Abstract
Introduction
Dolutegravir (DTG), a novel HIV-integrase strand transfer inhibitor (INSTI), is usually used with multiple antiretrovirals (ARVs) for treatment of HIV. DTG is now approved as Tivicay tablets in over 120 countries and Triumeq combination tablets (DTG/abacavir [ABC]/lamivudine [3TC]) in over 90 countries. In Japan, these formulations have been marketed since 2014 and 2015. The post-marketing prospective surveillance has been conducted as part of the HIV-Related Drug (HRD) cooperative survey aimed to collect actual drug use information in all of these DTG-treated patients in accordance with conditions for initial approvals.
Methods
The survey has been conducted to evaluate long-term safety and effectiveness of DTG since 2014, for approximately 6 years. The safety was evaluated by incidence of adverse drug reactions (ADRs) and change in body weight. The effectiveness was evaluated by plasma HIV RNA copies/mL and peripheral CD4+ cell counts.
Results
Of 2292 patients in 30 Japanese sites, 565 (24.65%) reported ADRs. The most common ADR was blood creatinine increased (4.28%). Incidence of ADRs was statistically significantly higher in patients with severe symptoms (Centers for Disease Control and Prevention [CDC] categories B and C) than those with category A, and in patients with comorbidities than those without comorbidities. Whereas incidence of ADRs was statistically significantly lower in antiretroviral therapy (ART)-experienced patients than that in ART-naïve patients. Incidence of ADRs related to suicide or self-injurious behavior was statistically significantly higher in patients with comorbidities of psychiatric disorders than those without comorbidities. The body weight tended to increase over time and those changes and percentage changes from baseline were greater in ART-naïve patients compared with ART-experienced patients. HIV RNA copies/mL and CD4+ cell counts showed favorable shifts from baseline in both ART-naïve and ART-experienced patients.
Conclusion
The results of the survey identified no new safety and effectiveness risks in Japanese patients with HIV/AIDS treated with DTG.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01842-3.
Keywords: Antiretroviral therapy, Dolutegravir, Effectiveness, Human immunodeficiency virus, Integrase strand transfer inhibitor, Post-marketing surveillance, Safety
Key Summary Points
| Why carry out this study? |
| Dolutegravir (DTG), an HIV-integrase strand transfer inhibitor (INSTI), is usually used with multiple antiretrovirals (ARVs) for treatment of HIV in the world; however, the safety and efficacy data for DTG has not been evaluated in clinical trials specifically targeting Japanese patients. |
| Post-marketing surveillance in Japan has been conducted since 2014 and aimed to collect domestic data on safety and effectiveness of DTG in clinical practice. |
| What was learned from the study? |
| Long-term treatment with DTG for up to 6 years had good safety and effectiveness profiles consistent with those observed in clinical trials. |
| The present results support the use of DTG for the treatment of patients with HIV in real-world clinical practice. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.14839548.
Introduction
An estimated 38 million adults and children worldwide were living with HIV/AIDS in 2019 according to the Joint United Nations Programme on HIV/AIDS (UNAIDS) estimates. The estimated 1.7 million people who acquired HIV worldwide in 2019 marked a 23% decline in new HIV infections since 2010. This was the lowest annual number of new infections since 1989, while the rate of new infections is still high in Eastern Europe and Central Asia. The number of patients is also increasing in the Middle East, North Africa, and Latin America [1].
The current initial treatment for HIV infections is antiretroviral therapy (ART), which generally consists of three or more antiretrovirals (ARVs). ART has greatly improved the prognosis of HIV-infected patients and early treatment for HIV infection has been promoted. However, problems with current anti-HIV therapies remain, including the emergence of resistant viruses, interactions with concomitant drugs, and the development of toxicity by long-term use. Therefore, a long-term, safe, and effective treatment is desired.
Dolutegravir sodium (DTG) is an HIV-integrase strand transfer inhibitor (INSTI) developed by a joint venture between Shionogi & Co., Ltd. and GlaxoSmithKline K.K. (currently ViiV Healthcare K.K.).
DTG was first approved as single-agent Tivicay® tablets in the USA in August 2013, in Europe in January 2014, and in Japan in March 2014. DTG was subsequently approved as Triumeq® combination tablets, a combination product containing abacavir sulfate (ABC) and lamivudine (3TC) in the USA in August 2014, in Europe in September 2014, and in Japan in March 2015; as Juluca® combination tablets, a combination product containing rilpivirine hydrochloride (RPV) in the USA in November 2017, in Europe in May 2018, and in Japan in November 2018; as Dovato® combination tablets, a combination product containing 3TC in the USA in April 2019, in Europe in July 2019, and in Japan in January 2020. DTG is now approved as Tivicay tablets in over 120 countries, Triumeq combination tablets in over 90 countries, Juluca combination tablets in over 40 countries, and Dovato combination tablets in over 30 countries.
In clinical trials of these formulations, DTG demonstrated high antiretroviral efficacy, high genetic barrier, and favorable safety profiles in both ART-naïve (except for DTG/RPV) and ART-experienced patients [2–14].
