analyze the potential challenges affecting schistosomiasis elimination, and propose the future research needs and priorities for the country, aiming to provide more universal insights into the structures needed for a global schistosomiasis elimination encompassing also other endemic regions.
Introduction
Schistosomiasis, caused by blood flukes of the genus Schistosoma that depend on snail intermediate hosts for their life cycle, is a neglected tropical parasitic disease of great public health and socioeconomic significance [1]. This zoonotic parasitic disease is estimated to affect around 240 million people in 78 tropical and subtropical countries across the world, with up to 780 million at risk [2]. For more than 30 years, the World Health Organization (WHO) has recommended a morbidity control strategy through mass drug administration (MDA) with the drug praziquantel [3]. This approach has resulted in strong progress toward schistosomiasis elimination, as evidenced by a remarkable decline in prevalence and intensity of the disease [4,5]. This success provides hope that the agenda set for elimination of schistosomiasis in “this wormy world” has been correct [6]. However, the ambitious goal of eliminating the infection as a public health problem in all endemic countries in 2030 remains challenging [7], notably in African countries where more than 90% of global schistosomiasis patients live and where over 85% of local populations are constantly at risk [8,9]. This is partly because the current strategy for schistosomiasis control, despite the success mentioned above, fails to prevent reinfection. In addition, there are still inadequate supplies of praziquantel pills, which are primarily supplied through donations from international pharmaceutical companies [10–12].
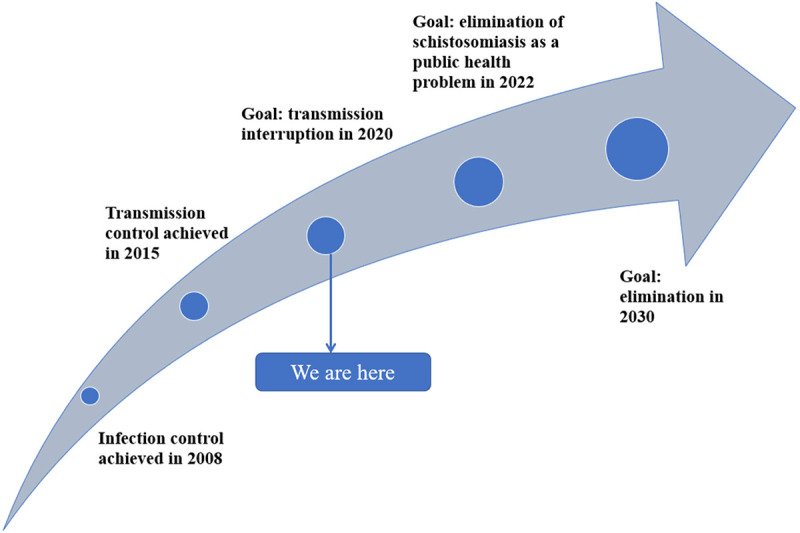
The schistosome species in China is Schistosoma japonicum, and its snail host is Oncomelania hupensis, and the description of the disease caused by schistosomiasis dates back more than 2,200 years [13]. Significant Schistosoma-associated morbidity was highly prevalent before the initiation of the national schistosomiasis control program in the mid-1950s. Indeed, China had the highest burden of schistosomiasis in the world and at that time [14,15]. Since then, the strong political will [16], an effective and flexible control strategy at different program stages, and the rigorous surveillance and monitoring of factors associated with the transmission of schistosomiasis have resulted in elimination of the disease as a public health problem in China as of 2015 (Table 1) [17].
Table 1. Criteria for the elimination of schistosomiasis.
| WHO criteria | Chinese criteria |
|---|---|
|
•
Disease control: prevalence of heavy-intensity infection <5% in all schistosomiasis-endemic setting of the country; • Elimination of schistosomiasis as a public health problem: prevalence of heavy-intensity infection <1% in all schistosomiasis-endemic setting of the country; • Elimination of transmission: reduction of infection incidence to zero. |
•
Infection control: prevalence of infection <5% in humans and livestock and absence of outbreaks of acute schistosomiasis; • Transmission control: prevalence of infection <1% in humans and livestock, including absence of both (i) local O. hupensis infected by S. japonicum (sampled for 2 consecutive days); and (ii) any local cases of acute schistosomiasis; • Transmission interruption: no S. japonicum infections identified in local residents or livestock for 5 consecutive years and absence of O. hupensis infected by S. japonicum for at least 5 years; • Elimination: absence of S. japonicum in local residents, livestock, or in O. hupensis for subsequent 5 years after transmission interruption. |
WHO, World Health Organization.
