TOP 10 TAKE-HOME MESSAGES FOR ADULTS WITH HEART FAILURE
This document describes performance measures for heart failure that are appropriate for public reporting or pay-for-performance programs.
The performance measures are from the 2017 American College of Cardiology/American Heart Association/Heart Failure Society of America heart failure guideline update and are selected from the strongest recommendations (Class 1 or 3).
Quality measures are also provided that are not yet ready for public reporting or pay for performance but might be useful for clinicians and healthcare organizations for quality improvement.
A new safety measure (laboratory monitoring for patients treated with mineralocorticoid receptor antagonists) is paired with a new treatment measure (mineralocorticoid receptor antagonists in patients with heart failure with reduced left ventricular ejection fraction).
Other additions to the performance measures include the new medication sacubitril/valsartan and use of cardiac resynchronization therapy.
To address frequent lack of titration of heart failure medications, 2 new performance measures are included based on dose, either reaching 50% of the recommended dose (e.g., beta blocker or angiotensin-converting enzyme inhibitor/angiotensin receptor antagonist/angiotensin receptor neprilysin inhibitor) or documenting that such a dose was not tolerated or otherwise inappropriate.
For all measures, if the clinician determines the care is inappropriate for the patient, that patient is excluded from the measure.
For all measures, patients who decline treatment or care are excluded.
A patient-centered discussion of the benefits and risks of implantable cardioverter-defibrillator treatment remains a performance measure.
To reflect the increasing importance of patient-reported outcome measures, 2 patient-reported outcomes quality measures were added that use heart failure patient-reported outcomes questionnaires currently accepted by the U.S. Food and Drug Administration.
PREAMBLE
The American College of Cardiology (ACC)/American Heart Association (AHA) performance measurement sets serve as vehicles to accelerate translation of scientific evidence into clinical practice. Measure sets developed by the ACC/AHA are intended to provide practitioners and institutions that deliver cardiovascular services with tools to measure the quality of care provided and identify opportunities for improvement.
Writing committees are instructed to consider the methodology of performance measure development (1,2) and to ensure that the measures developed are aligned with ACC/AHA clinical practice guidelines. The writing committees also are charged with constructing measures that maximally capture important aspects of care quality, including timeliness, safety, effectiveness, efficiency, equity, and patient-centeredness, while minimizing, when possible, the reporting burden imposed on hospitals, practices, and practitioners.
Potential challenges from measure implementation may lead to unintended consequences. The manner in which challenges are addressed is dependent on several factors, including the measure design, data collection method, performance attribution, baseline performance rates, reporting methods, and incentives linked to these reports.
The ACC/AHA Task Force on Performance Measures (Task Force) distinguishes quality measures from performance measures. Quality measures are those metrics that may be useful for local quality improvement but are not yet appropriate for public reporting or pay for performance programs (uses of performance measures). New measures are initially evaluated for potential inclusion as performance measures. In some cases, a measure is insufficiently supported by the guidelines. In other instances, when the guidelines support a measure, the writing committee may feel it is necessary to have the measure tested to identify the consequences of measure implementation. Quality measures may then be promoted to the status of performance measures as supporting evidence becomes available.
P. Michael Ho, MD, PhD, FACC, FAHA Chair, ACC/AHA Task Force on Performance Measures
1. INTRODUCTION
In 2019, the Task Force convened the writing committee to begin the process of revising the existing performance measures set for heart failure that was released in 2011 (3). The writing committee also was charged with the task of developing new measures to evaluate the care of patients in accordance with the 2017 ACC/AHA/HFSA heart failure guideline update (4).
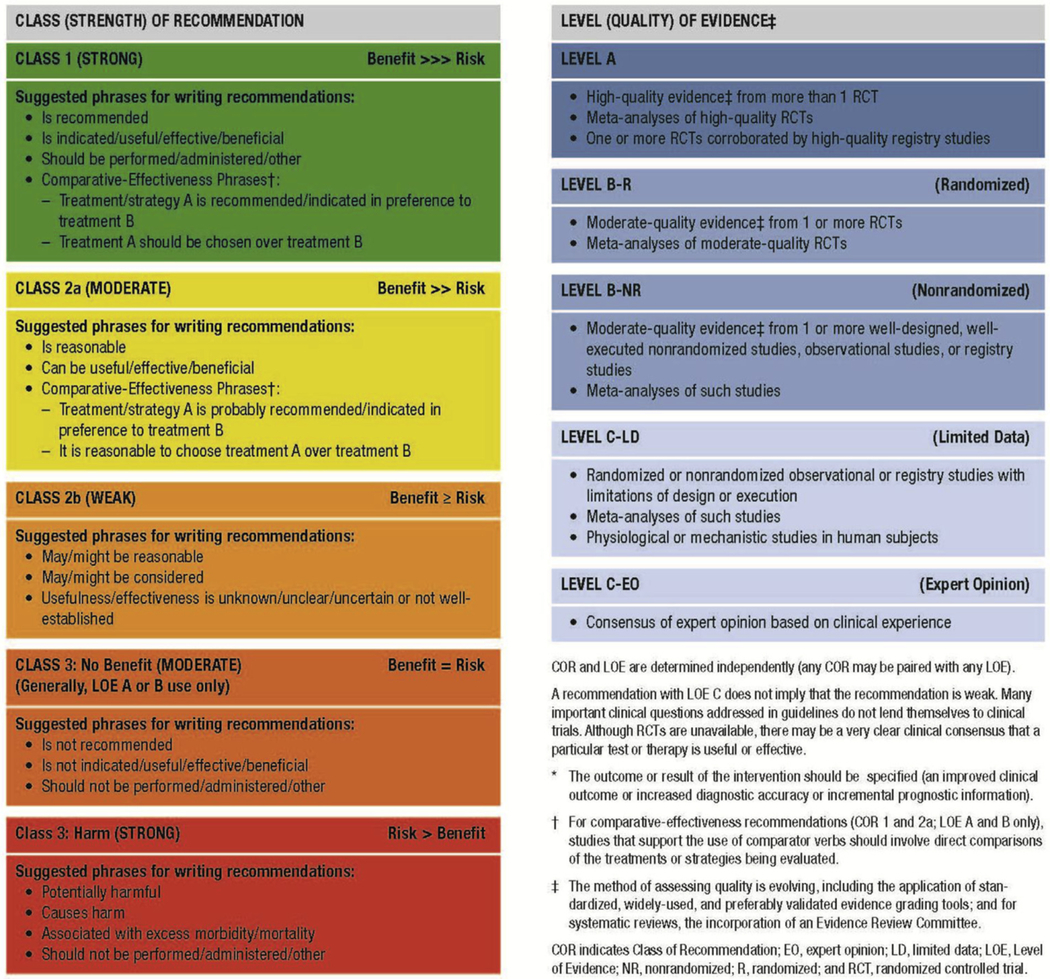
This updated performance measure set addresses in-hospital and continuing care in the outpatient setting. All Class 1 (strong) and 3 (no benefit or harmful, process to be avoided) guideline-recommended processes were considered for inclusion as performance measures. The current Class of Recommendation and Level of Evidence guideline classification scheme used by the ACC and AHA in their clinical guidelines is shown in Table 1. The value (benefit and cost) of a process of care was also considered. If high-quality, published, cost-effectiveness studies indicate that a Class 1 guideline recommendation for a process of care is considered a poor value by ACC/AHA standards, then it was not included as a performance measure (5). There were no Class 1 recommended processes of care judged to be of poor value. All ACC/AHA clinical practice guideline recommendations (including Class 2) were considered as potential quality measures. Ultimately, we selected measures based on their importance for health, existing gaps in care, ease of implementation, potential duplication with other performance measure lists, and risk for unintended consequences.
TABLE 1.
Applying Class of Recommendation and Level of Evidence to Clinical Strategies, Interventions, Treatments, or Diagnostic Testing in Patient Care* (Updated May 2019)
|
The writing committee developed a comprehensive heart failure measure set that includes 18 measures: 13 performance measures, 4 quality measures, 1 structural measure, and 2 rehabilitation performance measures (from the 2018 ACC/AHA performance measures for cardiac rehabilitation (6)), as reflected in Table 2 and Appendix A. The performance measures for heart failure included in the measure set are summarized in Table 2, which provides information on the measure number, measure title, and care setting. The measure specifications (Appendix A) provide information included in Table 2 and more detailed information including, the measure description, numerator, denominator (i.e., denominator exclusions and exceptions), rationale for the measure, clinical practice guideline that supports the measure, measurement period, source of data, and attribution.
TABLE 2.
ACC/AHA 2020 Heart Failure Clinical Performance, Quality and Structural Measures
| Measure No. | Measure Title | Care Setting | Attribution | Measure Domain | COR/LOE | |
|---|---|---|---|---|---|---|
| Performance Measures | ||||||
| PM-1 | LVEF assessment | Outpatient | Individual practitioner, Facility | Diagnostic | COR: 1, LOE: C; COR: 2a, LOE: C | |
| PM-2 | Symptom and activity assessment | Outpatient | Individual practitioner, Facility | Monitoring | COR: 1, LOE: C | |
| PM-3 | Symptom management | Outpatient | Individual practitioner, Facility | Treatment | See measure rationale in Appendix A for details | |
| PM-4 | Beta-blocker therapy for HFrEF | Outpatient, Inpatient | Individual practitioner, Facility | Treatment | COR: 1, LOE: A; COR: 1, LOE: B | |
| PM-5 | ACE inhibitor or ARB or ARNI therapy for HFrEF | Outpatient, Inpatient | Individual practitioner, Facility | Treatment | COR: 1, LOE: A; COR: 1, LOE: B-R | |
| PM-6 | ARNI therapy for HFrEF | Outpatient, Inpatient | Individual practitioner, Facility | Treatment | COR: 1, LOE: B-R | |
| PM-7 | Dose of beta-blocker therapy for HFrEF | Outpatient | Individual practitioner, Facility | Treatment | COR: 1, LOE: A | |
| PM-8 | Dose of ACE inhibitor, ARB, or ARNI therapy for HFrEF | Outpatient | Individual practitioner, Facility | Treatment | COR: 1, LOE: A; COR: 1, LOE: B-R | |
| PM-9 | MRA therapy for HFrEF | Outpatient, Inpatient | Individual practitioner, Facility | Treatment | COR: 1, LOE: A; COR: 1, LOE: B | |
| PM-10 | Laboratory monitoring in new MRA therapy | Outpatient, Inpatient | Individual practitioner, Facility | Monitoring | COR: 1, LOE: A | |
| PM-11 | Hydralazine/isosorbide dinitrate therapy for HFrEF in those self-identified as Black or African American | Outpatient, Inpatient | Individual practitioner, Facility | Treatment | COR: 1, LOE: A | |
| PM-12 | Counseling regarding ICD implantation for patients with HFrEF on guideline- directed medical therapy | Outpatient | Individual practitioner, Facility | Treatment | COR: 1, LOE: A | |
| PM-13 | CRT implantation for patients with HFrEF on guideline-directed medical therapy | Outpatient | Individual practitioner, Facility | Treatment | COR: 1, LOE: A; COR: 1, LOE: B | |
| Quality Measures | ||||||
| QM-1 | Patient self-care education | Outpatient | Individual practitioner, Facility | Self-Care | COR: 1, LOE: B | |
| QM-2 | Measurement of patient-reported outcome-health status | Outpatient | Individual practitioner, Facility | Monitoring | See measure rationale in Appendix A for details | |
| QM-3 | Sustained or improved health status in heart failure | Outpatient | Individual practitioner, Facility | Outcome | See measure rationale in Appendix A for details | |
| QM-4 | Postdischarge appointment for patients with heart failure | Inpatient | Individual practitioner, Facility | Treatment | COR: 2a, LOE: B | |
| Structural Measure | ||||||
| SM-1 | Heart failure registry participation | Outpatient, Inpatient | Facility | Structure | COR: 2a, LOE: B | |
|
Rehabilitation Performance Measures Related to Heart Failure (From the 2018 ACC/AHA performance measures for cardiac rehabilitation (6)) | ||||||
| Rehab PM-2 | Exercise training referral for HF from inpatient setting | Inpatient | Facility | Process | COR: 1, LOE: A | |
| Rehab PM-4 | Exercise training referral for HF from outpatient setting | Outpatient | Individual practitioner, Facility | Process | COR: 1, LOE: A | |
ACC indicates American College of Cardiology; ACE, angiotensin–converting enzyme; AHA, American Heart Association; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; COR, class of recommendation; CRT, cardiac resynchronization therapy; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter-defibrillator; LOE, level of evidence; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; PM, performance measure; QM, quality measure; and SM, structural measure.
The writing committee recognized that the 2018 ACC/AHA performance measures for cardiac rehabilitation have been published that address heart failure (6). The cardiac rehabilitation measure set includes performance measures for exercise training referral for inpatients and outpatients with heart failure and reduced left ventricular ejection fraction (Table 2). These rehabilitation measures also should be considered heart failure–related ACC/AHA performance measures.
A comprehensive list of contraindications to care is not provided. Instead, it is expected that clinical judgment will be used to determine if a contraindication exists. For example, certain patients with heart failure and congenital heart disease would not qualify for certain treatment measures and should be excluded from the denominator if documented by the clinician.
Although the measures are published as a set, their implementation can be individualized. It is not expected that all measures will be adopted simultaneously. Although all the measures are considered valuable in improving care, we recognize that organizations may only be able to focus on a limited number of measures. When implementing any measure that involves patient input, it is important to consider the patient’s health literacy and adapt data collection accordingly. Performance measures are a critical step in addressing disproportionately lower quality of care and potentially worse health status and outcomes among an underserved population.
1.1. Scope of the Problem
Heart failure is a major and growing public health problem in the United States with significant morbidity, mortality, and associated cost. A detailed discussion of the scope of the problem and opportunities to improve the quality of care that is provided to patients with this condition is available in the ACCF/AHA 2013 heart failure clinical practice guideline (7) and 2017 ACC/AHA/HFSA heart failure guideline update (4).
