Abstract
Age-related eye diseases, including cataract, glaucoma, diabetic retinopathy (DR), and age-related macular degeneration (AMD), are the leading causes of vision loss in the world. Several studies have shown that the occurrence and development of these diseases have an important relationship with oxidative stress in the eye. The Keap1-Nrf2-ARE pathway is a classical pathway that resists oxidative stress and inflammation in the body. This pathway is also active in the development of age-related eye diseases. A variety of drugs have been shown to treat age-related eye diseases through the Keap1-Nrf2-ARE (Kelch-like ECH-Associating protein 1- nuclear factor erythroid 2 related factor 2-antioxidant response element) pathway. This review describes the role of oxidative stress in the development of age-related eye diseases, the function and regulation of the Keap1-Nrf2-ARE pathway, and the therapeutic effects of drugs associated with this pathway on age-related eye diseases.
Keywords: oxidative stress, Keap1-Nrf2-ARE pathway, age-related eye diseases
INTRODUCTION
Age-related eye diseases, including cataract, glaucoma, diabetic retinopathy (DR), and age-related macular degeneration (AMD) are the main causes of blindness worldwide[1]. As a degenerative lesion of the eye, several studies have shown that the occurrence and development of these diseases have an important relationship with oxidative stress in the eye[1]–[4]. Keap1-Nrf2-ARE (Kelch-like ECH-Associating protein 1- nuclear factor erythroid 2 related factor 2-antioxidant response element) pathway is a classic pathway to resist oxidative stress in the body. Under normal conditions, Nrf2 binds to Keap1 and is inactivated by Keap1-dependent ubiquitination, thereby remaining at a low level. Under oxidative stress, the cysteine residue of Keap1 is modified and loses ubiquitin activity. Then, Nrf2 transfers to the nucleus, where it binds to ARE and mediates the expression of a series of antioxidant protein genes[5]. Modulation of this pathway has been widely recognized as a new pharmacological target for the treatment of many diseases. In recent years, the role of this pathway in the development of age-related eye diseases has also gained attention among researchers. A variety of drugs have been shown to treat age-related eye diseases or delay the progression through the Keap1-Nrf2-ARE pathway, such as calcium dobesilate (CaD), polyphenols, alpha-lipoic acid (ALA), Lutein/zeaxanthin isomers (L/Zi), probucol and coumarins[1],[6]–[8]. This review describes the role of oxidative stress in the development of age-related eye diseases, the function and regulation of the Keap1-Nrf2-ARE pathway, and the therapeutic effects of drugs associated with this pathway on age-related eye diseases.
OXIDATIVE STRESS AND AGE-RELATED EYE DISEASE
Oxidative stress occurs with the interference between the antioxidant and the pro-oxidant[9]. Antioxidant enzymes cannot quench free radicals, causing the overproduction of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which interact with many macromolecules in the cell. The excessive ROS and RNS injury cellular components including proteins, lipids, and DNA. These changes lead to a variety of diseases, including cancer, aging, cardiovascular and metabolic diseases[10]. In recent years, an increasing number of studies have shown that oxidative stress plays a key role in the occurrence and development of age-related eye diseases, such as cataracts, glaucoma, DR, and AMD[9].
Cataract
Cataract is the leading cause of global vision loss[10]. The etiology is the deposition of aggregated proteins in the lens and plasma membrane injury of lens fiber cells, leading to lens opacity, light scattering, and visual impairment[11]. The main symptoms include decreased vision, glare, and monocular diplopia[12]. The pathogenesis of cataract involves multiple mechanisms and oxidative stress is important in this process[13]. The lens is sensitive to oxidative stress from ultraviolet radiation, metabolic changes, and osmotic pressure[14]. Studies have shown that under oxidative stress, calcium ATPase in the plasma membrane is oxidized and loses its ion-transfer function. Calcium ions cannot be transported out of the lens epithelium, resulting in the activation of the m-calpain. The activated m-calpain causes dysfunction and precipitation of lens proteins[12]. Oxidative stress causes ROS to accumulate in the lens, leading to apoptosis and autophagy of the epithelial cells, and the opacity of len cortex[15]. Apoptosis associated with diabetic cataract formation occurs in human lens epithelial cells (HLE) after exposed to high glucose-induced oxidative stress for 24h[16]. The expression of let-7c-3p (a miRNA capable of inhibiting apoptosis and autophagy) is also downregulated in HLE[17]. Oxidative stress can also promote the development of cataract by destroying intracellular macromolecules, especially mitochondrial DNA, and disrupting the balance of electrolyte homeostasis[1]. Furthermore, the polyol pathway, which is also an important mechanism for the onset of diabetes, has crosstalk with oxidative stress. The accumulated polyol upregulates the level of aldose reductase enzyme (AR). Excessive consumption of nicotinamide adenine dinucleotide phosphate oxidase (NADPH) by AR inhibits the reduction of oxygen and increases the generation of ROS[13],[18].
Glaucoma
Glaucoma is a neurodegenerative disease characterized by progressive visual field defects. The pathological changes include progressive loss of retinal ganglion cells (RGCs) and structural changes in the optic papilla and disc[19]. Pathological increase in intraocular pressure (IOP) is an primary risk factor for glaucoma[20]. The common cause of hypertensive glaucoma is the oxidative stress of the trabecular meshwork (TM)[21], which is the classic aqueous outflow pathway and is important in regulation of IOP[22]. ROS causes TM mitochondrial dysfunction, inflammatory factor release, and extracellular matrix (ECM) composition changes, which causing dysfunction of TM[21]. Rotenone is a drug for establishing glaucoma model, it can inhibit mitochondrial electron chain complex I, cause the production of ROS and induce the senescence of human trabecular meshwork (HTM) cells[23]. Defects in mitochondrial complex I have been reported to be associated with TM cell degeneration in patients with primary open angle glaucoma (POAG)[24]–[25]. Under oxidative stress, F-actin cytoskeletal is reorganized, which changes the morphology and contractile function of TM tissues and increases aqueous outflow resistance[21],[26]. Oxidative stress-induced death of RGCs is also one of the pathogeneses of glaucoma[27]. Elevated level of ROS causes protein damage, lipid peroxidation, calcium overload, and mitochondrial dysfunction, ultimately leading to RGCs death and apoptosis[28]. The nuclear protein sirtuin 6 (SIRT6) promotes Nrf2/ARE signaling and decreases ROS generation via deacetylation of Nrf2, prevents hydrogen peroxide-induced apoptosis in RGCs[2]. Sestrin2 protein (a stress-inducible antioxidant protein) can downregulate Keap1 expression and activate Nrf2, alleviate RGC cell apoptosis under oxidative stress[29]. Lycium barbarum polysaccharides (LBP) protects RGC from chloride (CoCl2)-induced oxidative stress through inhibiting ROS generation and reducing RGC mitochondrial membrane potential[30].
Diabetic Retinopathy
DR is a common microvascular complications of diabetes and is also an important cause of non-traumatic blindness in adults[31]. The mechanism is hyperglycemia-induced inflammation and rupture of retinal microvasculature, causing distortion of the microvascular system and ultimately leading to retinal detachment[32]–[33]. Oxidative stress is the main pathological process of DR[34]–[35]. Persistent hyperglycemia induces many metabolic abnormalities in the retina, including inflammation, activation of protein kinase C (PKC), activation of hexosamine and polyol pathways[7]. These processes are all associated with mitochondrial overproduction of ROS, ultimately leading to increased oxidative stress[32]. Persistent hyperglycemia elevates the enzymatic activity of arginase. The activated arginase promotes activation of retinal angiogenesis through restoring NO and increasing oxidative stress[32]. Hyperglycemia-induced oxidative stress upregulates the expression of inflammatory factors[35] and induce apoptosis of pericytes through stimulating PKC-δ signaling[36]. Several studies demonstrated that hyperglycemia-induced oxidative stress can also increase mitochondrial permeability by opening permeability transition pores (MTP), leading to mitochondrial swelling and cytochrome C release[37]. Cheng et al[31] found that pancreatic kallikrein can significantly improve retinal pathological features in mouse models of type 2 diabetes by decreasing ROS production, downregulating NADPH oxidase 2 (NOX2), and upregulating the antioxidant superoxide dismutase 2 (SOD2). In addition, studies have shown that specific antioxidant supplementation can reduce the rate of DR progression by enhancing antioxidant defenses[38]–[39].
Age-related Macular Degeneration
AMD is a cumulative disease of the central retina. It is a major cause of blindness in the elderly and the prevalence increases with age[40]. It is characterized by injury to the retinal pigmental epithelium (RPE) cells in the macula, leading to foveal sensation damage[41]. RPE degeneration is mainly caused by long-term oxidative stress[42]. Retina is susceptible to oxidative stress on account of the high concentration of oxygen, the exposure to light and the high content of unsaturated fatty acids[43]–[44]. Excessive ROS and declining antioxidant systems result in oxidative stress, causing damage and apoptosis of RPE cells, photoreceptor cells, and choroidal capillary layers[1]. The mitochondrial DNA in human RPE cells exposed to oxidative stress is damaged and not effectively repaired[45]. The study by Alamri et al[46] showed that the loss of translocator protein (TSPO) can injury RPE cells through affecting multiple metabolism, while TSPO regulates mitochondria-mediated oxidative stress in RPE cells. Oxidative stress also causes lipid peroxidation and protein oxidation. The levels of malondialdehyde (MDA) and advanced oxidation protein products (AOPP) in the blood of AMD patients are higher than normal[47]. The activity of SOD (an antioxidant enzyme which can quench ROS) in the blood of AMD patients also decreases, aggravating oxidative stress[42]. In addition, studies by Sheu et al[44] and Du et al[48] found that the apoptosis of RPE cells can be reduced through inhibiting oxidative stress and activating antioxidant defense.
