Abstract
Introduction
Patients with cancer have an increased risk of cardiovascular disease including ischemic heart disease and vice versa. Anticancer drugs and radiotherapy are known to contribute to endothelial injury and vasospasm. However, the relations between vasospastic angina (VSA) and cancer or its treatment are poorly investigated.
Methods
A total of 786 patients underwent intracoronary acetylcholine (ACh) provocation tests to diagnose VSA. The positive ACh provocation test was defined as angiographic coronary artery spasm accompanied by chest pain and/or ischemic electrocardiographic changes. Patients were divided into active cancer, a history of cancer, and no cancer according to the status of malignancy. The impact of types of cancer, anticancer drugs, and radiotherapy on VSA was evaluated.
Results
Of 786 patients, 38 (4.8%) and 84 (10.7%) had active cancer and a history of cancer, respectively, and 401 (51.0%) were diagnosed as VSA. There was no significant difference in rates of positive ACh test among patients with active cancer, a history of cancer, and no cancer (39.5% vs. 57.1% vs. 50.9%, p = 0.20). Types of cancer and cancer treatment also had no impact on positive ACh provocation test.
Conclusions
In this cross-sectional observational study, we did not find an association of active and a history of cancer with the diagnosis of VSA. Anticancer treatment including chemotherapy and radiotherapy was not significantly associated with positive ACh provocation test.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01854-z.
Keywords: Vasospastic angina, Cancer, Anticancer drug, Acetylcholine provocation
Key Summary Points
| Why carry out this study? |
| Cancer and its treatment are considered to contribute to endothelial injury and vasospasm |
| However, relations between vasospastic angina (VSA) and cancer are poorly defined |
| What was learned from the study? |
| Cancer status and type had no impact on positive acetylcholine provocation test |
| Anticancer treatment was not significantly associated with VSA |
| Further studies are needed to elucidate the direct association of cancer with VSA |
Introduction
Vasospastic angina (VSA) is an important cardiac disorder that is associated with the deterioration of quality of life and can induce myocardial infarction and sudden cardiac death [1]. Endothelial dysfunction with deficiency of nitric oxide plays a key role in the mechanism of VSA [2], as well as inflammation, autonomic nerve system, and rho-kinase activity [3, 4].
Heart disease and cancer are the leading causes of death worldwide especially in developed countries [5], and patients with cancer have an increased risk for cardiovascular events and mortality from shared lifestyles, risk factors, and underlying mechanisms such as inflammation, endothelial dysfunction, and oxidative stress [6–11]. Toxicities of cancer treatment, including chemotherapy and radiotherapy, also contribute to endothelial injury and vasospasm, as described in the European Society of Cardiology position paper [12]. In this context, a significant association of anticancer drugs with VSA has been indicated. A case report demonstrated coronary spasm induced by 5-fluorouracil (5-FU) [13], and sorafenib, an inhibitor of the vascular endothelial growth factor (VEGF) receptors, was related to VSA with the potential mechanism of upregulation of rho-kinase activity [14, 15]. Thus, the presence or a history of cancer and chemotherapy and/or radiotherapy may be associated with coronary vasospasm. The aim of this study was to investigate the impact of cancer and its treatment on VSA.
Methods
Study Population
Between April 2012 and June 2020, a total of 809 consecutive patients underwent intracoronary acetylcholine (ACh) provocation test to diagnose VSA at Chiba University Hospital. Patients who had experienced out-of-hospital cardiac arrest (n = 23) were excluded. Thus, 786 patients were retrospectively included in the present study. Written informed consent for examination was obtained from all patients, and informed consent for the present study was obtained in the form of opt-out (no patients chose to opt out of participating in this study). This study was conducted in accordance with the Declaration of Helsinki, and the ethical committee of Chiba University Graduate School of Medicine approved the present study (Approval No. 3983).