1.2. Disclosure of Relationships With Industry and Other Entities
The Task Force makes every effort to avoid actual, potential, or perceived conflicts of interest that could arise as a result of relationships with industry or other entities (RWI). Information about the ACC/AHA policy on RWI can be found online. All members of the writing committee, as well as those selected to serve as peer reviewers of this document, were required to disclose all current relationships and those existing within the 12 months before the initiation of this writing effort. ACC/AHA policy also requires that the writing committee chair and at least 50% of the writing committee have no relevant RWI. Writing committee members are excluded from voting on sections to which their specific RWI may apply.
Any writing committee member who develops new RWI during his or her tenure on the writing committee is required to notify staff in writing. These statements are reviewed periodically by the Task Force and by members of the writing committee. Writing committee member and peer reviewer RWI, which are pertinent to the document, are included in the appendixes: Appendix B for relevant writing committee RWI and Appendix C for comprehensive peer reviewer RWI. Additionally, to ensure complete transparency, the writing committee members’ comprehensive disclosure information, including RWI not relevant to the present document, is available online. Disclosure information for the Task Force is also available online.
The work of the writing committee was supported exclusively by the ACC and the AHA without commercial support. Members of the writing committee volunteered their time for this effort. Meetings of the writing committee were confidential and attended only by writing committee members and staff from the ACC, AHA, and the Heart Failure Society of America, which served as a collaborator on this project.
2. METHODOLOGY
2.1. Literature Review
In developing the updated heart failure measure set, the writing committee reviewed evidence-based guidelines and statements that would potentially impact the construct of the measures. The clinical practice guidelines and scientific statements that most directly contributed to the development of these measures are shown in Table 3.
TABLE 3.
Associated ACC/AHA Clinical Practice Guidelines and Other Clinical Guidance Documents
| Clinical Practice Guidelines |
|---|
| 2017 ACC/AHA/HFSA heart failure guideline update (4) |
| 2016 ESC heart failure diagnosis and treatment guidelines (8) |
| 2013 ACCF/AHA heart failure clinical practice guideline (7) |
| 2017 AHA/ACC/HRS ventricular arrhythmias and prevention of sudden cardiac death guideline (9) |
| Performance Measures |
| 2011 ACCF/AHA/PCPI heart failure performance measurement set (3) |
| 2018 ACC/AHA performance measures for cardiac rehabilitation (6) |
| 2017 ACC expert consensus decision pathway for optimization of heart failure treatment (10) |
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; AHA, American Heart Association; ESC, European Society of Cardiology; HFSA, Heart Failure Society of America; HRS, Heart Rhythm Society; and PCPI, Physician Consortium for Performance Improvement.
2.2. Definition and Selection of Measures
The writing committee considered a number of additional factors, which are listed in Table 4. The potential impact, appropriateness for public reporting and pay for performance, validity, reliability, and feasibility were considered. The writing committee examined available information on current gaps in care. The term “heart failure” refers to stage C or D heart failure unless otherwise stated (4).
TABLE 4.
ACC/AHA Task Force on Performance Measures: Attributes for Performance Measures (11)
| 1. Evidence Based | |
|---|---|
| High-impact area that is useful in improving patient outcomes | a) For structural measures, the structure should be closely linked to a meaningful process of care that, in turn, is linked to a meaningful patient outcome. b) For process measures, the scientific basis for the measure should be well established, and the process should be closely linked to a meaningful patient outcome. c) For outcome measures, the outcome should be clinically meaningful. If appropriate, performance measures based on outcomes should adjust for relevant clinical characteristics through the use of appropriate methodology and high-quality data sources. |
| 2. Measure Selection | |
| Measure definition | a) The patient group to whom the measure applies (denominator) and the patient group for whom conformance is achieved (numerator) are clearly defined and clinically meaningful. |
| Measure exceptions and exclusions | b) Exceptions and exclusions are supported by evidence. |
| Reliability | c) The measure is reproducible across organizations and delivery settings. |
| Face validity | d) The measure appears to assess what it is intended to. |
| Content validity | e) The measure captures most meaningful aspects of care. |
| Construct validity | f) The measure correlates well with other measures of the same aspect of care. |
| 3. Measure Feasibility | |
| Reasonable effort and cost | a) The data required for the measure can be obtained with reasonable effort and cost. |
| Reasonable time period | b) The data required for the measure can be obtained within the period allowed for data collection. |
| 4. Accountability | |
| Actionable | a) Those held accountable can affect the care process or outcome. |
| Unintended consequences avoided | b) The likelihood of negative, unintended consequences with the measure is low. |
Reproduced with permission from Thomas et al (6). Copyright © 2018, American Heart Association, Inc., and American College of Cardiology Foundation.
ACC indicates American College of Cardiology; and AHA, American Heart Association.
3. ACC/AHA HEART FAILURE MEASURE SET
3.1. Discussion of Changes to 2011 Heart Failure Measure Set
After reviewing the existing clinical practice guidelines, and the 2011 ACCF/AHA/PCPI heart failure performance measurement set (3), the writing committee discussed which measures required revision to reflect updated science related to heart failure and identified which guideline recommendations could serve as the basis for new performance or quality measures. The writing committee also reviewed existing publicly available measure sets.
These subsections serve as a synopsis of the revisions that were made to previous measures and a description of why the new measures were created for both the inpatient and outpatient setting.
3.1.1. Retired Measures
The writing committee decided to retire the left ventricular ejection fraction assessment measure used in the inpatient setting due to >97% of use (12) (Table 5). Left ventricular ejection fraction assessment in the outpatient setting was retained.
TABLE 5.
Retired Heart Failure Measures From the 2011 Set (3)
| Measure No. | Care Setting | Measure Title | Rationale for Retiring the Measure |
|---|---|---|---|
| 2 | Inpatient | LVEF assessment | Inpatient documentation of LVEF is at >97% (12). |
LVEF indicates left ventricular ejection fraction.
3.1.2. Revised Measures
The writing committee reviewed and made changes to the patient self-care education, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy for left ventricular systolic dysfunction, and postdischarge appointment measures, as summarized in Table 6. Table 6 provides information on the updated measures including the care setting, title, and a brief rationale for revisions made to the measures.
TABLE 6.
Revised Heart Failure Measures
| Measure No. | Measure Title | Description of Revision | Rationale for Revision |
|---|---|---|---|
| 5 | Patient self-care education | Moved from Performance Measure to Quality Measure | Concern regarding the accuracy of self-care education documentation; limited evidence of improved outcomes with better documentation. |
| 7 | ACE inhibitor or ARB therapy for LVSD | Added ARNI | 2017 ACC/AHA/HFSA heart failure guideline update (4) made this revision to the recommendation. |
| 9 | Postdischarge appointment | Moved from Performance Measure to Quality Measure and included a time limit of 7 d | 2013 ACCF/AHA heart failure clinical practice guideline (7) lists postdischarge appointment from 7–14 d as a Class 2a recommendation. |
ACC indicates American College of Cardiology; ACE, angiotensin–converting enzyme; AHA, American Heart Association; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; HF, heart failure; and LVSD, left ventricular systolic dysfunction.
3.1.3. New Measures
The writing committee created 7 new performance measures (PM 6–11, 13), 2 quality measures (QM 2, 3), and 1 structural measure (SM-1) (Table 7). Six of the new performance measures were based on Class 1 guideline recommendations for therapies known to prolong survival. An additional performance measure (PM-10, measurement of potassium after a mineralocorticoid receptor antagonist prescription) is also guideline recommended and included as a safety measure to accompany prescription for mineralocorticoid receptor antagonist (PM-9). Two new measures based on dose were created (PM-7 and PM-8). These were chosen because of the gap between doses used in practice and those shown to provide survival benefit in clinical trials. They were designed to apply only to those patients without demonstrated intolerance at higher doses.
TABLE 7.
New Heart Failure Measures
| Measure No. | Care Setting | Measure Title | Rationale for Creating New Measure | Rationale for Designating as a Quality Measure Versus a Performance Measure |
|---|---|---|---|---|
| PM-6 | Outpatient, Inpatient | ARNI therapy for HFrEF | Important outcome benefit with large existing gap in care | N/A |
| PM-7 | Outpatient | Dose of beta-blocker therapy for HFrEF | Important outcome benefit and large existing gap in care | N/A |
| PM-8 | Outpatient | Dose of ACE inhibitor, ARB, or ARNI therapy for HFrEF | Important outcome benefit and large existing gap in care | N/A |
| PM-9 | Outpatient, Inpatient | MRA therapy for HFrEF | Important outcome benefit and large existing gap in care | N/A |
| PM-10 | Outpatient, Inpatient | Laboratory monitoring in new MRA therapy | Important outcome benefit and large existing gap in care | N/A |
| PM-11 | Outpatient, Inpatient | Hydralazine/isosorbide dinitrate therapy for HFrEF in those self-identified as Black or African American | Important outcome benefit and large existing gap in care | N/A |
| PM-13 | Outpatient | CRT implantation for patients with HFrEF on guideline-directed medical therapy | Important outcome benefit and large existing gap in care | N/A |
| QM-2 | Outpatient | Measurement of patient-reported, outcome-health status | Important outcome that is rarely measured | Best method of implementation is unclear |
| QM-3 | Outpatient | Sustained or improved health status in heart failure | Important outcome that is rarely measured | Needs validated risk-adjustment |
| SM-1 | Outpatient, Inpatient | Heart failure registry participation | Registries are a useful structure for measuring performance | Additional data needed to determine the impact of registry participation on quality |
ACE indicates angiotensin–converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; CRT, cardiac resynchronization therapy; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonists; N/A, not applicable; PM, performance measure; QM, quality measure; and SM, structural measure.
For more detailed information on each measure’s construct, refer to the specifications in Appendix A.
4. AREAS FOR FURTHER RESEARCH
There are multiple ways that cardiac rehabilitation and exercise prescriptions can be implemented (13). Further studies are needed to determine if there are differences in the magnitude of outcome improvements by approach. Similarly, although patient-reported outcomes are considered an important metric, the best way to measure these needs additional research. Two surveys are well validated: The Kansas City Cardiomyopathy Questionnaire (14) and the Minnesota Living with Heart Failure Questionnaire (15). However, risk-adjustment is required to fairly compare groups for use as an outcome measure. The collection of the measure (process of care) does not require risk-adjustment but will benefit from additional research to understand optimal timing of collection of patient-reported outcomes, including frequency and relation to the clinic visit. Finally, data supporting sodium-glucose cotransporter-2 inhibitors are emerging for heart failure treatment; however, with additional trials ongoing and having not been integrated into guideline recommendations at the time of generation of the measure set, the writing committee was unable to include them in the measure set.
ACC/AHA Task Force on Performance Measures
P. Michael Ho, MD, PhD, FACC, FAHA Chair‡
H. Vernon Anderson, MD, FACC‡
Ankeet S. Bhatt, MD, MBA§
Biykem Bozkurt, MD, PhD, FACC, FAHA, FHFSA§
Sandeep Das, MD, MPH, FACC§
Joao F. Monteiro Ferreira, MD, PhD, FACC‡
Gregg C. Fonarow, MD, FACC, FAHA, FHFSA, Ex Officio§
Stacy Garcia, RT(R), RN, BSN, MBA-HCM‡
Michael E. Hall, MD, MS, FACC, FAHA§
Hani Jneid, MD, FACC, FAHA§
Patricia A. Keegan, DNP, APRN, NP-C‡‖
Christopher Lee, PhD, RN, FAHA, FHFSA§
Leo Lopez, MD, FACC, FAHA‡
Jeffrey W. Olin, DO, FACC, FAHA§
Manesh R. Patel, MD, FACC§
Faisal Rahman, BM BCh‡
Matthew Roe, MD, FACC‡‖
Katherine B. Salciccioli, MD§
Alex Sandhu, MD, MS‡‖
Randal J. Thomas, MD, MS, FACC, FAHA‡‖
Siqin Kye Ye, MD, MS‡‖
Boback Ziaeian, MD, PhD, FACC, FAHA‡
‡American College of Cardiology Representative.
§American Heart Association Representative.
‖Former Task Force member; current member during the writing effort.
STAFF
American College of Cardiology
Athena Poppas, MD, FACC, President
Cathleen Gates, Interim Chief Executive Officer
John S. Rumsfeld, MD, PhD, FACC, Chief Science and Quality Officer
Lara Slattery, Division Vice President, Clinical Registry and Accreditation
Grace Ronan, Team Lead, Clinical Policy Publication
Timothy W. Schutt, MA, Clinical Policy Analyst
American College of Cardiology/American Heart Association
Abdul R. Abdullah, MD, Director, Guideline Science and Methodology
Rebecca L. Diekemper, MPH, Guideline Advisor, Performance Measures
American Heart Association
Robert A. Harrington, MD, FAHA, President
Nancy Brown, Chief Executive Officer
Mariell Jessup, MD, FAHA, Chief Science and Medical Officer
Radhika Rajgopal Singh, PhD, Vice President, Office of Science, Medicine, and Health
Paul St. Laurent, DNP, RN, Senior Science and Medicine Advisor
Melanie Shahriary, RN, BSN, Senior Manager, Performance Metrics, Quality and Health IT
Jody Hundley, Production and Operations Manager, Scientific Publications, Office of Science Operations
APPENDIX A. HEART FAILURE MEASURE SET
Performance Measures for Heart Failure
SHORT TITLE: PM-1 Left Ventricular Ejection Fraction Assessment (Outpatient Setting)
PM-1: Left Ventricular Ejection Fraction Assessment (Outpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure for whom the quantitative result of prior (any time in the past) LVEF assessment, using any imaging modality, is available in the medical record | |
| Numerator | Patients for whom the quantitative* results of prior (any time in the past) LVEF assessment, using any imaging modality, is available in the medical record (includes note documentation) *Single value or numerical range |
| Denominator | All patients age ≥18 y with a diagnosis of heart failure |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not evaluating LVEF (e.g., comfort care only) Documentation of patient reason(s) for not evaluating LVEF (e.g., patient refusal) |
| Measurement Period | 12 mo |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient |
| Rationale | |
| Evaluation of LVEF in patients with heart failure provides important information that is required to appropriately direct treatment. Several pharmacological therapies have demonstrated efficacy in slowing disease progression and improving survival in patients with left ventricular systolic dysfunction (3). Although most patients have an LVEF recorded, this remains a performance measure because knowledge of LVEF is required to determine eligibility for appropriate heart failure care. Patients post-heart transplant or with an LVAD are excluded, because these patients were excluded from clinical treatment trials for low LVEF heart failure. | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. A 2-dimensional echocardiogram with Doppler should be performed during initial evaluation of patients presenting with HF to assess ventricular function, size, wall thickness, wall motion, and valve function. (Class 1, Level of Evidence: C) 2. Repeat measurement of EF and measurement of the severity of structural remodeling are useful to provide information in patients with HF who have had a significant change in clinical status; who have experienced or recovered from a clinical event; or who have received treatment, including GDMT, that might have had a significant effect on cardiac function; or who may be candidates for device therapy. (Class 1, Level of Evidence: C) 3. Radionuclide ventriculography or magnetic resonance imaging can be useful to assess LVEF and volume when echocardiography is inadequate. (Class 2a, Level of Evidence: C) | |
ACCF indicates American College of Cardiology Foundation; AHA, American Heart Association; EF, ejection fraction; EHR, electronic health record; GDMT, guideline-directed medical therapy; HF, heart failure; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; and PM, performance measure.
