Abstract
Background
Nasal nitric oxide (nNO) levels in allergic rhinitis (AR), healthy people or nonallergic rhinitis (NAR) have shown contradicting results in previous studies. By meta‐analysis, we reviewed studies that measured nNO in AR patients to assess nNO's ability to discriminate AR from healthy people or NAR.
Methods
We systematically searched PubMed, Cochrane, Embase, Ovid, Web of Science, Wanfang Data, CNKI until December 15, 2020. Differences were expressed as standardized mean differences (SMD) with 95% confidence interval (CI), by random‐effects method.
Results
A total of 10 original studies with 561 AR patients, 327 healthy controls, 123 NAR patients were included in the narrative synthesis and 9 studies in the meta‐analysis. nNO in AR was significantly increased compared with healthy controls (SMD: 0.989; 95% CI: 0.402, 1.576; p = .001) or NAR (SMD: 0.680; 95% CI: 0.101, 1.259; p = 0.021). However, subgroup analysis based on measuring process and patient characteristics showed that no significant differences were detected in nNO between AR patients with nasal polyps or sinusitis or marked ostial obstruction and healthy controls.
Conclusions
nNO is a potential indicator for recognizing AR. Nasal polyps, sinusitis and marked ostial obstruction should be considered before nNO is applied to detect AR.
Keywords: allergic rhinitis, meta‐analysis, nasal nitric oxide
Graphical abstract
The meta analysis shows that nasal nitric oxide (nNO) in allergic rhinitis (AR) is significantly higher than healthy people and nonallergic rhinitis. However sinusitis, nasal polysis and marked ostial obstruction should be taken into consideration before nNO is applied to detect AR.

1. INTRODUCTION
Allergic rhinitis (AR) is a disease characterized by sneezing, itching, nasal congestion, and rhinorrhea following exposure of allergens. AR will detriment patients' efficiency of work and study, decline their quality of life and impact on asthma control level in AR patients combined with asthma, causing heavy healthcare economic burden. 1 Allergic Rhinitis and its Impact on Asthma (ARIA) states that golden standard in AR diagnosis include demonstration of skin‐prick testing for allergens or the serum immunoglobulin E (IgE) tests. 2
Nitric oxide (NO) is a free radical gas, playing an important role in many biological mechanisms. In respiratory system, NO is continuously released from upper and lower airway and soaringly released following proinflammatory cytokines and stimuli inducement. Fractional exhaled nitric oxide (FeNO) has been used as a noninvasive tool to reflect eosinophilic inflammation in lower airway diseases. For example, high level of FeNO suggests possibility of asthma. 3 Moreover, FeNO is also a good indicator to monitor glucocorticoid treatment.4, 5, 6 Similarly, several studies indicated that nasal nitric oxide (nNO) could be used to predict AR.7, 8, 9 Contradictorily, some studies suggested that nNO in AR was not significantly different from healthy people.10, 11 Therefore, we undertook a systematic review and meta‐analysis on the nNO's ability to discriminate AR from healthy controls or nonallergic rhinitis (NAR).
2. METHODS
2.1. Data sources and searches
Our methods have been described detailly in the published protocol (PROSPERO registration: CRD42020160578). We systematically searched following databases until December 15, 2020: PubMed, Cochrane, Embase, Ovid, Web of Science, Wanfang Data, CNKI in accordance with the Preferred Reporting Items for Systematic Review and Meta‐Analyses for Diagnostic Test Accuracy. The search strategy used the following terms: “allergic rhinitis” AND “nasal nitric oxide” found within all fields. There was no constraint on the publication language or study design during searching.
2.2. Study selection
Studies were included if they measured nNO in AR patients and healthy controls with information about nNO analyzer, sampling technique, sampling rate and AR diagnostic criteria. Studies were excluded if any of the following were presented (1) number of AR patients was less than 10; (2) the procedure of nNO measurement did not follow American Thoracic Society (ATS)/ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide, 2005; 12 (3) AR diagnosis did not meet criteria described in ARIA guidelines.2, 12
2.3. Selection process
After duplicate article exclusion, B.W. and Z.W., two of the authors, independently analyzed the found articles and carried out data extraction. Information like clinical characteristics of subjects, NO analyzer, NO sampling rate, sampling technique, AR diagnosis, nNO value and so forth was collected. If disagreement came up, a third investigator (Y.L.) was consulted, decision would be finally made by consensus.
2.4. Quality assessment
The Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool was used to evaluate the methodological quality of each study. 13 The tool was explicitly developed to estimate the quality of diagnostic test from four domains (patient selection, index test, reference standard and flow/timing). Each domain was graded as low, high, or unclear risk.