Cancer Definitions
Patients were divided into three groups according to the status of malignancy: active cancer, a history of cancer, and no cancer. Patients who were planned for surgery for cancer, were receiving anticancer drug(s) and/or radiotherapy and had recurrent, metastatic, or inoperable cancer were defined as having active cancer. Cancer type included oral cavity and pharynx, thyroid, lung, breast, esophagus, stomach, small intestine, colon and rectum, hepatobiliary pancreas, kidney, bladder, ovary, prostate, uterus, skin, and blood. Anticancer treatment included chemotherapy with anticancer drugs and radiotherapy. Anticancer drugs were categorized as follows: molecularly targeted drug (bevacizumab, sunitinib, and dasatinib), cytotoxic anticancer drug (cisplatin, docetaxel, fluorouracil, and tegafur/gimeracil/oteracil), and hormonal anticancer drug (anastrozole, leuprorelin, letrozole, and vicartamide).
Intracoronary Acetylcholine Provocation Tests
Intracoronary ACh provocation tests were performed to diagnose VSA based on the guidelines [16, 17], as previously reported [4, 18–23]. In brief, all vasodilators including calcium channel blockers and long-acting nitrates were discontinued at least 48 h before the examination in elective cases, except for sublingual nitroglycerin as needed. The radial artery and brachial vein were mainly used as approach sites [24]. After control angiography, a temporary pacing electrode was inserted in the right ventricle. Intracoronary ACh was administered in incremental doses of 20, 50, and 100 μg into the left coronary artery initially, and 20 and 50 μg into the right coronary artery subsequently, over a period of 20 s. One minute after the start of each injection, coronary angiography was performed to evaluate coronary vasospasm. After ACh provocation testing, 1–2 mg of isosorbide dinitrate was administered into the right and left coronary arteries, and coronary angiography was performed. Obstructive epicardial coronary artery disease (CAD) was defined as ≥ 50% stenosis on coronary angiography after administration of intracoronary isosorbide dinitrate. The positive ACh provocation test was defined as angiographic coronary artery spasm, a total or subtotal occlusion by the ACh administration, accompanied by chest pain and/or ischemic electrocardiographic changes. It was evaluated by two experienced cardiologists who were blinded to patients’ clinical characteristics.
Endpoint and Statistical Analysis
