Abstract
AIM
To investigate whether the axial length (AL)/total corneal refractive power (TCRP) ratio is a sensitive and simple factor that can be used for the early diagnosis of Marfan's syndrome (MFS) in children.
METHODS
The relationship between the AL/TCRP ratio and the diagnosis of MFS for 192 eyes in 97 children were evaluate. The biological characteristics, including age, sex, AL, and TCRP, were collected from medical records. Receiver operating characteristic (ROC) curve analysis was performed to investigate whether the AL/TCRP ratio effectively distinguishes MFS from other subjects. The Youden index was used to re-divide the whole population into two groups according to an AL/TCRP ratio of 0.59.
RESULTS
Of 96 subjects (mean age 7.46±3.28y) evaluated, 56 (110 eyes) had a definite diagnosis of MFS in childhood based on the revised Ghent criteria, 41 (82 eyes) with diagnosis of congenital ectopia lentis (EL) were included as a control group. AL was negatively correlated with TCRP, with a linear regression coefficient of -0.36 (R2=0.08). A significant correlation was found between age and the AL/TCRP ratio (P=0.023). ROC curve analysis showed that the AL/TCRP ratio distinguished MFS from the other patients at a threshold of 0.59. MFS patients were present in 24/58 (41.38%) patients with an AL/TCRP ratio of ≤0.59 and in 34/39 (87.18%) patients with an AL/TCRP ratio of >0.59.
CONCLUSION
An AL/TCRP ratio of >0.59 is significantly associated with the risk of MFS. The AL/TCRP ratio should be measured as a promising marker for the prognosis of children MFS. Changes in the AL/TCRP ratio should be monitored over time.
Keywords: axial length, total corneal refractive power ratio, diagnosis, Marfan's syndrome, children
INTRODUCTION
Marfan's syndrome (MFS) is a rare hereditary disease with an estimated prevalence of 2-3/10 000-20 000 individuals[1]. However, there are no clear ethnic, geographic or gender preference for MFS as MFS is an autosomal dominant disease. Mutations in the gene coding fibrillin-1 (FBN1) are main causes of MFS as FBN1 has an integral role in elastin deposition and microfibrils forming[2]–[4]. MFS is associated with systemic connective tissue disorders that frequently affect the eyes, cardiovascular system, and skeletal systems. Classical findings include aortic root dilation and mitral valve prolapse, which also are the most life-threatening presentations of MFS[5]. Ectopia lentis (EL) and aortic root dilation are the most common manifestation, affecting about 60% of patients[3].
Although the MFS phenotype in adults was well documented, characters of MFS in children were rarely reported according to incomplete phenotype in the young, marked phenotypic variability in different families, and the absence of molecular biology. As a life-threatening disease, it is crucial for early discovery, early diagnosis and early treatment at early childhood stage of MFS. As Faivre et al[6] reported EL was shown in 57% of MFS children under 10y as the earlist sign of MFS, the ocular characters of MFS may be potential marker for aiding diagnosis of MFS in childhood.
According to the 1996 Ghent criteria (Ghent-1 criteria) for the diagnosis of MFS, EL of any degree was the only ocular major criterion for MFS, and is found in about 60% patients with MFS. Three minor criteria were proposed, namely increased axial length (AL) of the globe, an abnormally flat cornea and a hypoplastic iris or hypoplastic ciliary muscle causing decreased miosis[3],[7].
However, in the current 2010 Revised Ghent Nosology (Ghent-2 criteria), EL was given more weight. To simplify the process and lower the cost associated with clinic tests, previous ocular criteria were replaced with myopia of greater than -3 diopters (D). In 2010 revision, another major change was the diagnosis of ‘isolated EL’ is excluded in patients who was under the age of 20 because cardiovascular development is incomplete before 20 years old. Chandra et al[8] reported that 57/123 (46.3%) cases of isolated EL caused by mutations of FBN1 classified were re-diagnosed as MFS by the revised Ghent criteria. During an observation period of 20y, 38.5% of patients with the diagnosis of isolated EL in their childhood had been described with aortic dilation with as they grow[8].
In our previous studies, AL and total corneal refractive power (TCRP) significantly differed between patients with MFS and control groups while the area under the curve (AUROC) was 0.85 for TCRP between the MFS and normal control groups[9]. The AL/corneal radius of curvature (CR) ratio was also higher in myopic eyes than in nonmyopic eyes which were shown ideal performance of myopic diagnosis[9]. Considering these findings, we investigated whether the AL/TCRP ratio, which represents AL, a flat cornea, and corneal astigmatism, might be useful for the identification of MFS.
We evaluated the predictive value of the AL/TCRP ratio in MFS patients, subjects with congenital EL patients without FBN1 mutation as control subjects.
Our primary objectives were as follows: 1) compare the AL/TCRP ratio, AL, TCRP, and other ocular characters between MFS and control groups in children patients; 2) evaluate the association between AL and TCRP; 3) explore the efficiency of using the AL/TCRP ratio as a diagnostic marker for MFS.