KEAP1-NRF2-ARE PATHWAY AND OXIDATIVE STRESS
To protect macromolecules from the adverse effects of oxidative stress, cells have developed multiple defense systems[33], as well as a range of antioxidant stress signaling pathways, including the JAK/STAT (Janus kinase-signal transducer and activator of transcription) signaling pathway, the PI3K/Akt (phosphatidylinositol-3-kinase-protein kinase B) signaling pathway, and Keap1-Nrf2-ARE pathway, etc. The Keap1-Nrf2-ARE signaling pathway plays a central role in the body's antioxidant stress. Studies have shown that, through the expression of Akt protein, the JAK/STAT signaling pathway induces the phosphorylation of Nrf2, which is then translocated to the nucleus, combined with an ARE, and finally activates the transcription of downstream genes[49]. When the PI3K/Akt signaling pathway is activated, phosphorylated Nrf2 can be released from Keap1 and translocated to the nucleus to activate expression of downstream antioxidant proteins[50]. The Keap1-Nrf2-ARE pathway is a multi-organ protection chain that is resistant to both exogenous and endogenous oxidative stress. It protects the body from damage or delays the progression of related diseases and is the main defense mechanism against oxidative stress and inflammation[51]–[52]. This pathway has already been a research hotspot in the development mechanism of oxidative stress diseases both at home and abroad.
Nrf2 is a transcription factor of the leucine zipper family that mediates the expression of a variety of antioxidant defense protein genes[53]–[54]. These genes all contain the cis-regulator element sequence ARE (5-GTTACnnnGC-3)[55] as a binding target for Nrf2. Human Nrf2 has six conserved domains, Neh1 to Neh6. A bZip motif in the Neh1 helps Nrf2 form a heterodimer with small Maf proteins, and then heterodimer binds to ARE, activating antioxidant gene transcription. The Neh2 contains a DLG and ETGE motif that binds to the Kelch domain of Keap1 with different affinities, downregulating the transcriptional activity of Nrf2[5],[56].
Keap1 is known as a Nrf2 inhibitor (INrf2). It consists of five domains: N-terminal region (NTR), bric-'a-brac domain (BTB), intervening region (IVR), Kelch region (also known as double glycine repeat, DGR), and C-terminal region (CTR)[1],[57]. The keap1 forms a dimer and binds to the Cullin3 (Cul3) through the BTB region[57]. Upon normal circumstances, Nrf2 binds to Keap1[58], inactivated by Keap1-dependent ubiquitination of Nrf2, and thus remains at a low level[5],[59]. The IVR region contains key cysteine residues that can sense oxidative signals and react with endogenous and exogenous electrophiles[60]–[61]. Under oxidative stress, cysteine residues in IVR are chemically modified by ROS or other electrophiles, preventing the ubiquitination and degradation of the Nrf2 protein. Nrf2 accumulates in the cytoplasm and transfers to the nucleus, where it binds to ARE and mediates a range of gene expressions of antioxidant and cytoprotective proteins[44],[52],[62]–[63], including glutathione peroxidase (GPx), heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO1), glutamate cysteine ligase (GCL), and glutathione transferase[64]–[65]. The Nrf2 protein is known to regulate nearly 200-600 cell protection genes[66] (Figure 1).
Figure 1. Protein domains of Keap1 and Nrf2.

Keap1 consists of five domains: NTR, BTB, IVR, Kelch region (also known as DGR), and CTR. The BTB domain can facilitate Keap1 forming a dimer and interacting with Cul3. The DGR and CTR are also known as the DC domain, which is the Keap1- and Nrf2-binding region. Nrf2 contains six Nrf2-ECH homology (Neh) domains. Neh1 contains a CNC-type basic leucine zipper DNA-binding motif, which is required for the heterodimerization of Nrf2 and proteins of the small Maf family. The Neh2 contains DLG and ETGE motifs. They bind to the Kelch domain of Keap1 with different affinities, regulating the activity of Nrf2. The Neh3 domain helps stabilize the Nrf2 protein. Neh4 and Neh5 are related to transcriptional activity. Neh6 contains a serine-rich conserved region that inhibits Nrf2 independent of Keap1.
Regarding this regulation of Keap1 on Nrf2, there are several models explaining its mechanism, and the more widely accepted one is the “hinge and latch” model[5]. In this model, the high-affinity ETGE motif acts as a “hinge” to fix Nrf2 to Keap1, and the low-affinity DLG motif is a “latch”. Upon normal condition, both the ETCG and DLG bind to keap1, and nrf2 is ubiquitinated and degraded[67]–[68]. Under oxidative stress, the covalently modification of the cysteine residues in Keap1's BTB and IVR domains cause the low-affinity DLG motif to detach from Keap1, while the high-affinity ETGE motif keeps binding to Keap1[69]. The Keap1-cul3 complex loses ubiquitin ligase activity to Nrf2. Nrf2 accumulates in the cytoplasm and transfers to the nucleus, mediating transcription of downstream genes[5],[70] (Figure 2).
Figure 2. The mechanisms involved in the Keap1-Nrf2-ARE pathway: the “hinge and latch” model.
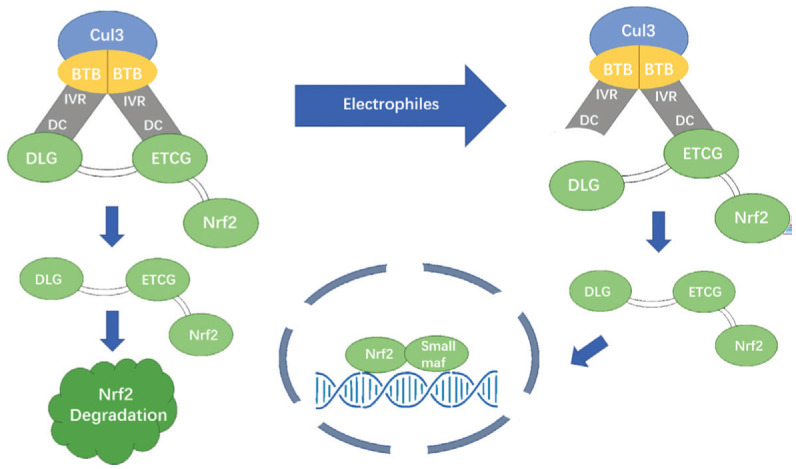
The ETGE and DLG motifs in the Neh2 domains bind to Keap1's DC domain with different affinities. Under normal circumstances, Nrf2 is isolated in the cytosol and keeps a low level through Keap1-dependent ubiquitination and protease degradation. Under oxidative stress, electrophiles (such as ROS) covalently modify the cysteine residues in Keap1's BTB and IVR domains, resulting in a conformational change in Keap1. The low-affinity DLG motif separates from Keap1, while the high-affinity ETGE motif keeps binding to Keap1. The Keap1-cul3 complex loses ubiquitin ligase activity to Nrf2. Nrf2 accumulates in the cytoplasm and metastasizes to the nucleus, upregulating transcription of downstream genes.
THERAPEUTIC EFFECT OF KEAP1-NRF2-ARE PATHWAY-RELATED DRUGS IN AGE-RELATED EYE DISEASES
The Keap1-Nrf2-ARE pathway, a major defense mechanism against oxidative stress and inflammation, has been widely recognized as a new pharmacological target for the treatment of many diseases, including cancer, neurodegenerative diseases, diabetes, reproductive system diseases, septic kidney injury, prion disease, and osteoarthritis[59],[71]–[76]. As the core pathway against oxidative stress, the role of regulation of this pathway in the treatment of age-related eye diseases has also attracted the attention of researchers. Cheng et al[77] evaluated the level of Nrf2 expression in glaucoma trabecular mesh (GTM) and HTM cells. Nrf2 was found to be downregulated in GTM cells relative to HTM cells, and the following experiments have shown that the over-expression of Nrf2 could stimulate cell proliferation and inhibit apoptosis in both GTM and HTM. Multiple studies have also indicated that upregulation of Nrf2 signaling and reduction of ROS production protect RGCs, retinal epithelial cells, and lenses from oxidative stress[2]–[3],[61],[78]–[80]. A variety of drugs have been proved to treat and protect age-related eye diseases through the Keap1-Nrf2-ARE pathway, including CaD, polyphenols, ALA, L/Zi, probucol, and coumarins.
Calcium Dobesilate
CaD is widely used as an angioprotective drug in the treatment of DR, diabetic nephropathy, and many other complications of diabetes. It can reduce blood viscosity, platelet activity and capillary permeability by inhibiting a variety of vasoactive substances[81]. Several studies have shown that CaD can inhibit the signaling of vascular endothelial growth factor (VEGF) and the activation of the autophagy-related PI3K/Akt/mTOR pathway, thereby inhibits endothelial cell migration and permeability change, reduces vascular damage in diabetic nephropathy[82]–[83].
The antioxidant effect of CaD has been demonstrated in several experiments[6],[81],[84]. Jafarey et al[85] demonstrated that CaD can can alleviate the oxidative stress in kidney tissue and protect rats against gentamicin-induced nephrotoxicity. They thought this protective effect of CaD comes from its antioxidant properties. Bogdanov et al[86] reported that CaD significantly decreased the level of dihydroethidium (DHE) and MDA (two oxidative stress markers), in the retina of db/db mice (a diabetic mouse model). It also inhibited interleukin (IL)-6, IL-8, nuclear factor (NF)-κB, tumor necrosis factor (TNF)-α, and other inflammatory factors expression. Leal et al[84] found that CaD can inhibit changes in the level and organization of tight junction protein and leukocyte adhesion to restore excessive vascular permeability in diabetic patients' retina. This protective effect of CaD may be related to the inhibition of p38 mitogen-activated protein kinase (MAPK) and NF-κB activation through alleviating oxidative/nitrosative stress[84]. Regarding the antioxidant mechanism of CAD, recent studies have shown that this may be related to the Keap1-Nrf2-ARE pathway[87]. Sun et al[6] found that CaD can increase the level of lens Nrf2 and HO-1, and inhibit the level of Keap1 in diabetic cataract rats, significantly decrease the degree of len opacity of D-galactose-induced cataract rat model. This suggests that CaD may be able to achieve therapeutic effects by adjusting the oxidant-antioxidant balance of the lens.