As can be seen in Table 1, the Chinese criteria regarding schistosomiasis elimination are even more stringent than those recommended by WHO [18]. The country remains currently at the “pre-elimination” stage [19], and we are aware that there are still challenges that may hinder the progress toward final disease elimination [20,21]. Here, we describe the current status of schistosomiasis in China, analyze the potential challenges affecting elimination, and propose the future research needs and priorities for the country. This discussion is also intended to provide more universal insights into the structures needed for a global schistosomiasis elimination program encompassing all endemic regions of the world.
Elimination of schistosomiasis in China: Current status
Following China’s concerted effort to control and eliminate schistosomiasis that has been ongoing for nearly 70 years [1], the endemic status is currently characterized by an extremely low prevalence and intensity of S. japonicum infections [22]. In 2019, only 30,000 individuals were estimated to have the disease across the country, with only 5 new infections detected that year [23]. This indicates a more than 99% reduction in the number of cases as compared to the situation in the 1950s (Fig 1) [23–27]. In addition, in 2019, the transmission of schistosomiasis had been controlled, or interrupted in approximately 95% of the 450 counties that were once considered endemic for S. japonicum, including 67 that have now achieved elimination (Figs 2 and 3). These data demonstrate that we are steadily approaching to final demise of the “God of Plague” [17]. It has been well known that experiences from the great success in schistosomiasis control in China include high-level political sustained commitment [28], persistent and adequate financial support [29], and integrated control mobilizing multi-sectoral resources [30,31]. Nevertheless, the following approaches have also contributed to this success: (1) government-led containment: In China, the national schistosomiasis control program is included in the annual governmental work plan at national, provincial, municipal, county, and township levels, and all schistosomiasis control approaches are implemented under governmental leadership, ensuring the smooth implementation of all activities [16]; (2) nationwide participation: All residents living in schistosomiasis-endemic foci are encouraged to participate in the schistosomiasis control program [30]; (3) a unique control strategy: Although there have been 3 major shifts in the national schistosomiasis control strategy, a unique strategy is employed across the whole country [28]; and (4) a powerful surveillance-response system: The strong monitoring capability and rapid response mechanism allows effective management of schistosomiasis epidemics, making eradication of the transmission risk possible [28].
Fig 1. Changes in the estimated number of schistosomiasis cases in China from 1950s to 2019.
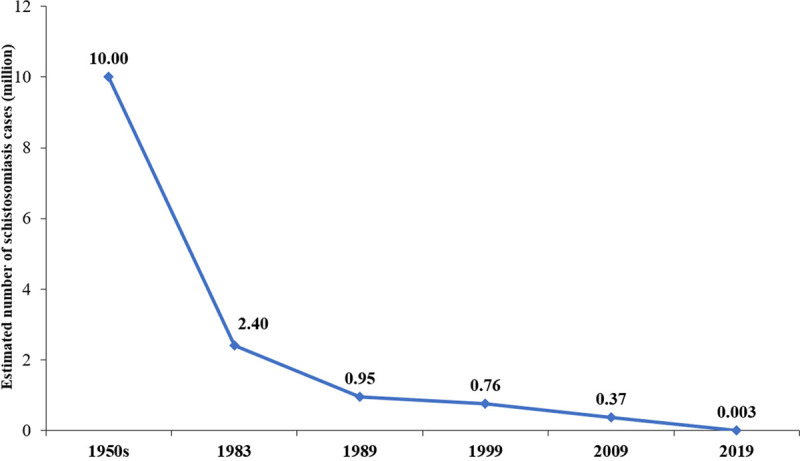
Fig 2.
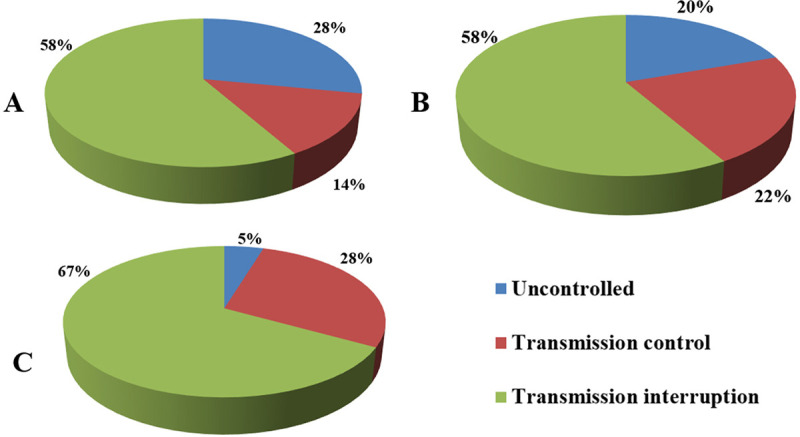
The changing proportions of infection control categories among schistosomiasis-endemic counties in different periods of schistosomiasis control in China from 1999 to 2019: (A) 1999, (B) 2009, and (C) 2019.
