Abstract
AIM
To investigate the association of axial length (AL) and ocular factors on AL elongation.
METHODS
A retrospective chart review of patients who underwent two or more AL examinations for more than two years. Totally 4 groups were divided according to initial AL (<24 mm, 24-26 mm, 26-28 mm, ≥28 mm). Initial fundus photograph was used to find risk factors associated AL elongation.
RESULTS
The mean age of the patients was 47.21±7.79y. AL remained almost unchanged in the groups with AL<24 mm and 24≤AL<26 mm. On the contrary, AL increased by 0.011 mm/y in the group with 26≤AL<28 mm and 0.035 mm/y in the group with AL≥28 mm (P<0.001). In high myopia, AL elongation increased in eye with longer AL (r=0.003, P=0.024), female gender (r=0.014, P=0.019), eye with larger peripapillary chorioretinal atrophic area (r=0.002, P=0.019), and smaller vascular arcade angle (r=-0.004, P=0.006). The risk of elongation 0.03 mm/y in high myopia was increased in female gender (P=0.040), and gradually increased in eye with large peripapillary chorioretinal atrophy area (P<0.01).
CONCLUSION
AL elongate significantly in the eye with longer AL, female gender, and the eye with larger atrophic area and smaller arcade angle on fundus photography.
Keywords: axial length elongation, fundus photography, high myopia, myopic complication
INTRODUCTION
The prevalence of myopia has been steadily increasing and myopia has been identified as a leading cause of visual impairment[1]–[4]. Myopic eye with a spherical equivalent more than -6.0 diopter (D) or an axial length (AL) longer than 26 mm is defined as high myopia[2],[5]. High myopia associated with posterior staphyloma and degenerative changes is characterized as pathologic myopia or degenerative myopia. Pathologic myopia is associated with pathologic features: tessellated fundus, diffuse or patchy chorioretinal atrophy, macular atrophy, lacquer crack, choroidal neovascularization (CNV), Fuchs spot, and posterior staphyloma[6]–[10].
Continuous elongation of AL has been identified in adults—particularly those with high myopia—and many studies have suggested that eyes with longer AL experience a significant axial length elongation (ALE) over time[11]–[15]. Several studies have suggested that ALE is related to the progression of posterior staphyloma in high myopia[12],[14], while others have reported that no differences in ALE with or without posterior staphyloma[11]. Ohsugi et al[13] have reported a significant increase in ALE among female gender and patients with myopic CNV, and they suggested hypothesis that there may be a relationship between ALE and collagen abnormalities. Likewise, it is reported that ALE is highly related to posterior staphyloma[12], and the progression of posterior staphyloma increases the risk of myopic maculopathy[16]. Furthermore, high myopia is a serious public health problem because the risk of uncorrectable visual impairment rises drastically with age in highly myopic patient[17].
The risk of uncorrectable visual impairment and ALE increases steadily with age in highly myopic patient. For this reason, early detection of patients who have a high risk of ALE, and proper explanation of ALE are important. However, no previous studies have investigated the risk of ALE according to AL difference. This study aims to compare the ALE according to AL in middle-aged adults (30-59y). Furthermore, this study investigates ocular factors associate ALE in high myopia (eyes with AL≥26 mm).
SUBJECTS AND METHODS
Ethical Approval
This study adhered to the tenets of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Severance Hospital, Yonsei University (4-2018-0985) on July 1st 2018, which waived the requirement for informed patient consent because of the retrospective study design.