DTG formulations except for DTG/RPV offer once-daily dosing without regard to meals, which could help drug adherence and reduces the risk of developing viral resistance mutations. In addition, DTG is less susceptible to the effects of cytochrome P450 (CYP) 3A4 and drug–drug interactions. On the basis of these benefits, DTG regimens (INSTI + two nucleoside reverse transcriptase inhibitors [NRTI], INSTI + one NRTI) are included in recommended regimens for the initial therapy in the Japanese Ministry of Health, Labour and Welfare (MHLW) Study Group Guidelines for Anti-HIV Therapy [15], the US Department of Health and Human Services Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV (the DHHS guidelines) [16], and the European AIDS Clinical Society (EACS) Guidelines [17].
As per the conditions for initial approval of these drugs, a post-marketing surveillance with all patients was required in principle, to collect information on the actual drug use in Japan. Consequently, the post-marketing surveillance has been conducted since April 2014 on Tivicay tablets and April 2015 on Triumeq combination tablets to collect information on the actual use of the drug from 30 sites in Japan. In this article, we evaluated the long-term safety and effectiveness information obtained by August 2020 in the post-marketing surveillance.
Methods
Subjects
A post-marketing prospective surveillance of DTG was conducted to evaluate safety and effectiveness of DTG based on the actual use as part of the HIV-Related Drug (HRD) cooperative survey in Japanese patients with HIV.
The HRD cooperative survey is a joint research program conducted by agreement with the MHLW. In this survey, a standardized survey form is used to reduce workload of healthcare professionals filling out different forms for each study sponsor because ART, a standard therapy for HIV, consists of the combination of multiple ARVs. The survey is conducted exclusively at sites with HIV specialists and a large number of HIV-infected patients.
This prospective surveillance is to be conducted for 10 years from initial marketing for Tivicay tablets. This interim report included all DTG-treated HIV-infected patients who registered to participate in the HRD cooperative survey between April 17, 2014 or April 10, 2015 (the day of initial marketing for Tivicay tablets or Triumeq combination tablets) and August 7, 2020 (the day of data lock). This article shows the post-marketing data collected from 30 sites in Japan. In addition, the start date of treatment with DTG was defined as the first date of treatment recorded on the survey forms. No patients received these drugs before the initial marketing release of Tivicay tablets or Triumeq combination tablets.
The adult participants received oral administration of DTG 50 mg once daily without regard to food. Tivicay tablets were also administrated to treatment-naïve children and adolescents aged 12 years and older whose body weight is 40 kg or more, or those treated with ARVs other than INSTIs. Administration of Tivicay tablets up to 50 mg twice daily was accepted for patients with INSTI-resistant HIV. Triumeq combination tablets were administrated to adult patients only. Each Triumeq tablet contains 600 mg of ABC and 300 mg of 3TC.
This post-marketing surveillance has been conducted in compliance with the Japanese ministry directive on Good Post-marketing Study Practice (GPSP). The study protocol was reviewed and approved by the ethics committee at each study site. In compliance with GPSP, consent for participation is not required in post-marketing surveillances; however, we defined the process of obtaining verbal consent in the study protocol. In line with the study protocol, use of DTG in treatment of HIV infection was explained to each potential subject, and verbal consent for the use was obtained prior to study initiation from all patients who would receive DTG.
Study Variables
Over the course of the survey, the following demographic, safety, and effectiveness information was collected: patient characteristics (age, sex, comorbidities, liver disorder, renal disorder); duration of treatment; prescription history of ARV backbone (drug name); usage of other concomitant drugs; severity (category of the Centers for Disease Control and Prevention [CDC] classification) at pretreatment; specific adverse events (AEs) as well as their dates of onset, clinical course, treatment, seriousness, outcome, causal relationship to disease or drugs, and presence or absence of abnormal changes in laboratory values (if measured); plasma HIV RNA copies/mL; and peripheral CD4+ cell counts.
Adverse drug reactions (ADRs) were defined as AEs suspected to be related to DTG evaluated by the reporting physician and the sponsor. The seriousness of ADRs was evaluated by the sponsor. The AE names shown in this article were reported according to Medical Dictionary for Regulatory Activities/Japanese version (MedDRA/J) version 23.0. In this survey, the MedDRA/J preferred term (PT)-specified ADR groups of single or multiple events that occurred were counted by time to onset of events. The list of events included in each ADR group is provided in Table S1 in the supplementary material. In this evaluation, events of the ADR group that occurred in the individual patient were counted as the event with the earlier onset. Neuropsychiatric ADRs and changes in body weight, which were considered to be risks of DTG, were also evaluated separately. For neuropsychiatric ADRs, events contained in the MedDRA/J system organ class (SOC) of psychiatric disorders and nervous system disorders were tabulated. The incidence of ADRs related to depression, suicide, or self-injurious behavior by the presence or absence of psychiatric disorders as comorbidities was also tabulated.
For changes in body weight, the amount of change (the mean difference of the body weight between at the time of start of DTG treatment [baseline] and each time of measurement) and the percentage change (the mean value of amount of change at each time of measurement divided by the baseline body weight) were also calculated.
Decrease in plasma HIV RNA copies/mL and increase in peripheral CD4+ cell counts were used for effectiveness evaluation.
Some of the safety data (change in body weight) and effectiveness data were shown separately for ART-naïve patients (data during the period of initial ART in patients with no prescription histories of ARVs at enrollment) and ART-experienced patients (data during the period of ART in this survey in patients with prescription histories of ARVs at enrollment, or the period of second or subsequent ART in this survey in patients with no prescription histories of ARVs at enrollment).
