Abstract
Background
Relative to nondiabetic patients, percutaneous coronary intervention (PCI) in patients with diabetes mellitus (DM) is associated with inferior clinical outcomes. We aimed to evaluate the outcomes of drug-coated balloon (DCB) in diabetic versus nondiabetic patients.
Methods and Results
In this observational, prospective, multicenter study, we compared the outcomes of patients with and without DM after undergoing PCI with DCBs. Target lesion failure (TLF) was analyzed as primary endpoint. Secondary endpoints were the rates of target lesion revascularization (TLR), major adverse cardiovascular events (MACE), cardiac death, myocardial infarction (MI), and any revascularization. Propensity score matching was used to assemble a cohort of patients with similar baseline characteristics. Among 2,306 eligible patients, 578 with DM and 578 without DM had similar propensity scores and were included in the analyses. During follow-up (366 ± 46 days), compared with DM patients, patients without DM were associated with a lower yearly incidence of TLF (2.77% vs. 5.36%; OR, 1.991; 95% CI, 1.077 to 3.681; P = 0.025) and TLR (1.90% vs. 4.15%; OR, 2.233; 95% CI, 1.083 to 4.602; P = 0.026). No significant differences were observed with regards to rates of MACE (OR: 1.580, 95% CI: 0.912-2.735; P = 0.100), cardiac death (OR: 1.608, 95% CI: 0.523-4.946; P = 0.403), MI (OR: 4.042, 95% CI: 0.855-19.117; P = 0.057), and any revascularization (OR: 1.534, 95% CI: 0.983-2.393; P = 0.058).
Conclusions
Diabetic patients experience higher TLF and TLR rates following DCB angioplasty without substantial increase in the risk of MACE, cardiac death, MI, or revascularization.
1. Introduction
Patients with diabetes mellitus (DM) are at increased risk of coronary artery disease (CAD). DM patients often present with a combination of diffuse coronary lesions and small vessel disease, indicating difficult stent delivery and high restenosis rates postpercutaneous coronary intervention (PCI). Regarding coronary revascularization, recent technological advances have expanded PCI use to more complex lesions [1, 2], especially drug eluting stents (DES), which have markedly reduced the rate of restenosis and repeat revascularization [3, 4]. However, CAD morbidity and mortality in DM patients remain high, even with current use of DES [5, 6].
Drug-coated balloon (DCB) is effective in treating instent restenosis (ISR) [7, 8]. Various clinical studies have revealed its effectiveness against de novo coronary lesions [9–11], especially in small vessel disease. Differences in study results are mainly due to differences in study approach, which may use the “DCB-only” or “hybrid” strategy. It also preserves access for future coronary artery bypass grafting (CABG).
To date, no studies have investigated the use of DCB angioplasty-only in diabetic patients with coronary artery disease. Here, we evaluated the outcomes of DCBs in diabetic versus nondiabetic patients.
2. Methods
2.1. Study Population
Patients were retrospectively enrolled at 3 Chinese centers from July 2014 to December 2019. Only patients with coronary vessel lesions sized between 2.0 and 4.0 mm, whether de novo or instent restenosis, and were eligible for the study. The exclusion criteria were ≥ type C dissection or >30% residual stenosis after lesion preparation, simultaneous ISR treatment and de novo lesions, revascularization within 1 month prior to index procedure, and unstable hemodynamics or cardiogenic shock (Figure 1).
Figure 1.
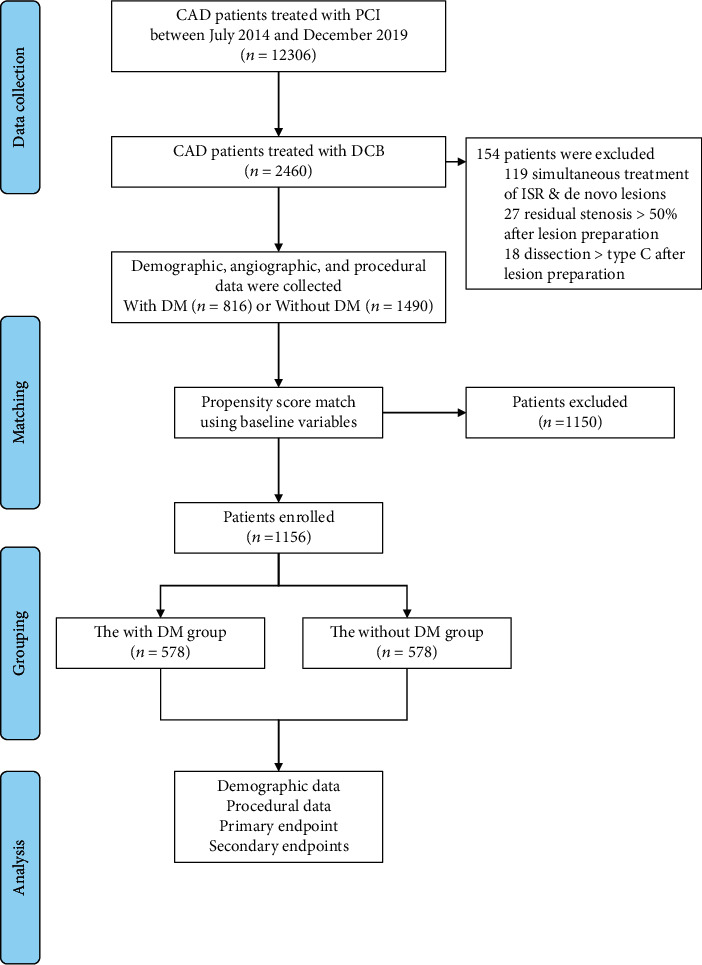
Study population. CAD: coronary artery disease; PCI: percutaneous coronary intervention; DCB: drug-coated balloon; DM: diabetes mellitus; ISR: instent restenosis.
