Abstract
Metformin (MET), a first-line oral agent used to treat diabetes, exerts its function mainly by activating adenosine monophosphate-activated protein. The accumulation of oxidized phospholipids in the outer layer of the retina plays a key role in retinal pigment epithelium (RPE) cells death and the formation of choroidal neovascularization (CNV), which mean the development of age-related macular degeneration (AMD). Recent studies have shown that MET can regulate lipid metabolism, inhibit inflammation, and prohibit retinal cell death and CNV formation due to various pathological factors. Here, newly discovered functions of MET that may be used for the prevention and treatment of AMD were reviewed.
Keywords: metformin, age-related macular degeneration, liver X receptor
INTRODUCTION
Metformin (MET) was first used to treat diabetes in 1957 and is currently one of the most widely used oral glucose-lowering drugs. Recent studies showed that MET can also decrease the risk of developing cardiovascular disease, exert antioxidant, anti-senescence, weight loss, nephroprotective and antineoplastic effects[1]–[4]. In the field of ophthalmology, it has been demonstrated that MET can reduce retinal cell death due to various pathological factors, alleviate diabetic retinopathy, and inhibit corneal and intraocular neovascularization[5]–[15]. The latest clinical studies revealed that MET can reduce the occurrence or delay the progression of age-related macular degeneration (AMD)[16]–[19]. However, the mechanisms of MET in this process remain unclear. This paper provides a literature review on newly discovered functions of MET that may be used for the prevention and treatment of AMD and the mechanisms involved.
ROLE OF OXIDIZED PHOSPHOLIPIDS IN PATHOGENESIS OF AMD
AMD is the primary cause of irreversible blindness in elderly people. There are an estimated 196 million patients with AMD globally in 2020[20]. AMD is classified as the early, moderate and late stages. The late stage of AMD is classified as dry (atrophic) or wet (neovascular) types according to pathological changes. The former occurs mainly because of the death of retinal pigment epithelium (RPE) cells, resulting in the degeneration of neuroretina, whereas the latter is due to choroidal neovascularization (CNV), which further causes bleeding and exudation.
Currently, AMD is thought to result from a combination of genetic, age-related, environmental, dietary, and other factors, among which age plays a crucial role[20]–[21]. With aging, the phagocytosis of RPE cells is reduced and detached outer segments of photoreceptor cells accumulate in RPE cells and beneath the RPE layer. This results in thickening of the Bruch's membrane and drusen formation[22], i.e., early AMD. Drusen contains abundant apolipoproteins, unsaturated fatty acid phospholipids, and cholesterol. Phospholipids are susceptible to oxidation and tend to form oxidized phospholipids (OxPLs). OxPLs mainly exists as oxidized low-density lipoprotein (OxLDL) in vivo. OxLDL enters RPE cells through CD36 receptors and further activates nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3)[23]–[24]. The activated NLRP3 inflammasome can induce the secretion of interleukin-1β (IL-1β) and IL-18, resulting in RPE cell pyroptosis. Secreted IL-1β can activate the inflammasome to further aggravate inflammation. Additionally, the activated NLRP3 inflammasome can promote CD36 expression and increase OxLDL uptake. These actions reduce ATP-binding cassette transporter A1 (ABCA1)-mediated cholesterol efflux, further exacerbating intracellular cholesterol overload[23],[25]–[26]. Given its strong oxidative and pro-inflammatory properties, OxPLs is considered as a key factor in AMD (Figure 1).
Figure 1. The role of OxPLs in pathogenesis of AMD and the main functions of MET to prevent and treat AMD.