Statistical Analyses
No statistical hypothesis testing was planned for both safety and effectiveness in this survey. In order to evaluate safety and effectiveness of DTG in Japanese patients, those were analyzed for DTG-treated patients (i.e., pool of patients who received Tivicay tablets and/or Triumeq combination tablets). The patients who received both Tivicay tablets and Triumeq combination tablets were counted only once in the pooled data.
Patients with collected survey form included all patients registered (patients registered by the day of lock date) and whose written survey form was collected. The safety analysis set comprised of all patients excluding those who did not visit the sites after the first visit, those who did not receive DTG (i.e., administration after the date of initial marketing could not be confirmed), and those for whom presence or absence of AE was unknown and follow-up of AEs could not be performed among the survey form collected patients. The effectiveness analysis set comprised of all patients excluding those who received DTG as off-label use or whose data of plasma HIV RNA copies/mL or peripheral CD4+ cell counts were not collected at pre and post DTG treatment among the safety analysis set.
In this survey, additional evaluation for ADRs was performed using univariate analysis with the following patient characteristics that were considered to affect the safety: age, sex, category of CDC classification at pretreatment, comorbidities, liver disorder, renal disorder, prescription history of ARV backbone. The relationship between patient characteristics and incidence of ADRs was examined by Fisher’s exact test for the factors with two categorical variables, or chi-square test for the factors with three categorical variables or more. Odds ratios with 95% confidence interval (CI) were estimated. Missing data were not imputed, and no adjustment for multiplicity was performed. All of the statistical analyses were conducted using SAS version 9.4.
Results
Surveyed Patients
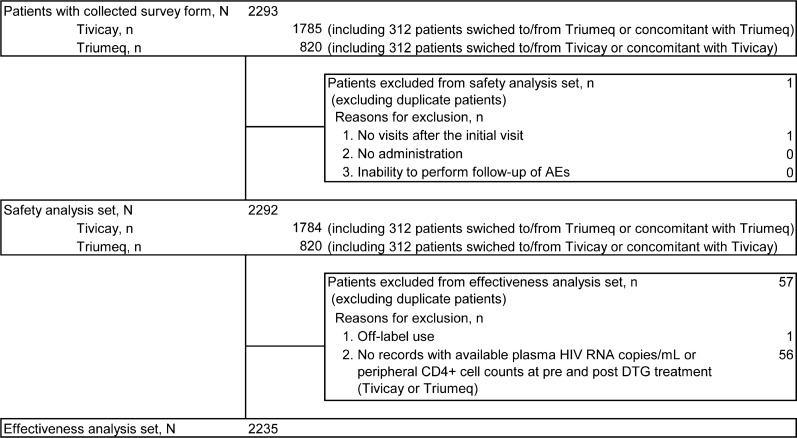
The patient disposition is shown in Fig. 1. A total of 2424 from 30 Japanese sites participating in this HRD cooperative survey were enrolled in the analysis; the required survey forms were collected from 2293 patients. The safety analysis set comprised of 2292 patients, excluding 1 patient who had no hospital visits after the initial visit. The effectiveness analysis set comprised of 2235 patients, excluding 1 patient who received DTG for off-label use (drug use via unapproved administration route: product use issue in PT) and 56 patients with no available HIV RNA copies/mL or CD4+ cell counts at pre and post DTG treatment.
Fig. 1.
Patient disposition. AE adverse event, DTG dolutegravir, HIV human immunodeficiency virus, RNA ribonucleic acid
Patient Characteristics
The patient characteristics is shown in Table 1. Of the 2292 patients in the safety analysis set, 2181 patients (95.16%) were male. The mean age at the initial treatment of DTG was 41.1 years (range 13–83 years); the vast majority (95.42%; 2187 patients) were adult (defined as non-elderly aged between 15 and 64 years). The proportion of patients with comorbidities was 62.48% (1432 patients), with hepatic disorder in 19.59% (449 patients) and renal disorder in 6.98% (160 patient). According to CDC classification at pretreatment, more than half of patients (66.71%, 1529 patients) had mild symptoms (category A); the proportion of patients with severe symptoms (category C) was 23.47% (538 patients). A total of 1542 patients (67.28%) received DTG treatment for more than 730 days. The proportion of patients with no treatment histories of ARV backbone at baseline (hereafter, ART-naïve patients) was 45.38% (1040 patients), and those with treatment histories of ARV backbone at baseline (hereafter, ART-experienced patients) was 53.18% (1219 patients). Disposition of the ARV backbone at baseline used was tenofovir disoproxil fumarate (TDF)+/emtricitabine (FTC) (41.80%; 958 patients), ABC+/3TC (39.35%; 902 patients), and tenofovir alafenamide fumarate (TAF)/FTC (13.70%; 314 patients) (Table 1).
Table 1.