2.5. Data synthesis and analysis
Studies reporting nNO values with mean and SD were included in the meta‐analysis. Otherwise, they would be excluded from meta‐analysis but still in qualitative synthesis. The reported nNO concentration (ppb) was converted into nl/min by formula ppb × sampling rate (L/min) to keep consistent between studies using different sampling rates. 14 Data were analyzed using STATA 16.0. Differences between AR and healthy controls or NAR were expressed as standardized mean differences (SMD) with 95% confidence interval (CI). Random‐effects models were used to calculate summary effects across the studies. We also assessed studies for heterogeneity by χ 2 Cochran's Q test and I 2 statics. In detail, I 2 = 0% indicates no heterogeneity; 25%, low; 25%–50%, moderate; and more than 50%, high heterogeneity. 15 Sensitivity analysis was performed by eliminating studies with high risk of bias. Subgroup analysis was applied in terms of patient characteristics and index test characteristics. Publication bias was assessed by funnel plot (SMD on the x‐axis against 1/SE of the SMD on the y‐axis), Egger test and the Begg and Mazumdar test. A p < .10 is considered statistically significant.16, 17
3. RESULTS
3.1. Study selection
In total, 1862 records were identified through a generalized search of all publications related to AR and nNO. After removing duplicates, 1092 records were screened by title and abstract. After screening titles and abstracts, 132 potentially eligible studies were selected for full review. Finally, 10 original research studies7, 8, 11, 18, 19, 20, 21, 22, 23, 24 were included in the narrative synthesis and 9 studies7, 8, 18, 19, 20, 21, 22, 23, 24 in the meta‐analysis (Figure 1).
Figure 1.
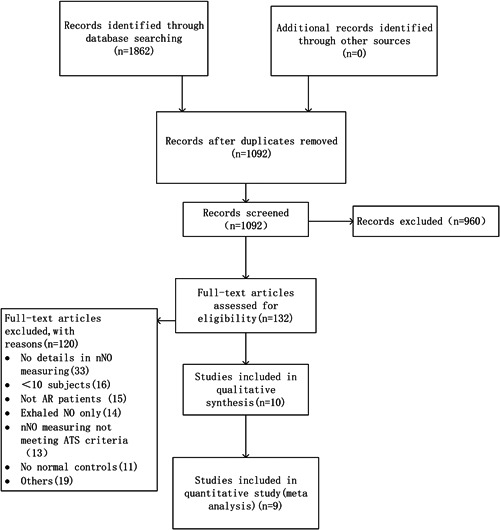
Summary of evidence search and selection
3.2. Study characteristics
The main demographic, study, index tests characteristics were presented in Tables 1 and 2. All studies included were case‐control studies. A total of 1011 participants were enrolled, 561 AR patients, 327 normal controls, 123 NAR patients. The age ranged from 4 to 50 years old. A total of 35.4%–73.1% was male gender. AR diagnosis was consistent with the guideline of ARIA, 2 including seasonal AR and perennial allergic rhinitis (PAR) types. The diagnostic criteria were a typical history of allergic symptoms and diagnostic tests including skin prick tests or the blood specific IgE. Although some patients were not mentioned the status of AR,7, 8, 20, 22, 23 the rest AR patients were clear stated symptomatic or not. The prevalence of asthma was 42.7%–100% in two studies,20, 22 while asthma patients were excluded in the rest studies. Most studies prohibited AR medication before measuring nNO, which included corticosteroids, antihistamines, etc.7, 8, 18, 19, 20, 21, 22, 23, 24
Table 1.
Patient and study characteristics
| Study, year | Location | Patients, total | AR status | Ages | Gender, n male (%) | Asthma (%) | Medication | |
|---|---|---|---|---|---|---|---|---|
| Henriksen (1999) 11 | Norway | AR 46 (Seasonal19, Seasonal + perennial27), HC 12 | Symptomatic | AR: 16.4 (13–20); HC: 17.8 (16–19) | 29/58 (50.0%) | 0 | Antihistamines | |
| Palm (2003) 18 | Sweden | AR 18, HC 18 | Symptomatic | AR: 32 (21–50); HC: 30 (20–46) | 18/36 (50%) | 0 | No steroid, no antihistamines | |
| Makris (2011) 19 | Greece | SAR 26, HC 15 | Symptomatic | AR 28.4 (16–47); HC 37.1 (27–56); | 22/41 (53.7%) | 0 | No corticosteroids, no antihistamines | |
| Lee (2012) 7 | Korea | AR 35, patients with deviated nasal septa as HC 34 | Not mentioned | AR: 22.7 ± 8.7; HC: 26.9 ± 11.0 | 42/69 (60.9%) | 0 | No nasal medication | |
| Suojalehto (2014) 20 | Finland | AR 89, NAR 44, HC 42 | Not mentioned | AR: 32.9 ± 1.3; NAR: 33.2 ± 1.5; HC: 33.5 ± 1.8 | 62/175 (35.4%) | 42.7 | No nasal steroids | |
| Nesic (2016) 8 | Serbia | AR 23, HC 10 | Not mentioned | AR: 33.4 ± 11.1; HC: 33.3 ± 8.4 | 18/33 (55%) | 0 | No steroids | |
| Hou (2018) 21 | China | AR 75, HC 31 | Symptomatic | AR: 36.24 ± 10.96; HC: 35.32 ± 12.11 | 65/106 (61.3%) | 0 | No nasal steroids, no antihistamine | |
| Mu (2019) 22 | China | AR concomitant with asthma 65, HC 40 | Not mentioned | AR 7.2 ± 1.9; HC7.2 ± 0.7 | 68/105 (64.8%) | 100 | Not mentioned | |
| Wen (2019) 23 | China | PAR 90, HC 79 | Not mentioned | AR: 9.7 ± 2.4; HC: 10.1 ± 1.9 | 116/169 (68.6%) | 0 | No corticosteroids, no leukotriene receptor antagonist | |
| Liu (2020) 24 | China | AR 94, NAR 79, patients diagnosed with pituitary tumor or lateral skull base mass as HC 46 | Symptomatic | AR: 30.5 ± 8.9; NAR: 37.7 ± 14.1; HC: 30.7 ± 5.5 | 160/219 (73.1%) | 0 | No corticosteroids | |
Note: Data is expressed in mean ± SD or mean (range).