SHORT TITLE: PM-2 Symptom and Activity Assessment (Outpatient Setting)
PM-2: Symptom and Activity Assessment (Outpatient Setting)
| Measure Description: Percentage of patient visits for those patients age ≥18 y with a diagnosis of heart failure with quantitative results of an evaluation of both current level of activity and clinical symptoms documented | |
| Numerator | Patient visits with quantitative results of an evaluation of both current level of activity and clinical symptoms documented* *Evaluation and quantitative results documented can include:
The NYHA functional classification reflects a subjective assessment by a healthcare provider of the severity of a patient’s symptoms. Patients are assigned to one of the following classes:
|
| Denominator | All patient visits for those patients age ≥18 y with a diagnosis of heart failure |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not evaluating both current level of activity and clinical symptoms (e.g., severe cognitive or functional impairment) Documentation of patient reason(s) for not evaluating both current level of activity and clinical symptoms |
| Measurement Period | 12 mo |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient |
| Rationale | |
| Initial and ongoing evaluations of patients with heart failure should include an assessment of symptoms and their functional consequences. These assessments serve as the basis for making treatment decisions, monitoring the effects of treatment, and modifying treatment as appropriate. Decreasing symptoms and improving function are 2 of the primary goals of heart failure treatment and represent important patient-centric outcomes for heart failure care. The ACC/AHA have not addressed PRO tool selection. However, the FDA has provided guidelines for an appropriate PRO tool (16) and, currently, 2 heart failure survey tools–the MLHFQ (15) and the KCCQ (14)–are considered qualified tools for FDA device use in heart failure (17). | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. A thorough history and physical examination should be obtained/performed in patients presenting with HF to identify cardiac and noncardiac disorders or behaviors that might cause or accelerate the development or progression of HF. (Class 1, Level of Evidence: C) 2. The NYHA functional classification gauges the severity of symptoms in those with structural heart disease. Although reproducibility and validity may be problematic (18), the NYHA functional classification is an independent predictor of mortality (19). It is widely used in clinical practice and research and for determining the eligibility of patients for certain healthcare services (7). However, NYHA functional class assessment is not reported in a significant number of patients in contemporary HF practices in the United States (20). 3. Evaluate general health status (see Figure 2, 2013 ACCF/AHA guideline) (7). Although no specific 2013 ACCF/AHA guideline recommendation is made regarding collection of NYHA or other quantitative result, knowledge of symptom status is needed to determine candidacy for appropriate HF treatments (7). | |
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; AHA, American Heart Association; EHR, electronic health record; FDA, U.S. Food and Drug Administration; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVAD, left ventricular assist device; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; PM, performance measure; and PRO, patient-reported outcome.
SHORT TITLE: PM-3 Symptom Management (Outpatient Setting)
PM-3: Symptom Management (Outpatient Setting)
| Measure Description: Percentage of patient visits for those patients age ≥18 y with a diagnosis of heart failure and with quantitative results of an evaluation of both level of activity and clinical symptoms documented in which patient symptoms have improved or remained consistent with treatment goals, or patient symptoms have worsened since last assessment and have a documented plan of care | |
| Numerator | Patient visits in which patient symptoms have improved or remained consistent with treatment goals since last assessment,* or patient symptoms have worsened since last assessment* and have a documented plan of care† *Examples of quantitative assessment:
|
| Denominator | All patient visits for those patients age ≥18 y with a diagnosis of heart failure and with quantitative results of an evaluation of both level of activity and clinical symptoms documented at the time of the encounter and at a prior time point 1 to 12 mo previously. |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | None |
| Measurement Period | 12 mo |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient |
| Rationale | |
| Heart failure significantly decreases HRQOL, especially in the areas of physical functioning and vitality (21,22). Lack of improvement in HRQOL after discharge from the hospital is a powerful predictor of rehospitalization and mortality (23,24). Women with heart failure have consistently been found to have worse HRQOL than men (22,25). Ethnic differences also have been found, with Mexican Hispanics reporting better HRQOL than other ethnic groups in the United States (26). Other determinants of poor HRQOL include depression, younger age, higher BMI, greater symptom burden, lower systolic blood pressure, sleep apnea, low perceived control, and uncertainty about prognosis (25,27–31). Objective data on symptoms and functional status from at least 2 time points are needed to decide if patients are benefitting from therapy. | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Goals of treatment in heart failure are to improve health-related quality of life and symptoms (see Figure 3, 2013 ACCF/AHA guideline) (7). | |
ACCF indicates American College of Cardiology Foundation; AHA, American Heart Association; BMI, body mass index; EHR, electronic health record; ICC, intraclass correlation coefficient; HRQOL, health-related quality of life; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVAD, left ventricular assist device; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; PM, performance measure; PRO, patient-reported outcome; VE/VCO2, ventilation and carbon dioxide; and VO2, oxygen consumption.
SHORT TITLE: PM-4 Beta-Blocker Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient and Inpatient Setting)
PM-4: Beta-Blocker Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient and Inpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤40% who were prescribed beta-blocker therapy either within a 12-mo period when seen in the outpatient setting or at hospital discharge | |
| Numerator | Patients who were prescribed* beta-blocker therapy† either within a 12-mo period when seen in the outpatient setting or at hospital discharge *Prescribed may include:
|
| Denominator | All patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤40% |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not prescribing beta-blocker therapy (e.g., intolerance) Documentation of patient reason(s) for not prescribing beta-blocker therapy (e.g., patient refusal) |
| Measurement Period | 12 mo |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient Inpatient |
| Rationale | |
| Beta blockers improve survival and reduce hospitalization for patients with stable heart failure and reduced LVEF (HFrEF) (7). Treatment should be initiated as soon as a patient is diagnosed with reduced LVEF and does not have prohibitively low systemic blood pressure, fluid overload, or recent treatment with an intravenous positive inotropic agent. Beta blockers have also been shown to lessen the symptoms of heart failure, improve the clinical status of patients, and reduce future clinical deterioration. Despite these benefits, use of beta blockers in eligible patients remains suboptimal (20). | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Use of 1 of the 3 beta blockers proven to reduce mortality (e.g., bisoprolol, carvedilol, and sustained-release metoprolol succinate) is recommended for all patients with current or prior symptoms of HFrEF, unless contraindicated, to reduce morbidity and mortality (32–37). (Class 1, Level of Evidence: A) 2. Initiation of beta-blocker therapy is recommended after optimization of volume status and successful discontinuation of intravenous diuretics, vasodilators, and inotropic agents. Beta-blocker therapy should be initiated at a low dose and only in stable patients. Caution should be used when initiating beta blockers in patients who have required inotropes during their hospital course (38–40). (Class 1, Level of Evidence: B) | |
ACCF indicates American College of Cardiology Foundation; AHA, American Heart Association; EHR, electronic health record; HFrEF, heart failure reduced ejection fraction; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; and PM performance measure.
SHORT TITLE: PM-5 Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker or Angiotensin Receptor-Neprilysin Inhibitor Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient and Inpatient Setting)
PM-5: Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker or Angiotensin Receptor-Neprilysin Inhibitor Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient and Inpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤40% who were prescribed ACE inhibitor, ARB, or ARNI either within a 12-mo period when seen in the outpatient setting or at hospital discharge | |
| Numerator | Patients who were prescribed* ACE inhibitor, ARB, or ARNI either within a 12-mo period when seen in the outpatient setting or at hospital discharge *Prescribed may include:
|
| Denominator | All patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤40% |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not prescribing ACE inhibitor, ARB, or ARNI (e.g., intolerance) Documentation of patient reason(s) for not prescribing ACE inhibitor, ARB, or ARNI (e.g., patient refusal) |
| Measurement Period | ACE inhibitor, ARB, or ARNI therapy initiated within a 12-mo period of being seen in the outpatient setting or from hospital discharge |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient Inpatient |
| Rationale | |
| Use of ACE inhibitor, ARB, or ARNI therapy has been associated with improved outcomes in patients with reduced LVEF (7). Long-term therapy with ARBs has also been shown to reduce morbidity and mortality, especially in ACE inhibitor-intolerant patients (41–44). More recently, ARNI therapy has also been shown to more significantly improve outcomes (45), such that the newest guidelines recommend replacement of ACE inhibitors or ARBs with ARNI therapy in eligible patients (4). However, despite the benefits of these drugs, use of ACE inhibitor, ARB, or ARNI remains suboptimal (20). | |
| Clinical Recommendation(s) | |
|
2017 ACC/AHA/HFSA heart failure guideline update (4) 1. The clinical strategy of inhibition of the renin-angiotensin system with ACE inhibitors (Class 1, Level of Evidence: A) (46–51), OR ARBs (Class 1, Level of Evidence: A) (41–44), OR ARNI (Class 1, Level of Evidence: B-R) (45) in conjunction with evidence-based beta blockers (7,33,52), and aldosterone antagonists in selected patients (53,54), is recommended for patients with chronic HFrEF to reduce morbidity and mortality. | |
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; ACE, angiotensin–converting enzyme; AHA, American Heart Association; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; EHR, electronic health record; HFrEF, heart failure reduced ejection fraction; HFSA, Heart Failure Society of America; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; and PM, performance measure.
SHORT TITLE: PM-6 Angiotensin Receptor-Neprilysin Inhibitor Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient and Inpatient Setting)
PM-6: Angiotensin Receptor-Neprilysin Inhibitor Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient and Inpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤40% who remained symptomatic at NYHA functional class II or class III despite ACE inhibitor or ARB therapy for a least 3 mo and were prescribed ARNI therapy either within a 12-mo period when seen in the outpatient setting or at hospital discharge | |
| Numerator | Patients who were prescribed* ARNI therapy either within a 12-mo period when seen in the outpatient setting or at hospital discharge *Prescribed may include:
|
| Denominator | All patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤40% after 3 mo of ACE inhibitor or ARB therapy |
| Denominator Exclusions | Heart transplant LVAD NYHA class I and class IV |
| Denominator Exceptions | Documentation of medical reason(s) for not prescribing ARNI therapy (e.g., intolerant) Documentation of patient reason(s) for not prescribing ARNI therapy (e.g., patient refusal, cost) Documentation of system reason(s) for not prescribing ARNI therapy |
| Measurement Period | ARNI therapy initiated within a 12-mo period of being seen in the outpatient setting or from hospital discharge |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient Inpatient |
| Rationale | |
| In a large randomized clinical trial, an ARNI (valsartan/sacubitril) was compared with an ACE inhibitor (enalapril) in symptomatic patients with HFrEF. The ARNI reduced the composite endpoint of cardiovascular death or heart failure hospitalization significantly, by 20% (45). The benefit was seen to a similar extent for both death and heart failure hospitalization and was consistent across subgroups. Since the initial large randomized clinical trial with ARNI, there has been additional clinical trial evidence (55,56), meta-analyses (57), and observational clinical effectiveness studies (58), which further support the use of valsartan/sacubitril in replacement of ACE inhibitor or ARB therapy to reduce mortality and morbidity. | |
| Clinical Recommendation(s) | |
|
2017 ACC/AHA/HFSA heart failure guideline update (4) 1. In patients with chronic symptomatic HFrEF NYHA class II or III who tolerate an ACE inhibitor or ARB, replacement by an ARNI is recommended to further reduce morbidity and mortality (45). (Class 1, Level of Evidence: ARNI: B-R) | |
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; ACE, angiotensin–converting enzyme; AHA, American Heart Association; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; EHR, electronic health record; HFrEF, heart failure reduced ejection fraction; HFSA, Heart Failure Society of America; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; and PM, performance measure.
SHORT TITLE: PM-7 Dose of Beta-Blocker Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient Setting)
PM-7: Dose of Beta-Blocker Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤40% who were prescribed a guideline-recommended beta blocker (e.g., bisoprolol, carvedilol, or sustained-release metoprolol succinate) at a dose that is at least 50% of the target dose (see Table A for target doses) | |
| Numerator | Patients who were prescribed a guideline-recommended beta blocker at a dose that is at least 50% of the target dose (see Table A for target doses) |
| Denominator | All patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤40% who were prescribed a recommended beta blocker |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of intolerance of higher dose or medical reason(s) for not prescribing higher dose of beta blocker Documentation of patient reason(s) for not prescribing higher dose of beta blocker Documentation of system reason(s) for not prescribing higher dose of beta blocker |
| Measurement Period | Annually |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient |
| Rationale | |
| Use of guideline-recommended beta blockers has been proven to reduce morbidity and mortality in patients with HFrEF, and studies have supported a dose-response relationship of beta blockers with improved outcomes (59–64). These findings suggest that, among HFrEF patients in whom target doses might be well tolerated, treating at less than the target dose may result in worse clinical outcomes. Despite guideline recommendations for clinicians to achieve target doses of beta blockers shown to be effective in major clinical trials, the percentage of patients achieving these doses is low and remains low over time (20,65,66). Treatment with a beta blocker should be initiated at very low doses, followed by gradual incremental increases in dose if lower doses have been well tolerated. Clinicians should make every effort to achieve the target doses of the beta blockers shown to be effective in major clinical trials (7). | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Use of 1 of the 3 beta blockers proven to reduce mortality (e.g., bisoprolol, carvedilol, and sustained-release metoprolol succinate) is recommended for all patients with current or prior symptoms of HFrEF, unless contraindicated, to reduce morbidity and mortality (32–37). (Class 1, Level of Evidence: A) 2017 ACC expert consensus decision pathway for optimization of heart failure treatment (10) 1. After a diagnosis of heart failure is made, GDMT should be initiated and therapies should be adjusted no more frequently than every 2 weeks to target doses (or maximally tolerated doses). 2. To achieve the maximal benefits of GDMT in patients with chronic HFrEF, therapies must be initiated and titrated to maximally tolerated doses (33,45,60,67). | |
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; AHA, American Heart Association; EHR, electronic health record; GDMT, guideline-directed medical therapy; HFrEF, heart failure reduced ejection fraction; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; and PM, performance measure.