Polyphenols
Polyphenolic compounds are a class of organic substances derived from plants, with several phenol structures in their molecular structure. Polyphenols can be divided into different sub-categories, including four families: flavonoids, tannins, phenolic acids, and anthocyanins. Many of these subtypes have been shown to have anti-inflammatory[88], antioxidant[89], anti-allergic, antibacterial, and anti-viral effects[90]. Recent studies have found that polyphenols can prevent the development of age-related eye diseases through the Keap1-Nrf2-ARE pathway[1],[7],[12],[79]. The polyphenol-enriched fraction of Vaccinium uliginosum L. (FH), which contains several flavonoids and anthocyanins, can significantly inhibit the decrease of Nrf2 and HO-1 in the lens of sulphate-induced cataract SD rats. In addition, FH also inhibits the consumption of glutathione (GSH) and the decrease in SOD and GPx activity[12]. Myricetin derivatives from syzygium malaccense is a flavonoid. It activates Nrf2 in ARPE-19 cells, increases the level of the downstream antioxidant protein SOD2, and reduces the production of hydrogen peroxide (H2O2) and ROS in cells. It also downregulates the expression of nitric oxide production factor (NOS2) at the transcriptional level, attenuate oxidative stress-induced apoptosis of ARPE-19 cells[7]. Hesperetin, which also belongs to flavonoid, can prevent H2O2-triggered oxidative damage through upregulating the Keap1-Nrf2/HO-1 signaling pathway in ARPE-19 cells. It inhibits ROS production, enhances the level of SOD and GSH, and reducs the formation of malondialdehyde (MDA). Hesperetin may play a role in the treatment of AMD and prevent further development[79].
Epigallocatechin gallate (EGCG) is a tannin that accounts for more than 50% of the whole tea polyphenols. Short-term supplementation with EGCG has been shown to treat glaucoma by protecting injured retinal neurons due to high IOP[1]. Tea polyphenols can protect the optic nerve by inducing the activation of Nrf2, regulating the activity of antioxidant enzymes and inhibiting the production of ROS. One plausible mechanism of EGCG-induced Nrf2 activation is that modification of cysteine thiols in Keap1 by active EGCG causes Keap1 to lose ubiquitin activity, resulting in activation of Nrf2[1].
Alpha-lipoic Acid
ALA is a natural mitochondrial disulfide complex. It is an important coenzyme of pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase. ALA can resist oxidative stress-mediated tissue damage by promoting the formation of endogenous antioxidants via activation of Nrf2[91]–[92]. Gu et al[91] reported that ALA alleviated oxidative damage of the liver induced by microcystins (MCs). ALA can decrease the level of MCs-induced ROS and promote the reduction of oxidized glutathione (GSSG), the oxidation product of GSH to reduced GSH (rGSH). However, knockdown of Nrf2 expression with shRNA remarkably attenuated α-LA-mediated rGSH regeneration. They speculated that Nrf2 is involved in the antioxidant process of ALA. Lee et al[73] reported that ALA prevented cisplatin cytotoxicity-induced oxidative stress via activation of the NRF2/HO-1 antioxidant pathway.
Recent studies have shown that ALA can protect retinal neurons[4],[8],[13]. Wang et al[8] found that ALA had a significant protective effect on RGC survival after optic nerve crush. Xia et al[93] confirmed that ALA can partially restore the functionality of retinal neurocircuitry and significantly improve the visual response properties of RGCs by activating the antioxidant pathway. Several theories explain the mechanism of ALA antioxidants. Some evidences supported ALA form lipoyl-cysteinyl mixed disulfides on Keap1, which increase Nrf2 activity and promote the transcription of downstream antioxidant genes[94]. Koriyama et al[4] demonstrated that ALA produced H2O2, which activated Keap1/Nrf2 signaling in RGC-5 cells, and induced the expression of the downstream antioxidant protein HO-1. Choi et al[95] established Nrf2-deficient epithelial cell models and found that ALA can still reduce ROS-induced cytotoxicity in these cells regardless of the Nrf2 status. The result suggested that ALA can activate the Nrf2-independent ROS detoxification pathway.
Zhao et al[96] found that lipoamide (LM), a neutral amide derivative of ALA, is more effective than ALA in protecting RPE cells from the acrolein-induced oxidative stress. They found that LM can more potently upregulate the expression of PPARGC1a (the pivotal transcriptional coactivator for mitochondrial biogenesis), increasing mitochondrial biosynthesis. It also more effectively induced the expression of Nrf2 and its translocation to the nucleus, leading to an increase in expression of phase II antioxidant enzymes. This may due to the stable structure of LM, and LM has a lower acidity, which makes it more suitable for physiological pH than ALA[97].
Lutein/zeaxanthin Isomers
L/Zi are oxidized forms of carotenoids (oxycarotenoids) that belong to natural pigments. In addition to a basic C40 isoprenoid structure similar to other carotenoids, they also have an ionone ring at each end[98]. The concentration of L/Zi in the retina, especially the fovea, is much higher than in other tissues, recommending the significant role of L/Zi in retina function and viability[98]. The Age-Related Eye Disease Study 2 (AREDS2) was a phase 3 study and controlled clinical trial performed by the National Institutes of Health's Eye Institute, which was conducted from 2006-2012, involving 4203 patients aged 50 to 85y. This study used L/Zi as a dietary supplement and found the risk of progression to advanced AMD has been reduced by 10% in the patients who received L/Zi compared with those who did not[99]. Until now, L/Zi is the only therapeutic intervention for AMD that succesfully underwent clinical trials.
Many studies have shown that carotenes can react with reactive ROS/RNS as a direct antioxidant, but because of the kinetic limitations due to their low scavenging rate constants, they cannot fully exert their antioxidant activity in vivo[100]–[101]. It is assumed that the major way carotenoids protect cells is inducing endogenous antioxidant defense through the Nrf2-Keap1 pathway[102]–[103]. Structural/functional studies have demonstrated that an electrophilic α, β-unsaturated carbonyl group (a C=C bond conjugated to -CHO aldehyde group) of the carotenoids or itheir cleavage and oxidation derivatives can undergo Michael reaction with Keap1, causing modification of redox sensitive SH groups in Keap1, which allows for Nrf2 release into the cytoplasm, activating downstream signaling[100]. Sahin et al[103] reported that supplementation of L/Zi attenuated the retina edema in mice induced by intense light emitting diode (LED) illumination. L/Zi supplementation also reduced the level of MDA and significantly increased the levels of Nrf2 and HO-1 in the mice retina. Yu et al[104] reported that L/Zi alleviated the apoptosis of the retina ONL layer in BALB/cJ mice induced by blue light exposure and significantly upregulated levels of Nrf2 and HO-1 in the retina. L/Zi can also protect the retina in other ways[100],[103]–[105]. L/Zi reduces inflammation by by quenching free radicals and blocking NF-κB pathway[105]. It has been reported that 415-455 nm of blue-violet light spectrum is the peak quantity for ROS generation, inducing mitochondrial dysfunction on RPE cells. L/Zi can absorb harmful blue light and protect against light-induced retinopathy[103]. L/Zi also reduces light-induced endoplasmic reticulum stress (ERS) in the retina of BALB/cJ mice. ERS can activate the unfolded protein response (UPR)[104]. It has been reported that excessive activation of UPR in the retina of elderly rats can significantly decrease the expression of Nrf2 and its downstream proteins, including rhodopsin and heme oxygenase-1 (HO-1)[106].
Probucol
Probucol is a bisphenol compound and has anti-inflammatory and antioxidant properties[107]–[108]. It is a potent free radical scavenger, which can inhibit lipid oxidation and increases low-density lipoprotein (LDL) catabolism[109]–[110], and is widely used in the treatment of atherosclerosis, xanthoma, and cardiovascular diseases[111]–[112]. Probucol can increase the expression of endogenous antioxidant enzymes and potently eliminate oxygen radical, thus mitigating oxidative stress-induced tissue damage[113]–[115]. Several studies have shown that probucol can treat some eye diseases through antioxidant [109],[115]–[117]. The combined treatment with probucol and insulin can prevent the development of diabetic cataract[116]. Probucol alone decreases the degree of lens opacity and suppresses the increase in protein carbonyls (a marker of reactive oxygen-mediated protein oxidation) in the lens of hyperglycemic rats[115]. The anti-cataract effect of probucol was due to its antioxidant capacity, not by ameliorating hyperglycemia and hyperlipidemia[115]. Experiments have shown that probucol can also inhibit high glucose-induced ROS generation, promote proliferation, and decrease apoptosis in Müller cells[117].
Probucol's antioxidant effect is related to Keap1/Nrf2 signaling pathway[109],[112]. Huang et al[112] demonstrated that probucol can decrease oxidative stress via upregulating the Keap1/Nrf2 signaling pathway, relieve mice cognitive deficits induced by D-galactose. Liu et al[109] found that probucol significantly inhibited retinopathy and retinal neuron apoptosis in streptozotocin (STZ)-induced diabetic mice. It upregulated the expression of Nrf2 in diabetic retina and inhibited the increase of ROS, indicating that probucol may reduce oxidative stress via upregulating the Nrf2 signaling pathway.
Regarding the antioxidant mechanism of probucol, Xiao et al[114] demonstrated that probucol can downregulate the expression of Keap1 in cells and upregulate the expression of nrf2 to alleviate oxidative stress. Huang et al[112] reported that probucol can increase the expression of Nrf2 in the brain of D-galactose-induced cognitive impairment mice, but does not affect the level of Keap1. They demonstrated that probucol can also promote the dissociation of Keap1/Nrf2 complex, preventing Nrf2 from degradation and upregulating gene expression of antioxidant enzymes. The underlying mechanism of this process requires further research.