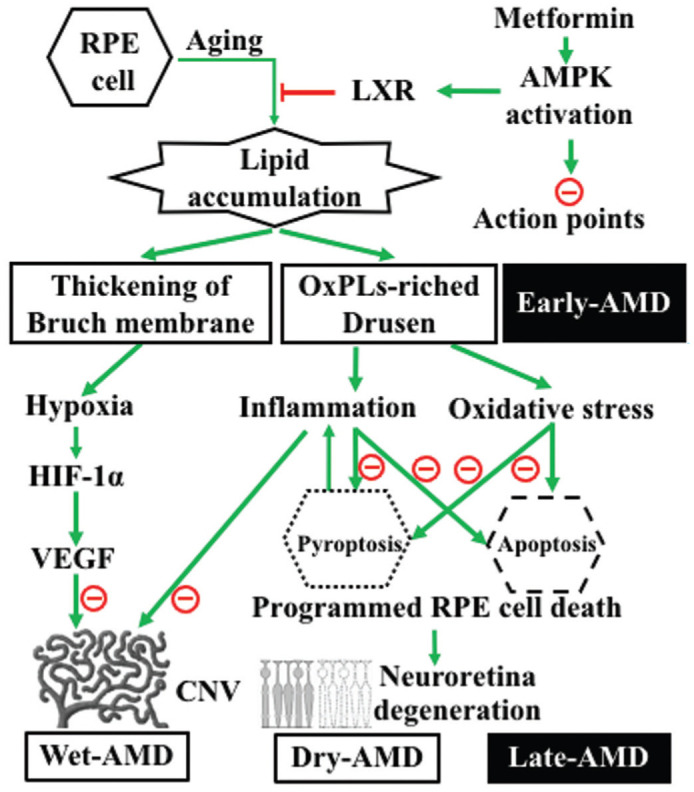
Lipid accumulation resulted from aging of RPE on one hand leads to the thickening of Bruch's membrane, which further leads to choroidal hypoxia, the expression of HIF-1 and VEGF, and ultimately the formation of CNV; on the other hand, it leads to the formation of drusen, which is the hallmark of early AMD. OxPLs in drusen induce inflammation and oxidative stress, which further cause programmed cell death, such as pyroptosis and apoptosis of RPE cells. Pyroptotic cell in turn aggravates inflammation by releasing pro-inflammation factors, and further promotes CNV formation. The death of RPE cells leads to the degeneration of the neuroretina. CNV formation and retinal degeneration mark the late stage of AMD. MET can activate AMPK. AMPK activation can reduce lipid accumulation through AMPK/LXR pathway, suppress inflammation, prohibit retinal cells from death, and inhibit CNV formation, thus to prevent and treat AMD. OxPLs: Oxidized phospholipids; RPE: Retinal pigment epithelium; AMPK: Adenosine monophosphate-activated protein kinase; LXR: Liver X receptor; HIF-1: Hypoxia inducible factor-1; VEGF: Vascular endothelial growth factor; AMD: Age-related macular degeneration; CNV: Choroidal neovascularization.
CURRENT STATUS OF AMD TREATMENT
Pathogenesis of AMD is not completely understood and its treatment remains unsatisfactory. Currently, treatments mainly target CNV in wet AMD, and available treatments include laser photocoagulation, photodynamic therapy, transpupillary thermotherapy, vitreous surgery for the excision of submacular neovascular membranes, as well as intravitreal injection of glucocorticoid and anti-vascular endothelial growth factor (VEGF) agents. Among these methods, intraocular injection of anti-VEGF agents is currently a mainstay treatment and has shown positive effects. However, the recurrence of CNV, high cost, injection-related complications, RPE cell death due to VEGF inhibition, vision loss, etc., should not be ignored[27]. In addition, no effective treatment is available for dry AMD, which accounts for more than 85% of AMD cases. The results of the AREDS study showed that antioxidant treatment can only limit disease progression[28]. Stem cell and complement-related treatments are still being researched[27],[29].
MAIN FUNCTIONS AND MECHANISM OF MET
MET was isolated from the extract of goat's rue (Galega officinalis) and was first used to treat diabetes 64 years ago. In addition to its glucose-lowering effects, an increasing number of studies have shown that MET can regulate lipid metabolism, reduce inflammatory and oxidative damage, and inhibit neovascularization[3]. Oxidative stress and inflammation induced by OxPLs are thought to play an important role in the pathogenesis of AMD, hyperglycemia is also considered as a risk factor for AMD. Therefore, it is reasonable to believe that MET may be used for the prevention and treatment of AMD through above effects.
About the mechanism of MET, it is thought that the glucose-lowering effect occur through inhibition of NADH: ubiquinone oxidoreductase (mitochondrial electron transport chain “complex I”) in the inner mitochondrial membrane to decrease ATP yield and increase the AMP/ATP ratio[30]. This metabolic transformation results in adenosine monophosphate-activated protein kinase (AMPK) activation, which is a key molecule in regulating energy metabolism and plays an important role in diabetes and other metabolic disorders. After activated, AMPK can shut down many downstream synthetic pathways that consume ATP and activate ATP degradation pathways to restore physiological energy equilibrium[31].
CRUCIAL ACTIONS OF MET FOR CONTROLLING AMD
Recently, retrospective studies[16]–[19] showed that MET decreased the incidence of AMD in a time- and dose-dependent manner. To exclude the effects of glucose-lowering on AMD occurrence, the authors simultaneously studied the effects of another diabetes drug on AMD occurrence. The results revealed no significant relationship between this drug and AMD. Currently, it is thought that the mechanisms involved are as follows:
MET Can Reduce Drusen Formation Through AMPK/LXR Pathway
Liver X receptor (LXR) is a ligand-activated nuclear transcription factor, its activation can induce the expression of lipid metabolism regulating genes and reduce cholesterol accumulation[32]. Studies in macrophage showed that MET depends on the AMPK/LXR pathway to increase the expression of inducible degrader of the LDL receptor, increase LDL receptor degradation, and reduce OxLDL uptake. Additionally, MET increases ABCA1/G1 expression to promote cholesterol efflux, thus to reduce intracellular cholesterol accumulation and drusen formation[33]–[37].