Fig 3. Overall progress of schistosomiasis elimination in the country.
Challenges
Although China is moving steadily toward complete elimination of schistosomiasis across the country, many challenges remain in order to eliminate this disease by 2030 (Table 2) [32]. Among them is financial support, the most urgent issue that must always be addressed. Indeed, one of the important lessons learned from the World Bank Loan Project (WBLP) for Chinese Schistosomiasis Control Program (1992 to 2001) is that sustained financial support is needed for effective program performance. The absence of adequate support after the end of the WBLP resulted in rebound of schistosomiasis transmission [29]. Since all interventions included in the national schistosomiasis elimination program are fully paid by the government and that reduced funding would fail to support the integrated control interventions and capability improvements agreed, it is essential that strong political support continues along with provision of commensurate financial support to maintain China’s elimination initiative.
Table 2. Potential challenges for elimination of schistosomiasis in China.
| Points of concern | Main challenge |
|---|---|
| • Financial support | Since the central government took over responsibility for the financial expenditure of the national schistosomiasis control program in 2018, local governments decreased or even ceased their support. This may make it difficult to sustain current control activities in the face of the continuous rise of the cost of products used for schistosomiasis elimination, manpower resources, and training. |
| • Snail control | Increasing rigorous environmental protection policies restrict the use of chemical snail control, which particularly affects activities in the endemic areas in the lake and mountainous regions. |
| • Emerging and reemerging sources of infections | Emerging S. japonicum reservoir hosts, such as goats, canines, and wild mice, are neglected but play an important role in the transmission of the parasite. In addition, protection of endangered species of deer, such as E. davidianus, poses a threat to continued control in certain places. |
| • Frequent natural disasters | Frequent flooding along the Yangtze River basin, the main schistosomiasis-endemic focus of China, and earthquakes can lead to alterations of snail-infested settings, resulting in snails to be spread into new areas. In addition, individuals infected with S. japonicum infections may be displaced into new transmission zones due to such events. |
| • Capability building | Grassroot staff remain the main force in the operation of the schistosomiasis elimination program; however, these workers currently suffer from problems related to old age, low education level, and minimum salaries, all of which have led to a high proportion of resignations, resulting in a reduced schistosomiasis control capability. In addition, governmental institution reforms have reduced the number of workers participating in schistosomiasis control, so many now work only part time. The reduced financial support further hinders the updating of hardware and restricts training of new control professionals. |
| • Integrated control | One of the most important lessons learned from the Chinese National Schistosomiasis Control Program is the need for mobilization of multi-sectoral resources and use of multiple interventions in an integrated manner; however, lack of sufficient financial support restricts collaboration of the health sector with the agriculture, water conservancy, forestry, and education sectors. |
| • Imported schistosomiasis | China’s policy of opening up, and its “Belt and Road” initiative in Africa, has recently resulted in imported cases of Schistosoma mansoni and Schistosoma haematobium infections not infrequently found also in China. Given the existence of Biomphalaria straminea in southern China and its capability of acting as intermediate host snail for S. mansoni, there is a growing concern about potential S. mansoni transmission in mainland China. In addition, the potential hybridization between S. japonicum and S. mansoni may affect the progress of the elimination program. |
| • Urban schistosomiasis | Schistosomiasis predominantly occurs in rural and poor regions. However, with the acceleration of China’s urbanization, the boundary between urban and rural regions has become increasingly unclear. In addition, due to the introduction of intermediate snail hosts resulting from construction of urban wetlands, the transmission of S. japonicum is more and more likely in urban regions. |
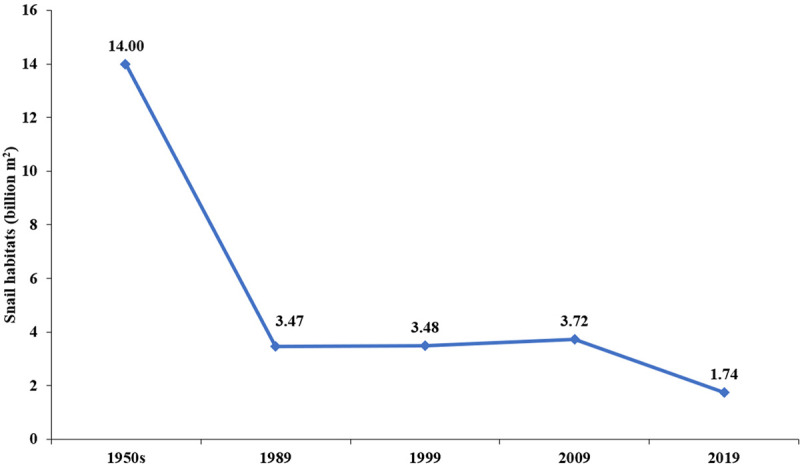
Snail control has proven to be one of the most effective tools for interruption of schistosomiasis [33–35], particularly with regard to S. japonicum, as its snail host is amphibious, and, therefore, more vulnerable than its African counterparts. This approach has been an important part of the Chinese national schistosomiasis control strategy since the 1950s (Fig 4) [36]. Although no S. japonicum infections have been identified in O. hupensis snails since 2014 [37], 1.74 billion m2 snail habitats were detected across China in 2019, including 642,000 m2 emerging snail-infested settings and 8.51 million m2 reemerging settings [23]. These widespread host snails are an important unknown factor during the stage when we move toward elimination of schistosomiasis.