Patients were given 300 mg aspirin before intervention, unless they were on long-term aspirin treatment. Clopidogrel or ticagrelor was administered at loading doses of 600 and 180 mg, respectively. Patients who underwent DCB only were put on dual antiplatelet therapy (DAPT) for at least 1 month after the procedure. Those receiving DCB and stent implantation received DAPT in accordance with established guidelines [12, 13]. Patients with contraindications or known hypersensitivity to DAPT, heparin, paclitaxel, or limus; women with childbearing potential; and those with a life expectancy below a year were excluded. Ethical approval for the study was granted by the First Affiliated Hospital of Zhengzhou University Institutional Review Board/Ethics Committee. All patients gave written informed consent. Data were collected using a common electronic case report form.
2.2. Study Procedure
During intervention, special emphasis was paid to adequate lesion preparation prior to DCB treatment as recommended in the latest expert consensus [14]. Predilatation with semicompliant balloon, noncompliant balloon, scoring balloon, or cutting balloon with a balloon-to-vessel ratio of 0.8-1.0 was mandatory (0.8-1.0 for de novo lesions and 1.0 for ISR lesions). Next, DCB angioplasty was done only in the absence of a major, flow-limiting dissection (≥ type C according to the NHLBI classification [15] and where residual stenosis was ≤30% based on at least 2 perpendicular angiographic views). In this study, DCB had a paclitaxel/iopromide matrix coating (SeQuent™ Please, B. Braun, Melsungen, Germany). To avoid geographic mismatch, DCB catheter length was designed to exceed that of the target lesion by at least 2 mm. DCB diameters were adapted to reference vessel diameters with a balloon-to-vessel ratio of 0.8-1.0. Recommended inflation time was at least 30 seconds at >7 bars. New-generation DESs were implanted if DCB-only outcomes were unsatisfactory due to severe residual stenosis or dissections.
2.3. Clinical Endpoints and Definitions
The study's primary outcome was one-year target lesion failure (TLF), a composite of cardiac death, target vessel myocardial infarction (MI), and target lesion revascularization (TLR). Various secondary outcomes were evaluated, including the rates of TLR, major adverse cardiovascular events (MACE, defined as the composite outcome of cardiac death, myocardial infarction (MI), and target vessel revascularization (TVR)), cardiac death, MI, and repeat revascularization (including PCI and CABG). Cardiac death is defined as death resulting from cardiovascular causes. And undetermined cause of death is defined as a death not attributable to any other category because of the absence of any relevant source documents. Such deaths will be classified as cardiovascular for end point determination [16, 17]. MI was defined by typical clinical symptoms, ECG changes, and/or elevated cardiac troponin values with at least one value above the 99th percentile upper reference limit (type 4b or 4c MI, except for perioperative MI) [18]. Patients' follow-up was by telephone or outpatient visits at 12 months.
2.4. Statistical Analysis
Data analysis was done using R statistics packages (http://www.r-project.org) and Empower (R) (http://www.empowerstats.com, X & Y Solutions Inc.). Categorical variables are presented as frequencies (percentages) and continuous variables as mean ± SD. Comparisons between patients with and without DM were done using Fisher's exact test for each variable. Mann–Whitney Wilcox nonparametric tests were used for continuous variables. Given the baseline characteristic differences between eligible participants in the 2 groups of the observational study, a 1 : 1 propensity score matching (PSM) was used to select patients with comparable baseline data. After evaluation of covariates that were clinically and/or statistically associated with the treatment group and removal of repeatedly defined or collinear variables, including baseline characteristics, risk factors, clinical conditions at admission, and treatment during operation, 12 variables (age, sex, hypertension, hyperlipidemia, renal insufficiency, acute coronary syndrome, family history of CAD, smoking history, PCI history, MI history, CABG history, and left ventricular ejection fraction) were included in the propensity score matching model using greedy nearest neighbor matching without replacement and a caliper of 0.02. Analyses of primary and secondary outcomes in the presence or absence of DM were done for the entire group and for the propensity matched cohort. Outcomes were compared using a log-rank test and presented as Kaplan-Meier curves. For all analyses reported, P values were 2-sided, and P < 0.05 indicated statistical significance.
3. Results
3.1. Patient Population
A total of 2,306 PCI patients treated with DCB met our inclusion and exclusion criteria (Figure 1). Of these 816 patients, (35.38%) had DM. Baseline characteristics are shown in Table 1. After matching, 578 patients were selected for each group. Statistical differences between the groups with regards to age, hypertension, hyperlipidemia, renal insufficiency, PCI history, and LVEF were reduced upon patient matching.
Table 1.