Abbreviations: AR, allergic rhinitis; HC, healthy controls; n, number; NAR, nonallergic rhinitis; PAR, perennial allergic rhinitis; SAR, seasonal allergic rhinitis.
Table 2.
Index test characteristics
| Study, year | Analyzer | Flow rate (L/min) | Method | AR diagnosis |
|---|---|---|---|---|
| Henriksen (1999) 11 | LR200; Logan Research, Rochester, UK | 0.25 | BH | Reported AR and had a positive allergy screening blood test with sensitization to seasonal allergens |
| Palm (2003) 18 | Aerocrine AB, Stockholm, Sweden | 0.5, 3, 9 | ER | Ongoing, symptomatic and reportedly SPT positive no steroid‐treated birch pollen AR |
| Makris (2011) 19 | ECOmedics CLD88sp | 3 | ER | The diagnosis was based on the typical clinical symptoms and the documentation of sensitization with SPTs |
| Lee (2012) 7 | Sievers 280i (GE Analytical Instruments, Boulder, CO) | 0.7 | ER | Diagnosed with AR by history‐taking and multiple antigen simultaneous tests or skin tests |
| Suojalehto (2014) 20 | NIOX (Aerocrine AB, Solna, Sweden) | 0.3 | BH | At least one positive SPT and relevant rhinitis symptoms to that allergen |
| Nesic (2016) 8 | NIOX MINO (Aerocrine AB, Solna, Sweden) | 0.3 | BH | A history of more than 3 years of AR and were positive for serum allergen‐specific IgE against house dust mite or pollen |
| Hou (2018) 21 | NIOX MINO (Aerocrine AB, Solna, Sweden) | 0.3 | ER | At least 1 positive pollen IgE measurement and the presence of AR symptoms |
| Mu (2019) 22 | sunvou‐SU‐02E Analyzer | 0.3 | ER | Have the above clinical manifestations (symptoms, signs), and also have a positive result of any one of the 2 SPTs or serum specific IgE tests |
| Wen (2019) 23 | NIOX MINO (Aerocrine AB, Solna, Sweden) | 0.3 | BH | Defined according to the ARIA guidelines |
| Liu (2020) 24 | NIOX (Aerocrine AB, Sweden) | 3 | ER | Defined as a history of any of typical clinical symptoms with a positive SPT or serum specific IgE |
Abbreviations: AR, allergic rhinitis; ARIA, allergic rhinitis and its impact on asthma; BH, breath hold; ER, exhalation against resistance; IgE, immunoglobulin E; SPT, skin prick test.
Different brands of NO analyzers were used in the included studies: NIOX, ECOmedics CLD88sp, LR200, NIOX MINO, Sievers 280i, Sunvou. Among them, NIOX MINO and Sunvou are electrochemical analyzers while the rest are chemiluminescence analyzers. During measurement of nNO, participants were required to obtain velum closure while gas was sampled from one nostril. Several methods including holding breath, exhaling against resistance can be achieved to ensure velum closure. Sampling flow rate is required to range from 0.25 to 3 L/min according to ATS recommendation. 12
3.3. Quality assessment
The QUADAS‐2 tool was used to evaluate the internal and external validity of each study. 13 The overall quality assessment was shown in the Figures 2 and 3. Because all studies recruited AR patients and healthy controls separately, the domain of patient selection had a high risk. Most studies did not mention the order of nNO measurement and AR diagnosis measurement. However, the domain of Index Test and Reference Standard had mostly low risk because those measurements were objective, a lack of blinding when evaluating these test results represented a smaller risk of bias. As for Flow and Timing domain, most studies did not mention the detailing time of nNO measurement and AR diagnosis measurement, so they mostly had unclear risk.
Figure 2.
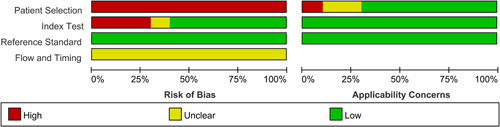
Methodological quality graph of each study with QUADAS‐2 tool for the 10 included studies. QUADAS‐2, Quality Assessment of Diagnostic Accuracy Studies‐2
Figure 3.
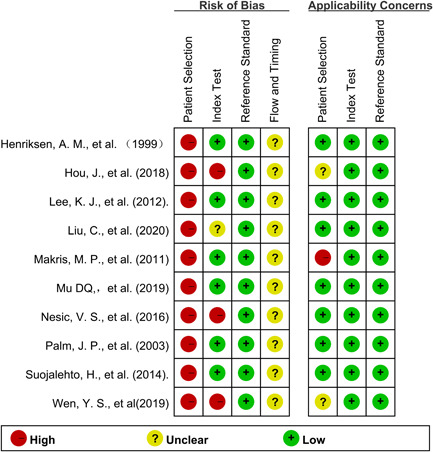
Methodological quality summary of each study with QUADAS‐2 tool for the 10 included studies. QUADAS‐2, Quality Assessment of Diagnostic Accuracy Studies‐2
3.4. Ability of nNO to discriminate AR from healthy controls or NAR
Because only 1 study reported nNO values in media (range), 11 9 studies were included for meta‐analysis,7, 8, 18, 19, 20, 21, 22, 23, 24 which involved 515 AR patients, 315 healthy controls, and 123 NAR patients (Table 3). As shown in the Figure 4, AR patients represented significantly increased nNO compared with healthy controls (SMD: 0.989; 95% CI: 0.402, 1.576; p = .001). The heterogeneity of this outcome was significant (I 2 = 92.7%) and it did not decrease after individually eliminating each study. It could be relevant with analyzer types, sampling rates, sampling techniques or population characteristics.