TABLE A.
Target Doses of Guideline-Directed Medical Therapies
| ACE Inhibitors | Target Dose | Total Daily Target Dose | 50% of Total Daily Target Dose |
|---|---|---|---|
| Captopril | 50 mg, three times daily | 150 mg | 75 mg |
| Enalapril | 10 mg, twice daily | 20 mg | 10 mg |
| Lisinopril | 20 mg, once daily | 20 mg | 10 mg |
| Ramipril | 10 mg, once daily | 10 mg | 5 mg |
| Perindopril | 8 mg, once daily | 8 mg | 4 mg |
| Trandolapril | 4 mg, once daily | 4 mg | 2 mg |
| Benazepril | 40 mg, once daily | 40 mg | 20 mg |
| Fosinopril | 40 mg, once daily | 40 mg | 20 mg |
| Quinapril | 20 mg, twice daily | 40 mg | 20 mg |
| ARB | |||
| Candesartan | 32 mg, once daily | 32 mg | 16 mg |
| Losartan | 100 mg, once daily* | 100 mg | 50 mg |
| Valsartan | 160 mg, twice daily | 320 mg | 160 mg |
| Irbesartan | 300 mg, once daily | 300 mg | 150 mg |
| Telmisartan | 80 mg, once daily | 80 mg | 40 mg |
| Olmesartan | 40 mg, once daily | 40 mg | 20 mg |
| Azilsartan | 80 mg, once daily | 80 mg | 40 mg |
| ARNI | |||
| Sacubitril/valsartan | 97/103 mg, twice daily | 194/206 mg | 98/102 mg† |
| Evidence-Based Beta-Blockers | |||
| Bisoprolol | 10 mg, once daily | 10 mg | 5 mg |
| Carvedilol | 25 mg, twice daily | 50 mg | 25 mg |
| Carvedilol extended release | 80 mg, once daily | 80 mg | 40 mg |
| Metoprolol succinate sustained release | 200 mg, once daily | 200 mg | 100 mg |
Sources for target doses include: 2013 ACCF/AHA heart failure clinical practice guideline (7), 2017 ACC/AHA/HFSA heart failure guideline update (4), 2017 ACC expert consensus decision pathway for optimization of heart failure treatment (10), and FDA-approved labels (68).
ACC/AHA Guidelines recommend losartan 150 mg as target dose. However, because current FDA-approved labeling has 100 mg as the maximal dose, the 100-mg dose is used in the performance measure.
The sacubitril 98 mg and valsartan 102 mg total daily dosing (49/51 mg twice daily) is considered fulfilling the 50% of target dosing criteria.
ACE indicates angiotensin–converting enzyme; ARB, angiotensin II receptor blocker; and ARNI, angiotensin receptor–neprilysin inhibitor.
SHORT TITLE: PM-8 Dose of Angiotensin-Converting Enzyme Inhibitor, Angiotensin Receptor Blocker, or Angiotensin Receptor-Neprilysin Inhibitor Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient Setting)
PM-8: Dose of Angiotensin-Converting Enzyme Inhibitor, Angiotensin Receptor Blocker, or Angiotensin Receptor-Neprilysin Inhibitor Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤40% who were prescribed an ACE inhibitor, ARB, or ARNI at a dose that is at least 50% of the target dose (see Table A for target doses) | |
| Numerator | Patients who were prescribed an ACE inhibitor, ARB, or ARNI at a dose that is at least 50% of the target dose (see Table A for target doses) |
| Denominator | All patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤40% who were prescribed an ACE inhibitor, ARB, or ARNI |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of intolerance of higher dose or medical reason(s) for not prescribing higher dose of ACE inhibitor, ARB, or ARNI Documentation of patient reason(s) for not prescribing higher dose of ACE inhibitor, ARB, or ARNI Documentation of system reason(s) for not prescribing higher dose of ACE inhibitor, ARB, or ARNI |
| Measurement Period | Annually |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient |
| Rationale | |
| Inhibition of the renin-angiotensin system with ACE inhibitor, ARB, or ARNI therapy has been proven to reduce morbidity and mortality in patients with HFrEF, and studies have supported a dose-response relationship of these therapies with improved outcomes (42,50,69,70). These findings suggest that, among HFrEF patients in whom target doses might be well tolerated, treating at less than the target dose may result in worse clinical outcomes. Despite guideline recommendations for clinicians to achieve target doses of ACE inhibitors, ARBs, or ARNIs, the number of patients achieving these doses is low and remains low over time (20,65,66). | |
| Clinical Recommendation(s) | |
|
2017 ACC/AHA/HFSA heart failure guideline update (4) 1. The clinical strategy of inhibition of the renin-angiotensin system with ACE inhibitors (Class 1, Level of Evidence: A) (46–51), OR ARBs (Class 1, Level of Evidence: A) (41–44), OR ARNI (Class 1, Level of Evidence: B-R) (45) in conjunction with evidence-based beta blockers (7,33,52), and aldosterone antagonists in selected patients (53,54), is recommended for patients with chronic HFrEF to reduce morbidity and mortality. 2. ACE inhibitors should be started at low doses and titrated upward to doses shown to reduce the risk of cardiovascular events in clinical trials. 3. ARBs should be started at low doses and titrated upward, with an attempt to use doses shown to reduce the risk of cardiovascular events in clinical trials. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment (10) 1. After a diagnosis of heart failure is made, GDMT should be initiated and therapies should be adjusted no more frequently than every 2 weeks to target doses (or maximally tolerated doses). 2. To achieve the maximal benefits of GDMT in patients with chronic HFrEF, therapies must be initiated and titrated to maximally tolerated doses (33,45,60,67). | |
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; ACE, angiotensin–converting enzyme; AHA, American Heart Association; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; EHR, electronic health record; GDMT, guideline-directed medical therapy; HFrEF, heart failure reduced ejection fraction; HFSA, Heart Failure Society of America; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; and PM, performance measure.
SHORT TITLE: PM-9 Mineralocorticoid Receptor Antagonist Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient and Inpatient Setting)
PM-9: Mineralocorticoid Receptor Antagonist Therapy for Heart Failure With Reduced Ejection Fraction (Outpatient and Inpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤35% who are NYHA class II through class IV despite attempts at treatment with beta blockers and ACE inhibitors, ARB, or ARNI | |
| Numerator | Patients who were prescribed* MRA either within a 12-mo period when seen in the outpatient setting or at hospital discharge *Prescribed may include:
|
| Denominator | All patients age ≥18 y with a diagnosis of heart failure with a current or prior LVEF ≤35% who are NYHA class II-IV despite attempts at treatment with beta blockers and ACE inhibitors, ARB, or ARNI, and have Cr ≤2.5 mg/dL for men and ≤2.0 mg/dL for women (or estimated glomerular filtration rate >30 mL/min/1.73 m2) and K <5.0 mEq/L |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not prescribing MRA therapy Documentation of patient reason(s) for not prescribing MRA therapy |
| Measurement Period | 12 mo |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient Inpatient |
| Rationale | |
| MRA therapy improves outcome in patients with heart failure and reduced LVEF (7). Use of MRA therapy in those without contraindications was 33% among 150 primary care and cardiology practices in the CHAMP-HF registry demonstrating a moderate to large treatment gap (20). | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Aldosterone receptor antagonists (or mineralocorticoid receptor antagonists) are recommended in patients with NYHA class II-IV HF and who have LVEF of 35% or less, unless contraindicated, to reduce morbidity and mortality. Patients with NYHA class II HF should have a history of prior cardiovascular hospitalization or elevated plasma natriuretic peptide levels to be considered for aldosterone receptor antagonists. Creatinine should be 2.5 mg/dL or less in men or 2.0 mg/dL or less in women (or estimated glomerular filtration rate >30 mL/min/1.73 m2), and potassium should be less than 5.0 mEq/L. Careful monitoring of potassium, renal function, and diuretic dosing should be performed at initiation and closely followed thereafter to minimize risk of hyperkalemia and renal insufficiency (54,71,72). (Class 1, Level of Evidence: A) 2. Aldosterone receptor antagonists are recommended to reduce morbidity and mortality following an acute MI in patients who have LVEF of 40% or less who develop symptoms of HF or who have a history of diabetes mellitus, unless contraindicated (73). (Class 1, Level of Evidence: B) | |
ACCF indicates American College of Cardiology Foundation; ACE, angiotensin–converting enzyme; AHA, American Heart Association; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; CHAMP-HF, CHAnge the Management of Patients with Heart Failure; Cr, creatinine; EHR, electronic health record; HF, heart failure; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; and PM, performance measure.
SHORT TITLE: PM-10 Laboratory Monitoring in New Mineralocorticoid Receptor Antagonist Therapy (Outpatient and Inpatient Setting)
PM-10: Laboratory Monitoring in New Mineralocorticoid Receptor Antagonist Therapy (Outpatient and Inpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure who were started on MRA therapy and had potassium and renal function checked within 1 wk of the patient initiation of the MRA prescription | |
| Numerator | Patients who had potassium and renal function checked within 1 wk of the patient initiation of the MRA prescription |
| Denominator | All patients age ≥18 y with a diagnosis of heart failure who filled a new prescription for MRA therapy |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | None |
| Measurement Period | 12 mo |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient Inpatient |
| Rationale | |
| The major risk associated with use of aldosterone receptor antagonists is hyperkalemia attributable to inhibition of potassium excretion, ranging from 2% to 5% in trials (54,72,73) to 24% to 36% in population-based registries (74,75). The development of potassium levels >5.5 mEq/L (approximately 12% in EMPHASIS-HF (72)) should trigger discontinuation or dose reduction of the aldosterone receptor antagonist unless other causes are identified. The development of worsening renal function should lead to careful evaluation of the entire medical regimen and consideration for stopping the aldosterone receptor antagonist (7). Close monitoring of serum potassium is required; potassium levels and renal function are most typically checked in 3 d and at 1 wk after initiating therapy and at least monthly for the first 3 mo (Table 17, 2013 ACCF/AHA guideline (7)). Despite the known risk of hyperkalemia with MRA initiation, the rate of measurement of potassium levels within 2 wk of initiation is low (76). Although the clinical guideline suggests checking in 3 d, this is not a formal recommendation. Thus, the writing committee chose a more conservative 7-d time period to allow patient and provider flexibility and acknowledge challenges with weekend and holiday laboratory assessments. | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Aldosterone receptor antagonists (or mineralocorticoid receptor antagonists) are recommended in patients with NYHA class II-IV HF and who have LVEF of 35% or less, unless contraindicated, to reduce morbidity and mortality. Patients with NYHA class II HF should have a history of prior cardiovascular hospitalization or elevated plasma natriuretic peptide levels to be considered for aldosterone receptor antagonists. Creatinine should be 2.5 mg/dL or less in men or 2.0 mg/dL or less in women (or estimated glomerular filtration rate >30 mL/min/1.73 m2), and potassium should be less than 5.0 mEq/L. Careful monitoring of potassium, renal function, and diuretic dosing should be performed at initiation and closely followed thereafter to minimize risk of hyperkalemia and renal insufficiency (54,71,72). (Class 1, Level of Evidence: A) 2. Inappropriate use of aldosterone receptor antagonists is potentially harmful because of life-threatening hyperkalemia or renal insufficiency when serum creatinine is greater than 2.5 mg/dL in men or greater than 2.0 mg/dL in women (or estimated glomerular filtration rate <30 mL/min/1.73 m2), and/or potassium greater than 5.0 mEq/L (74,75). (Class 3, Harm, Level of Evidence: B) | |
ACCF indicates American College of Cardiology Foundation; AHA, American Heart Association; EHR, electronic health record; EMPHASIS-HF, Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure; HF, heart failure; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; and PM, performance measure.
SHORT TITLE: PM-11 Hydralazine/Isosorbide Dinitrate Therapy for Heart Failure With Reduced Ejection Fraction in Those Self-Identified as Black or African American (Outpatient and Inpatient Setting)
PM-11: Hydralazine/Isosorbide Dinitrate Therapy for Heart Failure With Reduced Ejection Fraction in Those Self-Identified as Black or African American (Outpatient and Inpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure and a current or prior ejection fraction ≤40% who are self-identified as Black or African American and receiving ACE inhibitor, ARB, or ARNI therapy and beta-blocker therapy who were prescribed a combination of hydralazine and isosorbide dinitrate | |
| Numerator | Patients who were prescribed* hydralazine and isosorbide dinitrate or fixed dose combination of hydralazine/isosorbide dinitrate within a 12-mo period when seen in the outpatient setting or at hospital discharge *Prescribed may include:
|
| Denominator | All patients age ≥18 y with a diagnosis of heart failure (NYHA class III or class IV) with a current or prior LVEF ≤40% who are self-identified as Black or African American and receiving ACEI, ARB, or ARNI, and beta-blocker therapy |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not prescribing hydralazine/isosorbide dinitrate therapy Documentation of patient reason(s) for not prescribing hydralazine/isosorbide dinitrate therapy |
| Measurement Period | Hydralazine/isosorbide dinitrate therapy initiated within a 12-mo period of being seen in the outpatient setting or from hospital discharge |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient Inpatient |
| Rationale | |
| The combination of hydralazine and isosorbide dinitrate is recommended to improve outcomes for patients self-identified as African American or Black, who have moderate-to-severe symptoms on optimal medical therapy (7). Use of hydralazine and isosorbide dinitrate in self-identified African American or Black candidates for therapy has been suboptimal (77). | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. The combination of hydralazine and isosorbide dinitrate is recommended to reduce morbidity and mortality for patients self-described as African Americans with NYHA class III-IV HFrEF receiving optimal therapy with ACE inhibitors and beta blockers, unless contraindicated (78,79). (Class 1, Level of Evidence: A) | |
ACCF indicates American College of Cardiology Foundation; ACE, angiotensin–converting enzyme; AHA, American Heart Association; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; EHR, electronic health record; HFrEF, heart failure reduced ejection fraction; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; and PM, performance measure.