Demographic characteristics before and after propensity score matching.∗
| Variable | All patients | Propensity matched sample | ||||
|---|---|---|---|---|---|---|
| Without DM | With DM | P value | Without DM | With DM | P value | |
| Number of patients | 1490 | 816 | 578 | 578 | ||
| Age (years) | 58.71 ± 11.17 | 61.26 ± 10.54 | <0.001 | 59.99 ± 11.26 | 61.03 ± 10.34 | 0.237 |
| Sex (male) | 1077 (72.28%) | 567 (69.49%) | 0.156 | 409 (70.76%) | 404 (69.90%) | 0.748 |
| Hypertension | 697 (46.78%) | 487 (59.68%) | <0.001 | 337 (58.30%) | 359 (62.11%) | 0.186 |
| Hyperlipidemia | 391 (26.24%) | 309 (37.87%) | <0.001 | 200 (34.60%) | 219 (37.89%) | 0.245 |
| History of smoking | 490 (32.89%) | 267 (32.72%) | 0.936 | 207 (35.81%) | 202 (34.95%) | 0.758 |
| Renal insufficiency | 52 (3.49%) | 63 (7.72%) | <0.001 | 28 (4.84%) | 33 (5.71%) | 0.511 |
| Acute coronary syndrome | 1001 (67.18%) | 572 (70.10%) | 0.150 | 435 (75.26%) | 430 (74.39%) | 0.735 |
| Previous MI history | 146 (9.80%) | 89 (10.91%) | 0.400 | 63 (10.90%) | 65 (11.25%) | 0.851 |
| Previous PCI history | 406 (27.25%) | 292 (35.78%) | <0.001 | 190 (32.87%) | 210 (36.33%) | 0.216 |
| Previous CABG history | 28 (1.88%) | 19 (2.33%) | 0.465 | 12 (2.08%) | 12 (2.08%) | 1.000 |
| Family history of CAD | 265 (17.79%) | 166 (20.34%) | 0.132 | 127 (21.97%) | 122 (21.11%) | 0.721 |
| Previous stroke history | 146 (9.80%) | 204 (25.00%) | <0.001 | 99 (17.13%) | 121 (20.93%) | 0.099 |
| Peripheral artery disease | 119 (7.99%) | 177 (21.69%) | <0.001 | 81 (14.01%) | 104 (17.99%) | 0.065 |
| LVEF | 59.99 ± 7.55 | 58.95 ± 7.68 | 0.003 | 59.18 ± 7.61 | 59.25 ± 7.46 | 0.596 |
| Other vessel treated by DES only | 487 (32.68%) | 279 (34.19%) | 0.463 | 195 (33.74%) | 196 (33.91%) | 0.950 |
∗Plus–minus values are means ± SD. DM: diabetes mellitus; MI: myocardial infarction; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; CAD: coronary artery disease; LVEF: left ventricular ejection fraction; DES: drug-eluting stent.
3.2. PCI-Related Characteristics
Procedural baseline features are shown on Table 2. There were 2,660 lesions before matching, of which 424 (15.94%) were ISR and 2,236 (84.06%) were de novo lesions. After matching, 1,318 lesions remained, 649 in non-DM patients, and 669 in DM patients. A small proportion of patients (1.85% non-DM and 1.05% DM) also underwent DCB angioplasty in the left main and bypass graft vessel. Lesion preparation (predilatation with plain balloons, scoring balloons, cutting balloons, noncompliant balloons, or rotational atherectomy) was done for all lesions. DCB use was similar in the 2 groups. 1.11 ± 0.36 DCBs were used per lesion, the mean diameter was 2.75 ± 0.47 vs. 2.75 ± 0.48 mm, the total length was 25.03 ± 12.47 vs. 25.03 ± 12.16 mm, and inflation pressure was 8.26 ± 2.93 vs. 8.23 ± 1.35 bars (non-DM vs. DM group). The bailout stenting rate was low in the non-DM (3.08%) and DM group (3.59%).
Table 2.