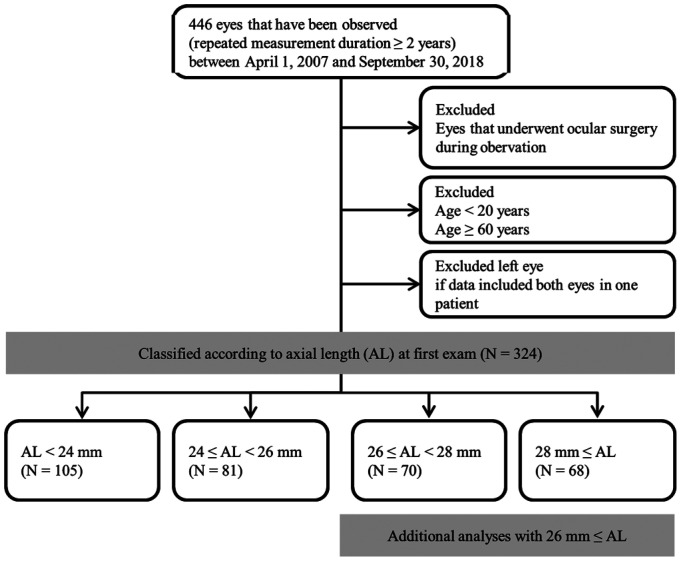
This retrospective, comparative study involved eyes that were observed for more than two years (repeated AL examinations for more than two years) and examination was performed using the IOL-Master (Carl Zeiss Meditec, Jena, Germany) from April 2007 to September 2018. We excluded all eyes that underwent an ophthalmologic surgery (e.g., refractive surgery, keratoplasty, cataract surgery, glaucoma surgery, retinal surgery) during the period of repeated measurement. Adults aged 20y or older patients were included in this study. Since most patients over than 60y had undergone ophthalmic surgery during the follow-up observation, 60y or older patients were excluded for statistical analysis. Eyes were divided into 4 groups based on the first measurement of AL: 1) AL<24 mm, 2) 24 mm≤AL<26 mm, 3) 26 mm≤AL<28 mm, and 4) AL≥28 mm. Age matching has been performed in all eye length classification groups. A total of 324 patients were included in the analysis. To study ocular factors that influence AL elongation in high myopia (AL≥26 mm), an additional investigation was performed. Ocular factors were identified based on fundus photography (Visucam; Carl Zeiss Meditec, Jena, Germany) obtained within 6mo from the date of first AL measurement (Figure 1). And mean intraocular pressure (IOP) was calculated form all IOPs measured during the observation period. All IOP was measured using a non-contact tonometer (TX-F; Canon, Tokyo, Japan). Regrettably, there were many patients who did not take optical coherence tomography (OCT) when they first visited the hospital, so OCT finding could not be included in this study.
Figure 1. Flow chart of study population.
This study included comparative analysis of 324 eyes after dividing them into 4 groups according to AL. Additional analyses were performed to elucidate factors that may affect AL elongation in eyes with an AL≥26mm.
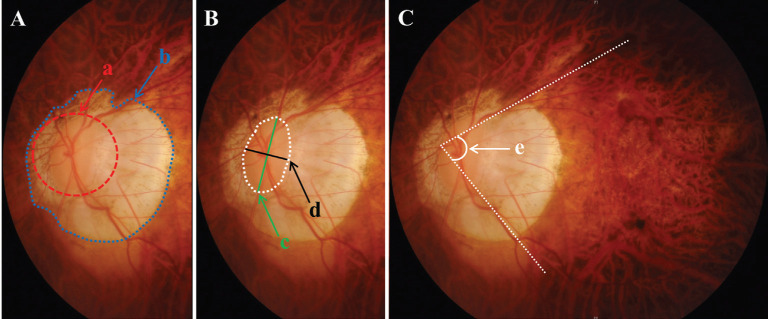
Ocular factors affecting ALE were examined according to the following three categories. First, atrophic area—defined as the ratio of circular area of chorioretinal atrophy to a circle having the longest axis of disc as the radius on fundus photography—was measured. By doing this, we have resolved individual size differences of fundus images. Second, tilted disc ratio—defined as the ratio of maximum to minimum disc diameter—was measured[18]–[19]. Third, vascular arcade angle—defined as the angel between two lines parallel to superior and inferior temporal arcades—was measured[20]–[21]. Atrophic area was categorized as follows: 1) 0 (area<2), 2) 1 (2≤area<3), 3) 2 (3≤area<4), 4) 3 (4≤area<6), and 5) 4 (area≥6). Arcade angle was rated as 0 (angle>90°), 1 (angle of 75°-90°), 2 (angle of 60°-75°), 3 (angle of 45°-60°), or 4 (angle≤45°; Figure 2).
Figure 2. Ocular factors determined from fundus photography.
A: Atrophic area=“b” area/ “a” area; B: Tilted disc ratio=“c” length/ “d” length; C: Vascular arcade angle=“e” angle.
Statistical Analysis
We assumed that AL changes linearly. Intercepts (of linear change of AL) were calculated using the date of first measurement and dates of repeated measurements (AL difference, mm per date difference, day). Based on these measures, changes in AL over one year (365d) were calculated. Data for continuous variables are expressed as the means± standard deviations. Intercepts were calculated from repeated measures of AL using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA). Atrophic area, tilted disc and arcade angle were measured with ImageJ® (National Institutes of Health, Bethesda, MD, USA) on fundus photographs. ANOVA was used to evaluate differences in AL changes among four groups and a post-hoc analysis was performed using Tukey's HSD. Univariate and multivariate linear regressions were carried out to identify factors having a correlation with the increase of AL in the group with AL≥26 mm. Univariate and multivariate binary Logistic regressions were conducted to investigate the risk of severe elongation. Two-tailed P-values less than 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA).