Coumarins
Coumarins are secondary compounds with a benzopyrone skeleton, which are widely distributed in the fruits, leaves and rhizomes of plants[118]. Coumarins mainly contain 4 major sub-types: simple coumarins, furanocoumarins, pyran-substituted coumarins, and pyranocoumarins[119]. Coumarins have many pharmacological activities including anti-inflammatory[120]–[121], antioxidant[120],[122], anticoagulant[118] and anticancer activities[123], and have been widely used in complementary and alternative medicine[118]. Several studies have found that coumarins can act as modulators of the Keap1-Nrf2-ARE signaling pathway and regulate oxidative stress in the body. Yao et al[122] found that coumarin derivatives can mitigate high-glucose induced oxidative stress and fibrosis in mesangial cells via activating Nrf2. The experiment of Hassanein et al[124] showed that umbelliferone (UBM, a coumarin derivative) can down-regulate Keap1 and up-regulate Nrf2, restore GSH and SOD activity in kidney cells, and reduce the kidney damage induced by methotrexate. Arora et al[123] demonstrated that Escletin (the 6,7-dihydroxy derivative of coumarin) can directly bind to Keap1, reduce the level of ROS in pancreatic cancer cells, and induce antiproliferative and apoptotic response. Hu et al[125] reported that imperatorin (a coumarin derivative) can induce Nrf2 activation, effectively upregulate the expression of downstream antioxidants, and protect cardiomyocytes from arsenic trioxide-induced damage.
Regarding the mechanism by which coumarins regulate the Keap1-Nrf2-ARE pathway, Hassanein et al[124] found that UMB can down-regulate the expression of Keap1 and up-regulate that of Nrf2. They thought that UMB causes the stabilization of Nrf-2, thereby accumulating in the nucleus and activating antioxidant and cytoprotective enzymes, terminally inhibiting oxidative damage. Using western blot analysis, Arora et al[123] found a remarkable loss of the interaction between Keap1 and Nrf2 after treatment with escletin. In vitro experiments also showed that under escletin treatment, Nrf2 can be released from Keap1, phosphorylated and transported to the nucleus. However, their results showed that escletin did not change the levels of the two proteins in the cell. Hassanein et al[118] used AutoDockVina1.5.6 for the molecular docking of coumarin derivatives and Keap1 protein. They found that coumarins can form hydrogen bonds with the side chains of polar, positively and negatively charged amino acids, binding to the binding pocket of Keap1 with high affinity and disrupting the interaction between Keap1 and Nrf2. This mechanism of action is considered to be superior to the modification of cysteine residues in Keap1 for the less toxic. The latter may modify thiol groups of other non-target proteins, leading to unpredictable toxic effects[126].
In recent years, many researches have studied the therapeutic effects of coumarins in ophthalmic diseases. Kim et al[127] found that escletin reduced activity of rat lens aldose reductase in vitro and inhibited the occurrence of galactose-induced cataract in rats. Angapelly et al[128] synthesized 4-sulfamoylphenyl/sulfocoumarin benzamides and evaluated its inhibitory effect on carbonic anhydrase. The results showed that this coumarin derivative can effectively inhibit carbonic anhydrase subtypes hCAII and XII. They thought the molecular structure of this compound can be further modified to develope effective carbonic anhydrase inhibitors for the treatment of epilepsy, cancer and glaucoma. Bucolo et al[129] found that cloricromene, a novel coumarin derivative, can reduce retinal inflammation in diabetic rats and preserve the blood-retinal barrier. However, there are few studies on the anti-oxidative stress ability of coumarins in the treatment of ophthalmic diseases. Ozal et al[121] reported that escletin can reduce the inflammatory response of human RPE cells induced by lipopolysaccharide. They found that escletin can inhibit NF-κB activation, extracellular signal-regulated kinase (ERK)1/2 phosphorylation and decrease the level of VEGF to undermine inflammation and oxidative stress. They also found that escletin can significantly up-regulate the expression of GPx and SOD2, which are downstream antioxidant enzymes of Nrf2. While they did not conduct further studies on whether this upregulation is through the Keap1-Nrf2-ARE pathway. Considering the ability of coumarins to regulate the Keap1-Nrf2-ARE pathway in other tissues and the crosstalk between the inflammation pathway and the anti-oxidative stress pathway, we believe that the ability of coumarins to regulate the Keap1-Nrf2-ARE pathway may take an important role in its treatment of ophthalmic diseases.
DISCUSSION
Age-related eye diseases, such as cataracts, AMD, glaucoma, and DR are the leading causes of progressive and irreversible vision loss all over the world. Although there are several surgical operations, such as intraocular lens (IOL) implantation, aqueous drainage device implant, and vitrectomy to treat these diseases, complications caused by surgery, including inflammation, hyphema, and corneal edema, cannot be ignored[130]–[131]. Therefore, it is necessary to find new therapeutic pathways and drug targets.
As an important mechanism of age-related eye diseases, oxidative stress has become a new research hotspot. Keap1-Nrf2-ARE, a classical pathway against oxidative stress and inflammation, plays a key role in combating oxidative stress and protecting the body from damage. It has been recognized as a new pharmacological target for the treatment of many diseases[5],[62]. However, the specific mechanism of antioxidants related to this pathway at the molecular level has yet to be further explored. It is currently known that probucol can upregulate the expression of Nrf2 and promote the transcription of downstream antioxidant genes, but whether it affects the expression of keap1 is controversial[112],[114]. EGCG, a tea polyphenol, is thought to modify cysteine thiols in Keap1, causing Keap1 to lose ubiquitin activity[1]. The mechanism of other phenols antioxidant effect remains unclear. We assume that the mechanism by which other phenols with similar structure to tea polyphenols activate Keap1-Nrf2 pathway is similar to that of EGCG.
In addition to activating the keap1-Nrf2-ARE pathway, many antioxidants can also affect inflammatory, apoptosis, autophagy pathways to protect the body. CaD can inhibit the activation of the autophagy-related PI3K/Akt/mTOR pathway[82]–[83], as well as downregulate the p38 MAPK /NF-κB inflammatory pathway through decreasing oxidative stress[84]. FH can inhibit the transcriptional activity of NF-κB and AP-1 induced by oxidative stress[132]. L/Zi alleviates inflammation by quenching free radicals and blocking NF-κB pathway[105]. It can also reduce light-induced ERS in the retina of BALB/cJ mice, inhibit the activation of UPR and apoptosis[104]. Coumarins have been proven to be able to inhibit the activation of NF-κB, and have anti-inflammatory, anti-cancer biological activities[120]–[123]. Moreover, metformin, which is widely used as a first-line medication for type 2 diabetes for decades, has been found that it can active nrf2 through AMPK/Sirt1 pathway and reduce oxidative stress in ARPE-19 cells induced by glyoxal. Metformin has been proposed as a candidate drug for AMD by both preclinical and clinical studies[133]. We speculate that this is because Keap1-Nrf 2-ARE is not a single antioxidant stress pathway, but has crosstalk with multiple pathways related to inflammation, apoptosis, and autophagy. The NF-κB pathway, which is widely present in a variety of metazoan cells, is a classic inflammation pathway and is critical in the regulation of many physiological and pathological processes including apoptosis, viral replication, tumorigenesis and inflammation[134]–[135]. NF-κB is normally linked to the inhibitory protein Iκ-B and remains an inactive state. Under the stimulation of pro-inflammatory substances, rapid phosphorylation of Iκ-B by I-κB kinase (IKK) causes IκB to dissociate from NF-κB and subsequently NF-κB translocate to the nucleus. In the nucleus, NF-κB induces the transcription of a variety of inflammatory factors including IL-6, COX-2, iNOS[136]–[137]. Keap1 can degrade IKKβ through ubiquitination, thereby inhibiting the activity of NF-κB[138]. The IKK complex is not only the key to for NF-κB activation, but also an interface for crosstalk between NF-κB pathways and other physiological processes. Several inflammatory mediators produced in the inflammatory process can react with Keap1 and activate Nrf2, causing downstream gene transcription initiating and inhibition of NF-κB activity[134]. NF-κB can also bind to the Nrf2 competitive transcription co-activator CBP[134]. However, the study of Lampiasi and Montana[136] showed that IKK/NF-κB did not affect the transcription and nuclear translocation of Nrf2 in LPS-stimulated RAW 264.7 cells. Chen et al[139] found human aortic endothelial cells treated with adenovirus vector Nrf2 can inhibit NF-κB downstream genes without affecting the activity of NF-κB. Therefore, whether there is corsstalk between Keap1-Nrf2-ARE pathway and NF-κB pathway remains to be further studied. The PI3K/Akt signaling pathway plays a key role in the regulation of apoptosis and autophagy[140]–[141]. It protects cells from apoptosis and autophagy through phosphorylation of Akt[142]. Besides activating Akt phosphorylation, PI3K can also promote nuclear translocation of Nrf2, increase the expression of downstream target genes of Nrf2 and enhance cell antioxidant capacity, thus promotes cell survival[143]. PI3K inhibitors can block the nuclear translocation of Nrf2 and the induction of stress proteins, indicates that the crosstalk between PI3K/Akt and Nrf2 pathway is very important to the cellular defense antioxidant system[144].
In addition, some antioxidants, such as dimethyl fumarate, have been reported to have some systemic side effects, such as lymphopenia[52]. Searching for agents with similar efficacy but less side effects in the structural analogues may be a way to avoid adverse influences of drugs. β-carotene, an antioxidant to treat AMD, has been shown to increase the incidence of lung cancer in smokers[145]. In AREDS2, the β-carotene in the AREDS-type supplements is replaced with L/Zi, which also belongs to carotenoid, although the effect of reducing the risk of progression to advanced AMD was not further improved compared to AREDS[99].
Potent antioxidant drugs are usually hydrophobic and have low water solubility[110]. Probucol is a non-ionizable, highly lipophilic, hydrophobic drug compound. It is the most poorly soluble hydrophobic antioxidant with an oral bioavailability <10%[146]. Mooranian et al[147] found that using ursodeoxycholic acid (UCDA) for the nano and micro encapsulation of probucol can improve probucol pharmacological properties, enhance probucol delivery and biological effects. Tale et al[148] used diblock terpolymers as an excipient platform to increase the solubility of probucol. Evidence suggested that at the same concentration, LM, a neutral amide derivative of ALA, can more effectively attenuate H2O2 or 6-hydroxydopamine-induced oxidative injury to PC12 cells than ALA[96]. This is considered to be due to the presence of the -CONH2 structure in LM. The resonance stabilization of CO-N helps stable LM. What's more, the LM has a lower acidity that makes it more adaptable to physiological pH[97]. These researches suggest that we can improve bioavailability and drug efficacy through encapsulating or modifying drugs or adding excipients.