SUBJECTS AND METHODS
Ethical Approval
This retrospective study enrolled subjects who underwent examinations between May 30, 2018 and May 30, 2019 at the Eye and ENT Hospital of Fudan University, Shanghai, China. The study obeyed the tenets of the Declaration of Helsinki. All of the subjects' guardian provided signed informed consent. The Human Research Ethics Committee of the Eye and ENT Hospital of Fudan University approved the study as the extension of our randomized controlled trial (ChiCTR2000039132).
Patient Selection
In total, 97 subjects <18 years of age were enrolled in this study. This included 56 patients diagnosed with MFS based on the Ghent-2 criteria, 41 subjects diagnosed as congenital EL without FBN1 mutation after gening genetic testing was enrolled in the control group. All participants underwent Pentacam AXL system measurements. Patients with keratoconus, microspherophakia, corneal disease, retinal detachment, glaucoma, uveitis, history of ocular surgery, or use of contact lenses <2wk before the examinations were excluded from this study. Both eyes in each subject were included in the study if data were available, otherwise data from one eye were used.
Examinations and Calculations
The family and medical histories were recorded for all subjects before examinations. FBN1 mutation screening was performed of all EL patients for diagnostic purposes if children presented at least one major and one minor criteria in two different systems.
The Cataract Pre OP pattern of the Pentacam AXL system (Oculus Inc., Wetzlar, Germany) with a rotating Scheimpflug camera was used to measure the oular characters of all the subjects such as the AL, mean keratometry of the anterior corneal surface (Km F), mean TCRP, and corneal astigmatism. The corneal aberration data included total corneal spherical aberrations (Z4,0) in the 6-mm zone around the corneal apex (WFA Z40), wave front aberration in the 4-mm zone around the corneal apex (WFA 4-mm zone), and the root mean square of the total corneal high order aberrations calculated in the 4-mm zone around the corneal apex (WFA HO RMS) were also collected by the Pentacam AXL system. The corneal diameter, thickness, mean radius of the posterior corneal surface/mean radius of the anterior corneal surface ratio (B/F ratio) and anterior chamber depth (ACD) were also recorded.
Subjects were examined by experienced ophthalmologists who were trained in the use of all devices. All values in individual subjects were taken as the means of five repeated measurements for each eye recorded by the equipment. The right and left eyes were analyzed separately.
Statistical Analysis
SPSS software version 23.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Two-sided P values of <0.05 were considered statistically significant. The Kolmogorov-Smirnov test was used to confirm whether the variables followed normal distributions. Demographic and ocular characteristics were compared among the MFS, control groups using the Chi-square test, Student's t-test, and Wilcoxon rank-sum test (Mann-Whitney U test). Linear regression analysis was used to determine the association between AL, Km TCRP, AL/TCRP ratio and age. To compare the diagnostic values of AL, TCRP, AL/TCRP ratio, Km F, ACD, WFA HO RMS, B/F ratio, we plotted receiver operating characteristic (ROC) curves for each parameter using data from the MFS and control groups. The Youden index was used to re-divide the study population into subgroups based on the cutoff value, and the sensitivity and specificity were determined.
RESULTS
Sample Population Characteristics
This retrospective study included 192 eyes from 97 subjects who underwent ocular examinations with a rotating Scheimpflug camera (Pentacam AXL, Oculus Inc.). This included 56 children (110 eyes) diagnosed with MFS based on the Ghent-2 criteria, 41 congenital EL children (82 eyes) as a control group. The demographic and ocular characteristics of two groups are presented in Table 1.
Table 1. Baseline characteristics of the MFS group and control group.