Patient characteristics patients with collected survey form
| Factors | Categories | Patients with collected survey form, n (%) | Safety analysis set, n (%) | Effectiveness analysis set, n (%) |
|---|---|---|---|---|
| Total | – | 2293 | 2292 | 2235 |
| Age (1) (years)a | < 15 | 1 (0.04) | 1 (0.04) | 1 (0.04) |
| ≥ 15, < 65 | 2188 (95.42) | 2187 (95.42) | 2134 (95.48) | |
| ≥ 65 | 104 (4.54) | 104 (4.54) | 100 (4.47) | |
| Age (2) (years) | < 10 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| ≥ 10, < 20 | 8 (0.35) | 8 (0.35) | 8 (0.36) | |
| ≥ 20, < 30 | 359 (15.66) | 359 (15.66) | 347 (15.53) | |
| ≥ 30, < 40 | 691 (30.14) | 690 (30.10) | 667 (29.84) | |
| ≥ 40, < 50 | 749 (32.66) | 749 (32.68) | 738 (33.02) | |
| ≥ 50, < 60 | 293 (12.78) | 293 (12.78) | 288 (12.89) | |
| ≥ 60, < 70 | 148 (6.45) | 148 (6.46) | 143 (6.40) | |
| ≥ 70 | 45 (1.96) | 45 (1.96) | 44 (1.97) | |
| n | 2293 | 2292 | 2235 | |
| Mean | 41.1 | 41.1 | 41.2 | |
| SD | 11.8 | 11.8 | 11.7 | |
| Min | 13 | 13 | 13 | |
| Max | 83 | 83 | 83 | |
| Sex | Male | 2182 (95.16) | 2181 (95.16) | 2128 (95.21) |
| Female | 111 (4.84) | 111 (4.84) | 107 (4.79) | |
| CDC classification at pretreatment | A | 1530 (66.72) | 1529 (66.71) | 1498 (67.02) |
| B | 125 (5.45) | 125 (5.45) | 121 (5.41) | |
| C | 538 (23.46) | 538 (23.47) | 529 (23.67) | |
| Unknown | 99 (4.32) | 99 (4.32) | 86 (3.85) | |
| P-0 | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| P-1 | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| P-2 | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Comorbidities | Absent | 751 (32.75) | 751 (32.77) | 732 (32.75) |
| Present | 1432 (62.45) | 1432 (62.48) | 1401 (62.68) | |
| Unknown | 110 (4.80) | 109 (4.76) | 102 (4.56) | |
| Liver disorder | Absent | 1703 (74.27) | 1703 (74.30) | 1660 (74.27) |
| Present | 449 (19.58) | 449 (19.59) | 443 (19.82) | |
| Unknown | 141 (6.15) | 140 (6.11) | 132 (5.91) | |
| Renal disorder | Absent | 2055 (89.62) | 2055 (89.66) | 2010 (89.93) |
| Present | 160 (6.98) | 160 (6.98) | 155 (6.94) | |
| Unknown | 78 (3.40) | 77 (3.36) | 70 (3.13) | |
| Duration of treatment (days) | ≤ 180 | 141 (6.15) | 140 (6.11) | 112 (5.01) |
| > 180, ≤ 365 | 218 (9.51) | 218 (9.51) | 211 (9.44) | |
| > 365, ≤ 730 | 368 (16.05) | 368 (16.06) | 361 (16.15) | |
| > 730 | 1542 (67.25) | 1542 (67.28) | 1536 (68.72) | |
| Unknown | 24 (1.05) | 24 (1.05) | 15 (0.67) | |
| N | 2269 | 2268 | 2220 | |
| Min | 1 | 2 | 2 | |
| Max | 2323 | 2323 | 2323 | |
| Prescription history of ARV at baseline | ART-naïve | 1040 (45.36) | 1040 (45.38) | 1025 (45.86) |
| ART-experienced | 1220 (53.21) | 1219 (53.18) | 1184 (52.98) | |
| Unknown | 33 (1.44) | 33 (1.44) | 26 (1.16) | |
| ARV backbone at baseline | ABC+/3TC | 902 (39.34) | 902 (39.35) | 878 (39.28) |
| TAF/FTC | 314 (13.69) | 314 (13.70) | 305 (13.65) | |
| TDF+/FTC | 959 (41.82) | 958 (41.80) | 942 (42.15) | |
| Other | 119 (5.19) | 119 (5.19) | 111 (4.97) |
ABC+/3TC abacavir sulfate/lamivudine, ARV antiretroviral, CDC Centers for Disease Control and Prevention, HIV human immunodeficiency virus, Max maximum, Min minimum, SD standard deviation, TAF/FTC tenofovir alafenamide fumarate/emtricitabine, TDF+/FTC tenofovir disoproxil fumarate/emtricitabine
aIn age group (1), patients were classified into three categories: adolescent (< 15 years), adult (≥ 15, < 64), elderly (≥ 65)
The patient characteristics of the effectiveness analysis set were similar to those of the safety analysis set.
ADRs
The incidence of ADRs by PT observed in at least 10 patients during this survey is shown in Table 2. The full list of the incidence of ADRs is provided in Table S2 in the supplementary material. Of the 2292 patients in the safety analysis set, a total of 565 patients (24.65%) reported at least one ADR. The most common ADRs by SOC (occurred in at least 2% of patients) were investigations (8.29%; 190 patients) which means laboratory test results, gastrointestinal disorders (4.97%; 114 patients), metabolism and nutrition disorders (3.71%; 85 patients), infections and infestations (3.40%; 78 patients), hepatobiliary disorders (3.23%; 74 patients), skin and subcutaneous tissue disorders (3.05%; 70 patients), psychiatric disorders (2.97%; 68 patients), and nervous system disorders (2.01%; 46 patients). The most common ADRs by PT (occurred in at least 1% of patients) were blood creatinine increased (4.28%; 98 patients), hepatic function abnormal (2.31%; 53 patients), nausea (2.05%; 47 patients), insomnia (1.48%; 34 patients), hypertension (1.18%; 27 patients), headache (1.13%; 26 patients), and hyperuricemia (1.05%; 24 patients).