Procedural characteristics before and after propensity score matching.∗
| Variable | All patients | Propensity matched sample | ||||
|---|---|---|---|---|---|---|
| Without DM | With DM | P value | Without DM | With DM | P value | |
| Number of lesions | 1704 | 956 | 649 | 669 | ||
| Lesion type | 0.610 | 0.587 | ||||
| Instent restenosis | 267 (15.67%) | 157 (16.42%) | 116 (17.87%) | 112 (16.74%) | ||
| De novo lesions | 1437 (84.33%) | 799 (83.58%) | 533 (82.13%) | 557 (83.26%) | ||
| Treated vessel | 0.541 | 0.729 | ||||
| Left anterior descending coronary artery | 775 (45.48%) | 410 (42.89%) | 274 (42.22%) | 284 (42.45%) | ||
| Left circumflex coronary artery | 545 (31.98%) | 310 (32.43%) | 200 (30.82%) | 215 (32.14%) | ||
| Left main coronary artery | 9 (0.53%) | 3 (0.31%) | 5 (0.77%) | 2 (0.30%) | ||
| Right coronary artery | 363 (21.30%) | 226 (23.64%) | 163 (25.12%) | 163 (24.36%) | ||
| Bypass graft | 12 (0.70%) | 7 (0.73%) | 7 (1.08%) | 5 (0.75%) | ||
| Number of lesions treated by DCB (per patient) | 0.417 | 0.372 | ||||
| 1 | 1294 (86.85%) | 689 (84.44%) | 511 (88.41%) | 497 (85.99%) | ||
| 2 | 180 (12.08%) | 115 (14.09%) | 63 (10.90%) | 72 (12.46%) | ||
| 3 | 14 (0.94%) | 11 (1.35%) | 4 (0.69%) | 8 (1.38%) | ||
| 4 | 2 (0.13%) | 1 (0.12%) | 0 (0.00%) | 1 (0.17%) | ||
| Total occlusion | 201 (11.80%) | 116 (12.13%) | 0.796 | 77 (11.86%) | 78 (11.66%) | 0.908 |
| Intracoronary thrombus | 8 (0.47%) | 4 (0.42%) | 0.850 | 4 (0.62%) | 3 (0.45%) | 0.722 |
| Diffuse vessel disease | 383 (22.48%) | 208 (21.76%) | 0.669 | 162 (24.96%) | 151 (22.57%) | 0.308 |
| Ostial lesion | 310 (18.19%) | 169 (17.68%) | 0.740 | 105 (16.18%) | 118 (17.64%) | 0.480 |
| Bifurcation lesion | 552 (32.39%) | 292 (30.54%) | 0.325 | 201 (30.97%) | 207 (30.94%) | 0.991 |
| Lesion preparation | 1704 (100%) | 956 (100%) | 649 (100%) | 669 (100%) | ||
| Semicompliant balloon | 1119 (65.67%) | 667 (69.77%) | 0.031 | 446 (68.72%) | 475 (71.00%) | 0.367 |
| NSE | 459 (26.94%) | 264 (27.62%) | 0.706 | 197 (30.35%) | 182 (27.20%) | 0.207 |
| Cutting balloon | 521 (30.58%) | 282 (29.50%) | 0.561 | 186 (28.66%) | 202 (30.19%) | 0.541 |
| DWB | 67 (3.93%) | 39 (4.08%) | 0.852 | 20 (3.08%) | 28 (4.19%) | 0.285 |
| Noncompliant balloon | 454 (26.64%) | 280 (29.29%) | 0.143 | 168 (25.89%) | 190 (28.40%) | 0.305 |
| ROTA | 28 (1.64%) | 26 (2.72%) | 0.059 | 12 (1.85%) | 17 (2.54%) | 0.392 |
| Number of DCBs used (per lesion) | 1.10 ± 0.36 | 1.11 ± 0.36 | 0.603 | 1.11 ± 0.36 | 1.11 ± 0.36 | 0.716 |
| Mean DCB diameter (mm) | 2.79 ± 0.47 | 2.75 ± 0.47 | 0.026 | 2.75 ± 0.47 | 2.75 ± 0.48 | 0.819 |
| Length of DCB balloon (mm) | 24.50 ± 12.34 | 24.98 ± 12.46 | 0.077 | 25.03 ± 12.47 | 25.03 ± 12.16 | 0.569 |
| Inflation pressure (bar) | 8.36 ± 2.84 | 8.32 ± 2.19 | 0.615 | 8.26 ± 2.93 | 8.23 ± 1.35 | 0.404 |
| Bailout stenting | 60 (3.52%) | 38 (3.97%) | 0.551 | 20 (3.08%) | 24 (3.59%) | 0.609 |
∗Plus–minus values are means ± SD. DM: diabetes mellitus; DCB: drug-coated balloon; NSE: noncompliant scoring balloon; DWB: dual wire balloon; ROTA: rotational atherectomy.
3.3. Clinical Outcomes
Overall population follow-up for a mean of 366 days revealed a TLF incidence rates of 4.53% and 2.42% in diabetic and nondiabetic patients, respectively (OR: 1.918, 95% CI: 1.203-3.060; P = 0.005). After PSM, relative to DM-patients, non-DM patients exhibited lower yearly TLF incidence (5.36% and 2.77%, respectively, OR: 1.991, 95% CI: 1.077-3.681; P = 0.025; log rank P = 0.023) (Table 3 and Figure 2(a)). Results were largely similar before and after PSM.
Table 3.
Risk of primary and secondary outcomes in the propensity score-matched cohort at one-year follow-up.∗
| Endpoint | All patients | Propensity matched sample | ||||||
|---|---|---|---|---|---|---|---|---|
| Without DM | With DM | Odds ratio (95% CI) | P value | Without DM | With DM | Odds ratio (95% CI) | P value | |
| Number of patients | 1490 | 816 | 578 | 578 | ||||
| TLF | 36 (2.42%) | 37 (4.53%) | 1.918 (1.203, 3.060) | 0.005 | 16 (2.77%) | 31 (5.36%) | 1.991 (1.077, 3.681) | 0.025 |
| TLR | 24 (1.61%) | 29 (3.55%) | 2.251 (1.302, 3.893) | 0.003 | 11 (1.90%) | 24 (4.15%) | 2.233 (1.083, 4.602) | 0.026 |
| MACE† | 45 (3.02%) | 44 (5.39%) | 1.830 (1.197, 2.798) | 0.005 | 22 (3.81%) | 34 (5.88%) | 1.580 (0.912, 2.735) | 0.100 |
| Cardiac death | 11 (0.74%) | 8 (0.98%) | 1.331 (0.533, 3.323) | 0.539 | 2 (0.87%) | 8 (1.38%) | 1.608 (0.523, 4.946) | 0.403 |
| MI | 3 (0.20%) | 10 (1.23%) | 6.150 (1.688, 22.409) | 0.002 | 2 (0.35%) | 8 (1.38%) | 4.042 (0.855, 19.117) | 0.057 |
| Any revascularization‡ | 81 (5.44%) | 68 (8.33%) | 1.581 (1.132, 2.209) | 0.007 | 35 (6.06%) | 52 (9.00%) | 1.534 (0.983, 2.393) | 0.058 |
∗DM: diabetes mellitus; CI: confidence interval; TLF: target lesion failure; TLR: target lesion revascularization; MACE: major adverse cardiovascular events; MI: myocardial infraction. †MACE defined as the composite outcome of cardiac death, myocardial infarction, and target vessel revascularization. ‡Any revascularization includes any percutaneous coronary intervention and coronary artery bypass grafting.
