Abstract
Group 2 Innate Lymphoid Cells (ILC2) play an important role in immune responses at barrier surfaces, notably in the lung during airway allergic inflammation or asthma. Several studies have described methods to isolate ILC2s from wild-type naive mice, most of them using cell sorting to obtain a pure population. Here, we describe in detail, a simple, efficient method for isolation and culture of lung mouse ILC2s. Lungs from Rag2-/- mice pretreated with IL-33 are collected and processed into single cell suspensions. Lymphoid cells are then recovered by density gradient separation. Lin-CD45+ cells are selected by depletion of lineage positive cells followed by positive selection of CD45+ cells. Culture of the isolated cells for several days results in a highly purified ILC2 population expressing typical cell surface markers (CD90.2, Sca1, CD25, CD127, and IL-33R). These cells can be expanded in culture for up to 10 days and used for diverse ex vivo assays or in vivo adoptive transfer experiments.
Keywords: Innate immunity, Allergic inflammation, ILC2, IL-33, Type 2 cytokine, Lung, Culture
Background
Group 2 Innate Lymphoid Cells (ILC2) are tissue resident cells that play crucial roles in anti-parasitic innate immunity as well as in the development of allergic inflammation. They respond to epithelial cell-derived cytokines such as interleukin-33 (IL-33) by producing large amounts of type 2 cytokines IL-5 and IL-13, which in turn induce eosinophilia and mucus production (Cayrol and Girard, 2018). In order to better characterize the function and regulation of these cells, numerous groups have sorted ILC2s from the lung of wild-type mice (WT) by fluorescence-activated cell sorting (FACS). Due to the low number of the ILC2s present in the lungs at steady state, this method results in a low yield of purified cells (1 x 104 per mouse) (for a review, see Moro et al., 2015 ). In the present protocol, we pretreat mice with IL-33, which triggers the in vivo expansion of lung ILC2s, resulting in a better yield of purified cells (1.3 x 106 per mouse). Moreover, we use Rag2-/- mice instead of WT mice because 1) the absence of B and T cells in these mice facilitates the purification of ILC2s, and 2) the number of ILC2s is greater in these mice. Culture of the isolated Lin-CD45+ cell population for a couple of days ex vivo provides highly purified lung ILC2s without the need to use a cell sorter. In summary, the procedure we describe is highly reproducible and provides abundant highly purified mouse lung ILC2s.
Materials and Reagents
Sterile disposable scalpel (labelians, NahitaTM, catalog number: SCMEC24)
96-Well polystyrene conical bottom MicroWellTM Plates (thermo fisher scientific, catalog number: 249570)
15 ml and 50 ml conical centrifuge polypropylene tubes (corning, Falcon®, catalog numbers: 352096 and 352070 respectively)
5 ml round-bottom polystyrene tubes (corning, Falcon®, catalog number: 352054)
Cell culture 60 x 15 mm Petri dishes (thermo fisher scientific, catalog number: 150288)
1 ml tuberculin syringes (with 25 G x 16 mm disposable) (Terumo, catalog number: SS-01T)
BD Micro-Fine+TM Insulin Syringes 0.3 ml; 30 G x 8 mm needle (BD, catalog number: 324826)
70 μm cell strainer (corning, Falcon®, catalog number: 352350)
Plunger of 2.5 ml syringes (Terumo, catalog number: SS*02SE1)
Hypodermic needles (Terumo, catalog number: NN-2516R)
Non cottoned open Pasteur pipettes (150 mm 2 ml) (Hilgenberg, catalog number: 3150102)
MS columns (Miltenyi Biotec, catalog number: 130-042-201)
6-well polystyrene (PS) multidish (thermo fisher scientific, catalog number: 140675)
1.2 ml Cluster Tubes loose (Thermo Fisher Scientific, Abgene®, catalog number: AB-0672)
Rag2-/- mice on a C57BL/6J background ( B6.129-Rag2tm1Fwa) (European Mouse Mutant Archive [EMMA])
-
Human recombinant Interleukin-33 (rIL-3395-270), natural form (home-made; previously described in Lefrançais et al., 2014 )
Note: Alternatively, recombinant IL-33 can be purchased from R&D systems (R&D systems, catalog number: 3625-IL).