Table 2.
Incidence of ADRs by PT in 10 or more patients: safety analysis set
| Status of post-marketing surveillance | |
|---|---|
| Surveyed sites, N | 30 |
| Safety analysis set, N | 2292 |
| Patients with ADRs, n | 565 |
| ADR events, N | 1065 |
| Percentage of patients with ADRs, % | 24.65 |
| PT | Patients with ADRs by PT, n (%) |
| Blood creatinine increased | 98 (4.28) |
| Hepatic function abnormal | 53 (2.31) |
| Nausea | 47 (2.05) |
| Insomnia | 34 (1.48) |
| Hypertension | 27 (1.18) |
| Headache | 26 (1.13) |
| Hyperuricemia | 24 (1.05) |
| Diarrhea | 22 (0.96) |
| Dyslipidemia | 20 (0.87) |
| Immune reconstitution inflammatory syndrome | 19 (0.83) |
| Renal impairment | 17 (0.74) |
| Liver disorder | 16 (0.70) |
| Beta 2 microglobulin urine increased | 15 (0.65) |
| Hepatic enzyme increased | 13 (0.57) |
| Blood triglycerides increased | 12 (0.52) |
| Hypercholesterolemia | 11 (0.48) |
| Hyperlipidemia | 11 (0.48) |
| Pruritus | 11 (0.48) |
| Tinea pedis | 11 (0.48) |
| Malaise | 10 (0.44) |
| Rash | 10 (0.44) |
Coded using MedDRA/J version 23.0
ADR adverse drug reaction, MedDRA/J Medical Dictionary for Regulatory Activities/Japanese version, PT preferred term
The incidence of serious ADRs is provided in Table S3 in the supplementary material. A total of 147 patients (6.41%) reported at least one serious ADR. The most common serious ADR was immune reconstitution inflammatory syndrome (0.83%; 19 patients), followed by renal impairment (0.74%; 17 patients). Other serious ADRs were reported in fewer than 10 patients. The outcomes of these serious ADRs were death in 7 patients (completed suicide in 2 patients and death, lung adenocarcinoma, lymphoma, myocardial infarction, and near drowning in 1 patient each), not resolved in 53 patients, and unknown in 4 patients; the remaining serious ADRs were recovered or resolving.
ADRs by Patient Characteristics
The incidence of ADRs by patient characteristics is shown in Table 3. There was a statistically significant difference in the incidence of ADRs by CDC classification at pretreatment, presence or absence of comorbidities, liver disorder, renal disorder, and prescription history of ARV backbone. There were no statistically significant differences in age and sex.
Table 3.
Incidence of ADRs by patient characteristics safety analysis set
| Factors | Categories | Patients, N | Patients with ADRs, n | Percentage of patients with ADRs, % | Odds ratio estimate | 95% CI for odds ratio | Chi-squared (X) or Fisher’s exact test (Y) |
|---|---|---|---|---|---|---|---|
| Total | – | 2292 | 565 | 24.65 | – | ||
| Age (1) (years)a | < 15 | 1 | 0 | 0.00 | 0.00 | 0.00 to > 999.999 | (X) p = 0.79 |
| > 15, < 65 | 2187 | 541 | 24.74 | 1 | – | ||
| ≥ 65 | 104 | 24 | 23.08 | 0.91 | 0.57 to 1.46 | ||
| Age (2) (years)b | < 50 | 1806 | 446 | 24.70 | 1 | – | (Y) p = 0.95 |
| ≥ 50 | 486 | 119 | 24.49 | 0.99 | 0.78 to 1.25 | ||
| Sex | Male | 2181 | 544 | 24.94 | 1 | – | (Y) p = 0.18 |
| Female | 111 | 21 | 18.92 | 0.70 | 0.43 to 1.14 | ||
| CDC classification at pretreatment | A | 1529 | 338 | 22.11 | 1 | – | (X) p < 0.001** |
| B | 125 | 58 | 46.40 | 3.05 | 2.10 to 4.42 | ||
| C | 538 | 151 | 28.07 | 1.37 | 1.10 to 1.72 | ||
| Unknown | 99 | 18 | 18.18 | ||||
| Comorbidities | Absent | 751 | 99 | 13.18 | 1 | – | (Y) p < 0.001** |
| Present | 1432 | 449 | 31.35 | 3.01 | 2.37 to 3.82 | ||
| Unknown | 109 | 17 | 15.60 | ||||
| Liver disorder | Absent | 1703 | 365 | 21.43 | 1 | – | (Y) p < 0.001** |
| Present | 449 | 156 | 34.74 | 1.95 | 1.56 to 2.45 | ||
| Unknown | 140 | 44 | 31.43 | ||||
| Renal disorder | Absent | 2055 | 504 | 24.53 | 1 | – | (Y) p = 0.004** |
| Present | 160 | 56 | 35.00 | 1.66 | 1.18 to 2.33 | ||
| Unknown | 77 | 5 | 6.49 | ||||
| Prescription history of ARV at baseline | ART-naïve | 1040 | 320 | 30.77 | 1 | – | (Y) p < 0.001** |
| ART-experienced | 1219 | 243 | 19.93 | 0.56 | 0.46 to 0.68 | ||
| Unknown | 33 | 2 | 6.06 |
ADR adverse drug reaction, ARV antiretroviral, CDC Centers for Disease Control and Prevention, Cl confidence interval, HIV human immunodeficiency virus
**p < 0.01
aIn age group (1), patients were classified into 3 categories: adolescent (< 15 years), adult (≥ 15, < 64), elderly (≥ 65)
bIn age group (2), patients were classified into 2 categories: non-older person (< 50 years), older person (≥ 50) in accordance with the Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV [16]
For CDC classification at pretreatment, the incidence of ADRs in patients with categories B (46.40% [odds ratio 3.05; 95% CI 2.10–4.42]) and C (28.07% [odds ratio 1.37; 95% CI 1.10–1.72]) was statistically significantly higher than that with category A (22.11%) (p < 0.001). For comorbidities, the incidences of ADRs in patients with comorbidities (31.35% [odds ratio 3.01; 95% CI 2.37–3.82), hepatic disorder (34.74% [odds ratio 1.95; 95%CI 1.56–2.45]), and renal disorder (35.00% [odds ratio 1.66; 95% CI 1.18–2.33]) were statistically significantly higher than those without comorbidities (13.18%; p < 0.001), hepatic disorder (21.43%; p < 0.001), and renal disorder (24.53%; p = 0.004). Whereas the incidence of ADRs was statistically significantly lower in ART-experienced patients (19.93% [odds ratio 0.56; 95% CI 0.46–0.68]) than that in ART-naïve patients (30.77%) (p < 0.001).