Figure 2.
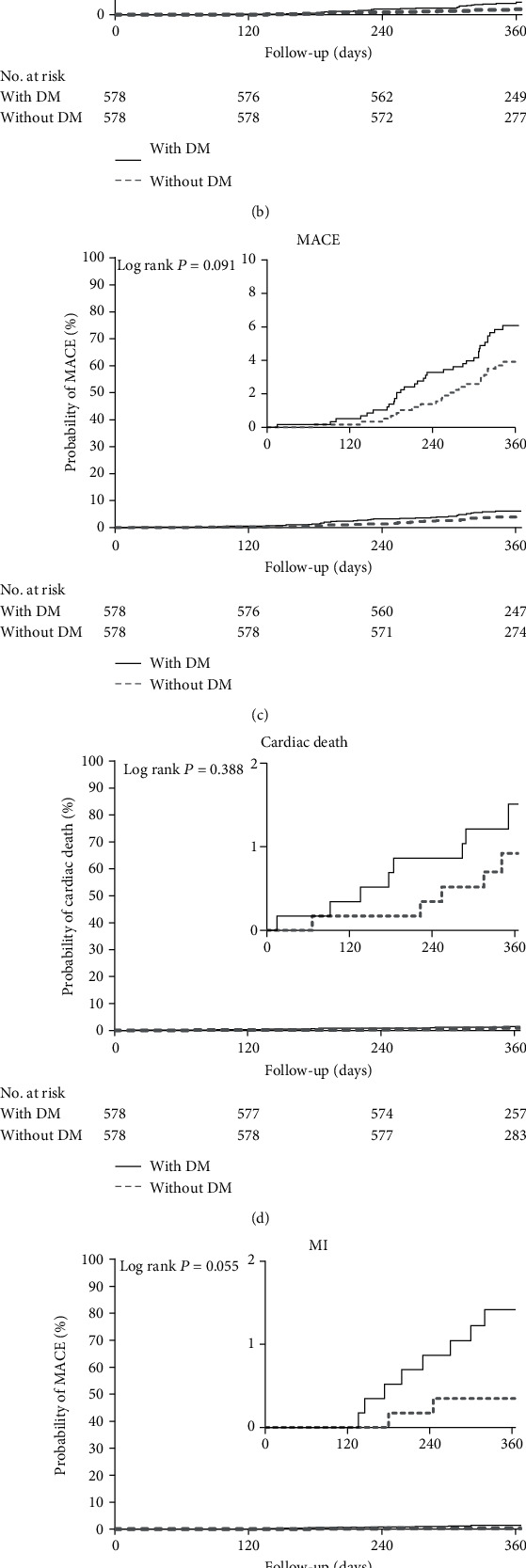
Cumulative risks of the study outcomes in the matched cohort (a), TLF (b), TLR (c), MACE (d), cardiac death (e), And MI (f). In any revascularization, DM: diabetes mellitus; TLF: target lesion failure; TLR: target lesion revascularization; MACE: major adverse cardiovascular events; MI: myocardial infraction. In each panel, the inset shows the same data on an enlarged y axis.
After matching, Kaplan-Meier analysis (Table 3, Figures 2(b)–2(f)) revealed that the cumulative rate of TLR was higher in the DM group at 1 year (with DM: 4.15% vs. without DM: 1.90%; OR, 2.233; 95% CI, 1.083 to 4.602; P = 0.026; log rank P = 0.022). MACE incidence was lower in the non-DM (3.81%) vs. DM (5.88%) group but without statistical difference (OR: 1.580; 95% CI: 0.912-2.735; P = 0.100; log rank P = 0.091). Additionally, in the non-DM vs DM group, there were no statistical differences with regards to incidence of cardiac death (OR: 1.608, 95% CI: 0.523-4.946, P = 0.403; log rank P = 0.388), MI (OR: 4.042, 95% CI: 0.855-19.117, P = 0.057; log rank P = 0.055), or any revascularization (OR: 1.534, 95% CI: 0.983-2.393, P = 0.058; log rank P = 0.052). However, the incidence of MI or any revascularization in the DM group trended upward over time.
3.4. Sensitivity Analysis
In the overall population, TLF exhibited good consistency before and after PSM. The incidence rates of TLR (OR: 2.251, 95% CI: 1.302-3.893), MACE (OR: 1.830, 95% CI: 1.197-2.798), MI (OR: 6.150, 95% CI: 1.688-22.409), and any revascularization (OR: 1.581, 95% CI: 1.132-2.209) in the diabetic group were higher than in the nondiabetic group (all P < 0.05, Table 3). Additionally, binary logistic regression analysis of TLF in the overall population revealed that DM increased TLF risk (OR: 1.721, 95% CI: 1.012-2.927, P = 0.045).