In conclusion, Keap1-Nrf2-ARE, an important pathway against oxidative stress, can be used as a new drug target for the treatment of age-related eye diseases. Further research is needed to determine the specific mechanism of drugs involved in this pathway inside and outside the cell. How to clear drug toxicology, how to avoid or reduce side effects, and how to modify the molecular structure of the drug to improve efficacy may be future research directions.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81970801; No.81670859); Natural Science Foundation of Hunan Province (No.2019JJ40001); Key Project of Changsha Science and Technology Bureau (No.kh1801229).
Conflicts of Interest: Cai ZY, None; Fu MD, None; Liu K, None; Duan XC, None.
REFERENCES
- 1.Bungau S, Abdel-Daim MM, Tit DM, Ghanem E, Sato S, Maruyama-Inoue M, Yamane S, Kadonosono K. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid Med Cell Longev. 2019;2019:9783429. doi: 10.1155/2019/9783429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J, Sun W, Song Y, Liu J, Xue F, Gong K, Yang X, Kang Q. SIRT6 protects retinal ganglion cells against hydrogen peroxide-induced apoptosis and oxidative stress by promoting Nrf2/ARE signaling via inhibition of Bach1. Chem Biol Interact. 2019;300:151–158. doi: 10.1016/j.cbi.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Himori N, Yamamoto K, Maruyama K, Ryu M, Taguchi K, Yamamoto M, Nakazawa T. Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death. J Neurochem. 2013;127(5):669–680. doi: 10.1111/jnc.12325. [DOI] [PubMed] [Google Scholar]
- 4.Koriyama Y, Nakayama Y, Matsugo S, Kato S. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2-dependent heme oxygenase-1 induction in the RGC-5 cellline. Brain Res. 2013;1499:145–157. doi: 10.1016/j.brainres.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Lu MC, Ji JA, Jiang ZY, You QD. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med Res Rev. 2016;36(5):924–963. doi: 10.1002/med.21396. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Wang B, Hao Y, Yang X. Effects of calcium dobesilate on Nrf2, Keap1 and HO-1 in the lenses of D-galactose-induced cataracts in rats. Exp Ther Med. 2018;15(1):719–722. doi: 10.3892/etm.2017.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arumugam B, Palanisamy UD, Chua KH, Kuppusamy UR. Protective effect of myricetin derivatives from Syzygium malaccense against hydrogen peroxide-induced stress in ARPE-19 cells. Mol Vis. 2019;25:47–59. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Wang W, Liu J, Huang X, Liu R, Xia H, Brecha NC, Pu M, Gao J. Protective effect of ALA in crushed optic nerve cat retinal ganglion cells using a new marker RBPMS. PLoS One. 2016;11(8):e0160309. doi: 10.1371/journal.pone.0160309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R, Lee C, Lin X, Zhao C, Li X. Novel function of VEGF-B as an antioxidant and therapeutic implications. Pharmacol Res. 2019;143:33–39. doi: 10.1016/j.phrs.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Mathew MC, Ervin AM, Tao J, Davis RM. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012(6):CD004567. doi: 10.1002/14651858.CD004567.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babizhayev MA, Yegorov YE. Reactive oxygen species and the aging eye: specific role of metabolically active mitochondria in maintaining lens function and in the initiation of the oxidation-induced maturity onset cataract—A novel platform of mitochondria-targeted antioxidants with broad therapeutic potential for redox regulation and detoxification of oxidants in eye diseases. Am J Ther. 2016;23(1):e98–e117. doi: 10.1097/MJT.0b013e3181ea31ff. [DOI] [PubMed] [Google Scholar]
- 12.Choi JI, Kim J, Choung SY. Polyphenol-enriched fraction of Vaccinium uliginosum L. protects selenite-induced cataract formation in the lens of Sprague-Dawley rat pups. Mol Vis. 2019;25:118–128. [PMC free article] [PubMed] [Google Scholar]
- 13.Sadik NAH, El-Boghdady NA, Omar NN, Al-Hamid HA. Esculetin and idebenone ameliorate galactose-induced cataract in a rat model. J Food Biochem. 2020;44(7):e13230. doi: 10.1111/jfbc.13230. [DOI] [PubMed] [Google Scholar]
- 14.Shoham A, Hadziahmetovic M, Dunaief JL, Mydlarski MB, Schipper HM. Oxidative stress in diseases of the human cornea. Free Radic Biol Med. 2008;45(8):1047–1055. doi: 10.1016/j.freeradbiomed.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Xu X, Liu Q, Huang H, Huang X, Lv H. Myricetin prevents cataract formation by inhibiting the apoptotic cell death mediated cataractogenesis. Med Sci Monit. 2020;26:e922519. doi: 10.12659/MSM.922519. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Du S, Shao J, Xie D, Zhang F. Decorin inhibits glucose-induced lens epithelial cell apoptosis via suppressing p22phox-p38 MAPK signaling pathway. PLoS One. 2020;15(4):e0224251. doi: 10.1371/journal.pone.0224251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li T, Huang YH, Zhou WK, Yan QC. Let-7c-3p regulates autophagy under oxidative stress by targeting ATG3 in lens epithelial cells. Biomed Res Int. 2020;2020:6069390. doi: 10.1155/2020/6069390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Đurašević S, Jasnić N, Prokić M, Grigorov I, Martinović V, Đorđević J, Pavlović S. The protective role of virgin coconut oil on the alloxan-induced oxidative stress in the liver, kidneys and heart of diabetic rats. Food Funct. 2019;10(4):2114–2124. doi: 10.1039/c9fo00107g. [DOI] [PubMed] [Google Scholar]
- 19.McMonnies CW. Glaucoma history and risk factors. J Optom. 2017;10(2):71–78. doi: 10.1016/j.optom.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waugh D. The contribution of fluoride to the pathogenesis of eye diseases: molecular mechanisms and implications for public health. Int J Environ Res Public Heal. 2019;16(5):856. doi: 10.3390/ijerph16050856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saccà SC, Tirendi S, Scarfì S, Passalacqua M, Oddone F, Traverso CE, Vernazza S, Bassi AM. An advanced in vitro model to assess glaucoma onset. ALTEX. 2020;37(2):265–274. doi: 10.14573/altex.1909262. [DOI] [PubMed] [Google Scholar]
- 22.Prasanna G, Li B, Mogi M, Rice DS. Pharmacology of novel intraocular pressure-lowering targets that enhance conventional outflow facility: Pitfalls, promises and what lies ahead? Eur J Pharmacol. 2016;787:47–56. doi: 10.1016/j.ejphar.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134(1):109–118. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 24.He Y, Leung KW, Zhang YH, Duan S, Zhong XF, Jiang RZ, Peng Z, Tombran-Tink J, Ge J. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: protection by antioxidants. Invest Ophthalmol Vis Sci. 2008;49(4):1447–1458. doi: 10.1167/iovs.07-1361. [DOI] [PubMed] [Google Scholar]
- 25.Maurya N, Agarwal NR, Ghosh I. Low-dose rotenone exposure induces early senescence leading to late apoptotic signaling cascade in human trabecular meshwork (HTM) cell line: an in vitro glaucoma model. Cell Biol Int. 2016;40(1):107–120. doi: 10.1002/cbin.10561. [DOI] [PubMed] [Google Scholar]
- 26.He JN, Zhang SD, Qu Y, Wang HL, Tham CC, Pang CP, Chu WK. Rapamycin removes damaged mitochondria and protects human trabecular meshwork (TM-1) cells from chronic oxidative stress. Mol Neurobiol. 2019;56(9):6586–6593. doi: 10.1007/s12035-019-1559-5. [DOI] [PubMed] [Google Scholar]
- 27.Bagnis A, Izzotti A, Saccà SC. Helicobacter pylori, oxidative stress and glaucoma. Dig Liver Dis. 2012;44(11):963–964. author reply 964. doi: 10.1016/j.dld.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Masuda T, Shimazawa M, Hara H. Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (edaravone) Oxid Med Cell Longev. 2017;2017:9208489. doi: 10.1155/2017/9208489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan YZ, Xing Y, Xiong L, Wang JM. Sestrin2 overexpression alleviates hydrogen peroxide-induced apoptosis and oxidative stress in retinal ganglion cells by enhancing Nrf2 activation via Keap1 downregulation. Chem Biol Interact. 2020;324:109086. doi: 10.1016/j.cbi.2020.109086. [DOI] [PubMed] [Google Scholar]
- 30.Zhong JX, Liu L, Sha XY, Wu YN, Chen MT. Lycium barbarum polysaccharides protects retinal ganglion cells against oxidative stress injury. Neural Regen Res. 2020;15(8):1526. doi: 10.4103/1673-5374.274349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng Y, Yu XC, Zhang J, Chang YP, Xue M, Li XY, Lu YH, Li T, Meng ZY, Su L, Sun B, Chen LM. Pancreatic kallikrein protects against diabetic retinopathy in KK Cg-Ây/J and high-fat diet/streptozotocin-induced mouse models of type 2 diabetes. Diabetologia. 2019;62(6):1074–1086. doi: 10.1007/s00125-019-4838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calderon GD, Juarez OH, Hernandez GE, Punzo SM, De la Cruz ZD. Oxidative stress and diabetic retinopathy: development and treatment. Eye (Lond) 2017;31(8):1122–1130. doi: 10.1038/eye.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Q, Wang J, Cheng Y, Dong X, Zhang M, Pei C. Palbinone alleviates diabetic retinopathy in STZ-induced rats by inhibiting NLRP3 inflammatory activity. J Biochem Mol Toxicol. 2020;34(7):e22489. doi: 10.1002/jbt.22489. [DOI] [PubMed] [Google Scholar]