SHORT TITLE: PM-12 Counseling Regarding Implantable Cardioverter-Defibrillator Implantation for Patients With Heart Failure With Reduced Ejection Fraction on Guideline-Directed Medical Therapy (Outpatient Setting)
PM-12: Counseling Regarding Implantable Cardioverter-Defibrillator Implantation for Patients With Heart Failure With Reduced Ejection Fraction on Guideline-Directed Medical Therapy (Outpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure with current LVEF ≤35% despite ACE inhibitor, ARB, or ARNI and beta-blocker therapy for at least 3 mo who were counseled regarding ICD implantation as a treatment option for the prophylaxis of sudden death | |
| Numerator | Patients who were counseled* regarding ICD implantation as a treatment option for the prophylaxis of sudden death *Counseling should be specific to each individual patient and include documentation of a discussion regarding the risk of sudden and non-sudden death and the efficacy, safety, and risks of an ICD. This will allow patients to be informed of the risks and benefits of ICD implantation and better able to make decisions based on the valuation of sudden cardiac death versus other risks. |
| Denominator | All patients age ≥18 y with a diagnosis of heart failure with current LVEF ≤35% despite ACE inhibitor, ARB, or ARNI and beta-blocker therapy for at least 3 mo |
| Denominator Exclusions | Functional ICD in situ Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not providing counseling regarding ICD implantation as a treatment option for the prophylaxis of sudden death (e.g., significant comorbidities, limited life expectancy, up titration of medical therapy is ongoing with anticipated LVEF improvement) |
| Measurement Period | 12 mo |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient |
| Rationale | |
| ICDs prevent sudden death due to ventricular tachyarrhythmias in select patients with HFrEF (7). However, frequent or inappropriate shocks from an ICD can lead to reduced quality of life. Patients may differ in the willingness to have an ICD implanted based on their preferences for quality and length of life. Given the significant risks and benefits of ICD implantation, eligible patients should be fully informed of this treatment option (7). Among 21,059 patients from 236 sites in the GWTG Registry, 23% received predischarge ICD counseling. Women were counseled less frequently than men, and racial and ethnic minorities were less likely to receive counseling than White patients (80). | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. ICD therapy is recommended for primary prevention of SCD to reduce total mortality in selected patients with nonischemic DCM or ischemic heart disease at least 40 days post-MI with LVEF of 35% or less and NYHA class II or III symptoms on chronic GDMT, who have reasonable expectation of meaningful survival for more than 1 year (81,82).† (Class 1, Level of Evidence: A) †Counseling should be specific to each individual patient and should include documentation of a discussion about the potential for sudden death and non-sudden death from HF or noncardiac conditions. Information should be provided about the efficacy, safety, and potential complications of an ICD and the potential for defibrillation to be inactivated if desired in the future, notably when a patient is approaching end of life. This will facilitate shared decision-making among patients, families, and the medical care team about ICDs (83). 2017 AHA/ACC/HRS ventricular arrhythmias and prevention of sudden cardiac death guideline (9) 1. Patients considering implantation of a new ICD or replacement of an existing ICD for a low battery should be informed of their individual risk of SCD and non-sudden death from HF or noncardiac conditions and the effectiveness, safety, and potential complications of the ICD in light of their health goals, preferences, and values (84–88). (Class 1, Level of Evidence: B-NR) | |
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; ACE, angiotensin–converting enzyme; AHA, American Heart Association; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; DCM, dilated cardiomyopathy; EHR, electronic health record; GWTG, Get With The Guidelines; GDMT, guideline-directed medical therapy; HF, heart failure; HFrEF, heart failure reduced ejection fraction; HRS, Heart Rhythm Society; ICD, implantable cardioverter-defibrillator; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PM, performance measure; and SCD, sudden cardiac death.
SHORT TITLE: PM-13 Cardiac Resynchronization Therapy Implantation for Patients With Heart Failure With Reduced Ejection Fraction on Guideline-Directed Medical Therapy (Outpatient Setting)
PM-13: Cardiac Resynchronization Therapy Implantation for Patients With Heart Failure With Reduced Ejection Fraction on Guideline-Directed Medical Therapy (Outpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure with current LVEF ≤35%, LBBB, QRS duration ≥150 ms, NYHA class II,III, and IV, despite ACE inhibitor, ARB, or ARNI and beta-blocker therapy for at least 3 mo who have undergone CRT implantation | |
| Numerator | Patients (meeting denominator criteria) who have undergone CRT implantation |
| Denominator | All patients age ≥18 y with a diagnosis of heart failure with current LVEF ≤35%, LBBB, QRS duration ≥150 ms, NYHA class II, III, and IV, despite ACE inhibitor, ARB, or ARNI and beta-blocker therapy for at least 3 mo |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not undergoing CRT implantation (e.g., multiple or significant comorbidities, limited life expectancy) Documentation of patient reason(s) for not undergoing CRT implantation (e.g., refusal) |
| Measurement Period | 12 mo |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient |
| Rationale | |
| CRT has been shown to improve survival and symptoms among symptomatic patients with heart failure and LVEF ≤35%, LBBB, and QRS duration ≥150 ms (7). CRT implantation (not just counseling) is recommended as CRT improves both quantity and quality of life, unlike ICDs, where there is no symptomatic benefit. In the GWTG database from 2014, 26% of eligible patients had CRT in place, implanted, or prescribed (89). Women were less likely to receive CRT, and this disparity increased over time. Black patients were less likely than White patients to have CRT. | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. CRT is indicated for patients who have LVEF of 35% or less, sinus rhythm, LBBB with a QRS duration of 150 ms or greater, and NYHA class II, III, or ambulatory IV symptoms on GDMT. (Class 1, Level of Evidence: A for NYHA class III/IV (90–93); Level of Evidence: B for NYHA class II (94,95)) | |
ACCF indicates American College of Cardiology Foundation; ACE, angiotensin–converting enzyme; AHA, American Heart Association; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; CRT, cardiac resynchronization therapy; EHR, electronic health record; GWTG, Get With The Guidelines; GDMT, guideline-directed medical therapy; ICD, implantable cardioverter-defibrillator; LBBB, left bundle branch block; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; and PM, performance measure.
Quality Measures for Heart Failure
SHORT TITLE: QM-1 Patient Self-Care Education (Outpatient Setting)
QM-1: Patient Self-Care Education (Outpatient Setting)
| Measure Description: Percentage of patients age ≥18 y with a diagnosis of heart failure who were provided with self-care education during ≥1 visits within a 12-mo period | |
| Numerator | Patients who were provided with self-care education* during $1 visits within a 12-mo period *Self-care education may include the following: Definition of heart failure (linking disease, symptoms, and treatment) and cause of patient’s heart failure; recognition of escalating symptoms and concrete plan for response to particular symptoms; indications and use of each medication; recommendations for modification of risks for heart failure progression; specific diet recommendations; individualized low-sodium diet; recommendation for alcohol intake; specific activity/exercise recommendations; importance of treatment adherence and behavioral strategies to promote treatment adherence; importance of monitoring weight daily at home. |
| Denominator | All patients age ≥18 y with a diagnosis of heart failure who were seen at least once for any visit within a 12-mo period |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not providing self-care education (e.g., comfort care only, dementia, or cognitive impairment) Documentation of patient reason(s) for not providing self-care education (e.g., patient refusal) |
| Measurement Period | 12 mo |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient |
| Rationale | |
| Patient self-care education is a useful nonpharmacological component to heart failure care. It may reduce the likelihood of nonadherence with recommended therapeutic strategies and lead to early identification of worsening clinical status and subsequent treatment. Heart failure disease management programs, in which patient education is an integral component, have been shown to be effective in improving self-care and reducing readmissions (96). This measure is intended to highlight the importance of providing appropriate self-care education to patients with heart failure. The form and manner of education (e.g., counseling, information in the form of pamphlets or booklets) is at the discretion of the individual clinician and should be specific to the needs of the patient. Data from the IMPROVE-HF registry indicate that only 61% of outpatients with heart failure were provided with education (including discussion of salt-restricted diet, monitoring of daily weight, warning signs of worsened heart failure, and activity recommendations), with rates of adherence ranging from 0% to 100% among practices (97). A number of consensus groups/patient advocacy organizations have developed educational materials that are recommended to aid implementation of the measure. These materials/tools include, but are not limited to:
| |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Patients with HF should receive specific education to facilitate HF self-care (103–108). (Class 1, Level of Evidence: B) | |
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; AHA, American Heart Association; EHR, electronic health record; HF, heart failure; HFSA, Heart Failure Society of America; IMPROVE-HF, Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting; LVAD, left ventricular assist device; and QM, quality measure.
SHORT TITLE: QM-2 Measurement of Patient-Reported Outcome-Health Status (Outpatient Setting)
QM-2: Measurement of Patient-Reported Outcome-Health Status (Outpatient Setting)
| Measure description: Percentage of outpatients age ≥18 y with a diagnosis of heart failure who have a disease-specific patient-reported health status measurement recorded within each 6-mo period | |
| Numerator | Patients with a disease-specific PRO reported in the medical record during a 6-mo period |
| Denominator | All patients age ≥18 y with a diagnosis of heart failure |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not reporting a disease-specific, patient-reported health status measurement (e.g., severe cognitive or functional impairment) Documentation of patient reason(s) for not reporting a disease-specific, patient-reported health status measurement |
| Measurement Period | 12 mo with at least 1 PRO reported in each 6 mo of the reporting cycle |
| Sources of Data | EHR data Clinical registry |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient |
| Rationale | |
| A fundamental goal of treating patients with heart failure is to improve symptoms, which is most accurately quantified by directly asking them. Disease-specific PROs (e.g., MLHFQ or KCCQ) are recommended as they are more sensitive to clinical change in heart failure than general health status measures. PROs are also predictive of other outcomes such as mortality, hospitalization, and costs (109–111) and often vary by sex, race/ethnicity, and socioeconomic status (112,113). Knowledge of a patient’s reported health status may prompt changes in medications that will further improve care (114). There are multiple disease-specific tools that have been developed to capture PROs in heart failure. The ACC/AHA have not addressed PRO tool selection. However, the FDA has provided guidelines for an appropriate PRO tool (16) and, currently, 2 heart failure survey tools–the MLHFQ (15) and the KCCQ (14)–are considered qualified tools for FDA device use in heart failure (17). As a process measure for capturing a clinically important outcome, no risk-adjustment methods are required. It is required as a foundation for outcomes-based performance measure and is paired with QM-3. | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. The ACC/AHA heart failure guideline modifies several recommendations based on the health status of the patient, usually quantified by the NYHA classification (7). | |
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; AHA, American Heart Association; EHR, electronic health record; FDA, U. S. Food and Drug Administration; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVAD, left ventricular assist device; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; PRO, patient-reported outcome; and QM, quality measure.
SHORT TITLE: QM-3 Sustained or Improved Health Status in Heart Failure (Outcome)
QM-3: Sustained or Improved Health Status (Patient-Reported Symptoms, Function, and Quality of Life) During the Reporting Period for All Patients With Heart Failure
| Measure Description: Percentage of patients age ≥18 y with heart failure whose patient-reported outcome score does not decline significantly (a decrease in scores of ≥5 points for the KCCQ or an increase of ≥7 points for the MLHFQ*) during a 12-mo period | |
| Numerator | Patients whose last score within the past 6 mo of the reporting period is not significantly worse (did not decrease by ≥5 points for the KCCQ or did not increase by ≥7 points for the MLHFQ*) than the first score in the first 6 mo of the reporting period *A clinically significant change in PROMIS-PLUS-HF is not established at the time of this writing. |
| Denominator | Heart failure patients age ≥18 y with at least 1 patient-reported outcome measurement in both the first and past 6 mo of the measurement period (12 mo) |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | None |
| Measurement Period | 12 mo |
| Sources of Data | Qualified EHR, QCDR, electronically or telephonically transmitted PROs |
| Attribution | Individual practitioner Facility |
| Care Setting | Outpatient |
| Rationale | |
| Unlike the other measures in this measure set, this is an outcome comparable to mortality. Although using each patient as their own control minimizes some of the need for risk adjustment, this measure has been designated as a quality metric because development of adequate risk-adjustment is needed prior to use as a performance measure (accountability). Two of the disease-specific PROs (KCCQ and MLHFQ) have published thresholds for change that are considered clinically meaningful (115,116). This outcome-based measure will enable comparison of the proportion of patients in each reporting unit that are not clinically worse over a year of treatment. Given that patients are expected to decline over time, this measure is not expected to be near 100%. | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Goals of treatment of heart failure preserved ejection fraction and heart failure reduced ejection fraction are to improve health-related quality of life and symptoms (Figure 3, 2013 ACCF/AHA guideline) (7). | |
ACCF indicates American College of Cardiology Foundation; AHA, American Heart Association; EHR, electronic health record; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVAD, left ventricular assist device; MLHFQ, Minnesota Living with Heart Failure Questionnaire; PRO, patient-reported outcome; PROMIS-PLUS-HF, Patient-Reported Outcomes Measurement Information System-Plus-Heart Failure; QCDR, Qualified Clinical Data Registry; and QM, quality measure.