4. Discussion
In this observational study, we evaluated the outcomes of PCI with DCB in diabetic versus nondiabetic patients treated for CAD at three participating centers. Our data suggest that relative to nondiabetic patients, diabetic patients treated with PCI with DCB exhibit higher incidence of TLF and TLR than nondiabetic patients. These findings enhance our current understanding of the safety and efficacy of DCB in all comers in contemporary clinical practice. However, the incidence rates of MACE, cardiac death, and any revascularization in the 2 groups were similar, suggesting that DCB angioplasty can serve as the default treatment option for such patients. Because our results are based on matching propensity scores, our findings are unlikely to result from negative confounding. Moreover, reliability of these findings was validated via sensitivity analysis methods like subgroup analysis.
CAD in the presence of DM has unique characteristics. The higher risk of ISR in patients with DM is secondary to complex pathophysiological mechanism, including endothelial dysfunction, serious vascular inflammation, high activated platelet levels, and higher levels of advanced end products of glycosylation [19, 20]. ISR risk within first-generation DES is higher due to sustained drug release and the inflammatory effects of the polymer [21]. On how to explain the increased risk of stent undersizing or underexpansion and subsequent increase in stent-related complications, a past study found that when compared to non-DM patients, diabetic patients treated with DES have greater residual plaque burden throughout the reference segment [22].
Development of second-generation DES with biocompatible polymers and thinner struts has improved diabetic patients' outcomes upon PCI [23]. However, diabetes remains an independent predictor of major adverse events [24]. In a recent study of 1,919 patients who received PCI with 2 different new-generation DES, diabetic patients had higher target lesion failure (TLF) risk (cardiac death, target vessel myocardial infarction, or ischemia-driven TLR) relative to non-DM patients (7.8% vs. 4.2%, P = 0.002), mainly due to a higher TLR rate (4.5% vs. 2.0%, P = 0.002) [6]. In diabetic patients, DCB has various advantages over DES, including even distribution of the antiproliferative drug along the vessel wall, resulting in better positive remodeling [25, 26]. In addition, local inflammatory reactions in diabetic patients are often severe, and DCB releases higher drug concentrations in the short-term, which is more conducive to inhibit inflammatory reactions. Although the new-generation DES uses more biocompatible or absorbable polymer, the continuous stimulation of the stent struts may cause the local inflammation to persist.
To our knowledge, no studies have investigated the use of DCB-only strategy in diabetic patients with CAD. The PEPCAD IV DM study randomly allocated 84 diabetic patients with significantly stenosed native coronary arteries to a paclitaxel-coated PTCA-balloon SeQuent™ Please, followed by deployment of the cobalt-chromium stent Coroflex™ Blue treatment group or to a paclitaxel-eluting stent Taxus™ Liberté treatment group [11]. At 9 months angiographic follow-up, the primary outcome of mean insegment late lumen loss was 0.37 ± 0.59 mm in the DCB + bare metal stents (BMS) group vs. 0.35 ± 0.63 mm in the DES group (P = 0.89). MACE rates were also similar in both groups (13.3% in DCB vs. 15.4% in DES, P = 0.96). Of note, although pr-dilatation was recommended in the study protocol, it was done in only 31.1% of DCB + BMS-treated patients. Additionally, that study was terminated prematurely due to slow patient enrolment. Further data on treating DM patients with DCB came from the DiabEtic Argentina Registry (DEAR), an observational, prospective, nonrandomized, open-label study [27]. A total of 92 patients at 3 centers were enrolled for DCB angioplasty with the DIOR™ II DCB. Subsequent BMS implantation was performed in 96% of the patients. These patients were compared with diabetic patients enrolled in other clinical trials treated at the same centers with BMS (n = 96) or DES (n = 29) implantation. At 1 year, MACE rates in the DCB-treated group were significantly lower relative to the BMS-treated group and similar to those of the DES-treated group (13.2% for DCB vs. 32.3% for BMS vs. 18.6% for DES). No angiographic measures were collected. Furthermore, a recent comprehensive meta-analysis compared DCB vs. DES outcomes in PCI against de novo CAD in diabetic patients [28]. Three studies involving 378 patients (440 lesions) were included. During a 17.3 ± 11.3 months follow-up, DCB's MACE risk (OR: 0.63, 95% CI: 0.36-1.12, P = 0.11), TLR (OR: 0.51, 95% CI: 0.25-1.06, P = 0.07), binary restenosis (OR: 0.42, 95% CI: 0.09-1.92, P = 0.26), and LLL (mean difference, -0.13 mm, 95% CI: -0.41-0.14, P = 0.34) were similar to those of DES. Finally, it is inferred that in diabetic patients with de novo coronary lesions undergoing PCI, DCBs are associated with similar outcomes relative to first-generation DES, with a signal toward potential benefit in lowering target lesion revascularization. In the early stage, BMS implantation after DCB angioplasty was to avoid safety problems caused by excessive local antiproliferative drug concentration. However, the safety of DES implantation at the same location of DCB angioplasty has been verified in those patients with bailout DES deployment [29, 30]. What needs to be pointed out is that whether it is the combination of DES + DCB or DES only, the incidence rates of ISR, stent thrombosis, and other adverse events that are lower than these in combination of DCB and BMS. In addition, the control groups of these studies are all 1st-generation DES. Although antiproliferative drug of DCB and 1st-generation DES is the same, as far as current clinical practice is concerned, the significance of such a comparison is relatively small.