- 34.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Q, Li S, Zhou Z, Fu M, Yang X, Hao K, Liu Y. HDAC6 inhibitor Cay10603 inhibits high glucose-induced oxidative stress, inflammation and apoptosis in retinal pigment epithelial cells via regulating NF-κB and NLRP3 inflammasome pathway. Gen Physiol Biophys. 2020;39(2):169–177. doi: 10.4149/gpb_2019058. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Miao X, Li F, Wang S, Liu Q, Wang Y, Sun J. Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxid Med Cell Longev. 2017;2017:9702820. doi: 10.1155/2017/9702820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramírez-Pérez G, Sánchez-Chávez G, Salceda R. Mitochondrial bound hexokinase type I in normal and streptozotocin diabetic rat retina. Mitochondrion. 2020;52:212–217. doi: 10.1016/j.mito.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 38.González de Vega R, García M, Fernández-Sánchez ML, González-Iglesias H, Sanz-Medel A. Protective effect of selenium supplementation following oxidative stress mediated by glucose on retinal pigment epithelium. Metallomics. 2018;10(1):83–92. doi: 10.1039/c7mt00209b. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Zuo Z, Lu S, Liu A, Liu X. Naringin attenuates diabetic retinopathy by inhibiting inflammation, oxidative stress and NF-κB activation in vivo and in vitro. Iran J Basic Med Sci. 2017;20(7):813–821. doi: 10.22038/IJBMS.2017.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Li R, Xie J, Hu J, Huang X, Ren F, Li L. Protective effect of hydrogen on sodium iodate-induced age-related macular degeneration in mice. Front Aging Neurosci. 2018;10:389. doi: 10.3389/fnagi.2018.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Votruba M, Gregor Z. Neovascular age-related macular degeneration: Present and future treatment options. Eye (Lond) 2001;15(3):424–429. doi: 10.1038/eye.2001.147. [DOI] [PubMed] [Google Scholar]
- 42.Yildirim Z, Ucgun NI, Yildirim F. The role of oxidative stress and antioxidants in the pathogenesis of age-related macular degeneration. Clinics (Sao Paulo) 2011;66(5):743–746. doi: 10.1590/S1807-59322011000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv Ophthalmol. 2006;51(5):461–481. doi: 10.1016/j.survophthal.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Sheu SJ, Chen JL, Bee YS, Lin SH, Shu CW. ERBB2-modulated ATG4B and autophagic cell death in human ARPE19 during oxidative stress. PLoS One. 2019;14(3):e0213932. doi: 10.1371/journal.pone.0213932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76(4):397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 46.Alamri A, Biswas L, Watson D, Shu XH. Deletion of TSPO resulted in change of metabolomic profile in retinal pigment epithelial cells. Int J Mol Sci. 2019;20(6):1387. doi: 10.3390/ijms20061387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Totan Y, Yağci R, Bardak Y, Ozyurt H, Kendir F, Yilmaz G, Sahin S, Sahin Tiğ U. Oxidative macromolecular damage in age-related macular degeneration. Curr Eye Res. 2009;34(12):1089–1093. doi: 10.3109/02713680903353772. [DOI] [PubMed] [Google Scholar]
- 48.Du Z, Zhang W, Wang S, Zhang J, He J, Wang Y, Dong Y, Huo M. Celastrol protects human retinal pigment epithelial cells against hydrogen peroxide mediated oxidative stress, autophagy, and apoptosis through sirtuin 3 signal pathway. J Cell Biochem. 2019;120(6):10413–10420. doi: 10.1002/jcb.28326. [DOI] [PubMed] [Google Scholar]
- 49.Kesarwani P, Murali AK, Al-Khami AA, Mehrotra S. Redox regulation of T-cell function: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2013;18(12):1497–1534. doi: 10.1089/ars.2011.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joo Choi R, Cheng MS, Shik Kim Y. Desoxyrhapontigenin up-regulates Nrf2-mediated heme oxygenase-1 expression in macrophages and inflammatory lung injury. Redox Biol. 2014;2:504–512. doi: 10.1016/j.redox.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu QY, Lei ZX, Huang AX, Wu QH, Xie SY, Awais I, Dai MH, Wang X, Yuan ZH. Toxic metabolites, MAPK and Nrf2/Keap1 signaling pathways involved in oxidative toxicity in mice liver after chronic exposure to Mequindox. Sci Rep. 2017;7:41854. doi: 10.1038/srep41854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satoh T, Lipton S. Recent advances in understanding NRF2 as a druggable target: development of pro-electrophilic and non-covalent NRF2 activators to overcome systemic side effects of electrophilic drugs like dimethyl fumarate. F1000Res. 2017;6:2138. doi: 10.12688/f1000research.12111.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13(11):1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deshmukh P, Unni S, Krishnappa G, Padmanabhan B. The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys Rev. 2017;9(1):41–56. doi: 10.1007/s12551-016-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hur W, Gray NS. Small molecule modulators of antioxidant response pathway. Curr Opin Chem Biol. 2011;15(1):162–173. doi: 10.1016/j.cbpa.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 56.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the Redox-sensitive Neh2 degron and the Redox-insensitive Neh6 degron. J Biol Chem. 2004;279(30):31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 57.Magesh S, Chen Y, Hu LQ. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev. 2012;32(4):687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Zhang J, Huang ZH, Huang XH, Zheng WB, Yin XF, Li YL, Li B, He QY. Isodeoxyelephantopin induces protective autophagy in lung cancer cells via Nrf2-p62-keap1 feedback loop. Cell Death Dis. 2017;8(6):e2876. doi: 10.1038/cddis.2017.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah SZA, Zhao D, Hussain T, Sabir N, Mangi MH, Yang L. p62-Keap1-NRF2-ARE pathway: a contentious player for selective targeting of autophagy, oxidative stress and mitochondrial dysfunction in prion diseases. Front Mol Neurosci. 2018;11:310. doi: 10.3389/fnmol.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Copple IM, Dinkova-Kostova AT, Kensler TW, Liby KT, Wigley WC. NRF2 as an emerging therapeutic target. Oxid Med Cell Longev. 2017;2017:8165458. doi: 10.1155/2017/8165458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Yuan ZL. Activation of Nrf2/HO-1 pathway protects retinal ganglion cells from a rat chronic ocular hypertension model of glaucoma. Int Ophthalmol. 2019;39(10):2303–2312. doi: 10.1007/s10792-018-01071-8. [DOI] [PubMed] [Google Scholar]
- 62.de Freitas Silva M, Pruccoli L, Morroni F, Sita G, Seghetti F, Viegas C, Tarozzi A. The Keap1/Nrf2-ARE pathway as a pharmacological target for chalcones. Molecules. 2018;23(7):1803. doi: 10.3390/molecules23071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dinkova-Kostova AT, Kazantsev AG. Activation of Nrf2 signaling as a common treatment of neurodegenerative diseases. Neurodegener Dis Manag. 2017;7(2):97–100. doi: 10.2217/nmt-2017-0011. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7(3-4):385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 65.Eggler AL, Gay KA, Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008;52(Suppl 1):S84–S94. doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- 66.Periyasamy P, Shinohara T. Age-related cataracts: Role of unfolded protein response, Ca2+ mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog Retin Eye Res. 2017;60:1–19. doi: 10.1016/j.preteyeres.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387(10/11):1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Q, Pi J, Woods CG, Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. 2010;244(1):84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26(8):2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uruno A, Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide. 2011;25(2):153–160. doi: 10.1016/j.niox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Wan H, Ge L, Li J, Zhang K, Wu W, Peng S, Zou X, Zhou H, Zhou B, Zeng X. Effects of a novel biflavonoid of Lonicera japonica flower buds on modulating apoptosis under different oxidative conditions in hepatoma cells. Phytomedicine. 2019;57:282–291. doi: 10.1016/j.phymed.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 72.Zheng H, Su Y, Sun Y, Tang T, Zhang D, He X, Wang J. Echinacoside alleviates hypobaric hypoxia-induced memory impairment in C57 mice. Phytother Res. 2019;33(4):1150–1160. doi: 10.1002/ptr.6310. [DOI] [PubMed] [Google Scholar]
- 73.Lee J, Jung SY, Yang KJ, Kim Y, Lee D, Lee MH, Kim DK. alpha-Lipoic acid prevents against cisplatin cytotoxicity via activation of the NRF2/HO-1 antioxidant pathway. PLoS One. 2019;14:e0226769. doi: 10.1371/journal.pone.0226769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin T, Ren Z, Liu XP, Luo Y, Long Y, Peng S, Chen SX, Zhang JW, Ma YF, Li J, Huang YF. Study of the selenizing Codonopsis pilosula polysaccharides protects RAW264.7 cells from hydrogen peroxide-induced injury. Int J Biol Macromol. 2019;125:534–543. doi: 10.1016/j.ijbiomac.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 75.Huang Y, Zhou F, Shen C, Wang H, Xiao Y. LBP reduces theinflammatory injury of kidney in septic rat and regulates the Keap1-Nrf2/ARE signaling pathway1. Acta Cir Bras. 2019;34(1):e20190010000003. doi: 10.1590/s0102-865020190010000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li B, Jiang T, Liu H, Miao Z, Fang D, Zheng L, Zhao J. Andrographolide protects chondrocytes from oxidative stress injury by activation of the Keap1-Nrf2-ARE signaling pathway. J Cell Physiol. 2018;234(1):561–571. doi: 10.1002/jcp.26769. [DOI] [PubMed] [Google Scholar]