| Parameters | MFS group | Control group | P |
| Subjects/eyes | 56/110 | 41/82 | |
| Sex (F:M) | 26:30 | 20:21 | 0.968 |
| Eyes (right:left) | 57/53 | 41/41 | 0.355 |
| Age (y) | 7.6±3.42 | 7.26±3.05 | 0.906 |
| AL (mm) | 24.73±2.54 | 22.84±1.33 | <0.01 |
| AL/TCRP (mm/D) | 0.63±0.07 | 0.56±0.04 | <0.01 |
| Km F (D) | 39.96±1.66 | 41.44±2.37 | <0.01 |
| Astig F (D) | 1.92±1.01 | 2.07±1 | 0.222 |
| Km TCRP (D) | 39.49±1.45 | 41±1.94 | <0.01 |
| Astig TCRP (D) | 2.07±1.07 | 2.2±1.07 | 0.426 |
| WFA 4-mm zone (D) | -1.67±1.33 | -2±1.13 | 0.081 |
| WFA Z40 (D) | 0.07±0.09 | 0.07±0.13 | 0.619 |
| WFA HO RMS (D) | 0.23±0.4 | 0.23±0.14 | 0.017 |
| ACD (mm) | 3.58±7.77 | 5.67±27.61 | 0.012 |
| B/F ratio | 81.38±10.94 | 83.53±2.7 | 0.029 |
| Cornea dia (mm) | 11.74±0.49 | 11.72±0.61 | 0.590 |
| Pupil dia (mm) | 4.18±1.66 | 4.47±1.51 | 0.264 |
| Pachy apex (µm) | 538.42±37.87 | 547.95±44.09 | 0.436 |
| Pachy pupil (µm) | 531.67±38.1 | 534.05±44.35 | 0.569 |
AL: Axial length; AL/TCRP: Axial length/total corneal refractive power ratio; F: Front (anterior corneal surface); Km: Mean keratometry; Astig: Astigmatism; WFA 4-mm zone: Wave front aberration in the 4-mm zone around the corneal apex; WFA Z40: Total corneal spherical aberrations (Z4,0) in the 6-mm zone around the corneal apex; WFA HO RMS: Root mean square of the total corneal high order aberrations calculated in the 4-mm zone around the corneal apex; B/F ratio: Mean radius of the posterior corneal surface/mean radius of the anterior corneal surface ratio; ACD: Anterior chamber depth; Cornea dia: Corneal diameter (horizontal); Pupil dia: Pupil diameter; Pachy apex: Corneal thickness at the apex; Pachy pupil: Corneal thickness at the pupil's center.
The mean ages of the MFS and control group was 7.6±3.42y and 7.26±3.05y, respectively (P=0.906). A significant difference in AL/TCRP was observed among the MFS and control groups, being significantly greater in the MFS group than the other group (0.63±0.07 and 0.56±0.04 mm/D, respectively, P<0.01). Furthermore, AL (24.73±2.54 and 22.84±1.33 mm, respectively, P<0.01) and Km TCRP (39.49±1.45 and 41±1.94 D, respectively, P<0.01) were significantly different between the MFS group and the control groups.
Correlations Among AL, TCRP, AL/TCRP, and Age
The Pearson correlation coefficient revealed a negative correlation between AL and TCRP of -0.36 (P<0.01) while AL was negatively correlated with TCRP, with a linear regression coefficient of -0.36, as shown in Figure 1 (R2=0.08, P<0.01). AL/TCRP ratio was shown significantly correlation with age (P=0.023) while it was also not correlated with sex, eye assessed in Table 2.
Figure 1. Linear regression coefficient of AL and TCRP.
AL: Axial length; Km: Mean keratometry; TCRP: Total corneal refractive power.
Table 2. The Pearson correlation coefficient between AL/TCRP ratio and other.
| Parameters | Pearson correlation coefficient | P |
| Sex (F:M) | -0.056 | 0.564 |
| Eyes (right:left) | -0.02 | 0.835 |
| Age (y) | 0.218 | 0.023 |
| AL (mm) | 0.949 | <0.01 |
| Km F (D) | -0.440 | <0.01 |
| Astig F (D) | -0.196 | 0.041 |
| Km TCRP (D) | -0.474 | <0.01 |
| Astig TCRP (D) | -0.166 | 0.085 |
| WFA 4-mm zone (D) | 0.131 | 0.176 |
| WFA Z40 (D) | -0.1 | 0.303 |
| WFA HO RMS (D) | -0.069 | 0.477 |
| ACD (mm) | -0.044 | 0.65 |
| B/F ratio | 0.066 | 0.496 |
| Cornea dia (mm) | 0.112 | 0.311 |
| Pupil dia (mm) | -0.067 | 0.488 |
| Pachy apex (µm) | -0.197 | 0.041 |
| Pachy pupil (µm) | -0.147 | 0.13 |
AL: Axial length; AL/TCRP: Axial length/total corneal refractive power ratio; F: Front (anterior corneal surface); Km: Mean keratometry; Astig: Astigmatism; WFA 4-mm zone: Wave front aberration in the 4-mm zone around the corneal apex; WFA Z40: Total corneal spherical aberrations (Z4,0) in the 6-mm zone around the corneal apex; WFA HO RMS: Root mean square of the total corneal high order aberrations calculated in the 4-mm zone around the corneal apex; B/F ratio: Mean radius of the posterior corneal surface/mean radius of the anterior corneal surface ratio; ACD: Anterior chamber depth; Cornea dia: Corneal diameter (horizontal); Pupil dia: Pupil diameter; Pachy apex: Corneal thickness at the apex; Pachy pupil: Corneal thickness at the pupil's center.
ROC Curve Analysis of AL, TCRP, AL/TCRP, and Other Ocular Characters for Detecting MFS
The ocular data showed significant differences in selecting potential predictors for diagnosis of MFS children. Based on the ROC curve analyses, AL/TCRP showed better diagnostic value for MFS compared with AL, Km TCRP, Km F, WFA HO RMS, ACD and B/F ratio (Figure 2). Table 3 shows the AUROC (95%CI), together with the max Youden index and best cut-off value. The AUROC of AL/TCRP ratio was the highest in the comparison between the MFS group and the control.