Ice
Dulbecco’s Modified Eagle Medium (DMEM, high glucose, GlutaMAXTM Supplement, pyruvate) (Thermo Fisher Scientific, GibcoTM, catalog number: 31966021)
Dulbecco’s Phosphate-Buffered Saline (DPBS, no calcium, no magnesium) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190169)
RPMI 1640 with high glucose, L-Glutamine, HEPES (ATCC, catalog number: 30-2001)
Penicillin/Streptomycin 100x liquid (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122)
2-Mercaptoethanol (Sigma-aldrich, catalog number: M3148)
Recombinant mouse IL-2 (Cys 160 Ser) Protein (R&D Systems, catalog number: 1150-ML-020; Reconstitute at 100 μg/ml in sterile DPBS containing 0.1% bovine serum albumin. Store stock solution at -70 °C)
Collagenase Type IV (Thermo Fisher Scientific, GibcoTM, catalog number: 17104019)
DNase I, from bovine pancreas (Roche Diagnostics, catalog number: 11284932001)
Fetal Bovine Serum (Thermo Fisher Scientific, GibcoTM, catalog number: 10270-106)
Normal mouse serum (Thermo Fisher Scientific, catalog number: 10410)
Lympholyte®-M (Cedarlane, catalog number: CL5035)
EasySepTM mouse hematopoietic progenitor cell isolation kit (StemCell Technologies, catalog number: 19856)
CD45 MicroBeads, mouse (Miltenyi Biotec, catalog number: 130-052-301)
-
FACS reagents
Note: dilutions have to be determined for each lot of reagent.
Fixable Viability Dye eFluor 506 (dilution: 1/1,000) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 65-0866-14)
Streptavidin, PE-CyTM 7 conjugated (dilution: 1/100) (BD PharmingenTM, catalog number: 557598)
-
FACS Monoclonal Antibodies
Note: dilutions have to be determined for each lot of antibody.
Rat anti-Mouse CD16/CD32 (mouse BD Fc BlockTM) (clone 2.4G2, dilution: 1/200) (BD PharmingenTM, catalog number: 553142)
Rat Anti-Mouse CD4, FITC conjugated (clone GK1.5, dilution: 1/2,000) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 11-0041-85)
Rat Anti-Mouse CD3, FITC conjugated (clone 17A2, dilution: 1/600) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 11-0032-80)
Rat Anti-Mouse CD19, FITC conjugated (clone 1D3, dilution: 1/2,000) (BD PharmingenTM, catalog number: 553785)
Rat Anti-Mouse CD45R/B220, FITC conjugated (clone RA3-6B2, dilution: 1/1,000) (BD PharmingenTM, catalog number: 553088)
Hamster Anti-Mouse CD11c, FITC conjugated (clone N418, dilution: 1/300) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 11-0114-82)
Rat Anti-Mouse CD11b, FITC conjugated (clone M1/70, dilution: 1/100) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 11-0112-85)
Rat Anti-Mouse Ter119, FITC conjugated (clone Ter119, dilution: 1/100) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 11-5921-85)
Rat Anti-Mouse Ly-6G/Ly-6C, FITC conjugated (clone RB6-8C5, dilution: 1/100) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 11-5931-85)
Hamster Anti-Mouse FceR1α, FITC conjugated (clone MAR-1, dilution: 1/100) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 11-5898-85)
Mouse Anti-Mouse NK1.1, FITC conjugated (clone PK136, dilution: 1/300) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 11-5941-85)
Rat Anti-Mouse CD45, PerCP conjugated (clone 30-F11, dilution: 1/1,000) (BD PharmingenTM, catalog number: 557235)
Rat Anti-Mouse CD90.2, APC-CyTM7 conjugated (clone 53-2.1, dilution: 1/600) (BD PharmingenTM, catalog number: 561641)
Rat Anti-Mouse CD25, eFluor 450 conjugated (clone PC61.5, dilution: 1/300) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 48-0251-82)
Rat Anti-Mouse CD127, PE conjugated (clone A7R34, dilution: 1/100) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 12-1271-83)
Rat Anti-Mouse Ly-6A/E (Sca-1), APC conjugated (clone D7, dilution: 1/300) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 17-5981-83)
Rat Anti-Mouse T1/ST2 (IL-33R), biotinylated (clone DJ8, dilution: 1/100) (MD Biosciences, catalog number: 101001B)
-
FACS Isotype controls
Note: Isotype controls are used at the same concentration as the specific antibody. So, dilutions have to be determined according to the concentration of the matched antibody.