Here, relative to non-DM patients, PCI with DCB in diabetic patients was associated with higher TLF risk before and after PSM. And binary logistic regression analysis of TLF in the overall population revealed that DM increased TLF risk. Most importantly, the incidences of TLR, MACE, cardiac death, and any revascularization in DCB-treated diabetic patients were much lower relative to DES-treated patients reported in past studies. If the answer is yes, the choice between DCB and DES, even PCI and CABG, should be made a great change.
4.1. Limitation
Because this was a nonrandomized, observational study, it suffers from potential selection and ascertainment bias despite our robust propensity score matching. Additionally, as part of PCI strategy, 32.6% of the patients received DES implantation in a different coronary artery in the same operation, which may affect the occurrence of clinical events. Lastly, this study only evaluated DCB application in patients with coronary heart disease and diabetes and could not be compared to DES over a same period. Thus, a prospective randomized clinical trial comparing the use of DCB and DES in diabetic coronary heart disease patients is highly warranted as it may have important clinical significance.
5. Conclusion
In conclusion, our findings suggest that relative to non-DM patients, DM patients experience higher TLF and TLR rates upon DCB angioplasty. However, there was no substantial increase in the risk of MACE, cardiac death, MI, and any revascularization attributable to DM.
Acknowledgments
This work has not been presented previously. We would like to thank all professors (especially Luosha Zhao, Feifei Zhang, Youyou Du and Guanghui Liu) and study students involved in this study. This work was financially supported by the Medical Science and Technique Research Plan of Henan Province (Provincial and Ministerial Co-construction Project) (Grant No. SB201901027).
Data Availability
The data sets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' Contributions
CGQ, WJL, ZYH, and LP performed the conceptualization. SCP, XW, PQ, YGS, YJZ, SZ, QWS, WCZ, SG, XLW, XLZ, RL, PSZ, and ZSQ performed the data curation. CGQ and LP performed the formal analysis. CGQ and ZYH performed the funding and acquisition. all authors contributed to the investigation. CGQ, WJL, and ZYH performed the methodology. CGQ and ZYH contributed to the project administration, resources, and supervision. CGQ, ZYH, GJS, XFQ, SCP, PSZ, and ZWH contributed to the resources. LP performed the visualization. LP and WJL performed the writing of the original draft: . all authors performed the writing, review, and editing. All authors read and approved final manuscript. Liang Pan and Wenjie Lu share the first authorship.
References
- 1.Stone G. W., Ellis S. G., Cox D. A., et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. The New England Journal of Medicine. 2004;350(3):221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 2.Stone G. W., Rizvi A., Newman W., et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. The New England Journal of Medicine. 2010;362(18):1663–1674. doi: 10.1056/NEJMoa0910496. [DOI] [PubMed] [Google Scholar]
- 3.Morice M. C., Serruys P. W., Sousa J. E., et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. The New England Journal of Medicine. 2002;346(23):1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 4.Palmerini T., Biondi-Zoccai G., Della Riva D., et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Journal of the American College of Cardiology. 2014;63(4):299–307. doi: 10.1016/j.jacc.2013.09.061. [DOI] [PubMed] [Google Scholar]
- 5.Bangalore S., Kumar S., Fusaro M., et al. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow-up from randomised trials. British Medical Journal. 2012;345(1, article e5170) doi: 10.1136/bmj.e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konigstein M., Ben-Yehuda O., Smits P. C., et al. Outcomes among diabetic patients undergoing percutaneous coronary intervention with contemporary drug-eluting stents: analysis from the bionics randomized trial. JACC: Cardiovascular Interventions. 2018;11(24):2467–2476. doi: 10.1016/j.jcin.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Lu W., Zhu Y., Han Z., Wang X., Wang X., Qiu C. Drug-coated balloon in combination with bare metal stent strategy for de novo coronary artery disease: a prisma-compliant meta-analysis of randomized clinical trials. Medicine. 2017;96(12, article e6397) doi: 10.1097/MD.0000000000006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., Lu W., Wang X., et al. Drug-coated balloon angioplasty: predicting outcomes based on different patterns of drug-eluting stent restenosis. The International Journal of Cardiovascular Imaging. 2020;36(2):171–178. doi: 10.1007/s10554-019-01681-y. [DOI] [PubMed] [Google Scholar]
- 9.Lu W., Zhu Y., Han Z., et al. Short-term outcomes from drug-coated balloon for coronary _de novo_ lesions in large vessels. Journal of Cardiology. 2019;73(2):151–155. doi: 10.1016/j.jjcc.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Jeger R. V., Farah A., Ohlow M. A., et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (basket-small 2): 3-year follow-up of a randomised, non-inferiority trial. The Lancet. 2020;396(10261):1504–1510. doi: 10.1016/S0140-6736(20)32173-5. [DOI] [PubMed] [Google Scholar]