Figure 2. ROC curve analysis for ocular characters of MFS children.

ROC: Receiver operating characteristic; AL: Axial length; AL/TCRP: Axial length/total corneal refractive power ratio; F: Front (anterior corneal surface); ACD: Anterior chamber depth; Km: Mean keratometry; WFA HO RMS: Root mean square of the total corneal high order aberrations calculated in the 4-mm zone around the corneal apex; B/F ratio: Mean radius of the posterior corneal surface/mean radius of the anterior corneal surface ratio.
Table 3. Results of the ROC curve analysis.
| MFS vs control | AUROC (95%CI) | Max Youden index | Best cut-off value |
| AL (mm) | 0.75 (0.68, 0.82) | 0.41 | 23.38 |
| AL/TCRP (mm/D) | 0.8 (0.74, 0.87) | 0.81 | 0.59 |
| Km F (D) | 0.61 (0.52, 0.69) | 0.37 | 41.15 |
| Km TCRP (D) | 0.72 (0.65, 0.79) | 0.36 | 40.15 |
| WFA HO RMS (D) | 0.73 (0.66, 0.8) | 0.212 | 0.16 |
| ACD (mm) | 0.59 (0.51, 0.67) | 0.195 | 2.38 |
| B/F ratio | 0.59 (0.51, 0.68) | 0.181 | 82.85 |
ROC: Receiver operating characteristic; AUROC: Area under the receiver operating characteristic curve; MFS: Marfan's syndrome; AL: Axial length; AL/TCRP: Axial length/total corneal refractive power ratio; F: Front (anterior corneal surface); Km: Mean keratometry; WFA HO RMS: Root mean square of the total corneal high order aberrations calculated in the 4-mm zone around the corneal apex; ACD: Anterior chamber depth; B/F ratio: Mean radius of the posterior corneal surface/mean radius of the anterior corneal surface ratio.
According to the best cutoff value of the AL/TCRP ratio determined using the Youden index, the eyes were divided into two groups. Group 1 comprised all eyes with an AL/TCRP ratio of ≤0.59 (59.38% of eyes) and group 2 comprised eyes with an AL/TCRP ratio >0.59 (40.62% of eyes). MFS was diagnosed in 24/58 (41.38%) subjects in group 1 compared with 34/39 (87.18%) eyes in group 2. The demographic and ocular characteristics of these two groups are presented in Table 4.
Table 4. Baseline characteristics of groups 1 and 2.
| Parameters | Group 1 | Group 2 | P |
| Subjects/eyes | 58/114 | 39/78 | |
| MFS/Un-MFS | 24/34 | 34/5 | <0.01 |
| Sex (F:M) | 28:30 | 19:20 | 0.184 |
| Eyes (right:left) | 62/52 | 36/42 | 0.306 |
| Age (y) | 7.13±2.91 | 7.93±3.7 | <0.01 |
| AL (mm) | 22.56±0.94 | 25.91±2.27 | <0.01 |
| AL/TCRP (mm/D) | 0.55±0.03 | 0.67±0.06 | <0.01 |
| Km F (D) | 41.47±2.15 | 39.3±1.25 | <0.01 |
| Astig F (D) | 2.15±1.03 | 1.75±0.91 | 0.032 |
| Km TCRP (D) | 40.97±1.68 | 38.91±1.27 | <0.01 |
| Astig TCRP (D) | 2.27±1.12 | 1.92±0.95 | 0.325 |
| WFA 4-mm zone (D) | -2.04±1.22 | -1.47±1.23 | <0.01 |
| WFA Z40 (D) | 0.07±0.12 | 0.07±0.09 | <0.01 |
| WFA HO RMS (D) | 0.26±0.39 | 0.18±0.14 | 0.486 |
| ACD (mm) | 3.33±7.7 | 6.11±28.09 | 0.225 |
| B/F ratio | 82.38±7.73 | 82.18±9.58 | 0.15 |
| Cornea dia (mm) | 11.68±0.53 | 11.79±0.54 | 0.272 |
| Pupil dia (mm) | 4.44±1.6 | 4.09±1.57 | 0.179 |
| Pachy apex (µm) | 550.31±39.66 | 531.38±40.22 | <0.01 |
| Pachy pupil (µm) | 536.18±39.22 | 528.33±42.94 | 0.064 |
AL: Axial length; AL/TCRP: Axial length/total corneal refractive power ratio; F: Front (anterior corneal surface); Km: Mean keratometry; Astig: Astigmatism; WFA 4-mm zone: Wave front aberration in the 4-mm zone around the corneal apex; WFA Z40: Total corneal spherical aberrations (Z4,0) in the 6-mm zone around the corneal apex; WFA HO RMS: Root mean square of the total corneal high order aberrations calculated in the 4-mm zone around the corneal apex; B/F ratio: Mean radius of the posterior corneal surface/mean radius of the anterior corneal surface ratio; ACD: Anterior chamber depth; Cornea dia: Corneal diameter (horizontal); Pupil dia: Pupil diameter; Pachy apex: Corneal thickness at the apex; Pachy pupil: Corneal thickness at the pupil's center.