Fig 4. Changes in the area of O. hupensis snail habitats in China from 1950s to 2019.
An unfortunate duality with respect to the need for snail control has arisen from the government’s call for environmental protection, which restricts the use of chemical treatment as part of snail control, while the development of China’s Yangtze River Economic Belt prohibits the use of environmental change, such as dams for snail control, along the Yangtze River basin—the main schistosomiasis-endemic focus of China. Meanwhile, the construction of large-scale wetlands has led to the introduction of O. hupensis snails into urban areas [38]. Agricultural developments, water conservancy, and deforestation programs have shown feasible for the improving snail-infested settings; however, these programs require costly investments [31,39]. To add fuel to the fire, the termination of financial support given to agriculture, water conservancy, and forestry sectors for schistosomiasis control programs since 2016 has resulted in ineffective multi-sectoral collaborations and cessation of environmental improvements for snail control. In addition, frequent floods along the Yangtze River basin [40–42] and earthquakes in the mountainous schistosomiasis-endemic areas may now lead to the spread of O. hupensis snails and expansion of schistosomiasis transmission as a result [43–45].
Unlike the other 2 main species of the genus Schistosoma infective to humans, S. japonicum is a zoonotic parasite with over 40 different mammalian species serving as reservoir hosts [46]. Although bovines are included in the Chinese National Schistosomiasis Control Program and given high priority for control interventions as a major source of infection [47], goats, dogs, and wild mice, which are also reservoirs for S. japonicum, are generally not targeted [48]. Previous studies have shown that these animals also play an increasingly important role in the transmission of schistosomiasis [20,49]. A recent meta-analysis showed 3.9% overall pooled prevalence of S. japonicum infections in 8,795 wild rodents based on 37 eligible publications, involving 61 field studies across mainland China from 1980 to 2020 [20]. No comparable overall change of the infection prevalence was detected in rodents with great reductions in the numbers of schistosomiasis cases in humans and bovines; however, importantly, there appeared to be an increase in S. japonicum prevalence in rodents over time within the hilly and mountainous endemic regions [20]. Since rodents were projected to become the dominant wildlife in human-driven environments and the main reservoir of zoonotic diseases in general within tropical zones, intensified monitoring and evaluation of potential S. japonicum infections within rodents is strongly recommended, particularly in hilly and mountainous regions [20]. A very recent, preprint publication also reports high prevalence of Schistosoma infections in rodents, highlighting the important role of rodents in schistosomiasis transmission across the world during the current stage of disease elimination [50]. Moreover, Elaphurus davidianus, a type of deer native to the river valleys of China, is a Grade 1 state-protected animal in China that can be seen grazing in marshlands around Poyang Lake and Dongting Lake areas, which are places where O. hupensis snails infected by S. japonicum have previously been found with high prevalence and intensity of infection [51–53]. In 2004, a field investigation detected 29.2% (38/130) of the wild-captured E. davidianus fecal samples positive for S. japonicum in Gudao section of the Yangtze River in Shishou City, central China [53]. In 2016, 16% (4/25) of the wild E. davidianus fecal samples captured from the Tian’e marshland of Shishou City were identified as infected by S. japonicum [52]. A recent study shows a 4.4% (2/46) detection of S. japonicum eggs in stool samples of E. davidianus captured from the grassland of Poyang Lake with a high intensity of infection based on the miracidial hatching test (+++) found [51]. This abundance of these deer poses a high risk of them becoming an emerging reservoir of S. japonicum infections, in particular since it is prohibited to investigate this animal or provide any kind of chemotherapy if needed. What makes matters worse is that fencing is also not allowed, while free grazing in the grasslands is encouraged [54]. Thus, the presence of this type of deer in areas still endemic for schistosomiasis is a big threat with respect to consolidation of the achievements gained from current concerted control efforts, and it endangers the progress toward elimination of schistosomiasis in China.