Table 3.
Summary of studies measuring nNO in AR, NAR, healthy controls groups following ATS standard
| Study | AR | HC | NAR | |||
|---|---|---|---|---|---|---|
| Subjects n | Mean ± SD nl/min | Subjects n | Mean ± SD nl/min | Subjects n | Mean ± SD nl/min | |
| Henriksen et al. (1999) 11 , a | 46 | Median (range): 276.25 (137.75–512.75) | 12 | Median (range): 253.5 (122.5–408) | ||
| Palm et al. (2003) 18 | 18 | 160 ± 75 (flow: 0.5 L/min); 211 ± 103 (flow: 3 L/min) | 18 | 153 ± 36 (flow: 0.5 L/min); 198 ± 45 (flow: 3 L/min) | ||
| Makris et al. (2011) 19 | 26 | 4162.4 ± 2895.9 (in pollen season); 3045.2 ± 1930.2 (out of pollen season) | 15 | 2324.9 ± 538.7 | ||
| Lee et al. (2012). 7 | 35 | 271.6 ± 83.4 | 34 | 193.5 ± 61.7 | ||
| Suojalehto et al. (2014). 20 | 89 | 292.1 ± 82.8 | 42 | 253.3 ± 57.5 | 44 | 260.4 ± 86.5 |
| Nesic et al. (2016) 8 | 23 | 209.0 ± 40.8 | 10 | 138.2 ± 40.0 | ||
| Hou et al. (2018) 21 | 75 | 61.7 ± 40.5 | 31 | 45.2 ± 14.5 | ||
| Mu et al. (2019) 22 | 65 | 236.4 ± 126 | 40 | 133.2 ± 38.4 | ||
| Wen et al. (2019) 23 | 90 | 229.6 ± 45.6 | 79 | 117.0 ± 29.2 | ||
| Liu et al. (2020) 24 | 94 | 2817 ± 1005 | 46 | 2550 ± 309 | 79 | 2010 ± 564 |
Abbreviations: AR, allergic rhinitis; ATS, American Thoracic Society; HC, healthy controls; NAR, nonallergic rhinitis.
Studies not included in meta‐analysis due to the data not represented with mean ± SD.
Figure 4.
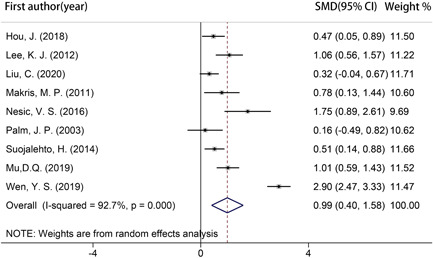
Forest plot showing the standardized mean differences in mean nasal nitric oxide between allergic rhinitis patients and healthy controls
Four studies8, 21, 23, 24 reported cut‐off values to discriminate between AR and healthy controls with their sensitivity and specificity (Table 4). Nesic et al. 8 and Wen et al. 23 used the same analyzer (NIOX MINO), the same flow rate (0.3 L/min) and the same method (BH), finally their nNO cut‐off value came out to be 169.4 and 161.4 nl/min, respectively. Their sensitivity was 83%, 100% and specificity was 80%, 94.9%, respectively.
Table 4.
Studies presenting with cut‐off values for discriminating allergic rhinitis patients and healthy controls
| Study | Analyzer | Flow rate (L/min) | Method | total subjects n | nNO cut‐off (nL/min) | sensitivity (%) | specificity (%) |
|---|---|---|---|---|---|---|---|
| Nesic et al. (2016) 8 | NIOX MINO | 0.3 | BH | AR 23, HC 10 | 169.4 | 83 | 80 |
| Hou et al. (2018) 21 | NIOX MINO | 0.3 | ER | AR 75, HC 31 | 51.9 | 54.7 | 67.7 |
| Wen et al. (2019) 23 | NIOX MINO | 0.3 | BH | AR 90, HC 79 | 161.4 | 100 | 94.9 |
| Liu et al. (2020) 24 | NIOX | 3 | ER | AR 94, HC 46 | 2541 | 53.2 | 54.3 |
Abbreviations: AR, allergic rhinitis; BH, breath hold; ER, exhalation against resistance; HC, healthy controls; nNO, nasal nitric oxide.
Two studies20, 24 reported nNO values of AR and NAR patients. As showed in the Figure 5, nNO value of AR patients was significantly higher than NAR (SMD: 0.680; 95% CI: 0.101, 1.259; p = .021). There was a high degree of heterogeneity (I 2 = 82.7%; p = .016).
Figure 5.