Correlations Among AL, TCRP, AL/TCRP, and Age in MFS Children
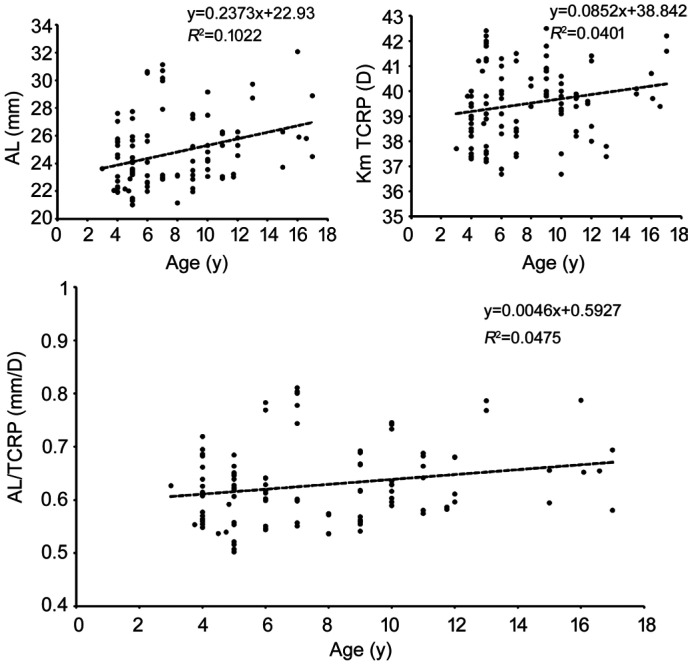
The Pearson correlation coefficient revealed a positive correlation between age and AL/TCRP ratio (P<0.01) with a linear regression coefficient of 0.0046, as shown in Figure 3 (R2=0.0475, P<0.01). The Pearson correlation coefficient revealed a positive correlation between age and AL of 0.32 (P<0.01) meanwhile a positive correlation was also shown between Km TCRP and age of 0.2 (P<0.01).
Figure 3. Correlations among AL, TCRP, AL/TCRP and age in MFS children.
AL: Axial length; AL/TCRP: Axial length/the total corneal refractive power ratio; F: Front (anterior corneal surface); Km: Mean keratometry; Astig: Astigmatism.
DISCUSSION
As an advanced inheritable disease, MFS occurs in about 2-3 individuals per 10 000 people. Because MFS is a progressive disease with a 1.1% mortality rate in youngs up to the age of 18y, the early detection of abnormal findings and early diagnosis and timely follow-up are essential for children. It was understood to be a genetic disorder with a mutation in the FBN1 dene affecting the connective tissue[10]. Ocular abnormalities in children with MFS include EL, increased AL, high corneal astigmatism, and decreased corneal curvature, for example[11]–[12]. The study[11] mentioned that EL was accured in 12.5% of children with MFS before 3 years old and in 45% children who was 4 to 5 years old. EL was introduced as the major ocular criterion for MFS in the Ghent-1 criteria, while increased AL and myopia may also occur in the early stages of MFS[3]. However, for accuracy and objectivity, the minor ocular criteria were modified and simplified with the introduction of the Ghent-2 criteria. Our research was looking forward to solve the missing of the ocular characters of MFS patients in childhood and the need of early diagnosis of MFS.
Previous Ocular Criteria for MFS
In 1988, the first international nosology, known as the Berlin nosology, was published. Since each organ system is poosibly affected by MFS, manifestations has been divided into major and minor criteria. Because eye manifestations occur in 60% MFS patients, ocular manifestations were included in the Berlin nosology, with EL as a major diagnostic criterion while minor criteria included a flat cornea, an elongated globe, retinal detachment, and myopia[13] (Table 5). The Ghent-1 criteria published in 1996 focused on FBN1 mutations as the underlying etiology of MFS, and these criteria subsequently replaced the Berlin nosology. An increased AL of the globe, an abnormally flat cornea, and a hypoplastic iris or hypoplastic ciliary muscle causing decreased miosis were included as the minor criteria in Ghent-1. High myopia and retinal detachment and were developed because an increased AL of the globe, so these cannot be considered as separate manifestations[14]–[16].
Table 5. Comparison of the Berlin, Ghent-1, and Ghent-2 nosology for MFS.
| Berlin nosology | Ghent-1 nosology | Ghent-2 nosology |
| Major diagnostic manifestations: ectopia lentis |
Major criterion: ectopia lentis of any agree |
In the absence of family history: ectopia lentis and causal FBN1 mutation with known aortic root dilatation=MFS In the presence of family history: ectopia lentis and family history of MFS (as defined above)=MFS |
| Flat cornea Elongated globe Retinal detachment Myopia |
Minor criterion: flat cornea; increased axial length of the globe (>23.5 mm); hypoplastic ciliary muscle causing decreased miosis |
Scoring of systemic features of MFS Myopia >3 D -1 point |
MFS: Marfan's syndrome.