Schistosomiasis elimination in China: Future requirements
The experience of many global schistosomiasis control programs has shown that MDA with praziquantel alone is unlikely to eliminate schistosomiasis [55], and an integrated strategy mobilizing resources from health, agriculture, water conservancy, forestry, and education sectors is required [56]. To tackle the challenges mentioned above, the complete eradication of potential risks for resurgence of the disease is of great and urgent need. During the stage of moving toward elimination of schistosomiasis, a more fine-grained precision control is recommended as the choice for managing interventions as part of the national schistosomiasis elimination program (Table 3) [57].
Table 3. Future research priorities for the elimination of schistosomiasis in China.
| Target | Intervention |
|---|---|
| • Precision identification | ➢ Identification of high-risk settings using geospatial tools; ➢ Monitoring of domestic and wild animals in snail-infested marshlands using AI and unmanned aerial vehicles; ➢ Assessing the role of reservoir hosts in the local transmission of schistosomiasis using epidemiological tools; ➢ Development of assays for early and sensitive detection of S. japonicum infections in humans, livestock, and O. hupensis snails; ➢ Identification and tracking of high-risk populations using high-level internet connection and approaches based on machine learning. |
| • Precision treatments | ➢ Development of health education materials based on social media, such as “WeChat”; ➢ Development of highly effective, minimally toxic, and environmentally friendly molluscicides; ➢ Development of orally administered agents for prevention of infections; ➢ Establishing a smart system to achieve real-time monitoring of schistosomiasis control data using cell phone technology; ➢ Building a sensitive and effective surveillance and forecast system as a response to emergency outbreaks and imported epidemics. |
| • Precision evaluations | ➢ Assessing the performance of praziquantel chemotherapy with regard to local transmission of schistosomiasis in order to avoid overtreatment; ➢ Assessing the performance of chemical treatment with regard to local transmission of schistosomiasis to avoid over-mollusciciding; ➢ Assessing the cost-effectiveness and cost-benefit of different packaging interventions to adjust optimal intervention combinations adapted to local epidemiological features and socioeconomic developments. |
AI, artificial intelligence.
Discussion and conclusions
Rapid social and economic development during the past decades has brought with it striking achievements in the Chinese National Schistosomiasis Control Program. Remarkably, in 2019, only 5 individuals tested positive for S. japonicum throughout the country [23]. Currently, the transmission of schistosomiasis is almost interrupted, and the country is steadily moving toward schistosomiasis elimination. In addition to the integrated strategy [58], Chinese experience and tools are now being transferred overseas and have been applied in countries affected by schistosomiasis in Africa and Southeast Asia [59–62]. It is clear that the experience gained and lessons learned over the past 7 decades of schistosomiasis control in China can provide valuable insights useful for the ongoing global schistosomiasis elimination program, as outlined in the new WHO Roadmap for Neglected Tropical Diseases [7].
Funding Statement
This study was funded by the grants from Jiangsu Provincial Department of Science and Technology (grant no. BZ2020003), Jiangsu Provincial Commission of Health (grant no. ZDRCA2016056, QNRC2016621 and H2018097) and Wuxi Municipal Bureau of Science and Technology (grant no. CSE31N1730). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383(9936):2253–64. doi: 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers. 2018;4(1):13. doi: 10.1038/s41572-018-0013-8 [DOI] [PubMed] [Google Scholar]
- 3.WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: WHO Technical Report Series N° 912. Report of a WHO expert committee. Geneva: WHO; 2002. [PubMed] [Google Scholar]
- 4.Mutapi F, Maizels R, Fenwick A, Woolhouse M. Human schistosomiasis in the post mass drug administration era. Lancet Infect Dis. 2017;17(2):e42–8. doi: 10.1016/S1473-3099(16)30475-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392(10159): 1789–1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deol AK, Fleming FM, Calvo-Urbano B, Walker M, Bucumi V, Gnandou I, et al. Schistosomiasis—Assessing progress toward the 2020 and 2025 global goals. N Engl J Med. 2019;381(26):2519–28. doi: 10.1056/NEJMoa1812165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization; 2020. [cited 2020 Oct 21]. Available from: https://www.who.int/neglected_diseases/WHONTD-roadmap-2030/en/ [Google Scholar]