The primary endpoint of the present study was positive ACh provocation test in patients with different cancer statuses. Relations between VSA and cancer type or treatment were also evaluated. All statistical analyses were conducted using JMP Pro 15.0.0 (SAS Institute, Cary, NC, USA). Data are expressed as median [interquartile range] or frequency (%). Continuous variables were compared using Kruskal-Wallis and Mann-Whitney U tests. Normal distribution was tested using Shapiro-Wilk test. Categorical variables were compared with Fisher’s exact test. Separate logistic regression analyses were performed to identify univariable predictors of positive ACh provocation test. The associated variables in univariable analyses (p < 0.20) and age, sex, and active cancer (irrespective of p value) were included in the model of multivariable logistic regression analysis. Because anticancer drug was highly correlated with active cancer, the two factors were not included in the multivariable model simultaneously. A value of p < 0.05 was considered statistically significant.
Results
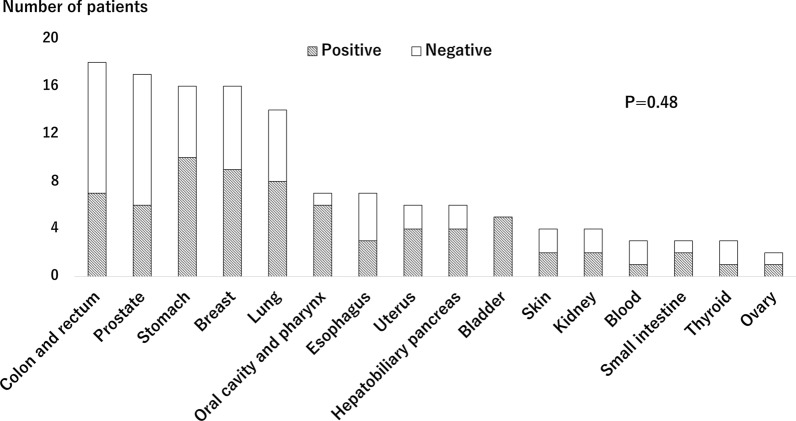
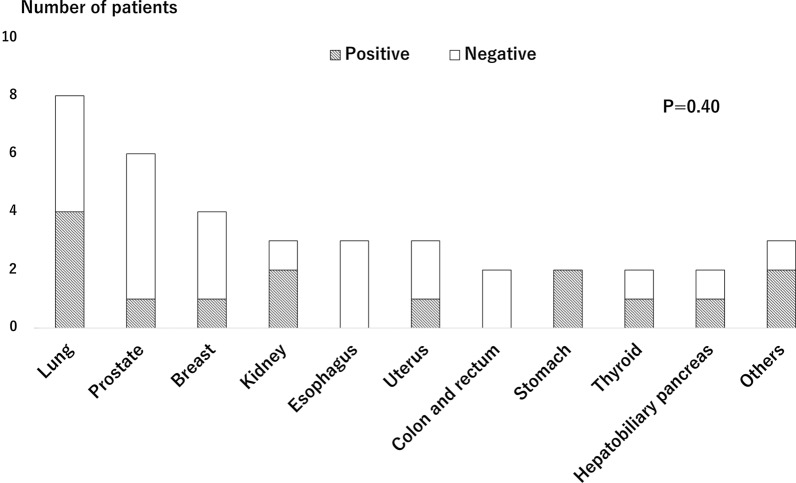
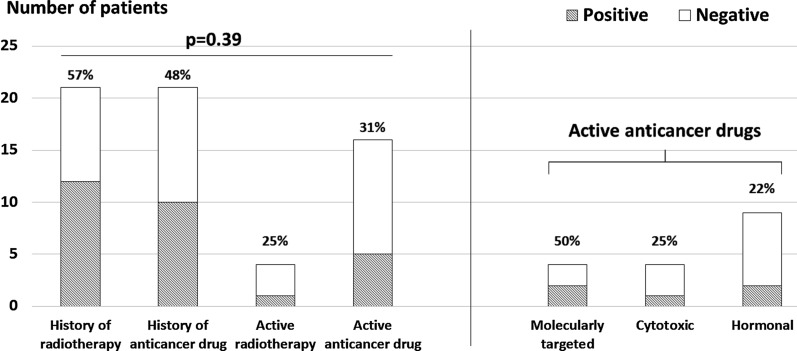
Of 786 patients, 38 (4.8%) and 84 (10.7%) had active cancer and a history of cancer, respectively, and 401 (51.0%) were diagnosed as VSA with positive ACh provocation test. Patients with cancer were older and had lower body mass index among the three groups (Table 1). In terms of ACh provocation test findings, the number of provoked angiographic coronary artery spasms was significantly fewer in patients with active cancer, while the rate of positive ACh provocation test was not different among the three groups (Table 2). Figure 1 shows relations between cancer type and VSA in patients with active and a history of cancer. The rates of positive ACh provocation test ranged from 33% to 100% among types of cancer with no significant differences. When focusing on only patients with active cancer, no significant differences were observed (Fig. 2). Among patients with active cancer, 16 were receiving anticancer drug (4 molecularly targeted, 4 cytotoxic, and 9 hormonal drugs) and 4 underwent ongoing radiotherapy. Cancer treatment also had no impact on positive ACh provocation test (Fig. 3). Multivariable analysis identified current smoking and obstructive epicardial CAD as factors associated with VSA, but cancer-related variables were not associated (Table 3).
Table 1.