The simplified and revised Ghent-2 criteria were introduced in 2010[14]–[16]. These criteria placed greater emphasis on EL, aortic root aneurysm or dissection and abnormally flat cornea as diagnostic criteria. Meanwhile, the minor ocular criteria, including anhypoplastic iris, or hypoplastic ciliary muscle causing decreased miosis were replaced with a single criterion, namely myopia greater than -3 D[14],[17]. The main reason for this substitution was that increased AL and a flat cornea show poor specificity and are not routinely measured by doctors. Considering the high sensitivity, early onset, high severity, and rapid progression of myopia, the criterion of myopia of greater than -3 D was allocated one point in the systemic score (Table 5).
Although the Ghent-2 criteria offers greater simplicity and affordability, no studies have shown that these criteria are more accurate than the Ghent-1 criteria[15]–[17]. In the Ghent-2 criteria, myopia of greater than -3 D was the only ocular criterion other than EL. This criterion can be easily measured and compared with the original three criteria in the Ghent-1 criteria, it shows high sensitivity and an early onset[8].
Although myopia of greater than -3 D is representative of ever-increasing in AL, it also can be corrupted by other effects. Likewise, the equivalent spherical lens degree may be affected by crystal astigmatism and corneal astigmatism. Additionally, optometry is not always objective because it is partly dependent on subjective retinoscopy. Following computed optometry combined with optometry, the ophthalmologist must make subjective assessments of the patient's vision. Myopia is also becoming more common, especially in Asian countries. For example, in some studies in Asia, myopia was reported in 31.1% of people overall and that 80%-90% of children who completed high school were myopic, of whom 10%-20% had high myopia[18]–[20]. Thus, in patients with MFS, myopia of greater than -3 D may actually represent axial myopia rather than specific ocular changes of MFS.
AL/TCRP Contributes to the Accurate Diagnosis of MFS
Luebke et al[21] evaluated Kmax as screening tools for MFS with a sensitivity of 73% and a specificity of 81% in a group of age- and AL matched people while Beene et al[22] found a Km lower than 41.40 D could be a good potential thresholds for diagnosing MFS. Both Kmax and km served as potential ocular screening values for MFS. In our recent studies, Chen et al[23] reported significant differences in ocular characteristics between MFS patients (20.5±14.2y) and control subjects in terms of anterior, posterior and total corneal refractive power, and that TCRP was a potential diagnostic marker for MFS based on an AUROC of 0.85[23]–[26]. Besides, previous studies showed that the AL/CR ratio was significantly greater in myopic eyes than in nonmyopic eyes[9],[27]–[30]. Therefore, He et al[31] proposed that the AL/CR ratio was a more sensitive and specific measurement for the diagnosis myopia.
To date, however, no researchers have considered using the AL/TCRP ratio, which provides an objective assessment of AL and corneal curvature, as a diagnostic criterion for MFS. After referring to a recent study concerning AL/CR ratio[9],[27]–[30] and confirmation of the inverse correlation between AL and TCRP, we investigated whether the AL/TCRP ratio which combines AL and corneal characteristics could be a potential marker for aiding the ocular diagnosis of MFS in childhood. In our study, we analyzed the ocular characteristics of 192 eyes in 97 patients. We found an inverse correlation between AL and TCRP in linear regression analysis. AL/TCRP also showed high sensitivity (0.66) and specificity (0.85) and an AUROC of 0.8 for detecting children MFS relative to the control group. We also confirmed that an AL/TCRP of >0.59 is a useful lower boundary, which can be easily used for the clinical diagnosis of chidren MFS.
Clinical Application of the AL/TCRP Ratio
About 50% of patients were diagnosed with MFS primarily as the first evaluation for ophthalmic complaints[32]. Chandra et al[8] found that 46.3% of patients classified as isolated EL were re-diagnosed as MFS by the revised Ghent criteria in the observation of 20y. Therefore, asymptomatic populations should to be addressed because of the severity and development of MFS.
For children with MFS who are still asymptomatic, the AL/TCRP ratio may help with early diagnosis and prevention of aggravated ocular symptoms.
With recent advances in eye testing equipment, the measurement of ocular characteristics has become much more accurate. In particular, the IOLmaster 700 provides a precise measurement of AL, and the Pentacam AXL system is reliable for measuring TCRP. Astigmatism on the posterior corneal surface may also be indicated by TCRP. Thus, the AL/TCRP ratio, which provides an index of changes in corneal parameters in patients with MFS, is an objective assessment that is independent of subjective evaluations by the patient.