RESULTS
No differences were observed between age, sex, and measurement duration among the 4 groups classified according to AL (P=0.172, 0.076, 0.423, respectively). AL remained almost unchanged in the groups with AL<24 mm and 24 mm ≤AL< 26 mm. AL increased by 0.011 mm/y in the group with 26 mm≤AL<28 mm, and 0.035 mm/y in the group with AL≥28 mm (P<0.001; Table 1).
Table 1. Difference among four groups classified by AL.
| Parameters | AL<24 mm (n=105) | 24 mm≤AL<26 mm (n=81) | 26 mm≤AL<28 mm (n=70) | AL≥28 mm (n=68) | Pa |
| Age at 1st examination, y | 48.28±7.52 | 47.43±7.77 | 45.63±7.86 | 46.94±8.06 | 0.172 |
| Sex, male, % | 45 (42.9) | 50 (61.7) | 38 (54.3) | 37 (54.4) | 0.076 |
| Observation duration, mo | 46.17±18.72 | 47.32±17.03 | 46.91±16.20 | 50.81±21.48 | 0.423 |
| AL change per year, mm | 0.000±0.03 | 0.000±0.03 | 0.011±0.03 | 0.035±0.04 | <0.001 |
AL: Axial length. aAnalysis of variance with post-hoc Turkey HSD.
Multivariate linear regression analyses revealed a significant AL increase with longer AL, female, larger chorioretinal atrophic areas, severe tilted disc ratios, and narrower vascular arcade angles (P<0.001, 0.002, <0.001, 0.003, <0.001, respectively). Age at initial examination, mean IOP were not correlated with ALE (P=0.312, 0.125, respectively; Table 2).
Table 2. Factors related to axial length elongation per year.
| Parameters | Unadjusted regression coefficienta |
Adjusted regression coefficientb |
||||
| r (95%CI) | Beta | P | r (95%CI) | Beta | P | |
| Axial length, mm | 0.012 (0.006 to 0.018) | 0.351 | <0.001 | 0.003 (0.001 to 0.006) | 0.177 | 0.024 |
| Age, y | 0 (0.000 to 0.001) | 0.086 | 0.312 | |||
| Sex, female | 0.019 (0.007 to 0.031) | 0.255 | 0.002 | 0.014 (0.003 to 0.026) | 0.161 | 0.019 |
| mean IOP, mm Hg | -0.002 (-0.004 to 0.001) | -0.13 | 0.125 | |||
| Atrophic area ratio | 0.004 (0.002 to 0.006) | 0.349 | <0.001 | 0.002 (0.000 to 0.004) | 0.195 | 0.019 |
| Tilted disc ratio | 0.037 (0.013 to 0.060) | 0.253 | 0.003 | |||
| Arcade angle, 10 degree | -0.007 (-0.011 to -0.004) | -0.353 | <0.001 | -0.004 (-0.008 to 0.001) | -0.22 | 0.006 |
aLinear regression of each variable, bLinear regression of all variables with backward elimination variable selection.
For assessing risk of severe ALE (defined as ≥0.03 mm per year, that was mean axial elongation per year on the group with AL≥28 mm), univariate binary Logistic regression was conducted in high myopia. Compared to AL 26-26.99 mm, the odds ratio (OR) for AL≥29 mm was 6.905 (95%CI=2.823 to 16.890, P<0.001). Females had an OR 2.583 higher when compared to male (95%CI=1.278 to 5.223, P=0.008). Compared to peripapillary atrophic area of less than 2-disc area, the OR for atrophic area between 2-2.99 was 5.750 (95%CI=2.004 to 16.173, P=0.001), 7.187 (95%CI=2.637 to 19.590, P<0.001) for atrophic area between 3-5.99, and 8.944 (95%CI=2.101 to 38.077, P=0.003) for atrophic area≥6. Compared to vascular arcade angle>90°, the OR for eyes with angle between 60°-75° was 3.750 (95%CI=1.476 to 9.529, P=0.005), and OR for narrower angle than 60° was 4.167 (0.963 to 18.023, P=0.056). Multivariate logistic regression analyses revealed that female and larger chorioretinal atrophic area was significantly increased the risk of severe ALE (Table 3).