- 11.Ali R. M., Degenhardt R., Zambahari R., et al. Paclitaxel-eluting balloon angioplasty and cobalt-chromium stents versus conventional angioplasty and paclitaxel-eluting stents in the treatment of native coronary artery stenoses in patients with diabetes mellitus. EuroIntervention. 2011;7:K83–K92. doi: 10.4244/EIJV7SKA15. [DOI] [PubMed] [Google Scholar]
- 12.Valgimigli M., Bueno H., Byrne R. A., et al. 2017 esc focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with Eacts: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (esc) and of the European Association for Cardio-Thoracic Surgery (Eacts) European Heart Journal. 2018;39(3):213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 13.Neumann F. J., Sousa-Uva M., Ahlsson A., et al. 2018 Esc/Eacts guidelines on myocardial revascularization. European Heart Journal. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 14.Jeger R. V., Eccleshall S., Wan Ahmad W. A., et al. Drug-coated balloons for coronary artery disease: third report of the international Dcb consensus group. JACC: Cardiovascular Interventions. 2020;13(12):1391–1402. doi: 10.1016/j.jcin.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 15.Huber M. S., Mooney J. F., Madison J., Mooney M. R. Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. The American Journal of Cardiology. 1991;68(5):467–471. doi: 10.1016/0002-9149(91)90780-O. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Garcia H. M., McFadden E. P., Farb A., et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation. 2018;137(24):2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- 17.Mansueto G., Costa D., Capasso E., et al. The dating of thrombus organization in cases of pulmonary embolism: an autopsy study. BMC Cardiovascular Disorders. 2019;19(1):p. 250. doi: 10.1186/s12872-019-1219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thygesen K., Alpert J. S., Jaffe A. S., et al. Fourth universal definition of myocardial infarction (2018) Journal of the American College of Cardiology. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 19.Aronson D., Bloomgarden Z., Rayfield E. J. Potential mechanisms promoting restenosis in diabetic patients. Journal of the American College of Cardiology. 1996;27(3):528–535. doi: 10.1016/0735-1097(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 20.Kereiakes D. J., Young J. J. Percutaneous coronary revascularization of diabetic patients in the era of drug-eluting stents. Reviews in Cardiovascular Medicine. 2005;6(Supplement 1):S48–S58. [PubMed] [Google Scholar]
- 21.Maluenda G., Lemesle G., Waksman R. A critical appraisal of the safety and efficacy of drug-eluting stents. Clinical Pharmacology and Therapeutics. 2009;85(5):474–480. doi: 10.1038/clpt.2009.8. [DOI] [PubMed] [Google Scholar]
- 22.Sakata K., Waseda K., Kume T., et al. Impact of diabetes mellitus on vessel response in the drug-eluting stent era. Circulation. Cardiovascular Interventions. 2012;5(6):763–771. doi: 10.1161/CIRCINTERVENTIONS.111.962878. [DOI] [PubMed] [Google Scholar]
- 23.Silber S., Serruys P. W., Leon M. B., et al. Clinical outcome of patients with and without diabetes mellitus after percutaneous coronary intervention with the resolute zotarolimus-eluting stent: 2-year results from the prospectively pooled analysis of the international global resolute program. JACC: Cardiovascular Interventions. 2013;6(4):357–368. doi: 10.1016/j.jcin.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Kedhi E., Gomes M. E., Lagerqvist B., et al. Clinical impact of second-generation everolimus-eluting stent compared with first-generation drug-eluting stents in diabetes mellitus patients: insights from a nationwide coronary intervention register. JACC: Cardiovascular Interventions. 2012;5(11):1141–1149. doi: 10.1016/j.jcin.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Kleber F. X., Schulz A., Waliszewski M., et al. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clinical Research in Cardiology. 2015;104(3):217–225. doi: 10.1007/s00392-014-0775-2. [DOI] [PubMed] [Google Scholar]
- 26.Ann S. H., Balbir Singh G., Lim K. H., Koo B. K., Shin E. S. Anatomical and physiological changes after paclitaxel-coated balloon for atherosclerotic de novo coronary lesions: serial Ivus-Vh and Ffr study. PLoS One. 2016;11(1, article e0147057) doi: 10.1371/journal.pone.0147057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mieres J., Fernandez-Pereira C., Risau G., et al. One-year outcome of patients with diabetes mellitus after percutaneous coronary intervention with three different revascularization strategies: results from the diabetic Argentina registry (Dear) Cardiovascular Revascularization Medicine. 2012;13(5):265–271. doi: 10.1016/j.carrev.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Megaly M., Ali A., Abraham B., et al. Outcomes with drug-coated balloons in percutaneous coronary intervention in diabetic patients. Cardiovascular Revascularization Medicine. 2020;21(1):78–85. doi: 10.1016/j.carrev.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Cortese B., Silva Orrego P., Agostoni P., et al. Effect of drug-coated balloons in native coronary artery disease left with a dissection. JACC: Cardiovascular Interventions. 2015;8(15):2003–2009. doi: 10.1016/j.jcin.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 30.Mitomo S., Jabbour R. J., Mangieri A., et al. Mid-term clinical outcomes after bailout drug-eluting stenting for suboptimal drug-coated balloon results: insights from a Milan registry. International Journal of Cardiology. 2018;263:17–23. doi: 10.1016/j.ijcard.2018.04.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.