Rat IgG1 κ Isotype Control, eFluor 450 (clone eBRG1, dilution: 1/300) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 48-4301-80)
Rat IgG2a κ Isotype Control, APC (clone eBR2a, dilution: 1/300) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 17-4321-81)
Rat IgG2a, κ Isotype Control, PE (clone eBR2a, dilution: 1/100) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 12-4321-82)
Rat IgG2a, κ Isotype Control, APC-CyTM 7 (Clone R35-95, dilution: 1/600) (BD PharmingenTM, catalog number: 552770)
Rat IgG1, κ Isotype Control, Biotin (Clone R3-34, dilution: 1/50) (BD PharmingenTM, catalog number: 553923)
Lung digestion solution (see Recipes)
PEF buffer (see Recipes)
PEB buffer (see Recipes)
ILC2 culture medium (see Recipes)
FACS buffer (see Recipes)
FACS staining buffer (see Recipes)
Equipment
MiniMACSTM separator (Miltenyi Biotec, catalog number: 130-042-102)
EasySepTM Magnet (StemCell Technologies, catalog number: 18000)
Malassez Hemocytometer
Water bath
Centrifuge (Eppendorf, model: 5804 R)
4 °C refrigerator
Flow cytometer (BD, model: LSR II)
Software
FlowJo software (Tree Star)
BD FACSDivaTM software (BD Biosciences)
Procedure
Note: In order to get enough ILC2 cells to perform various experiments, we generally purify ILC2s from 4 or 5 mice.
-
In vivo treatment of mice
Prepare purified human recombinant IL-3395-270 as previously described by Lefrançais et al. (2014) . Human IL-33 is a potent activator of mouse ST2+ cells ( Cayrol et al., 2018 ).
For each mouse, inject intraperitoneally 4 µg of human recombinant IL-3395-270 into a Rag2-/- mouse (diluted in DPBS; V = 100 μl/mouse), using an insulin syringe (0.3 ml; 30 G), five times at days 0, 1, 4, 5 and 6 (see Figure 1).
-
ILC2 Isolation
Notes:
Lympholyte®-M is a density separation medium. Put it at room temperature for at least 20 min before use, because the density of Lympholyte®-M is temperature dependent (Step B2).
At different steps during this procedure, 100 μl of cell suspensions is collected in a 96-well plate for subsequent FACS analysis. Keep that plate on ice, until Step B5.
-
Preparing lung single-cell suspension
For each mouse:
Sacrifice the mouse on Day 7, remove the lungs and place them into a 15 ml Falcon® tube filled with 5 ml DMEM.
Freshly prepare lung digestion solution (2 ml per mouse; see Recipes).
-
Transfer the lungs into a Petri dish containing 500 μl of lung digestion solution and smoothly inject 500 μl of lung digestion solution into the lobes of the lung, using a 25 G needle mounted on a 1 ml syringe. The lung must swell (see video 1)
Video 1. Procedure of lung digestion.
Download video file (9.4MB, mp4) The video shows the injection of digestion solution into the lung.
The video shows the injection of digestion solution into the lung. Cut the lungs into small pieces using a scalpel and transfer the crushed lungs into a Falcon® 50 ml tube. Rinse the Petri dish with 1 ml of lung digestion solution and transfer to the 50 ml tube.
Incubate the 50 ml tube containing the sample in a water bath at 37 °C for 1 h.
Put a 70 μm cell strainer on a 50 ml tube; apply the sample on it and rinse the tube used for the digestion with 2 ml DPBS.
In order to obtain a uniform single-cell suspension from tissue, grind the remaining tissue on the strainer with the plunger of a 2.5 ml syringe and wash the strainer with additional 2 ml DPBS.
-
Enrichment of leucocyte population by density gradient separation
-
Discard the cell strainer and transfer the cell suspension into a Falcon® 15 ml tube.
Centrifuge at 400 × g at room temperature for 5 min.
Remove the supernatant from the samples and resuspend the cell pellet in 5 ml DPBS.
Using Pasteur pipette, slowly add 5 ml Lympholyte®-M under the cell suspension (see Figure 2A).
Centrifuge at 1,300 × g at room temperature for 20 min without any brake.