- 77.Cheng J, Liang J, Qi J. Role of nuclear factor (erythroid-derived 2)-like 2 in the age-resistant properties of the glaucoma trabecular meshwork. Exp Ther Med. 2017;14(1):791–796. doi: 10.3892/etm.2017.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu XF, Hao JL, Xie T, Malik TH, Lu CB, Liu C, Shu C, Lu CW, Zhou DD. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell. 2017;16(5):934–942. doi: 10.1111/acel.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu C, Dong Y, Liu H, Ren H, Cui Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed Pharmacother. 2017;88:124–133. doi: 10.1016/j.biopha.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 80.Kowluru RA, Mishra M. Epigenetic regulation of redox signaling in diabetic retinopathy: role of Nrf2. Free Radic Biol Med. 2017;103:155–164. doi: 10.1016/j.freeradbiomed.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu J, Li S, Sun D. Calcium dobesilate and micro-vascular diseases. Life Sci. 2019;221:348–353. doi: 10.1016/j.lfs.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 82.Njau F, Shushakova N, Schenk H, Wulfmeyer VC, Bollin R, Menne J, Haller H. Calcium dobesilate reduces VEGF signaling by interfering with heparan sulfate binding site and protects from vascular complications in diabetic mice. PLoS One. 2020;15(1):e0218494. doi: 10.1371/journal.pone.0218494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, Lu YH, Tang C, Xue M, Li XY, Chang YP, Cheng Y, Li T, Yu XC, Sun B, Li CJ, Chen LM. Calcium dobesilate restores autophagy by inhibiting the VEGF/PI3K/AKT/mTOR signaling pathway. Front Pharmacol. 2019;10:886. doi: 10.3389/fphar.2019.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leal EC, Martins J, Voabil P, Liberal J, Chiavaroli C, Bauer J, Cunha-Vaz J, Ambrósio AF. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes. 2010;59(10):2637–2645. doi: 10.2337/db09-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jafarey M, Changizi Ashtiyani S, Najafi H. Calcium dobesilate for prevention of gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2014;8(1):46–52. [PubMed] [Google Scholar]
- 86.Bogdanov P, Solà-Adell C, Hernández C, García-Ramírez M, Sampedro J, Simó-Servat O, Valeri M, Pasquali C, Simó R. Calcium dobesilate prevents the oxidative stress and inflammation induced by diabetes in the retina of db/db mice. J Diabetes Complications. 2017;31(10):1481–1490. doi: 10.1016/j.jdiacomp.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 87.Kowluru RA, Mishra M, Kowluru A, Kumar B. Hyperlipidemia and the development of diabetic retinopathy: comparison between type 1 and type 2 animal models. Metabolism. 2016;65(10):1570–1581. doi: 10.1016/j.metabol.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khan M, Liu H, Wang J, Sun B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: a comprehensive review. Food Res Int. 2020;130:108933. doi: 10.1016/j.foodres.2019.108933. [DOI] [PubMed] [Google Scholar]
- 89.Saccà SC, Corazza P, Gandolfi S, Ferrari D, Sukkar S, Iorio EL, Traverso CE. Substances of interest that support glaucoma therapy. Nutrients. 2019;22;11(2):239. doi: 10.3390/nu11020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gothai S, Ganesan P, Park SY, Fakurazi S, Arulselvan P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: inflammation as a target. Nutrients. 2016;8(8):461. doi: 10.3390/nu8080461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gu L, Li S, Bai J, Zhang Q, Han Z. α-Lipoic acid protects against microcystin-LR induced hepatotoxicity through regeneration of glutathione via activation of Nrf2. Environ Toxicol. 2020;35(7):738–746. doi: 10.1002/tox.22908. [DOI] [PubMed] [Google Scholar]
- 92.Lee J, Jung SY, Yang KJ, Kim Y, Lee D, Lee MH, Kim DK. Α-Lipoic acid prevents against cisplatin cytotoxicity via activation of the NRF2/HO-1 antioxidant pathway. PLoS One. 2019;14(12):e0226769. doi: 10.1371/journal.pone.0226769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xia H, Nan Y, Huang X, Gao J, Pu M. Effects of tauroursodeoxycholic acid and alpha-lipoic-acid on the visual response properties of cat retinal ganglion cells: an in vitro study. Invest Ophthalmol Vis Sci. 2015;56(11):6638–6645. doi: 10.1167/iovs.15-17301. [DOI] [PubMed] [Google Scholar]
- 94.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99(18):11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi M, Park M, Lee S, Lee JW, Choi WJ, Lee C. Establishment of Nrf2-deficient HaCaT and immortalized primary human foreskin keratinocytes and characterization of their responses to ROS-induced cytotoxicity. Toxicol in Vitro. 2019;61:104602. doi: 10.1016/j.tiv.2019.104602. [DOI] [PubMed] [Google Scholar]
- 96.Zhao L, Liu Z, Jia H, Feng Z, Liu J, Li X. Lipoamide Acts as an indirect antioxidant by simultaneously stimulating mitochondrial biogenesis and phase II antioxidant enzyme systems in ARPE-19 cells. PLoS One. 2015;10(6):e0128502. doi: 10.1371/journal.pone.0128502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hou YN, Li XM, Peng SJ, Yao J, Bai FF, Fang JG. Lipoamide ameliorates oxidative stress via induction of Nrf2/ARE signaling pathway in PC12 cells. J Agric Food Chem. 2019;67(29):8227–8234. doi: 10.1021/acs.jafc.9b02680. [DOI] [PubMed] [Google Scholar]
- 98.Tuzcu M, Orhan C, Muz OE, Sahin N, Juturu V, Sahin K. Lutein and Zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC Ophthalmol. 2017;17(1):129. doi: 10.1186/s12886-017-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Age-Related Eye Disease Study 2 (AREDS2) Research Group. Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agrón E, Toth CA, Bernstein PS, Sperduto RD. Secondary analyses of the effects of lutein/Zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132(2):142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barros MP, Rodrigo MJ, Zacarias L. Dietary carotenoid roles in redox homeostasis and human health. J Agric Food Chem. 2018;66(23):5733–5740. doi: 10.1021/acs.jafc.8b00866. [DOI] [PubMed] [Google Scholar]
- 101.Ursini F, Maiorino M, Forman HJ. Redox homeostasis: the golden mean of healthy living. Redox Biol. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez-Concepcion M, Avalos J, Bonet ML, Boronat A, Gomez-Gomez L, Hornero-Mendez D, Limon MC, Meléndez-Martínez AJ, Olmedilla-Alonso B, Palou A, Ribot J, Rodrigo MJ, Zacarias L, Zhu C. A global perspective on carotenoids: metabolism, biotechnology, and benefits for nutrition and health. Prog Lipid Res. 2018;70:62–93. doi: 10.1016/j.plipres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 103.Sahin K, Gencoglu H, Akdemir F, Orhan C, Tuzcu M, Sahin N, Yilmaz I, Juturu V. Lutein and Zeaxanthin isomers may attenuate photo-oxidative retinal damage via modulation of G protein-coupled receptors and growth factors in rats. Biochem Biophys Res Commun. 2019;516(1):163–170. doi: 10.1016/j.bbrc.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 104.Yu MZ, Yan WM, Beight C. Lutein and Zeaxanthin isomers protect against light-induced retinopathy via decreasing oxidative and endoplasmic reticulum stress in BALB/cJ mice. Nutrients. 2018;10(7):842. doi: 10.3390/nu10070842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robles-Rivera RR, Castellanos-González JA, Olvera-Montaño C, Flores-Martin RA, López-Contreras AK, Arevalo-Simental DE, Cardona-Muñoz EG, Roman-Pintos LM, Rodríguez-Carrizalez AD. Adjuvant therapies in diabetic retinopathy as an early approach to delay its progression: the importance of oxidative stress and inflammation. Oxid Med Cell Longev. 2020;2020:3096470. doi: 10.1155/2020/3096470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lenox AR, Bhootada Y, Gorbatyuk O, Fullard R, Gorbatyuk M. Unfolded protein response is activated in aged retinas. Neurosci Lett. 2015;609:30–35. doi: 10.1016/j.neulet.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mooranian A, Zamani N, Luna G, Al-Sallami H, Mikov M, Goločorbin-Kon S, Stojanovic G, Arfuso F, Kovacevic B, Al-Salami H. Bile acid-polymer-probucol microparticles: protective effect on pancreatic β-cells and decrease in type 1 diabetes development in a murine model. Pharm Dev Technol. 2019;24(10):1272–1277. doi: 10.1080/10837450.2019.1665069. [DOI] [PubMed] [Google Scholar]
- 108.Yakushiji E, Ayaori M, Nishida T, Shiotani K, Takiguchi S, Nakaya K, Uto-Kondo H, Ogura M, Sasaki M, Yogo M, Komatsu T, Lu R, Yokoyama S, Ikewaki K. Probucol-oxidized products, spiroquinone and diphenoquinone, promote reverse cholesterol transport in mice. Arterioscler Thromb Vasc Biol. 2016;36(4):591–597. doi: 10.1161/ATVBAHA.115.306376. [DOI] [PubMed] [Google Scholar]
- 109.Liu HW, Luo Y, Zhou YF, Chen ZP. Probucol prevents diabetes-induced retinal neuronal degeneration through upregulating Nrf2. Biomed Res Int. 2020;2020:3862509. doi: 10.1155/2020/3862509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lau M, Giri K, Garcia-Bennett AE. Antioxidant properties of probucol released from mesoporous silica. Eur J Pharm Sci. 2019;138:105038. doi: 10.1016/j.ejps.2019.105038. [DOI] [PubMed] [Google Scholar]
- 111.Nakagawa S, Aruga J. Sphingosine 1-phosphate signaling is involved in impaired blood—brain barrier function in ischemia—reperfusion injury. Mol Neurobiol. 2020;57(3):1594–1606. doi: 10.1007/s12035-019-01844-x. [DOI] [PubMed] [Google Scholar]
- 112.Huang JL, Yu C, Su M, Yang SM, Zhang F, Chen YY, Liu JY, Jiang YF, Zhong ZG, Wu DP. Probucol, a “non-statin” cholesterol-lowering drug, ameliorates D-galactose induced cognitive deficits by alleviating oxidative stress via Keap1/Nrf2 signaling pathway in mice. Aging (Albany NY) 2019;11(19):8542–8555. doi: 10.18632/aging.102337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu BB, Wang H, Chi YF, Wang YM, Yao XM, Liu S, Qiu H, Fang J, Yin PH, Zhang XM, Peng W. Protective effects of probucol on Ox-LDL-induced epithelial-mesenchymal transition in human renal proximal tubular epithelial cells via LOX-1/ROS/MAPK signaling. Mol Med Rep. 2018;17(1):1289–1296. doi: 10.3892/mmr.2017.7935. [DOI] [PubMed] [Google Scholar]