MET Can Inhibit Inflammation
Macrophages are the most important inflammatory cells. Studies have shown that MET can activate AMPK to inhibit lipopolysaccharide (LPS)-induced synthesis of tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and reactive oxygen species (ROS)[38]–[39]. Kelly et al[40] found that MET can inhibit LPS-induced pro-IL-1β expression in a dose-dependent manner while simultaneously increasing the expression of anti-inflammatory IL-10 in macrophages.
In addition to its effects on macrophages, addition of MET to the culture medium of human umbilical vein endothelial cells (HUVEs) in vitro significantly inhibited TNF-α induced expression of vascular cell-adhesion molecule-1 (VCAM-1), intercellular cell-adhesion molecule-1 (ICAM-1), E-selectin, and MCP-1. AMPK played a central role in this process[41]–[42]. In vivo studies showed that treatment with MET in patients with type 2 diabetes for 3mo significantly reduced the levels of vascular endothelium-related factors, such as tissue plasminogen activator, VCAM-1, and ICAM-1, and improved vascular function[43]. In a model of ischemia reperfusion injury, MET decreased the expression of IL-1β, TNF-α, toll-like receptor 4 (TLR4), and chemokine C-C motif receptor 2 (CCR2), and reduced infiltration by monocytes and macrophages, thereby reducing inflammatory damage to the liver[44]. In addition, MET promotes a shift from the pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype in macrophages to exert its anti-inflammatory effects[45].
In experimental uveitis, the levels of IL-1β, TNF-α, MCP-1, IL-6, and IL-18 are increased in the aqueous humor and the outer segments of photoreceptor cells shrink. MET and AMPK agonists can activate AMPK, thereby inhibiting activation of cyclooxygenase 2, inducible nitric oxide synthase, and NF-κB to inhibit these changes[46]–[47].
MET Can Prohibit RPE and Photoreceptor from Death
RPE and photoreceptor death are main pathological changes of late stage AMD. Cell death can be classified as programmed cell death (apoptosis, autophagy, pyroptosis, necroptosis, ferroptosis etc.) and necrosis. Studies showed that pyroptosis and apoptosis are the main forms of cell death induced by OxPLs[48].
MET can maintain intracellular lipid metabolic equilibrium through AMPK/LXR pathway, prevent NLRP3 protein expression and inflammasome activation, and inhibit the release of inflammatory factors, thereby reducing cell death[49]–[51]. In addition, cardiac studies showed that MET can reduce sodium arsenite-induced secretion of IL-5, TNF-α, IL-1β, caspase-3 activation, and cardiomyocyte apoptosis[52]. Endothelial cell studies showed that MET can prevent high glucose-induced increased mitochondrial permeability and cytochrome C (Cyt-C) release to prevent endothelial cell apoptosis[53].
Studies have shown that MET can activate the AMPK and PI3K/Akt/mTOR/S6K pathways, increase superoxide dismutase (SOD) and glutathione levels, induce nuclear factor erythroid-2-related factor 2 (Nrf2) aggregation, and increase mitochondrial energy reserves to reduce retinal ganglion cell and RPE cell death in many oxidative stress and inflammation models[6],[8],[47],[54]–[63]. Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) regulates mitochondrial biosynthesis. AMPK activation can regulate mitochondrial function through PGC-1α and alleviate senescence and injury in RPE cells caused by hydrogen peroxide (H2O2), TNF-α, and ultraviolet light[56]–[57],[64], and photoreceptor cell death[65]. Other studies showed that the AMPK-mTOR pathway can induce autophagy in RPE cells which plays an important role in self-clearance and the maintenance of normal cellular function. Therefore, this pathway is also regarded as useful for AMD prevention and treatment[66]–[67]. In summary, MET can activate AMPK to reduce RPE and photoreceptor cell death in the prevention and treatment AMD.
MET Can Inhibit CNV Formation
The relationship between inflammation and neovascularization has been demonstrated[68]–[69]. OxLDL can induce cell pyroptosis and releasing of IL-1β, which promotes the expression of hypoxia-inducible factor-1 alpha (HIF-1α) and VEGF. IL-1β can also simulate mast cells to secrete IL-8, promoting the survival and proliferation of vascular endothelial cells and expression of matrix metalloproteinase (MMP)-2 and MMP-9, thereby promoting neovascularization[69]–[72]. In addition, the disrupted outer retinal barrier facilitates the spread of inflammatory factors and growth factors as well as the subsequent growth of CNV into the retina. In addition to increasing the release of inflammatory factors, OxLDL can also promote CNV by increasing VEGF, VEGF receptor 2 (VEGFR2), and transforming growth factor beta (TGF-β) expression of vascular endothelial cells[73]–[74].