- 8.Lai YS, Biedermann P, Ekpo UF, Garba A, Mathieu E, Midzi N, et al. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect Dis. 2015;15(8):927–40. doi: 10.1016/S1473-3099(15)00066-3 [DOI] [PubMed] [Google Scholar]
- 9.Hotez PJ, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl Trop Dis. 2009;3(9):e485. doi: 10.1371/journal.pntd.0000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Liang Y. Mass drug administration (MDA) for schistosomiasis. J Infect Dis. 2015;211(5):848–9. doi: 10.1093/infdis/jiu506 [DOI] [PubMed] [Google Scholar]
- 11.Lo NC, Addiss DG, Hotez PJ, King CH, Stothard JR, Evans DS, et al. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: the time is now. Lancet Infect Dis. 2017;17(2):e64–9. doi: 10.1016/S1473-3099(16)30535-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tchuem Tchuenté LA, Rollinson D, Stothard JR, Molyneux D. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infect Dis Poverty. 2017;6(1):42. doi: 10.1186/s40249-017-0256-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao SP, Shao BR. Schistosomiasis control in the People’s Republic of China. Am J Trop Med Hyg. 1982;31(1):92–9. [PubMed] [Google Scholar]
- 14.Chen MG. Progress and problems in schistosomiasis control in China. Trop Med Parasitol. 1989;40(2):174–6. [PubMed] [Google Scholar]
- 15.Song LG, Wu XY, Sacko M, Wu ZD. History of schistosomiasis epidemiology, current status, and challenges in China: on the road to schistosomiasis elimination. Parasitol Res. 2016;115(11):4071–81. doi: 10.1007/s00436-016-5253-5 [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Xu J, Bergquist R, Li SZ. Zhou XN. "Farewell to the God of Plague": The importance of political commitment towards the elimination of schistosomiasis. Trop Med Infect Dis. 2018;3(4):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Li SZ, Zhang LJ, Bergquist R, Dang H, Wang Q, et al. Surveillance-based evidence: elimination of schistosomiasis as a public health problem in the Peoples’ Republic of China. Infect Dis Poverty. 2020;9(1):63. doi: 10.1186/s40249-020-00676-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health. Control and elimination of schistosomiasis. Beijing: China Standard Publishing House, 2016; 1–4 (in Chinese). [Google Scholar]
- 19.Cao CL, Zhang LJ, Deng WP, Li YL, Lv C, Dai SM, et al. Contributions and achievements on schistosomiasis control and elimination in China by NIPD-CTDR. Adv Parasitol. 2020;110:1–62. doi: 10.1016/bs.apar.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 20.Zou HY, Yu QF, Qiu C, Webster JP, Lu DB. Meta-analyses of Schistosoma japonicum infections in wild rodents across China over time indicates a potential challenge to the 2030 elimination targets. PLoS Negl Trop Dis. 2020;14(9):e0008652. doi: 10.1371/journal.pntd.0008652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing X, Shan L, Chun-Li C, Shi-Zhu L, Xiao-Nong Z. Progress and challenges of schistosomiasis elimination in China. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(6):605–9. (in Chinese). doi: 10.16250/j.32.1374.2018249 [DOI] [PubMed] [Google Scholar]
- 22.Guo JY, Xu J, Zhang LJ, Lv S, Cao CL, Li SZ, et al. Surveillance on schistosomiasis in five provincial-level administrative divisions of the People’s Republic of China in the post-elimination era. Infect Dis Poverty. 2020;9(1):136. doi: 10.1186/s40249-020-00758-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang LJ, Xu ZM, Dang H, Li YL, Lü S, Xu J, et al. Endemic status of schistosomiasis in People’s Republic of China in 2019. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi.: 2020;32(6):551–558. (in Chinese). doi: 10.16250/j.32.1374.2020263 [DOI] [PubMed] [Google Scholar]
- 24.Basch PF. Schistosomiasis in China: an update. Am J Chin Med. 1986;14(1–2):17–25. doi: 10.1142/S0192415X86000041 [DOI] [PubMed] [Google Scholar]
- 25.Cen LP. A short review of the previous and current epidemiological situation of schistosomiasis in China. Rev Soc Bras Med Trop. 1997;30(1):57–60. doi: 10.1590/s0037-86821997000100011 [DOI] [PubMed] [Google Scholar]
- 26.Wang LY, Jiang QW, Liu JX, Zhao GM, Chen XY. Schistosomiasis situation in People’s Republic of China in 1999. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2000;12(6):321–3. (in Chinese). [PubMed] [Google Scholar]