- 114.Xiao X, Hou H, Lin V, Ho D, Tran K, Che B, May A, Zhang J, Lu Z, Lu Z, Shaw PX. Probucol protects rats from cardiac dysfunction induced by oxidative stress following cardiopulmonary resuscitation. Oxid Med Cell Longev. 2017;2017:1284804. doi: 10.1155/2017/1284804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Higashi K, Mori A, Sakamoto K, Ishii K, Nakahara T. Probucol slows the progression of cataracts in streptozotocin-induced hyperglycemic rats. Pharmacology. 2019;103(3-4):212–219. doi: 10.1159/000496055. [DOI] [PubMed] [Google Scholar]
- 116.Yoshida M, Kimura H, Kyuki K, Ito M. Combined effect of probucol and insulin on cataracts of diabetic rats fed a high cholesterol diet. Eur J Pharmacol. 2005;513(1-2):159–168. doi: 10.1016/j.ejphar.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 117.Zhou XX, Ai SB, Chen ZP, Li CX. Probucol promotes high glucose-induced proliferation and inhibits apoptosis by reducing reactive oxygen species generation in Müller cells. Int Ophthalmol. 2019;39(12):2833–2842. doi: 10.1007/s10792-019-01130-8. [DOI] [PubMed] [Google Scholar]
- 118.Hassanein EHM, Sayed AM, Hussein OE, Mahmoud AM. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. Oxidative Med Cell Longev. 2020;2020:1675957. doi: 10.1155/2020/1675957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Taira J, Ogi T. Induction of antioxidant protein HO-1 through Nrf2-ARE signaling due to pteryxin in peucedanum japonicum thunb in RAW264.7 macrophage cells. Antioxidants (Basel) 2019;5;8(12):621. doi: 10.3390/antiox8120621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wei X, Fan X, Feng Z, Ma Y, Lan X, Chen M. Ethyl acetate extract of Herpetospermum pedunculosum alleviates α-naphthylisothiocyanate-induced cholestasis by activating the farnesoid X receptor and suppressing oxidative stress and inflammation in rats. Phytomedicine. 2020;76:153257. doi: 10.1016/j.phymed.2020.153257. [DOI] [PubMed] [Google Scholar]
- 121.Ozal SA, Turkekul K, Gurlu V, Guclu H, Erdogan S. Esculetin protects human retinal pigment epithelial cells from lipopolysaccharide-induced inflammation and cell death. Curr Eye Res. 2018;43(9):1169–1176. doi: 10.1080/02713683.2018.1481517. [DOI] [PubMed] [Google Scholar]
- 122.Yao H, Zhang N, Zhang W, Li J, Hua H, Li Y. Discovery of a coumarin derivative as Nrf2 activator mitigating oxidative stress and fibrosis in mesangial cells under high glucose. Bioorg Med Chem Lett. 2020;30(20):127490. doi: 10.1016/j.bmcl.2020.127490. [DOI] [PubMed] [Google Scholar]
- 123.Arora R, Sawney S, Saini V, Steffi C, Tiwari M, Saluja D. Esculetin induces antiproliferative and apoptotic response in pancreatic cancer cells by directly binding to KEAP1. Mol Cancer. 2016;15(1):64. doi: 10.1186/s12943-016-0550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hassanein EHM, Mohamed WR, Shalkami AS, Khalaf MM, Hemeida RAM. Renoprotective effects of umbelliferone on methotrexate-induced renal injury through regulation of Nrf-2/Keap-1, P38MAPK/NF-κB, and apoptosis signaling pathways. Food Chem Toxicol. 2018;116(pt b):152–160. doi: 10.1016/j.fct.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 125.Hu L, Sun J, Li H, Wang L, Wei Y, Wang Y, Zhu Y, Huo H, Tan Y. Differential mechanistic investigation of protective effects from imperatorin and sec-O-glucosylhamaudol against arsenic trioxide-induced cytotoxicity in vitro. Toxicol in Vitro. 2016;37:97–105. doi: 10.1016/j.tiv.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 126.Raghunath A, Nagarajan R, Sundarraj K, Palanisamy K, Perumal E. Identification of compounds that inhibit the binding of Keap1a/Keap1b Kelch DGR domain with Nrf2 ETGE/DLG motifs in zebrafish. Basic Clin Pharmacol Toxicol. 2019;125(3):259–270. doi: 10.1111/bcpt.13222. [DOI] [PubMed] [Google Scholar]
- 127.Kim CS, Kim J, Lee YM, Sohn E, Kim JS. Esculetin, a coumarin derivative, inhibits aldose reductase activity in vitro and cataractogenesis in galactose-fed rats. Biomol Ther (Seoul) 2016;24(2):178–183. doi: 10.4062/biomolther.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Angapelly S, Angeli A, Khan AJ, Sri Ramya PV, Supuran CT, Arifuddin M. Synthesis and biological evaluation of 4-sulfamoylphenyl/sulfocoumarin carboxamides as selective inhibitors of carbonic anhydrase isoforms hCA II, IX, and XII. ChemMedChem. 2018;13(12):1165–1171. doi: 10.1002/cmdc.201800180. [DOI] [PubMed] [Google Scholar]
- 129.Bucolo C, Ward KW, Mazzon E, Cuzzocrea S, Drago F. Protective effects of a coumarin derivative in diabetic rats. Invest Ophthalmol Vis Sci. 2009;50(8):3846–3852. doi: 10.1167/iovs.08-3328. [DOI] [PubMed] [Google Scholar]
- 130.Bizrah M, Corbett MC. Intracameral phenylephrine to arrest intraoperative intraocular bleeding: a new technique. Ophthalmol Ther. 2019;8(1):137–141. doi: 10.1007/s40123-019-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wan WJ, Jiang L, Ji Y, Xun Y, Xiong L, Xiang YG, Li RN, Li ZY, Wang XQ, Stewart JM, Hu K. Effect of hypothermic perfusion on phacoemulsification in eyes with hard nuclear cataract: randomized trial. J Cataract Refract Surg. 2019;45(12):1717–1724. doi: 10.1016/j.jcrs.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 132.Jin HL, Choung S-Y, Jeong KW. Protective mechanisms of polyphenol-enriched fraction of Vaccinium uliginosum L. Against blue light-induced cell death of human retinal pigmented epithelial cells. J Funct Foods. 2017;39:28–36. [Google Scholar]
- 133.Qu S, Zhang C, Liu D, Wu J, Tian H, Lu L, Xu GT, Liu F, Zhang J. Metformin protects ARPE-19 cells from glyoxal-induced oxidative stress. Oxid Med Cell Longev. 2020;2020:1740943. doi: 10.1155/2020/1740943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 135.Zhuang Q, Ou J, Zhang S, Ming Y. Crosstalk between the CX3CL1/CX3CR1 axis and inflammatory signaling pathways in tissue injury. Curr Protein Pept Sci. 2019;20(8):844–854. doi: 10.2174/1389203720666190305165722. [DOI] [PubMed] [Google Scholar]
- 136.Lampiasi N, Montana G. An in vitro inflammation model to study the Nrf2 and NF-κB crosstalk in presence of ferulic acid as modulator. Immunobiology. 2018;223(4-5):349–355. doi: 10.1016/j.imbio.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 137.Linnewiel-Hermoni K, Motro Y, Miller Y, Levy J, Sharoni Y. Carotenoid derivatives inhibit nuclear factor kappa B activity in bone and cancer cells by targeting key thiol groups. Free Radic Biol Med. 2014;75:105–120. doi: 10.1016/j.freeradbiomed.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 138.Lee DF, Kuo HP, Liu M, Chou CK, Xia W, Du Y, Shen J, Chen CT, Huo L, Hsu MC, Li CW, Ding Q, Liao TL, Lai CC, Lin AC, Chang YH, Tsai SF, Li LY, Hung MC. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell. 2009;36(1):131–140. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen XL, Dodd G, Thomas S, Zhang XL, Wasserman MA, Rovin BH, Kunsch C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290(5):H1862–H1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 140.Wang HL, Liu FL, Li RQ, Wan MY, Li JY, Shi J, Wu ML, Chen JH, Sun WJ, Feng HX, Zhao W, Huang J, Liu RC, Hao WX, Feng XD. Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation. Neural Regen Res. 2021;16(6):1011–1016. doi: 10.4103/1673-5374.300454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yarmohammadi F, Hayes AW, Karimi G. Natural compounds against cytotoxic drug-induced cardiotoxicity: a review on the involvement of PI3K/Akt signaling pathway. J Biochem Mol Toxicol. 2021;35(3):e22683. doi: 10.1002/jbt.22683. [DOI] [PubMed] [Google Scholar]
- 142.Shao ZQ, Dou SS, Zhu JG, Wang HQ, Wang CM, Cheng BH, Bai B. Apelin-13 inhibits apoptosis and excessive autophagy in cerebral ischemia/reperfusion injury. Neural Regen Res. 2021;16(6):1044–1051. doi: 10.4103/1673-5374.300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qi HY, Han YF, Rong JH. Potential roles of PI3K/Akt and Nrf2-Keap1 pathways in regulating hormesis of Z-ligustilide in PC12 cells against oxygen and glucose deprivation. Neuropharmacology. 2012;62(4):1659–1670. doi: 10.1016/j.neuropharm.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 144.Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546(2-3):181–184. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- 145.Age-Related Eye Disease Study 2 Research Group. Lutein + Zeaxanthin and Omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 146.Nielsen FS, Petersen KB, Müllertz A. Bioavailability of probucol from lipid and surfactant based formulations in minipigs: Influence of droplet size and dietary state. Eur J Pharm Biopharm. 2008;69(2):553–562. doi: 10.1016/j.ejpb.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 147.Mooranian A, Zamani N, Mikov M, Goločorbin-Kon S, Stojanovic G, Arfuso F, Kovacevic B, Al-Salami H. Bio micro-nano technologies of antioxidants optimised their pharmacological and cellular effects, ex vivo, in pancreatic β-cells. Nanotechnol Sci Appl. 2020;13:1–9. doi: 10.2147/NSA.S212323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tale S, Purchel AA, Dalsin MC, Reineke TM. Diblock terpolymers are tunable and pH responsive vehicles to increase hydrophobic drug solubility for oral administration. Mol Pharm. 2017;14(11):4121–4127. doi: 10.1021/acs.molpharmaceut.7b00458. [DOI] [PubMed] [Google Scholar]