Baseline characteristics
| Variable | Active cancer (n = 38) | History of cancer (n = 84) | No cancer (n = 664) | p value |
|---|---|---|---|---|
| Age (years) | 74.0 [66.0, 78.3] | 72.0 [65.3, 78.0] | 65.0 [54.0, 72.0] | < 0.001 |
| Men | 22 (57.9%) | 53 (63.1%) | 329 (49.6%) | 0.049 |
| Body mass index (kg/m2) | 22.1 [21.3, 25.7] | 23.3 [20.3, 26.2] | 23.4 [21.3, 25.7] | 0.09 |
| Hypertension | 25 (65.8%) | 52 (61.9%) | 387 (58.3%) | 0.58 |
| Diabetes mellitus | 10 (26.3%) | 19 (22.6%) | 117 (17.6%) | 0.21 |
| Dyslipidemia | 24 (63.2%) | 51 (60.7%) | 428 (64.5%) | 0.77 |
| Current smoker | 6 (15.8%) | 15 (17.9%) | 114 (17.2%) | 0.97 |
| Prior myocardial infarction | 4 (10.5%) | 6 (7.2%) | 53 (8.1%) | 0.78 |
| ACS presentation | 9 (23.7%) | 18 (21.4%) | 129 (19.4%) | 0.69 |
| Clinical presentation | ||||
| Rest angina | 26 (68.4%) | 56 (66.7%) | 485 (73.0%) | 0.39 |
| Effort angina | 2 (5.3%) | 4 (4.8%) | 14 (2.1%) | 0.13 |
| Rest and effort angina | 7 (18.4%) | 20 (23.8%) | 145 (21.8%) | 0.82 |
| No angina | 3 (7.9%) | 4 (4.8%) | 20 (3.0%) | 0.16 |
| Medical treatment | ||||
| Calcium channel blocker | 17 (44.7%) | 37 (44.1%) | 301 (45.3%) | 0.99 |
| Long-acting nitrate | 12 (31.6%) | 18 (21.4%) | 110 (16.6%) | 0.048 |
| Antiplatelet | 12 (31.6%) | 29 (34.5%) | 194 (29.2%) | 0.55 |
| Statin | 10 (26.3%) | 30 (35.7%) | 255 (38.4%) | 0.32 |
| ACE-I or ARB | 18 (47.4%) | 28 (33.3%) | 218 (32.8%) | 0.19 |
| β-blocker | 9 (23.7%) | 13 (15.5%) | 97 (14.6%) | 0.30 |
ACS acute coronary syndrome, ACE-I angiotensin converting enzyme inhibitor, ACh acetylcholine, ARB angiotensin II receptor blocker
Table 2.
ACh provocation test findings
| Variable | Active cancer (n = 38) | History of cancer (n = 84) | No cancer (n = 664) | p value |
|---|---|---|---|---|
| Positive ACh provocation test | 15 (39.5%) | 48 (57.1%) | 338 (50.9%) | 0.20 |
| Signs of ischemia | ||||
| ECG change | 13 (34.2%) | 37 (44.1%) | 278 (41.9%) | 0.59 |
| Chest pain | 17 (44.7%) | 47 (56.0%) | 368 (55.4%) | 0.44 |
| Number of spasm vessels | 0 [0, 1] | 1 [0, 2] | 1 [0, 2] | 0.01 |
| Multivessel spasm | 8 (21.1%) | 31 (36.9%) | 179 (27.0%) | 0.11 |
| Obstructive epicardial CAD | 14 (36.8%) | 27 (32.1%) | 173 (26.1%) | 0.18 |
ACh acetylcholine, CAD coronary artery disease, ECG electrocardiogram
Fig. 1.
Positive rates of acetylcholine provocation test across types of cancer in patients with active and a history of cancer
Fig. 2.
Positive rates of acetylcholine provocation test across types of cancer in patients with active cancer
Fig. 3.
Positive rates of acetylcholine test across types of anticancer treatment. Active cancer treatment is further divided into three groups (molecularly targeted, cytotoxic, and hormonal drugs)
Table 3.
Factors associated with positive ACh provocation test
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (years) | 0.99 (0.98–1.01) | 0.37 | 1.00 (0.99–1.01) | 0.96 |
| Men | 1.53 (1.16–2.03) | 0.003 | 1.31 (0.97–1.76) | 0.08 |
| Body mass index (kg/m2) | 1.03 (0.99–1.07) | 0.16 | 1.02 (0.99–1.07) | 0.18 |
| Hypertension | 0.87 (0.65–1.15) | 0.33 | ||
| Diabetes mellitus | 0.70 (0.49–1.01) | 0.06 | 0.62 (0.42–0.91) | 0.01 |
| Dyslipidemia | 0.94 (0.71–1.26) | 0.70 | ||
| Current smoker | 1.95 (1.32–2.86) | < 0.001 | 1.74 (1.15–2.64) | 0.009 |
| Obstructive epicardial CAD | 1.54 (1.12–2.13) | 0.008 | 1.56 (1.11–2.18) | 0.01 |
| Active cancer | 0.61 (0.31–1.19) | 0.15 | 0.61 (0.31–1.21) | 0.16 |
| Active and a history of cancer | 1.03 (0.70–1.52) | 0.88 | ||
| Anticancer drug | 0.64 (0.33–1.26) | 0.19 | ||
| Radiotherapy | 1.04 (0.47–2.31) | 0.92 | ||
ACh acetylcholine, CAD coronary artery disease, CI confidence interval, OR odds ratio
Discussion
The present study included 5% and 11% of patients having active and a history of cancer in a cohort of patients with suspected VSA undergoing intracoronary ACh provocation test. Overall, 51% was diagnosed as VSA, but the status of cancer was significantly not associated with positive ACh provocation test. In addition, we did not find impact of type of cancer and anticancer treatment including chemotherapy and radiotherapy on positive ACh test. Multivariable analysis reinforced the findings. To the best of our knowledge, this is the first study investigating relations between VSA and cancer or its treatment.