SHORT TITLE: QM-4 Postdischarge Appointment for Patients With Heart Failure (Inpatient Setting)
QM-4: Postdischarge Appointment for Patients With Heart Failure (Inpatient Setting)
| Measure Description: Percentage of patients age ≥18 y discharged from an inpatient facility to ambulatory care or home health care with a principal discharge diagnosis of heart failure for whom a follow-up appointment was scheduled within 7 d and documented before discharge (as specified) | |
| Numerator | Patients for whom a follow-up appointment was scheduled within 7 d and documented before discharge including either:
|
| Denominator | All patients age ≥18 y discharged from an inpatient facility (e.g., hospital inpatient or observation) to ambulatory care (home or self-care) or home health care with a principal discharge diagnosis of heart failure |
| Denominator Exclusions | Heart transplant LVAD |
| Denominator Exceptions | Documentation of medical reason(s) for not documenting that a follow-up appointment was scheduled (e.g., patients transferring to another facility) Documentation of patient reason(s) for not documenting that a follow-up appointment was scheduled (e.g., patients who left against medical advice or discontinued or transferred care) |
| Measurement Period | 12 mo |
| Sources of Data | EHR data Administrative data/claims (inpatient or outpatient claims) Administrative data/claims expanded (multiple sources) Paper medical record |
| Attribution | Individual practitioner Facility |
| Care Setting | Inpatient |
| Rationale | |
| An observational study found that early outpatient follow-up (within 7 d) after discharge from a heart failure hospitalization is associated with a lower risk of 30-d readmission (117), although this has been an inconsistent finding (118). The writing committee agreed that more evidence is needed to support a short time period (<7 d) for the postdischarge appointment before this metric becomes a performance measure. | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Scheduling an early follow-up visit (within 7 to 14 days) and early telephone follow-up (within 3 days) of hospital discharge are reasonable (117,119). (Class 2a, Level of Evidence: B) | |
ACCF indicates American College of Cardiology Foundation; AHA, American Heart Association; EHR, electronic health record; LVAD, left ventricular assist device; and QM, quality measure.
Structural Measure for Heart Failure
SHORT TITLE: SM-1 Heart Failure Registry Participation
SM-1: Participation in ≥1 Regional or National Registries That Include Patients With Heart Failure
| Measure Description: Participation in a national or regional heart failure registry that provides regular performance reports based on benchmarked data | |
| Numerator | Does the facility participate in a national or regional heart failure registry* that provides regular performance reports based on benchmarked data? (yes/no) *Examples of such registries include the GWTG-HF, GWTG-360, PINNACLE Registry, and PINNACLE Registry Research Alliance. |
| Denominator | Not applicable |
| Denominator Exclusions | None |
| Denominator Exceptions | None |
| Measurement Period | Not applicable |
| Sources of Data | Facility attestation |
| Attribution | Measure reportable at the facility level only |
| Care Setting | Outpatient Inpatient |
| Rationale | |
| Participation in a registry allows measurement of performance for heart failure care, including benchmarking against other facilities. | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Participation in quality improvement programs and patient registries based on nationally endorsed, clinical practice guideline-based quality and performance measures can be beneficial in improving the quality of HF care (120,121). (Class 2a, Level of Evidence: B) | |
ACCF indicates American College of Cardiology Foundation; AHA, American Heart Association; GWTG, Get With The Guidelines; HF, heart failure; PINNACLE, Practice Innovation And Clinical Excellence; and SM, structural measure.
Rehabilitation Performance Measures Related to Heart Failure
(From the 2018 ACC/AHA performance measures for cardiac rehabilitation (6))
SHORT TITLE: PM-2 Exercise Training Referral for HFrEF From Inpatient Setting
PM-2: Exercise Training Referral for HFrEF From an Inpatient Setting
| Measure Description: Percentage of patients, age ≥18 y, hospitalized with a primary diagnosis of HFrEF in the previous 12 mo, who are referred for outpatient exercise training (or regular physical activity), typically delivered in the setting of an outpatient CR program | |
| Numerator | Patients hospitalized with primary diagnosis of HFrEF who have been referred to an outpatient CR program before hospital discharge Referral is defined as: 1. Documented communication* between the healthcare provider and the patient to recommend an outpatient CR program AND 2A. Official referral order† is sent to outpatient CR program OR 2B. Documentation of patient refusal to justify why patient information was not sent to the CR program‡ Note: Performance is met if steps 1 AND either 2A (official referral order transmitted) OR 2B (patient refusal documented in the patient’s medical record) are completed and documented. *All communications must maintain appropriate confidentiality as outlined by the Health Insurance Portability and Accountability Act of 1996 (HIPAA). †All patient information required for enrollment should be transmitted to the CR program. Necessary patient information may be found in the hospital discharge summary. ‡Patients who refuse a CR referral should not have their data transmitted to the receiving CR program against their will. |
| Denominator | All patients who have had HFrEF during the previous 12 mo, who are discharged from the hospital during the reporting period |
| Denominator Exclusions | Patients age <18 y Patients who leave during hospitalization against medical advice Patients who die during hospitalization Patients who are transferred to another hospital for inpatient care Patients who are already participating in a CR program before hospitalization |
| Denominator Exceptions | Documentation of a patient-oriented reason that precludes referral to CR (e.g., no traditional CR program available to the patient, within 60 min [travel time] from the patient’s home, or patient does not have access to an alternative model of CR delivery that meets all criteria for a CR program) Documentation of a medical reason that precludes referral to CR (e.g., patient deemed by a medical provider to have a medically unstable, life-threatening condition, or has other cognitive or physical impairments that preclude CR participation) Documentation of a healthcare system reason that precludes referral to CR (e.g., patient is discharged to a nursing care or long-term care facility, or patient lacks medical coverage for CR) |
| Measurement Period | Encounter |
| Sources of Data | Medical record or other database (e.g., administrative, clinical, registry) |
| Attribution | Measure reportable at facility level |
| Care Setting | Inpatient |
| Rationale | |
| Exercise training services have been shown to improve functional status and may help reduce morbidity and mortality in persons with stable chronic heart failure with reduced HFrEF. However, these services are used in a minority of eligible patients (122,123). A key component to outpatient exercise training (typically carried out in a CR program) is the appropriate and timely referral of patients. Generally, the most important time for this referral to take place is while the patient is hospitalized for a HFrEF. This performance measure has been developed to help healthcare systems implement effective steps in their systems of care that will optimize the appropriate referral of a patient to an outpatient exercise training program. This measure is designed to serve as a stand-alone measure or, preferably, to be included within other performance measurement sets that involve patients with HFrEF. This performance measure is provided in a format that allows for easy and flexible inclusion into such performance measurement sets. Effective referral of appropriate inpatients to an outpatient exercise training program is the responsibility of the healthcare team within a healthcare system that is primarily responsible for providing cardiovascular care to the patient with HFrEF during hospitalization. Published evidence suggests that automatic referral systems, accompanied by strong and supportive advice and guidance from a healthcare professional, can significantly help improve CR referral and enrollment, where exercise training typically takes place for patients with HFrEF. | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Exercise training (or regular physical activity) is recommended as safe and effective for patients with HF who are able to participate to improve functional status (124–130). (Class 1, Level of Evidence: A) 2011 AHA prevention of cardiovascular disease in women guideline update (131) 1. A comprehensive CVD risk-reduction regimen such as cardiovascular or stroke rehabilitation or a physician-guided home- or community-based exercise training program should be recommended to women with a recent acute coronary syndrome or coronary revascularization, new-onset or chronic angina, recent cerebrovascular event, peripheral arterial disease (Class 1; Level of Evidence A) or current/prior symptoms of heart failure, and an LVEF ≤35%. (Class 1; Level of Evidence B) | |
ACCF indicates American College of Cardiology Foundation; AHA, American Heart Association; CR, cardiac rehabilitation; CVD, cardiovascular disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; and PM, performance measure.
SHORT TITLE: PM-4 Exercise Training Referral for HFrEF From Outpatient Setting
PM-4: Exercise Training Referral for HFrEF From an Outpatient Setting
| Measure Description: Percentage of patients, age ≥18 y, evaluated in an outpatient setting who, within the previous 12 mo, have had a new HFrEF event or exacerbation and have not participated in an exercise training program, such as provided in CR programs, for the qualifying event/diagnosis, are to be referred for exercise training. | |
| Numerator | Patients in an outpatient clinical practice who have had a new HFrEF event or exacerbation and have not participated in a supervised exercise training program (e.g., as a CR program) during the previous 12 mo, who have been referred to an outpatient CR program Referral is defined as: 1. Documented communication* between the healthcare provider and the patient to recommend an outpatient CR program AND 2A. Official referral order† is sent to outpatient CR program OR 2B. Documentation of patient refusal to justify why patient information was not sent to the CR program‡ Note: Performance is met if steps 1 AND either 2A (official referral order transmitted) OR 2B (patient refusal documented in the patient’s medical record) are completed and documented. *All communications must maintain appropriate confidentiality as outlined by the Health Insurance Portability and Accountability Act of 1996 (HIPAA). †All patient information required for enrollment should be transmitted to the CR program. Necessary patient information may be found in the hospital discharge summary. ‡Patients who refuse a CR referral should not have their data transmitted to the receiving CR program against their will. |
| Denominator | All patients in an outpatient clinical practice who have had HFrEF during the previous 12 mo |
| Denominator Exclusions | Patients age <18 y Patients who leave clinic visit against medical advice Patients have already participated in or had already completed a CR program prior to clinic visit |
| Denominator Exceptions | Documentation of a patient-oriented reason that precludes referral to CR (e.g., no traditional CR program available to the patient, within 60 min [travel time] from the patient’s home, or patient does not have access to an alternative model of CR delivery that meets all criteria for a CR program) Documentation of a medical reason that precludes referral to CR (e.g., patient deemed by a medical provider to have a medically unstable, life-threatening condition, or has other cognitive or physical impairments that preclude CR participation) Documentation of a healthcare system reason that precludes referral to CR (e.g., patient resides in a nursing care or long-term care facility, or patient lacks medical coverage for CR) |
| Measurement Period | Encounter |
| Sources of Data | Medical record or other database (e.g., administrative, clinical, registry) |
| Attribution | Measure reportable at provider and facility level |
| Care Setting | Outpatient |
| Rationale | |
| CR services have been shown to help improve functional status and may help reduce morbidity and mortality in persons with stable chronic heart failure with reduced HFrEF. However, these services are used in a minority of eligible patients (122,123). A key component to outpatient CR program utilization is the appropriate and timely referral of patients. Generally, the most important time for this referral to take place is while the patient is hospitalized for a HFrEF. This performance measure has been developed to help healthcare systems implement effective steps in their systems of care that will optimize the appropriate referral of a patient to an outpatient CR program. This measure is designed to serve as a stand-alone measure or, preferably, to be included within other performance measurement sets that involve patients with HFrEF. This performance measure is provided in a format that allows for easy and flexible inclusion into such performance measurement sets. Effective referral of appropriate inpatients to an outpatient CR program is the responsibility of the healthcare team within a healthcare system that is primarily responsible for providing cardiovascular care to the patient with HFrEF during hospitalization. Published evidence suggests that automatic referral systems accompanied by strong and supportive advice and guidance from a healthcare professional can significantly help improve CR referral and enrollment. | |
| Clinical Recommendation(s) | |
|
2013 ACCF/AHA heart failure clinical practice guideline (7) 1. Exercise training (or regular physical activity) is recommended as safe and effective for patients with HF who are able to participate to improve functional status (124–130). (Class 1, Level of Evidence: A) 2011 AHA prevention of cardiovascular disease in women guideline update (131) 1. A comprehensive CVD risk-reduction regimen such as cardiovascular or stroke rehabilitation or a physician-guided home- or community-based exercise training program should be recommended to women with a recent acute coronary syndrome or coronary revascularization, new-onset or chronic angina, recent cerebrovascular event, peripheral arterial disease (Class 1; Level of Evidence A) or current/prior symptoms of heart failure and an LVEF ≤35%. (Class 1; Level of Evidence B) | |
ACCF indicates American College of Cardiology Foundation; AHA, American Heart Association; CR, cardiac rehabilitation; CVD, cardiovascular disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; and PM, performance measure.
APPENDIX B. AUTHOR RELATIONSHIPS WITH INDUSTRY AND OTHER ENTITIES (RELEVANT)–2020 ACC/AHA CLINICAL PERFORMANCE AND QUALITY MEASURES FOR HEART FAILURE
| Committee Member | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|
| Paul A. Heidenreich (Chair) | Stanford VA Palo Alto Health Care System–Professor of Medicine | None | None | None | None | None | None |
| Gregg C. Fonarow* (Vice Chair) | UCLA Medical Center–Professor of Medicine | None | None |
|
|
None | |
| Khadijah Breathett‡ | UA College of Medicine, Tucson–Assistant Professor of Medicine | None | None | None | None | None | None |
| Corrine Y. Jurgens | Boston College, School of Nursing–Associate Professor | None | None | None | None | None | None |
| Barbara A. Pisani* | Wake Forest Baptist Medical Center–Medical Director, Heart Failure, Heart Transplant, Mechanical Circulatory Support | None | None | None | None | None | |
| Bunny J. Pozehl | University of Nebraska Medical Center, Omaha Division–Professor College of Nursing | None | None | None | None | None | None |
| John A. Spertus* | Saint Luke’s Mid America Heart Institute–Director, Health Outcomes Research; University of Missouri-Kansas City–Professor | None | None | None | None | ||
| Kenneth G. Taylor‖ | Piedmont Heart Institute–Heart Failure and Interventional Cardiologist | None | None | None | None | None | None |
| Jennifer T. Thibodeau* | University of Texas Southwestern Medical Center–Associate Professor; Director, Heart Failure; Interim Section Chief, Heart Failure, Cardiac Transplantation, Ventricular Assist Devices | None | None | None |
|
None | None |
| Clyde W. Yancy | Northwestern University, Feinberg School of Medicine–Magerstadt Professor of Medicine; Division of Cardiology–Chief | None | None | None | None |
|
None |
| Boback Ziaeian | UCLA David Geffen School of Medicine–Assistant Professor; VA Greater Los Angeles Healthcare System–Director of Telecardiology | None | None | None | None | None | None |
This table represents the relationships of committee members with industry and other entities that were determined to be relevant to this document. These relationships were reviewed and updated in conjunction with all meetings and/or conference calls of the writing committee during the document development process. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5,000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. According to the ACC/AHA, a person has a relevant relationship IF: a) the relationship or interest relates to the same or similar subject matter, intellectual property or asset, topic, or issue addressed in the document; or b) the company/entity (with whom the relationship exists) makes a drug, drug class, or device addressed in the document or makes a competing drug or device addressed in the document; or c) the person, or a member of the person’s household, has a reasonable potential for financial, professional, or other personal gain or loss as a result of the issues/content addressed in the document.