Table 3. Factors related to axial length elongation ≥0.03 mm per year.
| Parameters | Unadjusted ORa (95%CI) | P | Adjusted ORb (95%CI) | P |
| Axial length, mm | ||||
| 26-26.99 | (reference) | |||
| 27-27.99 | 1.640 (0.508-5.296) | 0.408 | ||
| 28-28.99 | 1.262 (0.338-4.707) | 0.729 | ||
| ≥29 | 6.905 (2.823-16.890) | <0.001 | ||
| Sex | ||||
| Male | (reference) | (reference) | ||
| Female | 2.583 (1.278-5.223) | 0.008 | 2.265 (1.039-4.934) | 0.040 |
| Atrophic area ratio | ||||
| <2 | (reference) | (reference) | ||
| 2-2.99 | 5.750 (2.044-16.173) | 0.001 | 5.604 (1.953-16.076) | 0.001 |
| 3-5.99 | 7.187 (2.637-19.590) | <0.001 | 6.939 (2.500-19.263) | <0.001 |
| ≥6 | 8.944 (2.101-38.077) | 0.003 | 7.470 (1.708-32.670) | 0.008 |
| Tilted disc ratio | ||||
| < 1.30 | (reference) | |||
| 1.30-1.39 | 1.467 (0.588-3.657) | 0.411 | ||
| 1.40-1.49 | 3.048 (0.965-9.625) | 0.058 | ||
| ≥ 1.50 | 3.077 (1.227-7.715) | 0.017 | ||
| Arcade angle | ||||
| >90° | (reference) | |||
| 75.01°-90° | 2.024 (0.937-4.893) | 0.118 | ||
| 60.01°-75° | 3.750 (1.476-9.529) | 0.005 | ||
| ≤60° | 4.167 (0.963-18.023) | 0.056 |
aBinary logistic regression of “each variable”; bBinary logistic regression of “all variables” with backward elimination variable selection.
DISCUSSION
Pathologic myopia is defined as high myopia associated with degenerative changes: myopic maculopathy that included diffuse or patchy chorioretinal atrophy, lacquer cracks, and myopic CNV[22]. For simple classification of pathologic myopia, an international panel of myopia researchers developed a new classification of myopic maculopathy (META-PM classification; Table 4)[6].
Table 4. Summary of the classification of myopic maculopathy (META-PM classification).
| Classification | Fundus appearance |
| Category 0 | No macular degenerative lesion |
| Category 1 | Tessellated fundus only |
| Category 2 | Diffuse chorioretinal atrophy |
| Category 3 | Patchy chorioretinal atrophy |
| Category 4 | Macular atrophy |
| + Plus | Lacquer cracks, choroidal neovascularization, Fuchs spot, and posterior staphyloma |
Several studies were performed to explore the relationship between ALE and ocular factors. A previous study reported that AL was significantly correlated with tilted disc ratio, and eyes with a longer AL had more severe disc tilt[18]–[19]. In this study, ALE was correlated with tilted disc ratio before adjusting for AL, age, sex, and other variables, but no significance was found between ALE and tilted disc ratio after adjustment. While some studies had found a significant link between posterior staphyloma and ALE[12],[14], other studies obtained contradictory findings[11]. We did not analyze the correlation between ALE and posterior staphyloma, because it was challenging to assess posterior staphyloma based on fundus photography alone. Instead, we analyzed the correlation between ALE and chorioretinal atrophy area that had a significant relationship with posterior staphyloma. ALE was correlated with chorioretinal atrophy area, and this correlation was maintained after adjusting for several variables. Fledelius and Goldschmidt[20] suggested that myopia progression was correlated with both vascular arcade angle and its change. In the current study, a significant correlation was found between ALE and vascular arcade angle, and this correlation was maintained after adjustment for all variables.
There are contradictory findings regarding ALE and IOP. Some authors stated that AL increased significantly in groups with higher IOP[23]–[24], while others reported no relationship between them[11],[13].
The prevalence of open angle glaucoma in myopic eyes was higher than non-myopic eyes. One of the mechanisms was that optic nerve head in myopic eyes may be more susceptible to glaucomatous damage from elevated or normal IOP[25]. We supposed that ALE in myopic eyes may be associated with elevated or normal IOP, but no relationship was found in our study.
There are some contradictory studies that identified that ALE was more likely to be severe in older patients[14], while others revealed no association between ALE and age[11],[13]. In the studies that showed a significant correlation between ALE and age, AL was not adjusted for age and this had probably caused greater axial elongation in older individuals. Since AL is relatively longer in older individuals, ALE may be more severe as age increases without adjusting for AL. It is possible that there is no association between ALE and age, if they had adjusted for AL.