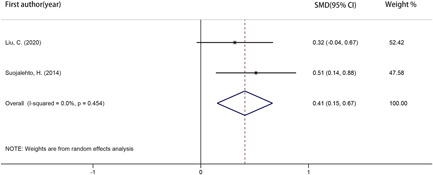
Forest plot showing the standardized mean differences in mean nasal nitric oxide between allergic rhinitis patients and nonallergic rhinitis patients
3.5. Sensitivity analysis
After quality assessment of all studies, there were no studies of low risk bias to perform sensitivity analysis.
3.6. Subgroup analysis
Subgroup analysis was performed for studies measuring nNO with different measuring process and different patient characteristics. It was detailly shown in the Table 5.
Table 5.
Subgroup analyses
| N of studies | N of participants | Effect size SMD (95% CI) in nNO | |
|---|---|---|---|
| Studies of the different kinds of analyzers | |||
| Stationary | 5 | 262 AR; 155 HC | SMD: 0.554; 95% CI: 0.260, 0.849; p = .010; I 2 = 46.3%, p = .114 |
| Handheld | 4 | 253 AR; 160 HC | SMD: 1.526; 95% CI: 0.361, 2.691; p = .010; I 2 = 95.6%, p = .000 |
| Studies of the different sampling techniques | |||
| BH | 3 | 202 AR; 131 HC | SMD: 1.717; 95% CI: 0.029, 3.404; p = .046; I 2 = 97.0%, p = .000 |
| ER | 6 | 313 AR; 184 HC | SMD: 0.638; 95% CI: 0.337, 0.938; p = .000; I 2 = 57.3%, p = .039 |
| Studies of the different sampling flow rates | |||
| 3 L/min | 3 | 138 AR; 79 HC | SMD: 0.374; 95% CI: 0.092, 0.656; p = .009; I 2 = 0.0%, p = .369 |
| 0.3 L/min | 5 | 342 AR; 202 HC | SMD: 1.316; 95% CI: 0.368, 2.264; p = .007; I 2 = 95.3%, p = .000 |
| Studies of the AR patients with/without asthma | |||
| With asthma | 1 | 65 AR; 40 HC | SMD: 1.011; 95% CI: 0.593, 1.428; p = .000; I 2 not applicable, p not applicable |
| Without asthma | 8 | 450 AR; 275 HC | SMD: 0.987; 95% CI: 0.310, 1.665; p = .004; I 2 = 93.6%, p = .000 |
| Studies of the AR patients with/without rhinitis symptoms | |||
| Having rhinitis symptoms | 4 | 213 AR; 110 HC | SMD: 0.404; 95% CI: 0.169, 0.638; p = .001; I 2 = 0.0%, p = .545 |
| Not sure having rhinitis symptoms | 5 | 302 AR; 205 HC | SMD: 1.438; 95% CI: 0.529, 2.346; p = .002; I 2 = 94.5%, p = .000 |
| Studies of the AR patients with/without nasal polyps | |||
| With nasal polyps | 1 | 10 AR; 42 HC | SMD: −0.215; 95% CI: −0.905, 0.476; p = .543; I 2 not applicable, p not applicable |
| Without nasal polyps | 5 | 361 AR; 208 HC | SMD: 1.195; 95% CI: 0.200, 2.189; p = .019; I 2 = 96%, p = .000 |
| Studies of the AR patients with/without sinusitis | |||
| With sinusitis | 2 | 77 AR; 125 HC | SMD: 0.972; 95% CI: −3.627, 5.571; p = .679; I 2 = 99.3%, p = .000 |
| Without sinusitis | 5 | 246 AR; 161 HC | SMD: 1.102; 95% CI: 0.689, 1.515; p = .000; I 2 = 70.5%, p = .009 |
| Studies of AR patients excluding/not excluding smoking | |||
| Excluding smoking | 4 | 142 AR; 74 HC | SMD: 0.723; 95% CI: 0.174, 1.272; p = .010; I 2 = 67.2%, p = .027 |
| Not excluding smoking | 5 | 373 AR; 241 HC | SMD: 1.157; 95% CI: 0.264, 2.049; p = .011; I 2 = 95.8%, p = .000 |
| Studies of AR patients with/without marked ostial obstruction | |||
| With marked ostial obstruction | 2 | 41 AR; 73 HC | SMD: −0.668; 95% CI: −1.498, 0.161; p = .114; I 2 = 72.5%, p = .057 |
| Without marked ostial obstruction | 2 | 123 AR; 73 HC | SMD: 0.950; 95% CI: 0.252, 1.647; p = .2016; I 2 = 79.3%, p = .028 |
Abbreviations: AR, allergic rhinitis; BH, breath hold; CI, confidence interval; ER, exhalation against resistance; HC, healthy controls; N, number; nNO, nasal nitric oxide; SMD, standardized mean differences.
Comparison 1: subgroup analysis by different NO analyzer types
In the subgroup analysis, we analyzed the effects of NO analyzer types on nNO's ability to discriminate AR. There was no evidence for different effects of NO analyzer types between subgroups.
Comparison 2: subgroup analysis by different NO sampling techniques
In the subgroup analysis, we analyzed the effects of NO sampling techniques on nNO's ability to discriminate AR. There was no evidence for different effects of NO sampling techniques between subgroups.
Comparison 3: subgroup analysis by different NO sampling flow rates
In the subgroup analysis, we analyzed the effects of NO sampling flow rates on nNO's ability to discriminate AR. There was no evidence for different effects of NO sampling flow rates between subgroups.