As MET can inhibit inflammation, it can reduce inflammation-related neovascularization[75]. In addition, experiments showed that AMPK activation can decrease MMP-2 and MMP-9 expression in endothelial cells and endothelial progenitor cells, and inhibit cell proliferation, migration, and tube formation[76]–[77]. Han et al[78] showed that MET can inhibit TNF-induced expression of ICAM-1 and MCP-1, as well as inhibit the proliferation, migration, and tube formation of endothelial cells, thereby preventing retinal neovascularization. MET can also reduce intracellular cholesterol in vascular endothelial cells, thus to disrupt the structure of lipid rafts, inhibit VEGFR2 dimerization and phosphorylation, and block VEGF-induced neovascularization[79]. Further analysis showed that MET can induce VEGF-A mRNA splicing to form VEGF120, thereby reducing VEGFR2 activation[5],[80]. Studies of oxygen-induced retinopathy (OIR) and very low-density lipoprotein receptor (VLDL-R) knockout animal models also showed that MET can decrease vascular inflammation and inhibit retinal neovascularization[5],[78]. In the laser-induced mouse CNV model, intraperitoneal injection of MET could inhibit CNV formation[9]. Clinical studies also showed that long-term MET treatment decreased the levels of VEGF and plasminogen activator inhibitor (PAI-1) in patients with type 2, thereby alleviating the severity of diabetic retinopathy and reducing the incidence of AMD[81].
CONCLUSION
In summary, oxidative stress and inflammation induced by OxPLs, a main component of drusen, can cause RPE cell death and CNV formation, which are the main pathological changes in late-stage AMD. MET was recently found to have the functions to regulate lipid metabolism, inhibit inflammation, prohibit retinal cells from death, and inhibit CNV formation (Figure 1). This makes MET a good candidate drug to prevent and treat AMD. Further in-depth investigation of the mechanism by which MET affects AMD and multicenter clinical studies are needed to validate the efficacy of MET and the way of its administration in AMD.
Acknowledgments
Foundation: Supported by the Natural Science Foundation of Shaanxi Province (No.2019SF-047).
Conflicts of Interest: Dang KR, None; Wu T, None; Hui YN, None; Du HJ, None.
REFERENCES
- 1.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122(6):253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ursini F, Russo E, Pellino G, D'Angelo S, Chiaravalloti A, De Sarro G, Manfredini R, De Giorgio R. Metformin and autoimmunity: a “new deal” of an old drug. Front Immunol. 2018;9:1236. doi: 10.3389/fimmu.2018.01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Massey S, Story D, Li LX. Metformin: an old drug with new applications. Int J Mol Sci. 2018;19(10):2863. doi: 10.3390/ijms19102863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao QS, Song W, Huang JQ, Wang D, Xu CW. Metformin decreased myocardial fibrosis and apoptosis in hyperhomocysteinemia -induced cardiac hypertrophy. Curr Res Transl Med. 2021;69(1):103270. doi: 10.1016/j.retram.2020.103270. [DOI] [PubMed] [Google Scholar]
- 5.Joe SG, Yoon YH, Choi JA, Koh JY. Anti-angiogenic effect of metformin in mouse oxygen-induced retinopathy is mediated by reducing levels of the vascular endothelial growth factor receptor Flk-1. PLoS One. 2015;10(3):e0119708. doi: 10.1371/journal.pone.0119708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A L, Zou T, He J, Chen X, Sun D, Fan X, Xu H. Rescue of retinal degeneration in rd1 mice by intravitreally injected metformin. Front Mol Neurosci. 2019;12:102. doi: 10.3389/fnmol.2019.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu CW, Tsen CL, Li MS, Bee YS, Lin SH, Sheu SJ. Metformin and rapamycin protect cells from vital dye-induced damage in retinal pigment epithelial cells and in vivo. Graefes Arch Clin Exp Ophthalmol. 2020;258(3):557–564. doi: 10.1007/s00417-019-04548-z. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Kong L, Wang JG, Ash JD. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2018;115(41):10475–10480. doi: 10.1073/pnas.1802724115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ying Y, Ueta T, Jiang S, Lin H, Wang Y, Vavvas D, Wen R, Chen YG, Luo Z. Metformin inhibits ALK1-mediated angiogenesis via activation of AMPK. Oncotarget. 2017;8(20):32794–32806. doi: 10.18632/oncotarget.15825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu S, Zhang C, Liu D, Wu J, Tian H, Lu L, Xu GT, Liu F, Zhang J. Metformin protects ARPE-19 cells from glyoxal-induced oxidative stress. Oxid Med Cell Longev. 