With respect to the relationship between ALE and sex, Ohsugi et al[13] suggested that AL significantly increased in female. In our study, there was a significant correlation between ALE and female sex, and this correlation remained significant even after adjusting for all variables. There was no clear explanation for this. Ohsugi et al[13] suggested the hypothesis that there may be a relationship between ALE and collagen abnormality, and many collagen abnormalities (connective tissue disease) more occurred in females than males, so we also suspect association between ALE collagen abnormality. Further research should be conducted.
There are some limitations to the present study. First, we assumed linear elongation of AL. It would be thought that there was some critical point associated AL elongation, but we assume that the AL change constantly. Second, this study has relatively short follow-up period. Since AL increases gradually, follow-up for more than 10y was meaningful. However, observation of AL using a single biometry instrument over a long period of time was challenging. Third, corneal thickness was not taken into consideration. Since IOP measure was deeply influenced by corneal thickness, we could not completely reveal the relationship between mean IOP and ALE, even though relationship was not observed in this study. Fourth, OCT data was not reviewed in this study. As the importance of choroidal thickness has been recently described, additional studies need to be carried out using OCT data. Finally, we could not analyze the relationship between ALE and systemic diseases. Based on the finding that AL elongated more significantly in females, it can be inferred that ALE is related with systemic conditions such as collagen abnormalities[13]. An additional study is currently in progress to clearly elucidate this relationship.
This study was meaningful in that it better explains difference in ALE according to AL. ALE was minimal or not observed in the group with AL<26 mm, however, it significantly progressed in the group with AL≥28 mm. Furthermore, this study determined risk factors associated with ALE in high myopia. When consulting with high myopic patient, even in middle-aged adult, it is necessary to detect the risk of ALE and carefully explain the risk to the patient with AL≥28 mm, female gender, and fundus finding with large chorioretinal atrophic areas and narrowing vascular arcade angle.
Acknowledgments
Conflicts of Interest: Kim HK, None; Kim SS, None.
REFERENCES
- 1.Ohno-Matsui K, Lai TYY, Lai CC, Cheung CMG. Updates of pathologic myopia. Prog Retin Eye Res. 2016;52:156–187. doi: 10.1016/j.preteyeres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Moreno JM, Flores-Moreno I, Ruiz-Medrano J. High myopia. Choroidal Disorders. Amsterdam: Elsevier; 2017. pp. 171–183. [Google Scholar]
- 3.Ueda E, Yasuda M, Fujiwara K, Hashimoto S, Ohno-Matsui K, Jun HT, Ishibashi T, Ninomiya T, Sonoda KH. Trends in the prevalence of myopia and myopic maculopathy in a Japanese population: the hisayama study. Invest Ophthalmol Vis Sci. 2019;60(8):2781–2786. doi: 10.1167/iovs.19-26580. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura S, Ching-Yu C, Saw SM. Global epidemiology of myopia. Updates on Myopia. Singapore: Springer Singapore; 2019. pp. 27–51. [Google Scholar]
- 5.Liu HH, Xu L, Wang YX, Wang S, You QS, Jonas JB. Prevalence and progression of myopic retinopathy in Chinese adults: the Beijing Eye Study. Ophthalmology. 2010;117(9):1763–1768. doi: 10.1016/j.ophtha.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CM, Saw SM, Verhoeven VJ, Klaver CC, Moriyama M, Shinohara K, Kawasaki Y, Yamazaki M, Meuer S, Ishibashi T, Yasuda M, Yamashita H, Sugano A, Wang JJ, Mitchell P, Wong TY, META-analysis for Pathologic Myopia (META-PM) Study Group International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159(5):877–883.e7. doi: 10.1016/j.ajo.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Ohno-Matsui K, Jonas JB. Posterior staphyloma in pathologic myopia. Prog Retin Eye Res. 2019;70:99–109. doi: 10.1016/j.preteyeres.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Ohno-Matsui K. What is the fundamental nature of pathologic myopia? Retina. 2017;37(6):1043–1048. doi: 10.1097/IAE.0000000000001348. [DOI] [PubMed] [Google Scholar]