Writing committee members were excluded from voting on sections to which their specific relationships with industry and other entities may apply. Dr. Fonarow was excluded from voting on PM-4, PM-5, PM-6, PM-7, PM-8, PM-9, PM-10, PM-12, PM-13, and SM-1. Dr. Pisani was excluded from voting on PM-5, PM-6, PM-8, PM-12, and PM-13. Dr. Spertus was excluded from voting on PM-2, PM-3, PM-4, PM-5, PM-6, PM-7, PM-8, PM-9, PM-10, PM-12, PM-13, QM-2, QM-3, and SM-1. Dr. Thibodeau was excluded from voting on PM-5, PM-6, and PM-8.
Significant relationship.
CMS reported payments to Dr. Breathett in 2019 related to food, beverage, travel, and lodging for Abbott; however, she disagrees with this report. Dr. Breathett was not the lead author on any measures.
No financial benefit.
CMS reported a food and beverage payment from Novartis to Dr. Taylor in 2019, however, he disagrees with this report. Novartis has marked the food and beverage entry for deletion. Dr. Taylor was not the lead author on any measures.
ACC indicates American College of Cardiology; AHA, American Heart Association; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; PI, principal investigator; UA, University of Arizona; UCLA, University of California, Los Angeles; UK, United Kingdom; and VA, Veterans Affairs.
APPENDIX C. REVIEWER RELATIONSHIPS WITH INDUSTRY AND OTHER ENTITIES (COMPREHENSIVE)–2020 ACC/AHA CLINICAL PERFORMANCE AND QUALITY MEASURES FOR HEART FAILURE
| Reviewer | Representation | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|---|
| Siqin Kye Ye | ACC/AHA TFPM Lead Reviewer | Center for Behavioral Cardiovascular Health–Assistant Professor of Medicine; Cardiology Inpatient Consultation Service–Director; Columbia Doctors–Associate Chief Medical Officer | None | None | None | None | None | None |
| John L. Jefferies | Official ACC | University of Tennessee Health Science Center–Jay M. Sullivan Distinguished Chair in Cardiovascular Medicine, Chief of Cardiology |
|
None | None | None | None | |
| Pam Peterson | Official ACC | University of Colorado Anschutz Medical Campus–Professor of Medicine, Denver Health Medical Center |
|
None | None |
|
None | None |
| Howard J. Eisen | Official AHA | Penn State College of Medicine–Chief, Division of Cardiology | None | None | None | None | None | None |
| Janet N. Scheel | Official AHA | Washington University School of Medicine/St. Louis Children’s Hospital–Professor of Pediatrics and Pediatric Cardiologist | None | None | None | None | None | None |
| Robert John Mentz | Official HFSA | Duke University School of Medicine–Associate Professor of Medicine, Associate Professor in Population Health Sciences, Member in the Duke Clinical Research Institute | None | None | None | None | ||
| Mary Norine Walsh | Content ACC | St. Vincent Heart Center of Indiana–Medical Director Heart Failure and Cardiac Transplantation | None | None | None | None | None | |
| Eldrin F. Lewis | Content AHA | Brigham and Women’s Hospital–Associate Physician; Harvard Medical School–Associate Professor of Medicine | None | None | None |
|
||
| Nancy M. Albert | Content ACC/AHA | Research and Innovation, Cleveland Clinic Health System & CNS/Kaufman Center for Heart Failure, Heart, Vascular & Thoracic Institute–Associate Chief Nursing Officer |
|
|
None |
|
|
None |
| Sana M. Al-Khatib | Content ACC/AHA | Duke University School of Medicine–Professor of Medicine, Member in the Duke Clinical Research Institute |
|
None | None |
|
|
|
| Anita Deswal | Content ACC/AHA | Baylor College of Medicine–Chief, Medicine-Cardiology, Michael E. DeBakey VA Medical Center & Baylor College of Medicine; Professor, Medicine-Cardiology, Winters Center for Heart Failure Research | None | None | None |
|
|
None |
| Mona Fiuzat | Content ACC/AHA | Duke University School of Medicine–Adjunct Associate Professor, Department of Medicine | None | None | None |
|
None | None |
| Adrian F. Hernandez | Content ACC/AHA | Duke University School of Medicine–Vice Dean for Clinical Research | None | None |
|
|
||
| J. Herb Patterson | Content ACC/AHA | UNC Eshelman School of Pharmacy–Interim Chair, Division of Pharmacotherapy and Experimental Therapeutics, Professor of Pharmacy, Research Professor of Medicine |
|
None | None | None | None | |
| Andrea Russo | Content ACC/AHA | Cooper Medical School of Rowan University–Professor of Medicine and Director; Cardiac Electrophysiology and Arrhythmia Services–Director; Cardiovascular Research Cooper University Hospital Dorrance #393–Program Director, Clinical Cardiac Electrophysiology Fellowship | None | None | None | None | None | |
| Paul Varosy | Content ACC/AHA | VA Eastern Colorado Health Care System–Director of Cardiac Electrophysiology | None | None | None |
|
|
None |
This table represents all relationships of reviewers with industry and other entities that were reported at the time of peer review, including those not deemed to be relevant to this document, at the time this document was under review. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5,000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. Names are listed in alphabetical order within each category of review. Please refer to http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/relationships-with-industry-policy for definitions of disclosure categories or additional information about the ACC/AHA Disclosure Policy for Writing Committees.
Significant (greater than $5,000) relationship.
No financial relationship.
This disclosure was entered under the Clinical Trial Enroller category in the ACC’s disclosure system.
ACC indicates American College of Cardiology; AHA, American Heart Association; CNS, Clinical Nurse Specialist; DSMB, Data Safety and Monitoring Board; FDA, U. S. Food and Drug Administration; HFSA, Heart Failure Society of America; NHLBI, National Heart, Lung, and Blood Institute; NIH, National Institutes of Health; PCORI, Patient-Centered Outcomes Research Institute; PI, principal investigator; SCD, sudden cardiac death; TFPM, Task Force on Performance Measures; UNC, University of North Carolina; VA, Veterans Administration; and VT, ventricular tachycardia.
Footnotes
This Physician Performance Measurement Set (PPMS) and related data specifications were developed by the Physician Consortium for Performance Improvement (the Consortium), including the American College of Cardiology (ACC), the American Heart Association (AHA), and the American Medical Association (AMA), to facilitate quality-improvement activities by physicians. The performance measures contained in this PPMS are not clinical guidelines, do not establish a standard of medical care, and have not been tested for all potential applications. Although copyrighted, they can be reproduced and distributed, without modification, for noncommercial purposes, for example, use by health care providers in connection with their practices. Commercial use is defined as the sale, license, or distribution of the performance measures for commercial gain, or incorporation of the performance measures into a product or service that is sold, licensed, or distributed for commercial gain. Commercial uses of the PPMS require a license agreement between the user and the AMA (on behalf of the Consortium) or the ACC or the AHA. Neither the AMA, ACC, AHA, the Consortium, nor its members shall be responsible for any use of this PPMS.
The measures and specifications are provided “as is” without warranty of any kind.
Limited proprietary coding is contained in the measure specifications for convenience. Users of the proprietary code sets should obtain all necessary licenses from the owners of the code sets. The AMA, the ACC, the AHA, the National Committee for Quality Assurance (NCQA), and the Physician Consortium for Performance Improvement (PCPI) and its members disclaim all liability for use or accuracy of any Current Procedural Terminology (CPT) or other coding contained in the specifications.
CPT contained in the measures specifications is copyright 2004–2012 American Medical Association. LOINC copyright 2004–2012 Regenstrief Institute, Inc. This material contains SNOMED CLINICAL TERMS (SNOMED CT) copyright 2004–2012 International Health Terminology Standards Development Organization. All rights reserved.
This document was approved by the American College of Cardiology Clinical Policy Approval Committee on June 29, 2020; by the American Heart Association Science Advisory and Coordinating Committee on June 11, 2020; the American Heart Association Executive Committee on July 22, 2020; and by the Heart Failure Society of America on July 23, 2020.
The American College of Cardiology requests that this document be cited as follows: Heidenreich PA, Fonarow GC, Breathett K, Jurgens CY, Pisani BA, Pozehl BJ, Spertus JA, Taylor KG, Thibodeau JT, Yancy CW, Ziaeian B. 2020 ACC/AHA clinical performance and quality measures for adults with heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol 2020;76:2527-64.
Copies: This document is available on the websites of the American College of Cardiology (www.acc.org) and the American Heart Association (professional.heart.org). For copies of this document, please contact Elsevier Inc. Reprint Department via fax (212-633-3820) or (reprints@elsevier.com).
Developed in Collaboration With the Heart Failure Society of America
Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, American Society of Health-System Pharmacists, Heart Rhythm Society, and the International Society for Heart and Lung Transplantation
REFERENCES
- 1.Spertus JA, Bonow RO, Chan P, et al. ACCF/AHA new insights into the methodology of performance measurement: a report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures. J Am Coll Cardiol. 2010;56:1767–82. [DOI] [PubMed] [Google Scholar]
- 2.Spertus JA, Eagle KA, Krumholz HM, et al. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. J Am Coll Cardiol. 2005;45:1147–56. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Ganiats TG, Beam CT, et al. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure. J Am Coll Cardiol. 2012;59:1812–32. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–22. [DOI] [PubMed] [Google Scholar]
- 6.Thomas RJ, Balady G, Banka G, et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2018;71:1814–37. [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American College of Chest Physicians, Heart Rhythm Society and International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 8.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 9.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91–220. [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW, Januzzi JL, Allen LA, et al. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;71:201–30. [DOI] [PubMed] [Google Scholar]
- 11.Normand SL, McNeil BJ, Peterson LE, et al. Eliciting expert opinion using the Delphi technique: identifying performance indicators for cardiovascular disease. Int J Qual Health Care. 1998;10:247–60. [DOI] [PubMed] [Google Scholar]
- 12.Patel DB, Shah RM, Bhatt DL, et al. Guideline-appropriate care and in-hospital outcomes in patients with heart failure in teaching and nonteaching hospitals: findings from Get With The Guidelines-Heart Failure. Circ Cardiovasc Qual Outcomes. 2016;9:757–66. [DOI] [PubMed] [Google Scholar]
- 13.Taylor RS, Long L, Mordi IR, et al. Exercise-based rehabilitation for heart failure: Cochrane systematic review, meta-analysis, and trial sequential analysis. J Am Coll Cardiol HF. 2019;7:691–705. [DOI] [PubMed] [Google Scholar]
- 14.Green CP, Porter CB, Bresnahan DR, et al. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 15.Rector TS, Kubo SH, Cohn JN. Patients’ self-assessment of their congestive heart failure. Part 2: content, reliability and validity of a new measure, the Minnesota Living with Heart Failure Questionnaire. Heart Failure. 1987;Oct/Nov:198–209. [Google Scholar]
- 16.U.S. Food and Drug Administration. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims. Accessed June 24, 2020. [DOI] [PMC free article] [PubMed]
- 17.U.S. Food and Drug Administration. Medical device development tools (MDDT). Available at: https://www.fda.gov/medical-devices/science-and-research-medical-devices/medical-device-development-tools-mddt#Qualified_Tools. Accessed June 24, 2020.