Limitations
There are some limitations of our study to discuss. First, only 56 MFS children were enrolled in the study, so more data might be necessary in the future. To solve this problem, we started to built multicenter to gain more participates. Second, all the MFS children in our study had EL, while none of the MFS subjects without EL. There may have some ouclar difference between MFS with or without EL. Third, a longitudinal study is needed to determine the changes of the AL/TCRP ratio changes from time to time or whether there is a significant difference in the AL/TCRP ratio between children and adults with MFS.
In conclusion, we found that an AL/TCRP ratio >0.59 is a potential diagnostic factor for MFS children. Therefore, we suggest that the AL/TCRP ratio can be evaluated as a effective clinically significant criterion for the diagnosis of MFS children. In future follow-up studies, it will be important to monitor changes in the AL/TCRP ratio over time.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81770908); the Shanghai Science and Technology Commission (Scientific Innovation Project, No.20Y11911000).
Conflicts of Interest: Chen TH, None; Miao AZ, None; Wang YL, None; Zhang M, None; Chen JH, None; Zheng JL, None; Deng M, None; Ji YH, None; Jiang YX, None.
REFERENCES
- 1.Yuan SM, Jing H. Marfan's syndrome: an overview. Sao Paulo Med J. 2010;128(6):360–366. doi: 10.1590/S1516-31802010000600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitterman AD, Sponseller PD. Marfan syndrome: a clinical update. J Am Acad Orthop Surg. 2017;25(9):603–609. doi: 10.5435/JAAOS-D-16-00143. [DOI] [PubMed] [Google Scholar]
- 3.Ho NC, Tran JR, Bektas A. Marfan's syndrome. Lancet. 2005;366(9501):1978–1981. doi: 10.1016/S0140-6736(05)66995-4. [DOI] [PubMed] [Google Scholar]
- 4.Coucke P, Van Acker P, De Paepe A. Mutation analysis of the FBN1 gene in patients with Marfan syndrome. Methods Mol Med. 2006;126:81–95. doi: 10.1385/1-59745-088-X:81. [DOI] [PubMed] [Google Scholar]
- 5.Radke RM, Baumgartner H. Diagnosis and treatment of Marfan syndrome: an update. Heart Br Cardiac Soc. 2014;100(17):1382–1391. doi: 10.1136/heartjnl-2013-304709. [DOI] [PubMed] [Google Scholar]
- 6.Faivre L, Masurel-Paulet A, Collod-Beroud G, et al. Clinical and molecular study of 320 children with Marfan syndrome and related type I fibrillinopathies in a series of 1009 probands with pathogenic FBN1 mutations. Pediatrics. 2009;123(1):391–398. doi: 10.1542/peds.2008-0703. [DOI] [PubMed] [Google Scholar]
- 7.Gehle P, Goergen B, Pilger D, Ruokonen P, Robinson PN, Salchow DJ. Biometric and structural ocular manifestations of Marfan syndrome. PLoS One. 2017;12(9):e0183370. doi: 10.1371/journal.pone.0183370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra A, Patel D, Aragon-Martin JA, Pinard A, Collod-Béroud G, Comeglio P, Boileau C, Faivre L, Charteris D, Child AH, Arno G. The revised Ghent nosology; reclassifying isolated ectopia lentis. Clin Genet. 2015;87(3):284–287. doi: 10.1111/cge.12358. [DOI] [PubMed] [Google Scholar]
- 9.Jong M, Sankaridurg P, Naduvilath TJ, Li W, He MG. The relationship between progression in axial length/corneal radius of curvature ratio and spherical equivalent refractive error in myopia. Optom Vis Sci. 2018;95(10):921–929. doi: 10.1097/OPX.0000000000001281. [DOI] [PubMed] [Google Scholar]
- 10.Bollero P, Arcuri L, Miranda M, Ottria L, Franco R, Barlattani A. Marfan Syndrome: oral implication and management. Oral Implantol (Rome) 2017;10(2):87–96. doi: 10.11138/orl/2017.10.2.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salchow DJ, Gehle P. Ocular manifestations of Marfan syndrome in children and adolescents. Eur J Ophthalmol. 2019;29(1):38–43. doi: 10.1177/1120672118761333. [DOI] [PubMed] [Google Scholar]
- 12.Drolsum L, Rand-Hendriksen S, Paus B, Geiran OR, Semb SO. Ocular findings in 87 adults with Ghent-1 verified Marfan syndrome. Acta Ophthalmol. 2015;93(1):46–53. doi: 10.1111/aos.12448. [DOI] [PubMed] [Google Scholar]
- 13.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 14.Faivre L, Collod-Beroud G, Adès L, et al. The new Ghent criteria for Marfan syndrome: what do they change? Clin Genet. 2012;81(5):433–442. doi: 10.1111/j.1399-0004.2011.01703.x. [DOI] [PubMed] [Google Scholar]
- 15.Penpattharakul W, Pithukpakorn M. Revised Ghent criteria is comparable to original diagnostic criteria for Marfan syndrome with increased ability to clinically diagnose related disorders. J Med Assoc Thail. 2016;99(1):34–39. [PubMed] [Google Scholar]