For each sample, collect the cells of interest from the Lympholyte®-M/DPBS interface with a Pasteur pipette (see Figure 2B). Combine all samples in a single 15 ml Falcon® conical tube filled with 5 ml DPBS. This step enables the recovery of lymphoid cells and eliminates erythrocytes, dead cells and debris.
-
-
Enrichment of lineage negative population
-
Centrifuge at 400 × g at 4 °C for 5 min to pellet the cells.
Note: Work under sterile conditions (hood) from this step.
-
Remove the supernatant and resuspend the cell pellet in 10 ml PEF buffer.
Note: Take 100 μl of cell suspension for subsequent FACS analysis (see Figure 3A for gating strategy and Figure 3B-before purification).
Count the cells using a hemocytometer. The typical recovered cell number is 20 x 106 per mouse.
Centrifuge again at 400 × g at 4 °C for 5 min.
Proceed with negative selection: deplete lineage positive cells using The EasySepTM Mouse Hematopoietic Cell Isolation Kit. This method uses biotinylated antibodies directed against non-hematopoietic stem cells and non-progenitor cells (CD5, CD11b, CD19, CD45R/B220, Ly6G/C(Gr-1), TER119) and streptavidin-coated magnetic particles. Follow the kit protocol to separate unlabeled cells (Lin-) using an EasySepTM magnet. At the end of this step, the cells of interest (Lin-) are recovered in a 15 ml tube.
-
Repeat the depletion step with the EasySepTM magnet using the negative fraction containing Lin-ILC2s
Note: Take 100 μl of each cellular fraction for subsequent FACS analysis (see Figure 3B-lineage depletion; first and second depletion).
-
-
Selection of CD45+ population
Centrifuge at 400 × g at 4 °C for 5 min.
Remove the supernatant, resuspend the cell pellet in 5 ml of PEB buffer and determine cell number using a hemocytometer. The typical recovered cell number is 3.7 x 106 per mouse.
-
Proceed with CD45 positive selection: select CD45+ cells by using magnetic beads conjugated to anti-mouse CD45 monoclonal antibody, MS columns, MiniMACSTM separator and follow the recommendations of the manufacturer (Miltenyi Biotech). At the end of this step, the cells of interest (Lin-CD45+) are recovered.
Note: Take 100 μl of cell suspension for subsequent FACS analysis (see Figure 3B-CD45 selection).
Centrifuge at 400 × g at 4 °C for 5 min.
Remove the supernatant, resuspend cell pellet in 1 ml of ILC2 culture medium (see Recipes).
Count cells by using a hemocytometer. Typically, we obtain 1.3 x 106 ILC2s per mouse.
-
Proceed with ILC2s staining for FACS analysis
Fill wells of the 96-well plate containing cells from subsequent isolation steps with DPBS.
Centrifuge the plate at 400 × g at 4 °C for 5 min and remove the supernatant.
Perform the viability staining: resuspend cell pellets with 100 μl/well of Viability Dye-eFluor506 diluted to 1/1,000 in DPBS. Incubate for 30 min at 4 °C.
Wash the cells: complete wells with FACS buffer (see Recipes), centrifuge the plate at 400 × g at 4 °C for 5 min and remove the supernatant.
Resuspend cell pellets in 50 μl/well of the different antibodies (listed in Materials and Reagents #31-33) diluted in FACS staining buffer (containing Fc block and mouse serum). Incubate for 30 min at 4 °C.
Repeat the washing step.
Resuspend cell pellets in 100 μl of FACS buffer, transfer each sample to annotated FACS tubes and add 200 μl of FACS buffer (300 μl total volume).
-
Proceed to FACS analyses (on a LSR II for example). We generally obtain an ILC2 population with about 90% of cells expressing typical ILC2s markers (Lin-CD45+ CD25+ CD90.2+ CD127+ Sca1+ ST2+).
Note: Cell surface markers analysis after ILC2 isolation–see an example in Figures 3C and 3D.
-
ILC2 culture
This step of ILC2 culture leads to increased numbers of ILC2s and a better degree of cell purity.
Dilute cell suspension (from step B4) in order to get 0.3 x 106 cells/ml.
Distribute 3 ml of ILC2 cell suspension/well in a 6-well plate (Day 7).
The day after (Day 8), in the morning, add another 3 ml ILC2 medium to each well. In the evening, pipet the cells up and down, and split them into 3 wells and add again 3 ml medium per well (total volume per well = 5 ml).