Comparison 4: subgroup analysis by AR patients with/without asthma
In the subgroup analysis, we analyzed the concomitant of asthma on nNO's ability to discriminate AR. There was no evidence for different effects of asthma between subgroups.
Comparison 5: subgroup analysis by AR patients with/without rhinitis symptoms
In the subgroup analysis, we analyzed the existence of rhinitis symptoms on nNO's ability to discriminate AR. There was no evidence for different effects of rhinitis symptoms between subgroups.
Comparison 6: subgroup analysis by AR patients with/without nasal polyps
In the subgroup analysis, we analyzed the existence of nasal polyps on nNO's ability to discriminate AR. There was evidence for different effects of nasal polyps between subgroups. No significant differences of nNO were detected between AR patients with nasal polyps and healthy controls.
Comparison 7: subgroup analysis by AR patients with/without sinusitis
In the subgroup analysis, we analyzed concomitant of sinusitis on nNO's ability to discriminate AR. To be more specific, sinusitis meant acute unilateral maxillary sinusitis or sinus inflammation in the studies enrolling AR patients with sinusitis.23, 24 There was evidence for different effects of sinusitis between subgroups. No significant differences of nNO were detected between AR patients with sinusitis and healthy controls.
Comparison 8: subgroup analysis by AR patients excluding/not excluding smoking
In the subgroup analysis, we analyzed smoking on nNO's ability to discriminate AR. There was no evidence for different effects of smoking between subgroups.
Comparison 9: subgroup analysis by AR patients with/without marked ostial obstruction
In the subgroup analysis, we analyzed the existence of marked ostial obstruction on nNO's ability to discriminate AR. The ostial obstruction was measured through a semiquantitative computed tomography scoring system or active anterior rhinomanometry.20, 21There was evidence for different effects of marked ostial obstruction between subgroups. No significant differences of nNO were detected between AR patients with marked ostial obstruction and healthy controls.
3.7. Publication bias
Publication bias was detected by visual examination to funnel plot (Figure 6). While the Egger test (p = .7251) and the Begg and Mazumdar test (p = .1179) indicated no publication bias.
Figure 6.
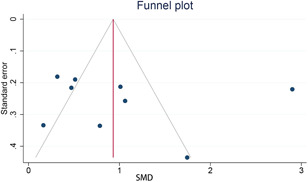
Funnel plot for studies evaluating nasal nitric oxide in allergic rhinitis patients and healthy controls
4. DISCUSSION
In this systematic review and meta‐analysis, we have shown that nNO in AR patients was significantly higher than healthy controls and NAR. The nNO measurement in accordance with ATS recommended standardized procedure fits for kids older than 4 years old and adults who are able to cooperate with sampling techniques to ensure velum closure. Subgroup analysis showed that when AR patients were concomitant with nasal polyps, sinusitis or marked ostial obstruction, it was hard for nNO to detect them.
Gustafsson's group found endogenous NO was present in the exhaled air of humans and other mammals in 1991. 25 NO is synthesized from l‐arginine by NO synthase (NOS) in the respiratory system, which has three isoforms: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). 26 iNOS is induced by proinflammatory cytokines and/or bacterial products in almost every epithelial cell, while the other two isoforms are constitutively expressed.6, 26, 27 So the level of exhaled NO is usually increased and regulated by iNOS enzyme. 28 Studies discovered that NO in the exhaled air of patients with asthma was higher than healthy controls.29, 30 Now, serving as an indicator of eosinophil inflammation of the lower respiratory tract, high level of FeNO suggests possibility of asthma in National Institute for Health and Care Excellence (NICE): Clinical Guidelines. 3 Furthermore, FeNO has been used to guide inhaled corticosteroid treatment in asthma patients and chronic cough patients.5, 31, 32 Compared with FeNO value in lower airway, nNO has a far higher level in upper airway, which had been proofed by Alving in 1993. 33 Most studies indicated that the main production of nNO was in paranasal sinuses. As the epithelium of sinuses produces a large amount of NO,34, 35, 36 it may explain the reason why nNO value is far higher than FeNO.
There are two different ways of measuring the fractional concentration of nasal NO. If the measurement is obtained by nasal exhalation, it is called nasal FeNO. If the measurement is obtained by transnasal flow in series, it is called nNO. 6 Our study only focused on nNO because it is recommended by ATS. 12 In this meta‐analysis, we found that nNO in AR was significantly higher than healthy controls or NAR. It was consistent with the finding in eosinophilic chronic rhinosinusitis that higher levels of nasal FeNO may reflect the persistence of eosinophilic inflammation in sinus mucosa with concomitant iNOS upregulation. 37 However, some studies reported nNO was not statistically different in AR compared with healthy controls. Swelling of nasal mucosa may lead to occluded sinus ostia and then prevent NO distributing to nasal cavity, which may explain the contradicting results. Wen et al. 23 found that nNO level in PAR patients with acute maxillary sinusitis was negatively correlated to total nasal resistance. Hou et al. 21 found that nNO in AR patients with nasal obstruction score more than 7 was significantly decreased compared with healthy subjects, while nNO in AR patients with nasal obstruction score less than 7 was significantly increased compared with healthy subjects. 21 These studies and our finding may explain the reason of current controversial study results. We did subgroup analysis for different patient characteristics and measuring process. Subgroup analysis in different patient characteristics showed that nNO could not detect AR patients concomitant with nasal polys, sinusitis or marked ostial obstruction. The rest factors, including different analyzer types, sampling flow rates, sampling techniques, concomitant asthma, rhinitis symptoms and smoking, do not impair nNO's ability in discriminating AR from healthy controls.