Cancer and Ischemic Heart Disease
Patients with cancer have an increased risk of cardiovascular disease including ischemic heart disease and vice versa [8–11]. A large-scale national registry including > 6.5 million participants showed that in patients with acute myocardial infarction, 2.8% had active cancer and 6.2% had a history of cancer [25]. In this registry, patients with active cancer had higher mortality as expected, but they were also at a higher risk for major adverse cardiovascular and cerebrovascular events compared with patients with no cancer [25]. VSA, a part of ischemic heart disease, reportedly accounts for approximately 40% of all angina in East Asian populations [16]. Given that patients with cancer and ischemic heart disease including VSA have shared lifestyle and risk factors (e.g., smoking and metabolic syndrome) and underlying mechanisms (e.g., inflammation, endothelial dysfunction, and oxidative stress) [9, 10], it seems biologically plausible that patients with cancer are likely to have coronary vasospasm. However, no study has addressed this issue.
Vasospastic Angina in Cancer
In a position paper from the European Society of Cardiology, chemotherapy including 5-FU and VEGF inhibitors and radiotherapy are listed as having effects on endothelial injury, vasospasm, and coronary atherosclerosis [12]. 5-FU is used to treat patients with gastrointestinal and other malignancies and is reported to induce myocardial ischemia through vasospasm and endothelial injury in up to 10% of cases [12]. Indeed, patients treated with 5-FU experience chest pain in 1–18% [26, 27]. VEGF inhibitors are another class of drugs associated with angina at an incidence of 1–15%. Inhibition of VEGF receptor impairs stimulation of endothelial nitric oxide activity, increases oxidative stress, and upregulates rho-kinase activity, contributing to coronary vasospasm [26]. In fact, several case reports have demonstrated coronary spasm induced by 5-FU and VEGF inhibitors [13–15]. However, it is difficult to determine in clinical practice whether chest pain in patients treated with anticancer drugs is induced by VSA. A recent retrospective single-center study demonstrated that 87 out of 4019 (2.2%) patients treated with 5-FU were adjudicated as having VSA according to the presence of “typical chest pain” without any other diagnostic criteria [27]. In the present study, VSA was diagnosed by invasive intracoronary ACh provocation test, which has good diagnostic ability [16], and no significant relations between VSA and cancer or its treatment were shown. Even in patients actively treated with anticancer drugs, no impact was found with a positive ACh provocation rate of 31%. Although patients who were receiving molecularly targeted drugs had a numerically higher rate of positive ACh test than those with cytotoxic and hormonal drugs (50% vs. 25% vs. 22%), all percentages were lower than the overall rate of positive ACh test in the present study (51%). These findings may be robust because of consistent rates of positive ACh test across cancer types and multivariable analysis findings. Since patients with cancer often experience chest pain by numerous etiologies including myocardial ischemia and others [26], a pretest probability of having VSA might have been low in cancer patients in the present study. Further studies are needed to elucidate the impact of cancer and its treatment on coronary vasospasm. Beyond the relation between VSA and cancer, multivariable analysis showed current smoking and obstructive epicardial CAD as factors associated with coronary vasospasm in the present study. Smoking is a well-recognized risk factor for coronary spasm [16, 28], and a close relationship between coronary atherosclerosis and VSA has been shown in previous reports [29].