At Day 11, collect cells by pipetting into 15 ml Falcon® tube, centrifuge at 400 × g for 5 min.
-
Count cells with a hemocytometer. ILC2s are ready to be used for ex vivo or in vivo experiments. They can also be maintained in culture for up to 10 days at 0.4 x 106 cells/ml.
Note: Take 100 μl of ILC2 cell suspension for subsequent FACS analysis (see Figure 4).
-
Proceed to FACS analysis as described above (step B5). We generally obtain an ILC2 population (13.6 x 106 cells per mouse) with > 95% of cells expressing typical ILC2s markers (Lin-CD45+ CD25+ CD90.2+ CD127+ Sca1+ ST2+).
Note: See an example in Figure 4–Cell surface markers analysis after ILC2 isolation and culture.
Figure 1. A schematic view of the whole procedure.
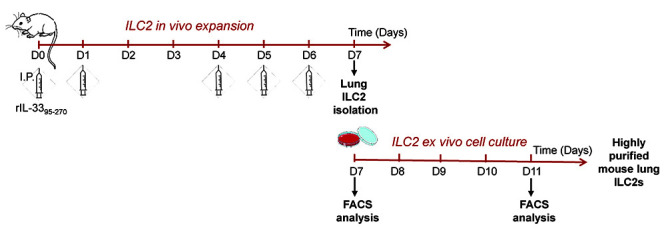
Rag2-/- mice were first treated with rIL-3395-270 to expand ILC2s in vivo (see Procedure A). Lin-CD45+ ILC2s are then isolated (see Procedure B) and cultured for several days (see Procedure C), before being used for subsequent ex vivo or in vivo experiments.
Figure 2. Density gradient separation procedure.
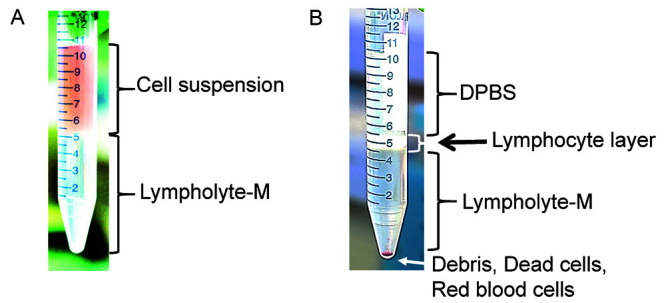
A. Before centrifugation, lymphoid cells are located in the cell suspension phase above the Lympholyte®-M. B. After centrifugation, lymphoid cells are concentrated in the interface between the DPBS buffer and the Lympholyte®-M. Red blood cells, dead cells and debris are found at the bottom of the tube.
Figure 3. Isolation of mouse lung ILC2s.
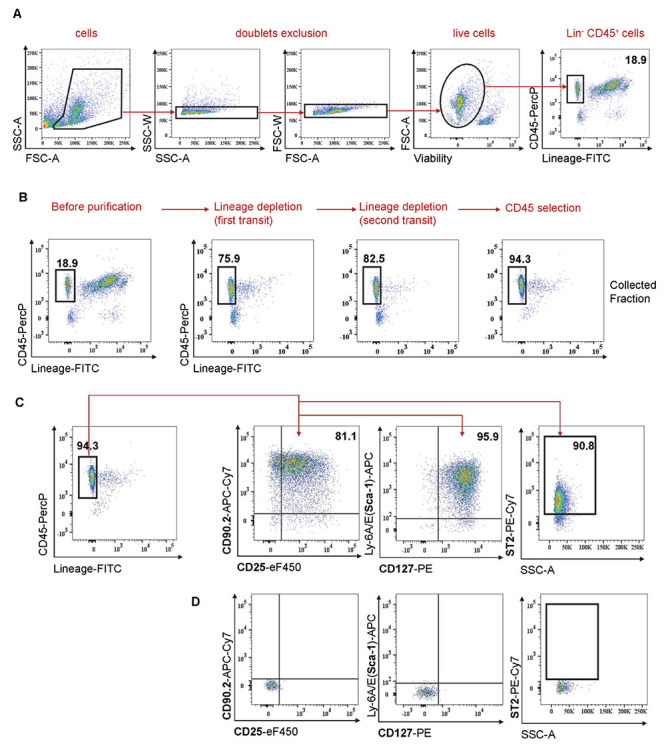
A. Gating strategy used to identify ILC2s. Doublet cells and dead cells are first excluded. ILC2s lack lineage markers and express CD45 surface marker. Number indicates % among live cells. B. ILC2 purification is analyzed by FACS at different steps: before purification, after depletion of lineage + cells, and after CD45 positive selection. Cells are gated on live single cells. Numbers indicate % among live cells. C. Analysis of cell surface markers after ILC2 isolation (Day 7). Cells are gated on live single cells. Numbers indicate % among Lin-CD45+ cells. D. Isotype controls are shown.