Neuropsychiatric ADRs
The incidence of neuropsychiatric ADRs is shown in Table 4. A total of 107 patients (4.67%) reported at least one neuropsychiatric ADR. The incidence of neuropsychiatric ADRs by SOC was 2.97% for psychiatric disorders (68 patients) and 2.01% for nervous system disorder (46 patients). The most common neuropsychiatric ADR by PT in each SOC was insomnia (1.48%; 34 patients) and headache (1.13%; 26 patients).
Table 4.
Incidence of neuropsychiatric ADRs safety analysis set
| Status of post-marketing surveillance | |
|---|---|
| Surveyed sites, N | 30 |
| Safety analysis set, N | 2292 |
| Patients with ADRs, n | 107 |
| ADR events, N | 133 |
| Percentage of patients with ADRs, % | 4.67% |
| SOC/PT | Patients with ADRs by SOC/PT, n (%) |
| Psychiatric disorders | 68 (2.97) |
| Insomnia | 34 (1.48) |
| Sleep disorder | 6 (0.26) |
| Depression | 5 (0.22) |
| Depressed mood | 4 (0.17) |
| Depressive symptom | 4 (0.17) |
| Adjustment disorder | 3 (0.13) |
| Anger | 3 (0.13) |
| Anxiety | 3 (0.13) |
| Abnormal dreams | 2 (0.09) |
| Anxiety disorder | 2 (0.09) |
| Completed suicide | 2 (0.09) |
| Irritability | 2 (0.09) |
| Middle insomnia | 2 (0.09) |
| Nightmare | 2 (0.09) |
| Suicide attempt | 2 (0.09) |
| Alcoholism | 1 (0.04) |
| Enuresis | 1 (0.04) |
| Mental disorder | 1 (0.04) |
| Schizophrenia | 1 (0.04) |
| Social anxiety disorder | 1 (0.04) |
| Suicidal ideation | 1 (0.04) |
| Nervous system disorders | 46 (2.01) |
| Headache | 26 (1.13) |
| Dizziness | 7 (0.31) |
| Facial paralysis | 3 (0.13) |
| Hypoesthesia | 3 (0.13) |
| Somnolence | 3 (0.13) |
| Migraine | 2 (0.09) |
| Tension headache | 2 (0.09) |
| Balance disorder | 1 (0.04) |
| Cerebral hemorrhage | 1 (0.04) |
| Head discomfort | 1 (0.04) |
| Neuropathy peripheral | 1 (0.04) |
| Taste disorder | 1 (0.04) |
Coded using MedDRA/J version 23.0
ADR adverse drug reaction, MedDRA/J Medical Dictionary for Regulatory Activities/Japanese version, PT preferred term, SOC system organ class
The incidence of ADRs related to depression, suicide, or self-injurious behavour is shown by presence or absence of psychiatric disorders comorbidities in Table 5. The incidence of ADRs related to depression was higher in patients with comorbidities of psychiatric disorders than that without those comorbidities (1.12% vs 0.51%); however, the difference was not statistically significant. Whereas the incidence of ADRs related to suicide or self-injurious behavour was statistically significantly higher in patients with comorbidities of psychiatric disorders (1.12% vs 0.05%; p = 0.006).
Table 5.