2020;2020:1740943. doi: 10.1155/2020/1740943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alomar SY, M Barakat B, Eldosoky M, Atef H, Mohamed AS, Elhawary R, El-Shafey M, Youssef AM, Elkazaz AY, Gabr AM, Elaskary AA, Salih MAK, Alolayan SO, Zaitone SA. Protective effect of metformin on rat diabetic retinopathy involves suppression of toll-like receptor 4/nuclear factor-k B expression and glutamate excitotoxicity. Int Immunopharmacol. 2021;90:107193. doi: 10.1016/j.intimp.2020.107193. [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Wu Q, Zhu Y, Liu Y, Xie X, Li S, Lin H, Chen W, Zhu F. Co-delivery of metformin and levofloxacin hydrochloride using biodegradable thermosensitive hydrogel for the treatment of corneal neovascularization. Drug Deliv. 2019;26(1):522–531. doi: 10.1080/10717544.2019.1609623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XR, Leng Y, Jiang QZ, Wang ZW, Luo P, Zhang C, Chen L, Wang YW, Wang HL, Yue XF, Shen CX, Zhou Y, Shi CM, Xie L. Eye drops of metformin prevents fibrosis after glaucoma filtration surgery in rats via activating AMPK/Nrf2 signaling pathway. Front Pharmacol. 2020;11:1038. doi: 10.3389/fphar.2020.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan YP, Wu CT, Lin JL, Hsiung CA, Liu HY, Lai JN, Yang CC. Metformin treatment is associated with a decreased risk of nonproliferative diabetic retinopathy in patients with type 2 diabetes mellitus: a population-based cohort study. J Diabetes Res. 2020;2020:9161039. doi: 10.1155/2020/9161039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Qi YH. Application prospects of metformin in ophthalmology. Guoji Yanke Zazhi(Int Eye Sci) 2017;17(4):673–676. [Google Scholar]
- 16.Brown EE, Ball JD, Chen Z, Khurshid GS, Prosperi M, Ash JD. The common antidiabetic drug metformin reduces odds of developing age-related macular degeneration. Invest Ophthalmol Vis Sci. 2019;60(5):1470–1477. doi: 10.1167/iovs.18-26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YY, Shen YC, Lai YJ, Wang CY, Lin KH, Feng SC, Liang CY, Wei LC, Chou P. Association between metformin and a lower risk of age-related macular degeneration in patients with type 2 diabetes. J Ophthalmol. 2019;2019:1649156. doi: 10.1155/2019/1649156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blitzer AL, Ham SA, Colby KA, Skondra D. Association of metformin use with age-related macular degeneration: a case-control study. JAMA Ophthalmol. 2021;139(3):302–309. doi: 10.1001/jamaophthalmol.2020.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart JM, Lamy R, Wu F, Keenan JD. Relationship between oral metformin use and age-related macular degeneration. Ophthalmol Retina. 2020;4(11):1118–1119. doi: 10.1016/j.oret.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong WL, Su XY, Li X, Cheung CMG, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Heal. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Bedell M, Zhang K. Age-related macular degeneration: genetic and environmental factors of disease. Mol Interv. 2010;10(5):271–281. doi: 10.1124/mi.10.5.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu XR, Neric NJ, Crabb JS, Crabb JW, Bhattacharya SK, Rayborn ME, Hollyfield JG, Bonilha VL. Age-related changes in the retinal pigment epithelium (RPE) PLoS One. 2012;7(6):e38673. doi: 10.1371/journal.pone.0038673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gnanaguru G, Choi AR, Amarnani D, D'Amore PA. Oxidized lipoprotein uptake through the CD36 receptor activates the NLRP3 inflammasome in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2016;57(11):4704–4712. doi: 10.1167/iovs.15-18663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoppe G, O'Neil J, Hoff HF, Sears J. Accumulation of oxidized lipid-protein complexes alters phagosome maturation in retinal pigment epithelium. Cell Mol Life Sci. 2004;61(13):1664–1674. doi: 10.1007/s00018-004-4080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandstetter C, Patt J, Holz FG, Krohne TU. Inflammasome priming increases retinal pigment epithelial cell susceptibility to lipofuscin phototoxicity by changing the cell death mechanism from apoptosis to pyroptosis. J Photochem Photobiol B. 2016;161:177–183. doi: 10.1016/j.jphotobiol.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Shaw PX, Stiles T, Douglas C, Ho D, Fan W, Du HJ, Xiao X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci. 2016;3(2):196–221. doi: 10.3934/molsci.2016.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernández-Zimbrón LF, Zamora-Alvarado R, Ochoa-De la Paz L, Velez-Montoya R, Zenteno E, Gulias-Cañizo R, Quiroz-Mercado H, Gonzalez-Salinas R. Age-related macular degeneration: new paradigms for treatment and management of AMD. Oxidative Med Cell Longev. 