- 16.von Kodolitsch Y, de Backer J, Schüler H, et al. Perspectives on the revised Ghent criteria for the diagnosis of Marfan syndrome. Appl Clin Genet. 2015:137. doi: 10.2147/TACG.S60472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang JH, Han H, Jang SY, Moon JR, Sung K, Chung TY, Lee HJ, Ki CS, Kim DK. A comparison of the Ghent and revised Ghent nosologies for the diagnosis of Marfan syndrome in an adult Korean population. Am J Med Genet. 2012;158A(5):989–995. doi: 10.1002/ajmg.a.34392. [DOI] [PubMed] [Google Scholar]
- 18.Morgan IG, French AN, Ashby RS, Guo XX, Ding XH, He MG, Rose KA. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134–149. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Walline J, Smith M. Controlling myopia progression in children and adolescents. Adolesc Heal Med Ther. 2015:133. doi: 10.2147/AHMT.S55834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu PC, Huang HM, Yu HJ, Fang PC, Chen CT. Epidemiology of myopia. Asia Pac J Ophthalmol (Phila) 2016;5(6):386–393. doi: 10.1097/APO.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 21.Luebke J, Boehringer D, Eberwein P, Reinhard T. Corneal K-values as a diagnostic screening tool for Marfan syndrome. Cornea. 2017;36(6):700–703. doi: 10.1097/ICO.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 22.Beene LC, Traboulsi EI, Seven I, Ford MR, Sinha Roy A, Butler RS, Dupps WJ., Jr Corneal deformation response and ocular geometry: a noninvasive diagnostic strategy in Marfan syndrome. Am J Ophthalmol. 2016;161:56–64.e1. doi: 10.1016/j.ajo.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Jing Q, Tang Y, Qian D, Lu Y, Jiang Y. Corneal curvature, astigmatism, and aberrations in Marfan syndrome with lens subluxation: evaluation by pentacam HR system. Sci Rep. 2018;8(1):4079. doi: 10.1038/s41598-018-22358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Jing Q, Tang Y, Qian D, Lu Y, Jiang Y. Age differences in axial length, corneal curvature, and corneal astigmatism in Marfan syndrome with ectopia lentis. J Ophthalmol. 2018;2018:1436834. doi: 10.1155/2018/1436834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T, Deng M, Zhang M, Chen J, Chen Z, Jiang Y. Visual outcomes of lens subluxation surgery with Cionni modified capsular tension rings in Marfan syndrome. Sci Rep. 2021;11(1):2994. doi: 10.1038/s41598-021-82586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen TH, Chen JH, Jin GM, Zhang M, Chen ZX, Zheng DY, Jiang YX. Clinical ocular diagnostic model of Marfan syndrome in patients with congenital ectopia lentis by pentacam AXL system. Trans Vis Sci Tech. 2021;10(7):3. doi: 10.1167/tvst.10.7.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashemi H, Khabazkhoob M, Miraftab M, Emamian MH, Shariati M, Abdolahi-Nia T, Fotouhi A. Axial length to corneal radius of curvature ratio and refractive errors. J Ophthalmic Vis Res. 2013;8(3):220–226. [PMC free article] [PubMed] [Google Scholar]
- 28.Seo KY, Yang H, Kim WK, Nam SM. Calculations of actual corneal astigmatism using total corneal refractive power before and after myopic keratorefractive surgery. PLoS One. 2017;12(4):e0175268. doi: 10.1371/journal.pone.0175268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Xiao L, Meng X, Wang L, Wang D. Development of corneal astigmatism (CA) according to axial length/corneal radius (AL/CR) ratio in a one-year follow-up of children in Beijing, China. J Ophthalmol. 2018;2018:4209236. doi: 10.1155/2018/4209236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badmus SA, Ajaiyeoba AI, Adegbehingbe BO, Onakpoya OH, Adeoye AO. Axial length/corneal radius of curvature ratio and refractive status in an adult Nigerian population. Niger J Clin Pract. 2017;20(10):1328–1334. doi: 10.4103/njcp.njcp_183_16. [DOI] [PubMed] [Google Scholar]
- 31.He XG, Zou HD, Lu LN, Zhao R, Zhao HJ, Li QQ, Zhu JF. Axial length/corneal radius ratio: association with refractive state and role on myopia detection combined with visual acuity in Chinese schoolchildren. PLoS One. 2015;10(2):e0111766. doi: 10.1371/journal.pone.0111766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strider D, Moore T, Guarini J, Fallin B, Ivey J, Kron I. Marfan's syndrome: a family affair. J Vasc Nurs. 1996;14(4):91–98. doi: 10.1016/s1062-0303(96)80024-0. [DOI] [PubMed] [Google Scholar]