- 27.Hao Y, Zheng H, Zhu R, Guo JG, Wang LY, Chen Z, et al. Schistosomiasis situation in People’s Republic of China in 2009. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2010;12(6):521–7. (in Chinese). [PubMed] [Google Scholar]
- 28.Wang L, Utzinger J, Zhou XN. Schistosomiasis control: experiences and lessons from China. Lancet. 2008;372(9652):1793–935. doi: 10.1016/S0140-6736(08)61358-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Dai JR, Liang YS. Apropos: factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasit Vectors. 2014;7:408. doi: 10.1186/1756-3305-7-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu H, Yap P, Utzinger J, Jia TW, Li SZ, Huang XB, et al. Policy support and resources mobilization for the National Schistosomiasis Control Programme in the People’s Republic of China. Adv Parasitol. 2016;92:341–83. doi: 10.1016/bs.apar.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Zhou YB, Song XX, Li SZ, Zhong B, Wang TP, et al. Integrated control strategy of schistosomiasis in the People’s Republic of China: Projects involving agriculture, water conservancy, forestry, sanitation and environmental modification. Adv Parasitol. 2016;92:237–68. doi: 10.1016/bs.apar.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 32.Brattig NW, Bergquist R, Qian MB, Zhou XN, Utzinger J. Helminthiases in the People’s Republic of China: Status and prospects. Acta Trop. 2020;212:105670. doi: 10.1016/j.actatropica.2020.105670 [DOI] [PubMed] [Google Scholar]
- 33.Lo NC, Gurarie D, Yoon N, Coulibaly JT, Bendavid E, Andrews JR, et al. Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. Proc Natl Acad Sci U S A. 2018;115(4):E584–91. doi: 10.1073/pnas.1708729114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokolow SH, Wood CL, Jones IJ, Swartz SJ, Lopez M, Hsieh MH, et al. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl Trop Dis. 2016;10(7):e0004794. doi: 10.1371/journal.pntd.0004794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokolow SH, Wood CL, Jones IJ, Lafferty KD, Kuris AM, Hsieh MH, et al. To reduce the global burden of human schistosomiasis, use ’old fashioned’ snail control. Trends Parasitol. 2018;34(1):23–40. doi: 10.1016/j.pt.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian C, Zhang Y, Zhang X, Yuan C, Gao Z, Yuan H, et al. Effectiveness of the new integrated strategy to control the transmission of Schistosoma japonicum in China: a systematic review and meta-analysis. Parasite. 2018;25:54. doi: 10.1051/parasite/2018058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lei ZL, Zhang LJ, Xu ZM, Dang H, Xu J, Lv S, et al. Endemic status of schistosomiasis in People’s Republic of China in 2014. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2015;27(6):563–9. (in Chinese). [PubMed] [Google Scholar]
- 38.Huang YX. Wetland protection and Oncomelania hupensis control. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013;25(5):533–7. (in Chinese). [PubMed] [Google Scholar]
- 39.Yang X, Zhang Y, Sun QX, Zhou JX, Zhou XN. SWOT analysis on snail control measures applied in the national schistosomiasis control programme in the People’s Republic of China. Infect Dis Poverty. 2019;8(1):13. doi: 10.1186/s40249-019-0521-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao W, Yue-Ling X, Jia-Jing Z, Yang L, Yu-Ting Z, Ming-Xing X. Assessment of schistosomiasis transmission risk after flood damage in Wuhan City. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(4):410–4. (in Chinese). doi: 10.16250/j.32.1374.2018046 [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Zheng SB, Yang Y, Cheng WT, Pan X, Dai QQ, et al. The Three Gorges Dam: Does the flooding time determine the distribution of schistosome-transmitting snails in the middle and lower reaches of the Yangtze River, China? Int J Environ Res Public Health. 2018;15(7):1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Y, Qiu J, Li R, Shen Q, Huang D. Identification of potential high-risk habitats within the transmission reach of Oncomelania hupensis after floods based on SAR techniques in a plane region in China. Int J Environ Res Public Health. 2017;14(9):986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia X, Bo Z, Yang L, Zi-Song W, Yi Z, Lin C. Impact of earthquake disaster on schistosomiasis transmission and emergency prevention and control in Sichuan Province. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019;31(3):333–6. (in Chinese). doi: 10.16250/j.32.1374.2019072 [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Xu BH, Chen L, Wu ZS, Xiao ZY, Wang CF, et al. Mid-term evaluation on schistosomiasis control effect in Lushan earthquake-stricken areas in Sichuan Province. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013;25(5):467–72. (in Chinese). [PubMed] [Google Scholar]