Using the same analyzer, same flow rate and same method, Nesic et al. 8 and Wen et al. 23 reported similar cut‐off value (169.4 and 161.4 nl/min, respectively) with good specificity and sensitivity, which means experts could set a specific cut‐off value under single specific nNO measuring procedure for AR screening.
Our study presented with some limitations. First, high degree heterogeneity significantly influences our results. Although it is hard to determine the exact source of heterogeneity, here are some possible sources: included studies were held in different countries and different inclusion and exclusion criteria were set, leading to diverse demographic and clinical characteristics; few studies gave detailed description on AR patients such as their AR symptoms; kids were involved in meta‐analysis, while nNO were age‐related in kids younger than 12 years old. 38 Second, all included studies were case‐control designed, studies reporting cut‐off values did not prespecify threshold, both causing it potentially overestimate the accuracy of a diagnostic test.
All considered, our meta‐analysis found that nNO in AR patients are significantly higher than healthy controls and NAR. nNO serves as a potential indicator for discriminating AR. However, nasal polyps, sinusitis and marked ostial obstruction are supposed to be taken into consideration before nNO is applied to detect AR. In addition, referring to the role of FeNO played in asthma, it remains to be seen whether nNO could be used as an indicator of AR treatment responsiveness in future studies.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All author drafted the manuscript. Conception was from Bingbing Wang, Zhenchao Wu, and Yi Liu. Bingbing Wang designed the study protocol, performed data extraction and checked the manuscript. Zhenchao Wu performed data extraction, data analysis and interpretation, revised the manuscript to make it more fluent. Feifei Wang, Zuojuan Yin and Lei Shi did the literature search and study selection. Administrative support from Yi Liu who was responsible for any disagreement arising from the whole work.
ETHICS STATEMENT
I confirm that the manuscript has been submitted solely to this journal and is not published, in press, or currently submitted elsewhere.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This work was supported by grants from Clinical Medical Science and Technology Innovation Program of Jinan City (202019162) and Natural Science Foundation of China (Project number: 82071569).
Wang B, Wu Z, Wang F, Yin Z, Shi L, Liu Y. Nasal nitric oxide testing for allergic rhinitis patients: Systematic review and meta‐analysis. Immun Inflamm Dis. 2021;9:635‐648. 10.1002/iid3.439
Systematic Review Registration: PROSPERO [CRD42020160578]
Bingbing Wang and Zhenchao Wu should be considered joint first author.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Meltzer EO. Allergic rhinitis: burden of illness, quality of life, comorbidities, and control. Immunol Allergy Clin North Am. 2016;36(2):235‐248. [DOI] [PubMed] [Google Scholar]
- 2. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8‐160. [DOI] [PubMed] [Google Scholar]
- 3. National Institute for Health and Care Excellence: Clinical Guidelines Asthma: diagnosis, monitoring and chronic asthma management. London: National Institute for Health and Care Excellence (UK)Copyright © NICE; 2020. [Google Scholar]
- 4. Hoyte FCL, Gross LM, Katial RK. Exhaled nitric oxide: an update. Immunol Allergy Clin North Am. 2018;38(4):573‐585. [DOI] [PubMed] [Google Scholar]
- 5. Song WJ, Won HK, Moon SD, et al. Could fractional exhaled nitric oxide test be useful in predicting inhaled corticosteroid responsiveness in chronic cough? A systematic review. J Allergy Clin Immunol Pract. 2017;5(1):135‐143 e131. [DOI] [PubMed] [Google Scholar]
- 6. Jorissen M, Lefevere L, Willems T. Nasal nitric oxide. Allergy. 2001;56(11):1026‐1033. [DOI] [PubMed] [Google Scholar]
- 7. Lee KJ, Cho SH, Lee SH, et al. Nasal and exhaled nitric oxide in allergic rhinitis. Clin Exp Otorhinolaryngol. 2012;5(4):228‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nesic VS, Djordjevic VZ, Tomic‐Spiric V, Dudvarski ZR, Soldatovic IA, Arsovic NA. Measuring nasal nitric oxide in allergic rhinitis patients. J Laryngol Otol. 2016;130(11):1064‐1071. [DOI] [PubMed] [Google Scholar]
- 9. Wang PP, Wang GX, Ge WT, Tang LX, Zhang J, Ni X. Nasal nitric oxide in allergic rhinitis in children and its relationship to severity and treatment. Allergy Asthma Clin Immunol. 2017;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moody A, Fergusson W, Wells A, Bartley J, Kolbe J. Nasal levels of nitric oxide as an outcome variable in allergic upper respiratory tract disease: influence of atopy and hayfever on nNO. Am J Rhinol. 2006;20(5):425‐429. [DOI] [PubMed] [Google Scholar]
- 11. Henriksen AM, Sue‐Chu M, Lingaas Holmen T, Langhammer A, Bjermer L. Exhaled and nasal NO levels in allergic rhinitis: relation to sensitization, pollen season and bronchial hyperresponsiveness. Eur Respir J. 1999;13(2):301‐306. [DOI] [PubMed] [Google Scholar]