2018;2018:8374647. doi: 10.1155/2018/8374647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119(10):1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jemni-Damer N, Guedan-Duran A, Fuentes-Andion M, Serrano-Bengoechea N, Alfageme-Lopez N, Armada-Maresca F, Guinea GV, Perez-Rigueiro J, Rojo F, Gonzalez-Nieto D, Kaplan DL, Panetsos F. Biotechnology and biomaterial-based therapeutic strategies for age-related macular degeneration. part II: cell and tissue engineering therapies. Front Bioeng Biotechnol. 2020;8:588014. doi: 10.3389/fbioe.2020.588014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martín-Rodríguez S, de Pablos-Velasco P, Calbet JAL. Mitochondrial complex I inhibition by metformin: drug-exercise interactions. Trends Endocrinol Metab. 2020;31(4):269–271. doi: 10.1016/j.tem.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Stein BD, Calzolari D, Hellberg K, Hu YS, He L, Hung CM, Toyama EQ, Ross DS, Lillemeier BF, Cantley LC, Yates JR, Shaw RJ. Quantitative in vivo proteomics of metformin response in liver reveals AMPK-dependent and-independent signaling networks. Cell Rep. 2019;29(10):3331–3348.e7. doi: 10.1016/j.celrep.2019.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulman IG. Liver X receptors link lipid metabolism and inflammation. FEBS Lett. 2017;591(19):2978–2991. doi: 10.1002/1873-3468.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325(5936):100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sáenz J, Alba G, Reyes-Quiroz ME, Geniz I, Jiménez J, Sobrino F, Santa-María C. Curcumin enhances LXRα in an AMP-activated protein kinase-dependent manner in human macrophages. J Nutr Biochem. 2018;54:48–56. doi: 10.1016/j.jnutbio.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 35.He X, Chen X, Wang L, Wang W, Liang Q, Yi L, Wang Y, Gao Q. Metformin ameliorates Ox-LDL-induced foam cell formation in raw264.7 cells by promoting ABCG-1 mediated cholesterol efflux. Life Sci. 2019;216:67–74. doi: 10.1016/j.lfs.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Abdul NS, Chuturgoon AA. Fumonisin B1 regulates LDL receptor and ABCA1 expression in an LXR dependent mechanism in liver (HepG2) cells. Toxicon. 2021;190:58–64. doi: 10.1016/j.toxicon.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Hiebl V, Schachner D, Ladurner A, Heiss EH, Atanasov AG, Dirsch VM. Soraphen A enhances macrophage cholesterol efflux via indirect LXR activation and ABCA1 upregulation. Biochem Pharmacol. 2020;177:114022. doi: 10.1016/j.bcp.2020.114022. [DOI] [PubMed] [Google Scholar]
- 38.Bułdak Ł, Machnik G, Bułdak RJ, Łabuzek K, Bołdys A, Okopień B. Exenatide and metformin express their anti-inflammatory effects on human monocytes/macrophages by the attenuation of MAPKs and NFκB signaling. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(10):1103–1115. doi: 10.1007/s00210-016-1277-8. [DOI] [PubMed] [Google Scholar]
- 39.Bai B, Chen HB. Metformin: a novel weapon against inflammation. Front Pharmacol. 2021;12:622262. doi: 10.3389/fphar.2021.622262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly B, Tannahill GM, Murphy MP, O'Neill LAJ. Metformin inhibits the production of reactive oxygen species from NADH: ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J Biol Chem. 2015;290(33):20348–20359. doi: 10.1074/jbc.M115.662114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor κB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47(6):1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 42.Jansen T, Kvandová M, Daiber A, Stamm P, Frenis K, Schulz E, Münzel T, Kröller-Schön S. The AMP-activated protein kinase plays a role in antioxidant defense and regulation of vascular inflammation. Antioxidants. 2020;9(6):525. doi: 10.3390/antiox9060525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skrha J, Prázný M, Hilgertová J, Kvasnicka J, Kalousová M, Zima T. Oxidative stress and endothelium influenced by metformin in type 2 diabetes mellitus. Eur J Clin Pharmacol. 2007;63(12):1107–1114. doi: 10.1007/s00228-007-0378-1. [DOI] [PubMed] [Google Scholar]
- 44.Cahova M, Palenickova E, Dankova H, Sticova E, Burian M, Drahota Z, Cervinkova Z, Kucera O, Gladkova C, Stopka P, Krizova J, Papackova Z, Oliyarnyk O, Kazdova L. Metformin prevents ischemia reperfusion-induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am J Physiol Gastrointest Liver Physiol. 2015;309(2):G100–G111. doi: 10.1152/ajpgi.00329.2014. [DOI] [PubMed] [Google Scholar]
- 45.Jing YY, Wu F, Li D, Yang L, Li Q, Li R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol Cell Endocrinol. 2018;461:256–264. doi: 10.1016/j.mce.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 46.Kalariya NM, Shoeb M, Ansari NH, Srivastava SK, Ramana KV. Antidiabetic drug metformin suppresses endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2012;53(7):3431–3440. doi: 10.1167/iovs.12-9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamoshita M, Fujinami K, Toda E, Tsubota K, Ozawa Y. Neuroprotective effect of activated 5′-adenosine monophosphate-activated protein kinase on cone system function during retinal inflammation. BMC Neurosci. 2016;17(1):32. doi: 10.1186/s12868-016-0268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan K, Abukhousa IMO, Wang YD. Research progress of a new type of programmed cell death-pyroptosis. Progress in Modern Biomedicine. 2019;19(9):1793–1796. [Google Scholar]