- 45.Shi YH, Wang BD. Schistosomiasis control effect in Mianyang City in ten years after Wenchuan earthquake. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;31(5):549–51. (in Chinese). doi: 10.16250/j.32.1374.2018069 [DOI] [PubMed] [Google Scholar]
- 46.Ding C, Qiu Z, Zhu H. Multi-host transmission dynamics of schistosomiasis and its optimal control. Math Biosci Eng. 2015;12(5):983–1006. doi: 10.3934/mbe.2015.12.983 [DOI] [PubMed] [Google Scholar]
- 47.Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, et al. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360(2):121–8. doi: 10.1056/NEJMoa0800135 [DOI] [PubMed] [Google Scholar]
- 48.Jiao-Jiao L. Endemic status and control of animal schistosomiasis in China. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019;31(1):40–6. (in Chinese). doi: 10.16250/j.32.1374.2018313 [DOI] [PubMed] [Google Scholar]
- 49.VAN Dorssen CF, Gordon CA, Li Y, Williams GM, Wang Y, Luo Z, et al. Rodents, goats and dogs—their potential roles in the transmission of schistosomiasis in China. Parasitology. 2017;144(12):1633–42. doi: 10.1017/S0031182017000907 [DOI] [PubMed] [Google Scholar]
- 50.Liang S, Ponpetch K, Zhou Y, Guo J, Erko B, Stothard JR, et al. Diagnosis of Schistosoma infection in non-human animal hosts: A systematic review and meta-analysis. Preprints. 2021:2021050075. doi: 10.20944/preprints202105.0075.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lv SB, Liu YW, Liu YM, Xu SG, Li YF, Yuan M, et al. Impact of “Elaphurus davidianus return home project” on the transmission of schistosomiasis in Poyang Lake areas. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2020;32(5):498–501. (in Chinese). doi: 10.16250/j.32.1374.2020039 [DOI] [PubMed] [Google Scholar]
- 52.Ma XP, Zhao HM, Chen YC. The epidemiological status of schistosomiasis of wapitis in Shishou City of Hubei Province. Chin J Vet Parasitol. 2007;15(5):28–30. [Google Scholar]
- 53.Yuan DS, Liu W, Zhang YS, Liu ZQ, Li Q. Investigation of epidemic situation of schistosomiasis in Gudao region of Changjiang in Shishou City. Chin J Vet Parasitol. 2006;14(1):20–2. [Google Scholar]
- 54.Li SM, Deng WC, Cheng XH, He HB, Zhou YB, Zhou J, et al. Challenges and countermeasures of schistosomiasis control in Hunan Province in the new era. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2020;32(3):225–9. (in Chinese). doi: 10.16250/j.32.1374.2020051 [DOI] [PubMed] [Google Scholar]
- 55.Secor WE. Early lessons from schistosomiasis mass drug administration programs. F1000Res. F1000 Faculty Rev 2015;4:1157. doi: 10.12688/f1000research.6826.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray DJ, McManus DP, Li Y, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis. 2010;10(10):733–6. doi: 10.1016/S1473-3099(10)70099-2 [DOI] [PubMed] [Google Scholar]
- 57.Zhou XN. Implementation of precision control to achieve the goal of schistosomiasis elimination in China. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2016;28(1):1–4. (in Chinese). [PubMed] [Google Scholar]
- 58.Wang W, Yang K. Schistosomiasis and the global goals. N Engl J Med. 2020;382(16):1575–6. doi: 10.1056/NEJMc2002117 [DOI] [PubMed] [Google Scholar]
- 59.Wang XY, He J, Juma S, Kabole F, Guo JG, Dai JR, et al. Efficacy of China-made praziquantel for treatment of schistosomiasis haematobium in Africa: A randomized controlled trial. PLoS Negl Trop Dis. 2019;13(4):e0007238. doi: 10.1371/journal.pntd.0007238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang K, Yang HT, Liang YS, Dai JR. Li Wei, Zhang JF, et al. A path analysis on China’s participation in global health governance: a case study of China Aid of Schistosomiasis Control in Zanzibar. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019;31(1):14–8. (in Chinese). doi: 10.16250/j.32.1374.2019041 [DOI] [PubMed] [Google Scholar]
- 61.Xu J, Yu Q, Tchuenté LA, Bergquist R, Sacko M, Utzinger J, et al. Enhancing collaboration between China and African countries for schistosomiasis control. Lancet Infect Dis. 2016;16(3):376–83. doi: 10.1016/S1473-3099(15)00360-6 [DOI] [PubMed] [Google Scholar]
- 62.Xu J, Bergquist R, Qian YJ, Wang Q, Yu Q, Peeling R, et al. China-Africa and China-Asia collaboration on schistosomiasis control: A SWOT analysis. Adv Parasitol. 2016;92:435–66. doi: 10.1016/bs.apar.2016.02.005 [DOI] [PubMed] [Google Scholar]