- 12. American Thoracic Society; European Respiratory Society . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912‐930. [DOI] [PubMed] [Google Scholar]
- 13. Whiting PF. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529‐536. [DOI] [PubMed] [Google Scholar]
- 14. Collins SA, Gove K, Walker W, Lucas JS. Nasal nitric oxide screening for primary ciliary dyskinesia: systematic review and meta‐analysis. Eur Respir J. 2014;44(6):1589‐1599. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
- 18. Palm JP, Alving K, Lundberg JO. Characterization of airway nitric oxide in allergic rhinitis: the effect of intranasal administration of L‐NAME. Allergy. 2003;58(9):885‐892. [DOI] [PubMed] [Google Scholar]
- 19. Makris MP, Gratziou C, Aggelides XS, Koulouris SP, Koti I, Kalogeromitros DC. Exhaled nitric oxide, bronchial hyperresponsiveness and spirometric parameters in patients with allergic rhinitis during pollen season. Iran J Allergy Asthma Immunol. 2011;10(4):251‐260. [PubMed] [Google Scholar]
- 20. Suojalehto H, Vehmas T, Lindström I, et al. Nasal nitric oxide is dependent on sinus obstruction in allergic rhinitis. Laryngoscope. 2014;124(6):E213‐E218. [DOI] [PubMed] [Google Scholar]
- 21. Hou J, Lou H, Wang Y, et al. Nasal ventilation is an important factor in evaluating the diagnostic value of nasal nitric oxide in allergic rhinitis. International Forum of Allergy & Rhinology. 2018;8(6):686‐694. [DOI] [PubMed] [Google Scholar]
- 22. Mu DQ, Pan JH. [Correlation of fractional exhaled nitric oxide in the upper and lower airways with the level of asthma control]. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21(5):426‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wen YS, Lin CY, Yang KD, Hung CH, Chang YJ, Tsai YG. Nasal nitric oxide is a useful biomarker for acute unilateral maxillary sinusitis in pediatric allergic rhinitis: a prospective observational cohort study. World Allergy Organ J. 2019;12(4):100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu C, Zheng K, Liu X, et al. Use of nasal nitric oxide in the diagnosis of allergic rhinitis and nonallergic rhinitis in patients with and without sinus inflammation. J Allergy Clin Immunol Pract. 2020;8(5):1574‐1581.e4. [DOI] [PubMed] [Google Scholar]
- 25. Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991;181(2):852‐857. [DOI] [PubMed] [Google Scholar]
- 26. Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84(3):731‐765. [DOI] [PubMed] [Google Scholar]
- 27. Antosova M, Mokra D, Pepucha L, et al. Physiology of nitric oxide in the respiratory system. Physiol Res. 2017;66(Suppl 2):S159‐S172. [DOI] [PubMed] [Google Scholar]
- 28. Sato S, Wang X, Saito J, et al. Exhaled nitric oxide and inducible nitric oxide synthase gene polymorphism in Japanese asthmatics. Allergol Int. 2016;65(3):300‐305. [DOI] [PubMed] [Google Scholar]
- 29. Persson MG, Zetterström O, Agrenius V, Ihre E, Gustafsson LE. Single‐breath nitric oxide measurements in asthmatic patients and smokers. Lancet. 1994;343(8890):146‐147. [DOI] [PubMed] [Google Scholar]
- 30. Sacco O, Sale R, Silvestri M, et al. Total and allergen‐specific IgE levels in serum reflect blood eosinophilia and fractional exhaled nitric oxide concentrations but not pulmonary functions in allergic asthmatic children sensitized to house dust mites. Pediatr Allergy Immunol. 2003;14(6):475‐481. [DOI] [PubMed] [Google Scholar]
- 31. Essat M, Harnan S, Gomersall T, et al. Fractional exhaled nitric oxide for the management of asthma in adults: a systematic review. Eur Respir J. 2016;47(3):751‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sandrini A, Taylor DR, Thomas PS, Yates DH. Fractional exhaled nitric oxide in asthma: an update. Respirology. 2010;15(1):57‐70. [DOI] [PubMed] [Google Scholar]
- 33. Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6(9):1368‐1370. [PubMed] [Google Scholar]
- 34. Lundberg JO, Rinder J, Weitzberg E, Lundberg JM, Alving K. Nasally exhaled nitric oxide in humans originates mainly in the paranasal sinuses. Acta Physiol Scand. 1994;152(4):431‐432. [DOI] [PubMed] [Google Scholar]
- 35. Lundberg JON, Farkas‐Szallasi T, Weitzberg E, et al. High nitric oxide production in human paranasal sinuses. Nat Med. 1995;1(4):370‐373. [DOI] [PubMed] [Google Scholar]
- 36. Lewandowski K, Busch T, Lohbrunner H, et al. Low nitric oxide concentrations in exhaled gas and nasal airways of mammals without paranasal sinuses. J Appl Physiol (1985). 1998;85(2):405‐410. [DOI] [PubMed] [Google Scholar]
- 37. Takeno S, Taruya T, Ueda T, Noda N, Hirakawa K. Increased exhaled nitric oxide and its oxidation metabolism in eosinophilic chronic rhinosinusitis. Auris Nasus Larynx. 2013;40(5):458‐464. [DOI] [PubMed] [Google Scholar]
- 38. Struben VMD, Wieringa MH, Mantingh CJ, et al. Nasal NO: normal values in children age 6 through to 17 years. Eur Respir J. 2005;26(3):453‐457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