Figure 4. Analysis of ILC2 cell surface markers by flow cytometry after 4 days in culture (Day 11).
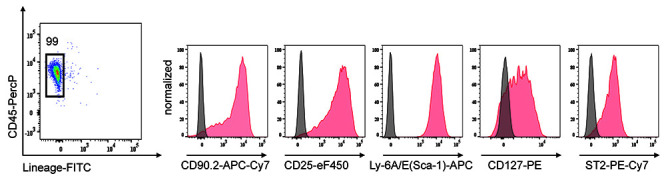
Representative histograms of CD90.2, CD25, Sca-1, CD127 and ST2 markers at the surface of purified ILC2s. Number indicates % among live cells. Isotype controls are shown (grey histograms).
Data analysis
Samples are acquired on a LSR II flow cytometer using FACSDiVaTM software, before (see Figure 3) and after (see Figure 4) ILC2 cell culture. Flow cytometry data are analyzed using FlowJo to gate, quantify, and analyze ILC2 population.
Recipes
-
Lung digestion solution
Freshly prepare 2 ml/sample of DMEM containing 2 mg/ml collagenase IV and 0.1 mg/ml DNase I
-
PEF buffer
DPBS containing 2% filtered FBS and 1 mM EDTA
Keep sterile and cold during the experiment and store at 4 °C
-
PEB buffer
DPBS containing 0.5% BSA and 2 mM EDTA
Keep sterile and cold during the experiment and store at 4 °C
-
ILC2 culture medium
RPMI-1640 medium supplemented with 10% filtered FBS, 1% penicillin/streptomycin, 50 μM 2-mercaptoethanol and 20 ng/ml recombinant mouse IL-2
Prepare freshly, keep sterile and store at 4 °C during the time of the experiment only
-
FACS buffer
DPBS containing 5% filtered FBS and 5 mM EDTA
Store at 4 °C
-
FACS staining buffer
FACS buffer containing 5% mouse serum and 1/200 anti-mouse CD16/CD32 (Fc block)
Store at 4 °C
Acknowledgments
This protocol was adapted from our publication ( Cayrol et al., 2018 ). This work was supported by Agence Nationale de la Recherche (A.D.; ANR-12-BSV3-0005-01 and ANR-16-CE15-0009-01 to J.-P.G.), the French Ministry of Research (P.S.; E.M.), and the “Fondation ARC” (E.M.). We thank members of the Girard's laboratory for help with animal experiments and Françoise Viala for iconography and the video. We would like to thank the ANEXPLO-IPBS facility and Imaging Core Facility TRI-IPBS. We are grateful to Nathalie Ortega for help with animal ethics.
Competing interests
The authors declare no conflict of interest.
Ethics
All mice experiments were handled according to institutional guidelines under protocols approved by the French Ministry of Research and the FRBT (C2EA-01) animal care committee (projects 00663.02 and APAFIS#3873-2016020116301837v3).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Cayrol C., Duval A., Schmitt P., Roga S., Camus M., Stella A., Burlet-Schiltz O., A. Gonzalez-de-Peredo and Girard J. P.(2018). Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol 19(4): 375-385. [DOI] [PubMed] [Google Scholar]
- 2. Cayrol C. and Girard J. P.(2018). Interleukin-33(IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev 281(1): 154-168. [DOI] [PubMed] [Google Scholar]
- 3. Lefrançais E., Duval A., Mirey E., Roga S., Espinosa E., Cayrol C. and Girard J. P.(2014). Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A 111(43): 15502-15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moro K., Ealey K. N., Kabata H. and Koyasu S.(2015). Isolation and analysis of group 2 innate lymphoid cells in mice. Nat Protoc 10(5): 792-806. [DOI] [PubMed] [Google Scholar]