Incidence of ADRs related to depression, suicide, or self-injurious behavour by psychiatric disorders comorbidities safety analysis set
| Comorbidities of psychiatric disorders | Patients with ADRs related to depression, n/patients, N (%) | Odds ratio estimate (95% CI for odds ratio) | Fisher’s exact test |
|---|---|---|---|
| Absent | 10/1945 (0.51) | 1 (–) | p = 0.20 |
| Present | 3/268 (1.12) | 2.19 (0.60–8.01) | |
| Unknown | 0/79 (0.00) |
| Comorbidities of psychiatric disorders | Patients with ADRs related to suicide and self-injurious behavour, n/patients, N (%) | Odds ratio estimate (95% CI for odds ratio) | Fisher’s exact test |
|---|---|---|---|
| Absent | 1/1945 (0.05) | 1 (–) | p = 0.006** |
| Present | 3/268 (1.12) | 22.00 (2.28–212.29) | |
| Unknown | 1/79 (1.27) |
ADR adverse drug reaction, CI confidence interval
**p < 0.01
Time to Onset of ADRs After DTG Treatment
The times to onset of events after treatment with DTG for the ADR groups (at least 10 patients) and ADR groups by company’s interest (suicide- and hepatitis-related) are shown in Table 6. In more than half of the ADR groups (12 of 22 groups), the events occurred within 180 days (median time to ADR onset) after the start of DTG treatment. Of these, the events contained in the following five ADR groups occurred as early as within 30 days (median time to ADR onset): nausea and vomiting (2.0 days), malaise (9.0 days), headache-related (10.0 days), diarrhea (20.0 days), and immune reconstitution inflammatory syndrome-related (22.0 days). Whereas the events contained in the following four ADR groups occurred late (occurred 365 days after the start of DTG treatment): hepatitis-related (370.0 days), blood cholesterol-related (547.5 days), blood pressure-related (568.0 days), and hyperlipidemia-related (790.0 days).
Table 6.
Time to onset of events after treatment with DTG by ADR group safety analysis set
| Period | ≤ 30 days | > 30 to ≤ 90 days | > 90 to ≤ 180 days | > 180 days ≤ 1 years | > 1 to ≤ 2 years | > 2 to ≤ 3 years | > 3 to ≤ 4 years | > 4 to ≤ 5 years | > 5 to ≤ 6 years | > 6 years | Unknown | Total | Median (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A: Number of patients up to each period | 2268 | 2234 | 2188 | 2128 | 1910 | 1542 | 1226 | 828 | 365 | 3 | 24 | 2292 | – |
| B: Number of patients with a specific group of ADRs (first occurrence) [n, B/A (%)] | 167 (7.36) | 80 (3.58) | 30 (1.37) | 56 (2.63) | 47 (2.46) | 21 (1.36) | 10 (0.82) | 11 (1.33) | 0 (0.00) | 0 (0.00) | 18 (75.00) | 440 (19.20) | 57.0 |
| Nausea and vomiting | 34 (1.50) | 4 (0.18) | 2 (0.09) | 3 (0.14) | 1 (0.05) | 2 (0.13) | 0 (0.00) | 2 (0.24) | 0 (0.00) | 0 (0.00) | 3 (12.50) | 51 (2.23) | 2.0 |
| Malaise | 6 (0.26) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.05) | 1 (0.06) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (8.33) | 10 (0.44) | 9.0 |
| Events related to headache | 16 (0.71) | 2 (0.09) | 3 (0.14) | 2 (0.09) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (0.24) | 0 (0.00) | 0 (0.00) | 3 (12.50) | 28 (1.22) | 10.0 |
| Diarrhea | 11 (0.49) | 4 (0.18) | 1 (0.05) | 1 (0.05) | 2 (0.10) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (12.50) | 22 (0.96) | 20.0 |
| Events related to immune reconstitution inflammatory syndrome | 12 (0.53) | 7 (0.31) | 1 (0.05) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 20 (0.87) | 22.0 |