Study Limitations
The present study has several limitations. This was a single-center, retrospective, cross-sectional study. The overall sample size was not small, but subgroups of cancer and treatment had only small number of patients. Thus, larger, prospective studies are warranted. In patients with a history of cancer, data on the duration and dosage of chemotherapy and radiotherapy were not available. In addition, patient characteristics were different among the three groups. Even though a case report showed that intracoronary ACh provocation test confirmed the diagnosis of VSA in a patient with sorafenib-induced coronary artery spasm [14], whether ACh provocation test can identify patients with chemotherapy-induced coronary vasospasm as well as VSA patients who are not associated with anticancer therapies remains unclear.
Conclusions
Active and a history of cancer were not significantly associated with the diagnosis of VSA. We did not find an association of anticancer treatment including chemotherapy and radiotherapy with positive ACh provocation test in this cross-sectional observational study.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Author Contributions
TM: writing, draft preparation, data gathering, formal analysis. YS: conceptualization, methodology, investigation, writing—review and editing, formal analysis. KS: investigation. KT: investigation. KK: conceptualization. HK: supervision. YK: project administration, writing—review and editing.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
The authors (Tadahiro Matsumoto, Yuichi Saito, Kan Saito, Kazuya Tateishi, Ken Kato, Hideki Kitahara, and Yoshio Kobayashi) declare that they have nothing to disclose.
Compliance with Ethics Guidelines
The informed consent was obtained from all participants, and the study was reviewed and approved by the ethics committee of Chiba University Graduate School of Medicine. The study was conducted in accordance with this approval and adhered to the tenets of the Declaration of Helsinki revised in 2013.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Peter O, Ahmed A, Henrik SH, Eva P, Anastasios A, Udo S. Structural and functional coronary artery abnormalities in patients with vasospastic angina pectoris. Circ J. 2015;79:1431–1438. doi: 10.1253/circj.CJ-15-0520. [DOI] [PubMed] [Google Scholar]
- 2.Kugiyama K, Yasue H, Okumura K, et al. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996;94:266–271. doi: 10.1161/01.CIR.94.3.266. [DOI] [PubMed] [Google Scholar]
- 3.Ohyama K, Matsumoto Y, Takanami K, et al. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J Am Coll Cardiol. 2018;71:414–425. doi: 10.1016/j.jacc.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Saito Y, Kitahara H, Shoji T, Nakayama T, Fujimoto Y, Kobayashi Y. Decreased double product at rest in patients with severe vasospasm. Heart Lung Circ. 2020;29:1511–1516. doi: 10.1016/j.hlc.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71. [DOI] [PMC free article] [PubMed]
- 6.Koczwara B, Meng R, Miller MD, et al. Late mortality in people with cancer: a population-based Australian study. Med J Aust. 2021;214:318–323. doi: 10.5694/mja2.50879. [DOI] [PubMed] [Google Scholar]
- 7.Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armenian SH, Xu L, Ky B, et al. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol. 2016;34:1122–1130. doi: 10.1200/JCO.2015.64.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derek R, Lan P, Nanette CK, Gabor G, Edith P. Prevention of cardiovascular disease among cancer survivors: the role of pre-existing risk factors and cancer treatments. Curr Epidemiol Rep. 2017;4:239–247. doi: 10.1007/s40471-017-0117-9. [DOI] [Google Scholar]
- 10.Handy CE, Quispe R, Pinto X, et al. Synergistic opportunities in the interplay between cancer screening and cardiovascular disease risk assessment: together we are stronger. Circulation. 2018;138:727–734. doi: 10.1161/CIRCULATIONAHA.118.035516. [DOI] [PubMed] [Google Scholar]