- 9.Ohno-Matsui K, Fang Y, Shinohara K, Takahashi H, Uramoto K, Yokoi T. Imaging of Pathologic Myopia. Asia Pac J Ophthalmol (Phila) 2019 doi: 10.22608/APO.2018494. [DOI] [PubMed] [Google Scholar]
- 10.Ohno-Matsui K, Jonas JB. Understanding pathologic myopia. Updates on Myopia. Singapore: Springer Singapore; 2019. pp. 201–218. [Google Scholar]
- 11.Saka N, Moriyama M, Shimada N, Nagaoka N, Fukuda K, Hayashi K, Yoshida T, Tokoro T, Ohno-Matsui K. Changes of axial length measured by IOL master during 2y in eyes of adults with pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):495–499. doi: 10.1007/s00417-012-2066-9. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi A, Ito Y, Iguchi Y, Yasuma TR, Ishikawa K, Terasaki H. Axial length increases and related changes in highly myopic normal eyes with myopic complications in fellow eyes. Retina. 2012;32(1):127–133. doi: 10.1097/IAE.0b013e318214d094. [DOI] [PubMed] [Google Scholar]
- 13.Ohsugi H, Ikuno Y, Shoujou T, Oshima K, Ohsugi E, Tabuchi H. Axial length changes in highly myopic eyes and influence of myopic macular complications in Japanese adults. PLoS One. 2017;12(7):e0180851. doi: 10.1371/journal.pone.0180851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saka N, Ohno-Matsui K, Shimada N, Sueyoshi S, Nagaoka N, Hayashi W, Hayashi K, Moriyama M, Kojima A, Yasuzumi K, Yoshida T, Tokoro T, Mochizuki M. Long-term changes in axial length in adult eyes with pathologic myopia. Am J Ophthalmol. 2010;150(4):562–568.e1. doi: 10.1016/j.ajo.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Fledelius HC, Goldschmidt E. Oculometry findings in high myopia at adult age: considerations based on oculometric follow-up data over 28y in a cohort-based Danish high-myopia series. Acta Ophthalmol. 2010;88(4):472–478. doi: 10.1111/j.1755-3768.2008.01472.x. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W, Yasuzumi K, Nagaoka N, Saka N, Yoshida T, Tokoro T, Mochizuki M. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117(8):1595–1611.e4. doi: 10.1016/j.ophtha.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Tideman JW, Snabel MC, Tedja MS, van Rijn GA, Wong KT, Kuijpers RW, Vingerling JR, Hofman A, Buitendijk GH, Keunen JE, Boon CJ, Geerards AJ, Luyten GP, Verhoeven VJ, Klaver CC. Association of axial length with risk of uncorrectable visual impairment for Europeans with myopia. JAMA Ophthalmol. 2016;134(12):1355–1363. doi: 10.1001/jamaophthalmol.2016.4009. [DOI] [PubMed] [Google Scholar]
- 18.Tay E, Seah SK, Chan SP, Lim AT, Chew SJ, Foster PJ, Aung T. Optic disk ovality as an index of tilt and its relationship to myopia and perimetry. Am J Ophthalmol. 2005;139(2):247–252. doi: 10.1016/j.ajo.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 19.Hyung SM, Kim DM, Hong C, Youn DH. Optic disc of the myopic eye: relationship between refractive errors and morphometric characteristics. Korean J Ophthalmol. 1992;6(1):32. doi: 10.3341/kjo.1992.6.1.32. [DOI] [PubMed] [Google Scholar]
- 20.Fledelius HC, Goldschmidt E. Optic disc appearance and retinal temporal vessel arcade geometry in high myopia, as based on follow-up data over 38y. Acta Ophthalmol. 2010;88(5):514–520. doi: 10.1111/j.1755-3768.2009.01660.x. [DOI] [PubMed] [Google Scholar]
- 21.Jonas JB, Weber P, Nagaoka N, Ohno-Matsui K. Temporal vascular arcade width and angle in high axial myopia. Retina. 2018;38(9):1839–1847. doi: 10.1097/IAE.0000000000001786. [DOI] [PubMed] [Google Scholar]
- 22.Ohno-Matsui K. Pathologic myopia. Asia Pac J Ophthalmol. 2016;5(6):415–423. doi: 10.1097/APO.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 23.Jensen H. Myopia progression in young school children and intraocular pressure. Documenta Ophthalmol. 1992;82(3):249–255. doi: 10.1007/BF00160772. [DOI] [PubMed] [Google Scholar]
- 24.Pruett RC. Progressive myopia and intraocular pressure: what is the linkage? A literature review. Acta Ophthalmol Suppl. 1988;185:117–127. doi: 10.1111/j.1755-3768.1988.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106(10):2010–2015. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]