- 18.Goldman L, Hashimoto B, Cook EF, et al. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 1981;64:1227–34. [DOI] [PubMed] [Google Scholar]
- 19.Madsen BK, Hansen JF, Stokholm KH, et al. Chrome congestive heart failure: description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J. 1994;15:303–10. [DOI] [PubMed] [Google Scholar]
- 20.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72:351–66. [DOI] [PubMed] [Google Scholar]
- 21.Heo S, Doering LV, Widener J, et al. Predictors and effect of physical symptom status on health-related quality of life in patients with heart failure. Am J Crit Care. 2008;17:124–32. [PubMed] [Google Scholar]
- 22.Lesman-Leegte I, Jaarsma T, Coyne JC, et al. Quality of life and depressive symptoms in the elderly: a comparison between patients with heart failure and age- and gender-matched community controls. J Card Fail. 2009;15:17–23. [DOI] [PubMed] [Google Scholar]
- 23.Moser DK, Yamokoski L, Sun JL, et al. Improvement in health-related quality of life after hospitalization predicts event-free survival in patients with advanced heart failure. J Card Fail. 2009;15:763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez-Artalejo F, Guallar-Castillón P, Pascual CR, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165:1274–9. [DOI] [PubMed] [Google Scholar]
- 25.Heo S, Moser DK, Widener J. Gender differences in the effects of physical and emotional symptoms on health-related quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2007;6:146–52. [DOI] [PubMed] [Google Scholar]
- 26.Riegel B, Moser DK, Rayens MK, et al. Ethnic differences in quality of life in persons with heart failure. J Card Fail. 2008;14:41–7. [DOI] [PubMed] [Google Scholar]
- 27.Bosworth HB, Steinhauser KE, Orr M, et al. Congestive heart failure patients’ perceptions of quality of life: the integration of physical and psychosocial factors. Aging Ment Health. 2004;8:83–91. [DOI] [PubMed] [Google Scholar]
- 28.Carmona-Bernal C, Ruiz-García A, Villa-Gil M, et al. Quality of life in patients with congestive heart failure and central sleep apnea. Sleep Med. 2008;9:646–51. [DOI] [PubMed] [Google Scholar]
- 29.Lewis EF, Lamas GA, O’Meara E, et al. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;9:83–91. [DOI] [PubMed] [Google Scholar]
- 30.Masoudi FA, Rumsfeld JS, Havranek EP, et al. Age, functional capacity, and health-related quality of life in patients with heart failure. J Card Fail. 2004;10:368–73. [DOI] [PubMed] [Google Scholar]
- 31.Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits and health-related quality of life in chronic heart failure. J Cardiovasc Nurs. 2010;25:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xamoterol in severe heart failure. The Xamoterol in Severe Heart Failure Study Group. Lancet. 1990;336:1–6. [PubMed] [Google Scholar]
- 33.Merit-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 34.Australia-New Zealand Heart Failure Research Collaborative Group. Effects of carvedilol, a vasodilator-beta-blocker, in patients with congestive heart failure due to ischemic heart disease. Circulation. 1995;92:212–8. [PubMed] [Google Scholar]
- 35.Dargie H Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–90. [DOI] [PubMed] [Google Scholar]
- 36.Poole-Wilson PA, Swedberg K, Cleland JGF, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. [DOI] [PubMed] [Google Scholar]
- 37.The Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–67. [DOI] [PubMed] [Google Scholar]
- 38.Butler J, Young JB, Abraham WT, et al. Beta-blocker use and outcomes among hospitalized heart failure patients. J Am Coll Cardiol. 2006;47:2462–9. [DOI] [PubMed] [Google Scholar]
- 39.Fonarow GC, Abraham WT, Albert NM, et al. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE-HF program. J Am Coll Cardiol. 2008;52:190–9. [DOI] [PubMed] [Google Scholar]
- 40.Metra M, Torp-Pedersen C, Cleland JGF, et al. Should beta-blocker therapy be reduced or withdrawn after an episode of decompensated heart failure? Results from COMET. Eur J Heart Fail. 2007;9:901–9. [DOI] [PubMed] [Google Scholar]
- 41.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. [DOI] [PubMed] [Google Scholar]
- 42.Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840–8. [DOI] [PubMed] [Google Scholar]
- 43.Pfeffer MA, McMurray JJV, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906. [DOI] [PubMed] [Google Scholar]
- 44.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–66. [DOI] [PubMed] [Google Scholar]
- 45.McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 46.Group CTS. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–35. [DOI] [PubMed] [Google Scholar]
- 47.Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 48.Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet. 1993;342:821–8. [PubMed] [Google Scholar]
- 49.Køber L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting–enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–6. [DOI] [PubMed] [Google Scholar]
- 50.Packer M, Poole-Wilson P, Armstrong P, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999;100:2312–8. [DOI] [PubMed] [Google Scholar]
- 51.Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trial. The SAVE Investigators. N Engl J Med. 1992;327:669–77. [DOI] [PubMed] [Google Scholar]
- 52.Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–9. [DOI] [PubMed] [Google Scholar]
- 53.Eschalier R, McMurray JJ, Swedberg K, et al. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). J Am Coll Cardiol. 2013;62:1585–93. [DOI] [PubMed] [Google Scholar]
- 54.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. [DOI] [PubMed] [Google Scholar]
- 55.Morrow DA, Velazquez EJ, DeVore AD, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/valsartan or enalapril in the PIONEER-HF Trial. Circulation. 2019;139:2285–8. [DOI] [PubMed] [Google Scholar]
- 56.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–48. [DOI] [PubMed] [Google Scholar]
- 57.Solomon SD, Claggett B, McMurray JJ, et al. Combined neprilysin and renin-angiotensin system inhibition in heart failure with reduced ejection fraction: a meta-analysis. Eur J Heart Fail. 2016;18:1238–43. [DOI] [PubMed] [Google Scholar]
- 58.Tan NY, Sangaralingham LR, Sangaralingham SJ, et al. Comparative effectiveness of sacubitril-valsartan versus ACE/ARB therapy in heart failure with reduced ejection fraction. J Am Coll Cardiol HF. 2020;8:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campodonico J, Piepoli M, Clemenza F, et al. Dose-dependent efficacy of beta-blocker in patients with chronic heart failure and atrial fibrillation. Int J Cardiol. 2018;273:141–6. [DOI] [PubMed] [Google Scholar]
- 60.CIBIS-II Investigators Committee. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 61.Paolillo S, Mapelli M, Bonomi A, et al. Prognostic role of beta-blocker selectivity and dosage regimens in heart failure patients. Insights from the MECKI score database. Eur J Heart Fail. 2017;19:904–14. [DOI] [PubMed] [Google Scholar]
- 62.Bristow MR, Gilbert EM, Abraham WT, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996;94:2807–16. [DOI] [PubMed] [Google Scholar]
- 63.Fiuzat M, Wojdyla D, Kitzman D, et al. Relationship of beta-blocker dose with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial. J Am Coll Cardiol. 2012;60:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiuzat M, Wojdyla D, Pina I, et al. Heart rate or beta-blocker dose? Association with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF-ACTION trial. J Am Coll Cardiol HF. 2016;4:109–15. [DOI] [PubMed] [Google Scholar]
- 65.Gheorghiade M, Albert NM, Curtis AB, et al. Medication dosing in outpatients with heart failure after implementation of a practice-based performance improvement intervention: findings from IMPROVE HF. Congest Heart Fail. 2012;18:9–17. [DOI] [PubMed] [Google Scholar]
- 66.Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. [DOI] [PubMed] [Google Scholar]
- 68.U.S. National Library of Medicine. DailyMed. Available at: https://dailymed.nlm.nih.gov/dailymed/. Accessed June 24, 2020.
- 69.Khan MS, Fonarow GC, Ahmed A, et al. Dose of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and outcomes in heart failure: a meta-analysis. Circ Heart Fail. 2017;10:e003956. [DOI] [PubMed] [Google Scholar]
- 70.Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vizzardi E, D’Aloia A, Giubbini R, et al. Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class I or II heart failure. Am J Cardiol. 2010;106:1292–6. [DOI] [PubMed] [Google Scholar]
- 72.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [DOI] [PubMed] [Google Scholar]
- 73.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. [DOI] [PubMed] [Google Scholar]
- 74.Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41:211–4. [DOI] [PubMed] [Google Scholar]
- 75.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–51. [DOI] [PubMed] [Google Scholar]
- 76.Chauhan V, Dev S, Pham M, et al. Facility variation and predictors of serum potassium monitoring after initiation of a mineralocorticoid receptor antagonist in patients with heart failure. Am Heart J. 2015;170:543–9. [DOI] [PubMed] [Google Scholar]
- 77.Ziaeian B, Fonarow GC, Heidenreich PA. Clinical effectiveness of hydralazine-isosorbide dinitrate in African-American patients with heart failure. J Am Coll Cardiol HF. 2017;5:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carson P, Ziesche S, Johnson G, et al. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trial Study Group. J Card Fail. 1999;5:178–87. [DOI] [PubMed] [Google Scholar]
- 79.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–57. [DOI] [PubMed] [Google Scholar]
- 80.Hess PL, Hernandez AF, Bhatt DL, et al. Sex and race/ethnicity differences in implantable cardioverter-defibrillator counseling and use among patients hospitalized with heart failure: findings from the Get With The Guidelines-Heart Failure Program. Circulation. 2016;134:517–26. [DOI] [PubMed] [Google Scholar]
- 81.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 82.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 83.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hauptman PJ, Chibnall JT, Guild C, et al. Patient perceptions, physician communication, and the implantable cardioverter-defibrillator. JAMA Intern Med. 2013;173:571–7. [DOI] [PubMed] [Google Scholar]
- 85.Lewis KB, Stacey D, Matlock DD. Making decisions about implantable cardioverter-defibrillators from implantation to end of life: an integrative review of patients’ perspectives. Patient. 2014;7:243–60. [DOI] [PubMed] [Google Scholar]
- 86.Ottenberg AL, Mueller PS, Topazian RJ. “It’s not broke, so let’s not try to fix it”: why patients decline a cardiovascular implantable electronic device. Pacing Clin Electrophysiol. 2014;37:1306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stewart GC, Weintraub JR, Pratibhu PP, et al. Patient expectations from implantable defibrillators to prevent death in heart failure. J Card Fail. 2010;16:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuhas J, Mattocks K, Gravelin L, et al. Patients’ attitudes and perceptions of implantable cardioverter-defibrillators: potential barriers to appropriate primary prophylaxis. Pacing Clin Electrophysiol. 2012;35:1179–87. [DOI] [PubMed] [Google Scholar]
- 89.Randolph TC, Hellkamp AS, Zeitler EP, et al. Utilization of cardiac resynchronization therapy in eligible patients hospitalized for heart failure and its association with patient outcomes. Am Heart J. 2017;189:48–58. [DOI] [PubMed] [Google Scholar]
- 90.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. [DOI] [PubMed] [Google Scholar]
- 91.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. [DOI] [PubMed] [Google Scholar]
- 92.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- 93.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–90. [DOI] [PubMed] [Google Scholar]
- 94.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. [DOI] [PubMed] [Google Scholar]
- 95.Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. [DOI] [PubMed] [Google Scholar]
- 96.Stromberg A The crucial role of patient education in heart failure. Eur J Heart Fail. 2005;7:363–9. [DOI] [PubMed] [Google Scholar]
- 97.Fonarow GC, Yancy CW, Heywood JT. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE registry. Arch Intern Med. 2005;165:1469–77. [DOI] [PubMed] [Google Scholar]
- 98.American Heart Association. Health topics heart failure. Available at: https://www.heart.org/en/health-topics/heart-failure. Accessed June 24, 2020.
- 99.American College of Cardiology. CardioSmart heart failure. Available at: https://www.cardiosmart.org/Heart-Conditions/Heart-Failure. Accessed June 24, 2020.
- 100.HFSA Learning Center. Patient education heart failure. Available at: https://learningcenter.hfsa.org/Public/Catalog/Home.aspx?Search=heartþfailure&Criteria=18&tab=2. Accessed June 24, 2020.
- 101.NIH National Heart, Lung, and Blood Institute. Health topics heart failure. Available at: https://www.nhlbi.nih.gov/health-topics/heart-failure. Accessed June 24, 2020.
- 102.European Society of Cardiology. Heart failure matters: practical information for patients, families and caregivers. Available at: https://www.heartfailurematters.org/en_GB. Accessed June 24, 2020.
- 103.Aguado O, Morcillo C, Delas J, et al. Long-term implications of a single home-based educational intervention in patients with heart failure. Heart Lung. 2010;39:S14–22. [DOI] [PubMed] [Google Scholar]
- 104.Boren SA, Wakefield BJ, Gunlock TL, et al. Heart failure self-management education: a systematic review of the evidence. Int J Evid Based Healthc. 2009;7:159–68. [DOI] [PubMed] [Google Scholar]
- 105.Gwadry-Sridhar FH, Arnold JM, Zhang Y, et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150:982. [DOI] [PubMed] [Google Scholar]
- 106.Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179–85. [DOI] [PubMed] [Google Scholar]
- 107.Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–63. [DOI] [PubMed] [Google Scholar]
- 108.VanSuch M, Naessens JM, Stroebel RJ, et al. Effect of discharge instructions on readmission of hospitalised patients with heart failure: do all of the Joint Commission on Accreditation of Healthcare Organizations heart failure core measures reflect better care? Qual Saf Health Care. 2006;15:414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chan PS, Soto G, Jones PG, et al. Patient health status and costs in heart failure: insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS). Circulation. 2009;119:398–407. [DOI] [PubMed] [Google Scholar]
- 110.Heidenreich PA, Spertus JA, Jones PG, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47:752–6. [DOI] [PubMed] [Google Scholar]
- 111.Soto GE, Jones P, Weintraub WS, et al. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110:546–51. [DOI] [PubMed] [Google Scholar]
- 112.Khariton Y, Hernandez AF, Fonarow GC, et al. Health status variation across practices in outpatients with heart failure: insights from the CHAMP-HF (Change the Management of Patients With Heart Failure) registry. Circ Cardiovasc Qual Outcomes. 2018;11:e004668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khariton Y, Nassif ME, Thomas L, et al. Health status disparities by sex, race/ethnicity, and socioeconomic status in outpatients with heart failure. J Am Coll Cardiol HF. 2018;6:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thomas M, Khariton Y, Fonarow GC, et al. Association of changes in heart failure treatment with patients’ health status: real-world evidence from CHAMP-HF. J Am Coll Cardiol HF. 2019;7:615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dreyer RP, Jones PG, Kutty S, et al. Quantifying clinical change: discrepancies between patients’ and providers’ perspectives. Qual Life Res. 2016;25:2213–20. [DOI] [PubMed] [Google Scholar]
- 116.Nassif ME, Tang Y, Cleland JG, et al. Precision medicine for cardiac resynchronization: predicting quality of life benefits for individual patients-an analysis from 5 clinical trials. Circ Heart Fail. 2017;10:e004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–22. [DOI] [PubMed] [Google Scholar]
- 118.Grafft CA, McDonald FS, Ruud KL, et al. Effect of hospital follow-up appointment on clinical event outcomes and mortality. Arch Intern Med. 2010;170:955–60. [DOI] [PubMed] [Google Scholar]
- 119.Krumholz HM, Chen YT, Wang Y, et al. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000;139:72–7. [DOI] [PubMed] [Google Scholar]
- 120.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585–96. [DOI] [PubMed] [Google Scholar]
- 121.Fonarow GC, Heywood JT, Heidenreich PA, et al. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2007;153:1021–8. [DOI] [PubMed] [Google Scholar]
- 122.Cortes O, Arthur HM. Determinants of referral to cardiac rehabilitation programs in patients with coronary artery disease: a systematic review. Am Heart J. 2006;151:249–56. [DOI] [PubMed] [Google Scholar]
- 123.Park LG, Schopfer DW, Zhang N, et al. Participation in cardiac rehabilitation among patients with heart failure. J Card Fail. 2017;23:427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Austin J, Williams R, Ross L, et al. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail. 2005;7:411–7. [DOI] [PubMed] [Google Scholar]
- 125.Austin J, Williams WR, Ross L, et al. Five-year follow-up findings from a randomized controlled trial of cardiac rehabilitation for heart failure. Eur J Cardiovasc Prev Rehabil. 2008;15:162–7. [DOI] [PubMed] [Google Scholar]
- 126.Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12:706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Piepoli MF, Davos C, Francis DP, et al. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ. 2004;328:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–25. [DOI] [PubMed] [Google Scholar]
- 130.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116:693–706. [DOI] [PubMed] [Google Scholar]
- 131.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