- 49.Zhang L, Lu L, Zhong X, Yue Y, Hong Y, Li Y, Li Y. Metformin reduced NLRP3 inflammasome activity in Ox-LDL stimulated macrophages through adenosine monophosphate activated protein kinase and protein phosphatase 2A. Eur J Pharmacol. 2019;852:99–106. doi: 10.1016/j.ejphar.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Chen B, Li J, Zhu H. AMP-activated protein kinase attenuates oxLDL uptake in macrophages through PP2A/NF-κB/LOX-1 pathway. Vascul Pharmacol. 2016;85:1–10. doi: 10.1016/j.vph.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Huangfu N, Wang Y, Cheng J, Xu Z, Wang S. Metformin protects against oxidized low density lipoprotein-induced macrophage apoptosis and inhibits lipid uptake. Exp Ther Med. 2018;15(3):2485–2491. doi: 10.3892/etm.2018.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Shi W, Gao X, SreeHarsha N, Zhang D. Cardioprotective role of metformin against sodium arsenite-induced oxidative stress, inflammation, and apoptosis. IUBMB Life. 2020;72(4):749–757. doi: 10.1002/iub.2174. [DOI] [PubMed] [Google Scholar]
- 53.Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, Leverve X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54(7):2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 54.Rotermund C, Machetanz G, Fitzgerald JC. The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol. 2018;9:400. doi: 10.3389/fendo.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harun-Or-Rashid M, Inman DM. Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J Neuroinflammation. 2018;15(1):313. doi: 10.1186/s12974-018-1346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S, Gaur U, Chong CM, Lin SF, Fang JK, Zeng ZW, Wang HT, Zheng WH. Berberine protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of AMPK. Int J Mol Sci. 2018;19(6):1736. doi: 10.3390/ijms19061736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li XF, Liu XM, Huang DR, Cao HJ, Wang JY. PF-06409577 activates AMPK signaling to protect retinal pigment epithelium cells from UV radiation. Biochem Biophys Res Commun. 2018;501(1):293–299. doi: 10.1016/j.bbrc.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Kaarniranta K, Kajdanek J, Morawiec J, Pawlowska E, Blasiak J. PGC-1α protects RPE cells of the aging retina against oxidative stress-induced degeneration through the regulation of senescence and mitochondrial quality control. the significance for AMD pathogenesis. Int J Mol Sci. 2018;19(8):2317. doi: 10.3390/ijms19082317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar A, Giri S, Kumar A. 5-Aminoimidazole-4-carboxamide ribonucleoside-mediated adenosine monophosphate-activated protein kinase activation induces protective innate responses in bacterial endophthalmitis. Cell Microbiol. 2016;18(12):1815–1830. doi: 10.1111/cmi.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawashima H, Ozawa Y, Toda E, Homma K, Osada H, Narimatsu T, Nagai N, Tsubota K. Neuroprotective and vision-protective effect of preserving ATP levels by AMPK activator. FASEB J. 2020;34(4):5016–5026. doi: 10.1096/fj.201902387RR. [DOI] [PubMed] [Google Scholar]
- 61.Zhu X, Wang K, Zhou F, Zhu L. Paeoniflorin attenuates atRAL-induced oxidative stress, mitochondrial dysfunction and endoplasmic reticulum stress in retinal pigment epithelial cells via triggering Ca2+/CaMKII-dependent activation of AMPK. Arch Pharm Res. 2018;41(10):1009–1018. doi: 10.1007/s12272-018-1059-6. [DOI] [PubMed] [Google Scholar]
- 62.Kim SH, Park JW. Morin hydrate attenuates CSE-induced lipid accumulation, ER stress, and oxidative stress in RPE cells: implications for age-related macular degeneration. Free Radic Res. 2019;53(8):865–874. doi: 10.1080/10715762.2019.1637862. [DOI] [PubMed] [Google Scholar]