- 11.Okura Y, Ozaki K, Tanaka H, Takenouchi T, Sato N, Minamino T. The impending epidemic of cardiovascular diseases in patients with cancer in Japan. Circ J. 2019;83:2191–2202. doi: 10.1253/circj.CJ-19-0426. [DOI] [PubMed] [Google Scholar]
- 12.Zamorano JL, Lancellotti P, Rodriguez MD, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 13.Luwaert RJ, Descamps O, Majois F, Chaudron JM, Beauduin M. Coronary artery spasm induced by 5-fluorouracil. Eur Heart J. 1991;12:468–470. doi: 10.1093/oxfordjournals.eurheartj.a059919. [DOI] [PubMed] [Google Scholar]
- 14.Arima Y, Oshima S, Noda K, et al. Sorafenib-induced acute myocardial infarction due to coronary artery spasm. J Cardiol. 2009;54:512–515. doi: 10.1016/j.jjcc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Naib T, Steingart RM, Chen CL. Sorafenib-associated multivessel coronary artery vasospasm. Herz. 2011;36:348–351. doi: 10.1007/s00059-011-3444-5. [DOI] [PubMed] [Google Scholar]
- 16.JCS Joint Working Group Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013) Circ J. 2014;78:2779–2801. doi: 10.1253/circj.CJ-66-0098. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki S, Kaikita K, Yamamoto E, Jinnouchi H, Tsujita K. Role of acetylcholine spasm provocation test as a pathophysiological assessment in nonobstructive coronary artery disease. Cardiovasc Interv Ther. 2021;36:52–53. doi: 10.1007/s12928-020-00735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tateishi K, Saito Y, Kitahara H, et al. Vasospastic angina and overlapping cardiac disorders in patients resuscitated from cardiac arrest. Heart Vessels. 2021;36:321–329. doi: 10.1007/s00380-020-01705-x. [DOI] [PubMed] [Google Scholar]
- 19.Saito Y, Shoji T, Tateishi K, Kitahara H, Fujimoto Y, Kobayashi Y. Mental health status in patients undergoing intracoronary acetylcholine provocation test. Adv Ther. 2020;37:3807–3815. doi: 10.1007/s12325-020-01424-9. [DOI] [PubMed] [Google Scholar]
- 20.Saito Y, Kitahara H, Nishi T, Fujimoto Y, Kobayashi Y. Decreased resting coronary flow and impaired endothelial function in patients with vasospastic angina. Coron Artery Dis. 2019;30:291–296. doi: 10.1097/MCA.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 21.Tateishi K, Saito Y, Kitahara H, et al. Safety and usefulness of acetylcholine provocation test in patients with no culprit lesions on emergency coronary angiography. Int J Cardiol. 2018;269:27–30. doi: 10.1016/j.ijcard.2018.06.108. [DOI] [PubMed] [Google Scholar]
- 22.Saito Y, Kitahara H, Shoji T, et al. Relation between severity of myocardial bridge and vasospasm. Int J Cardiol. 2017;248:34–38. doi: 10.1016/j.ijcard.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Kitahara H, Shoji T, et al. Intracoronary acetylcholine provocation testing—omission of the 20-µg dose is feasible in patients without coronary artery spasm in the other coronary artery. Circ J. 2016;80:1820–1823. doi: 10.1253/circj.CJ-16-0344. [DOI] [PubMed] [Google Scholar]
- 24.Sueda S, Kohno H. Transitional changes of acetylcholine spasm provocation test procedures. Cardiovasc Interv Ther. 2020;35:321–326. doi: 10.1007/s12928-019-00624-7. [DOI] [PubMed] [Google Scholar]
- 25.Bharadwaj A, Potts J, Mohamed MO, et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J. 2020;41:2183–2193. doi: 10.1093/eurheartj/ehz851. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann J, Yang EH, Iliescu CA, et al. Vascular toxicities of cancer therapies: the old and the new—an evolving avenue. Circulation. 2016;133:1272–1289. doi: 10.1161/CIRCULATIONAHA.115.018347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafar A, Drobni ZD, Mosarla R, et al. The incidence, risk factors, and outcomes with 5-fluorouracil–associated coronary vasospasm. JACC CardioOncol. 2021;3:101–109. doi: 10.1016/j.jaccao.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrow JD, Frei B, Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 29.Tsujita K, Sakamoto K, Kojima S, et al. Coronary plaque component in patients with vasospastic angina: a virtual histology intravascular ultrasound study. Int J Cardiol. 2013;168:2411–2415. doi: 10.1016/j.ijcard.2013.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.