- 63.Khallaghi B, Safarian F, Nasoohi S, Ahmadiani A, Dargahi L. Metformin-induced protection against oxidative stress is associated with AKT/mTOR restoration in PC12 cells. Life Sci. 2016;148:286–292. doi: 10.1016/j.lfs.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 64.Chung EJ, Efstathiou NE, Konstantinou EK, Maidana DE, Miller JW, Young LH, Vavvas DG. AICAR suppresses TNF-α-induced complement factor B in RPE cells. Sci Rep. 2017;7(1):17651. doi: 10.1038/s41598-017-17744-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Athanasiou D, Aguila M, Opefi CA, South K, Bellingham J, Bevilacqua D, Munro PM, Kanuga N, Mackenzie FE, Dubis AM, Georgiadis A, Graca AB, Pearson RA, Ali RR, Sakami S, Palczewski K, Sherman MY, Reeves PJ, Cheetham ME. Rescue of mutant rhodopsin traffic by metformin-induced AMPK activation accelerates photoreceptor degeneration. Hum Mol Genet. 2017;26(2):305–319. doi: 10.1093/hmg/ddw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hyttinen JM, Petrovski G, Salminen A, Kaarniranta K. 5′-Adenosine monophosphate-activated protein kinase—mammalian target of rapamycin axis as therapeutic target for age-related macular degeneration. Rejuvenation Res. 2011;14(6):651–660. doi: 10.1089/rej.2011.1220. [DOI] [PubMed] [Google Scholar]
- 67.Zhang ZY, Bao XL, Cong YY, Fan B, Li GY. Autophagy in age-related macular degeneration: a regulatory mechanism of oxidative stress. Oxidative Med Cell Longev. 2020;2020:2896036. doi: 10.1155/2020/2896036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohr T, Haudek-Prinz V, Slany A, Grillari J, Micksche M, Gerner C. Proteome profiling in IL-1β and VEGF-Activated human umbilical vein endothelial cells delineates the interlink between inflammation and angiogenesis. PLoS One. 2017;12(6):e0179065. doi: 10.1371/journal.pone.0179065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim GY, Lee JW, Ryu HC, Wei JD, Seong CM, Kim JH. Proinflammatory cytokine IL-1beta stimulates IL-8 synthesis in mast cells via a leukotriene B4 receptor 2-linked pathway, contributing to angiogenesis. J Immunol. 2010;184(7):3946–3954. doi: 10.4049/jimmunol.0901735. [DOI] [PubMed] [Google Scholar]
- 70.Argaw AT, Zhang YT, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177(8):5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- 71.Lavalette S, Raoul W, Houssier M, Camelo S, Levy O, Calippe B, Jonet L, Behar-Cohen F, Chemtob S, Guillonneau X, Combadière C, Sennlaub F. Interleukin-1β inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am J Pathol. 2011;178(5):2416–2423. doi: 10.1016/j.ajpath.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li AH, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170(6):3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 73.Wu T, Xu WQ, Wang YF, Tao MZ, Hu ZC, Lv B, Hui YN, Du HJ. OxLDL enhances choroidal neovascularization lesion through inducing vascular endothelium to mesenchymal transition process and angiogenic factor expression. Cell Signal. 2020;70:109571. doi: 10.1016/j.cellsig.2020.109571. [DOI] [PubMed] [Google Scholar]
- 74.Dandapat A, Hu CP, Sun LQ, Mehta JL. Small concentrations of oxLDL induce capillary tube formation from endothelial cells via LOX-1-dependent redox-sensitive pathway. Arterioscler Thromb Vasc Biol. 2007;27(11):2435–2442. doi: 10.1161/ATVBAHA.107.152272. [DOI] [PubMed] [Google Scholar]
- 75.Bharath LP, Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol. 2021;320(5):C873–C879. doi: 10.1152/ajpcell.00604.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Esfahanian N, Shakiba Y, Nikbin B, Soraya H, Maleki-Dizaji N, Ghazi-Khansari M, Garjani A. Effect of metformin on the proliferation, migration, and MMP-2 and -9 expression of human umbilical vein endothelial cells. Mol Med Rep. 2012;5(4):1068–1074. doi: 10.3892/mmr.2012.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li WD, Li NP, Song DD, Rong JJ, Qian AM, Li XQ. Metformin inhibits endothelial progenitor cell migration by decreasing matrix metalloproteinases, MMP-2 and MMP-9, via the AMPK/mTOR/autophagy pathway. Int J Mol Med. 2017;39(5):1262–1268. doi: 10.3892/ijmm.2017.2929. [DOI] [PubMed] [Google Scholar]
- 78.Han J, Li Y, Liu X, Zhou T, Sun H, Edwards P, Gao H, Yu FS, Qiao X. Metformin suppresses retinal angiogenesis and inflammation in vitro and in vivo. PLoS One. 2018;13(3):e0193031. doi: 10.1371/journal.pone.0193031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noghero A, Perino A, Seano G, Saglio E, Lo Sasso G, Veglio F, Primo L, Hirsch E, Bussolino F, Morello F. Liver X receptor activation reduces angiogenesis by impairing lipid raft localization and signaling of vascular endothelial growth factor receptor-2. Arterioscler Thromb Vasc Biol. 2012;32(9):2280–2288. doi: 10.1161/ATVBAHA.112.250621. [DOI] [PubMed] [Google Scholar]
- 80.Yi QY, Deng G, Chen N, Bai ZS, Yuan JS, Wu GH, Wang YW, Wu SJ. Metformin inhibits development of diabetic retinopathy through inducing alternative splicing of VEGF-A. Am J Transl Res. 2016;8(9):3947–3954. [PMC free article] [PubMed] [Google Scholar]
- 81.Ersoy C, Kiyici S, Budak F, Oral B, Guclu M, Duran C, Selimoglu H, Erturk E, Tuncel E, Imamoglu S. The effect of metformin treatment on VEGF and PAI-1 levels in obese type 2 diabetic patients. Diabetes Res Clin Pract. 2008;81(1):56–60. doi: 10.1016/j.diabres.2008.02.006. [DOI] [PubMed] [Google Scholar]