| Blood creatinine increased | 35 (1.54) | 31 (1.39) | 10 (0.46) | 10 (0.47) | 12 (0.63) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 98 (4.28) | 56.5 |
| Events related to drug eruption | 17 (0.75) | 14 (0.63) | 3 (0.14) | 8 (0.38) | 5 (0.26) | 3 (0.19) | 1 (0.08) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (4.17) | 52 (2.27) | 62.0 |
| Tinea pedis | 1 (0.04) | 4 (0.18) | 1 (0.05) | 2 (0.09) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (12.50) | 11 (0.48) | 65.0 |
| Events related to renal function abnormal | 16 (0.71) | 9 (0.40) | 4 (0.18) | 10 (0.47) | 3 (0.16) | 2 (0.13) | 3 (0.24) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (12.50) | 50 (2.18) | 73.0 |
| Events related to blood triglyceride | 6 (0.26) | 4 (0.18) | 3 (0.14) | 3 (0.14) | 2 (0.10) | 2 (0.13) | 1 (0.08) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 21 (0.92) | 98.0 |
| Events related to hepatic function abnormal | 26 (1.15) | 17 (0.76) | 9 (0.41) | 15 (0.70) | 18 (0.94) | 7 (0.45) | 3 (0.24) | 1 (0.12) | 0 (0.00) | 0 (0.00) | 2 (8.33) | 98 (4.28) | 127.0 |
| Pruritus | 4 (0.18) | 0 (0.00) | 2 (0.09) | 2 (0.09) | 2 (0.10) | 1 (0.06) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 11 (0.48) | 141.0 |
| Events related to suicide | 0 (0.00) | 1 (0.04) | 1 (0.05) | 3 (0.14) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 5 (0.22) | 190.0 |
| Events related to insomnia | 8 (0.35) | 4 (0.18) | 2 (0.09) | 8 (0.38) | 6 (0.31) | 3 (0.19) | 0 (0.00) | 2 (0.24) | 0 (0.00) | 0 (0.00) | 3 (12.50) | 36 (1.57) | 239.0 |
| Events related to blood uric acid | 6 (0.26) | 2 (0.09) | 2 (0.09) | 7 (0.33) | 5 (0.26) | 4 (0.26) | 0 (0.00) | 3 (0.36) | 0 (0.00) | 0 (0.00) | 2 (8.33) | 31 (1.35) | 243.0 |
| Dyslipidemia | 4 (0.18) | 4 (0.18) | 0 (0.00) | 1 (0.05) | 2 (0.10) | 2 (0.13) | 3 (0.24) | 0 (0.00) | 1 (0.27) | 0 (0.00) | 3 (12.50) | 20 (0.87) | 301.0 |
| Events related to glycemia | 1 (0.04) | 1 (0.04) | 2 (0.09) | 6 (0.28) | 2 (0.10) | 1 (0.06) | 2 (0.16) | 2 (0.24) | 1 (0.27) | 0 (0.00) | 2 (8.33) | 20 (0.87) | 304.0 |
| Events related to depression | 3 (0.13) | 0 (0.00) | 0 (0.00) | 3 (0.14) | 3 (0.16) | 2 (0.13) | 1 (0.08) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (4.17) | 13 (0.57) | 363.0 |
| Events related to hepatitis | 0 (0.00) | 1 (0.04) | 0 (0.00) | 1 (0.05) | 2 (0.10) | 0 (0.00) | 1 (0.08) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 5 (0.22) | 370.0 |
| Events related to blood cholesterol | 1 (0.04) | 1 (0.04) | 1 (0.05) | 1 (0.05) | 3 (0.16) | 2 (0.13) | 2 (0.16) | 1 (0.12) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 12 (0.52) | 547.5 |
| Events related to blood pressure | 2 (0.09) | 1 (0.04) | 2 (0.09) | 2 (0.09) | 8 (0.42) | 2 (0.13) | 4 (0.33) | 2 (0.24) | 2 (0.55) | 0 (0.00) | 3 (12.50) | 28 (1.22) | 568.0 |
| Hyperlipidemia | 1 (0.04) | 1 (0.04) | 1 (0.05) | 1 (0.05) | 1 (0.05) | 3 (0.19) | 1 (0.08) | 2 (0.24) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 11 (0.48) | 790.0 |
Common events (occurred in 10 or more patients) and company’s special interest events (occurred in fewer than 10 patients) were included
ADR adverse drug reaction, DTG dolutegravir
Changes in Body Weight
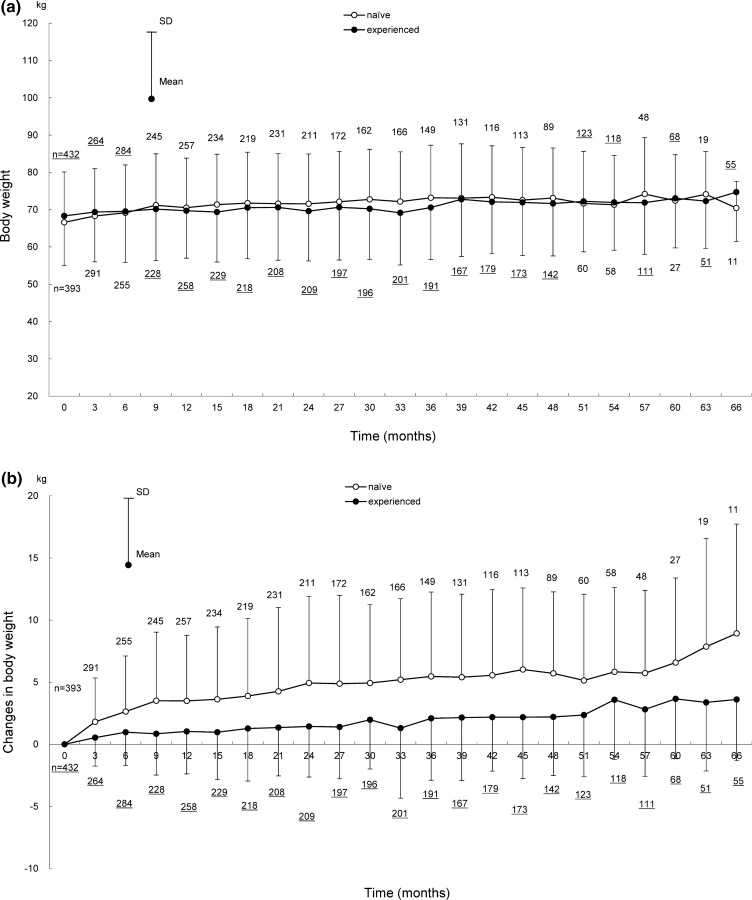
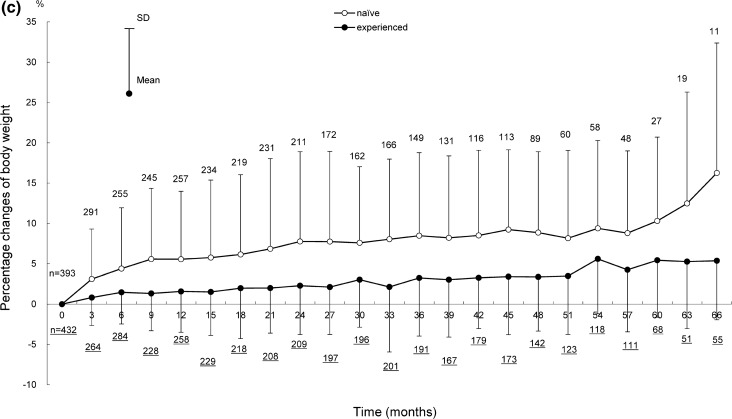
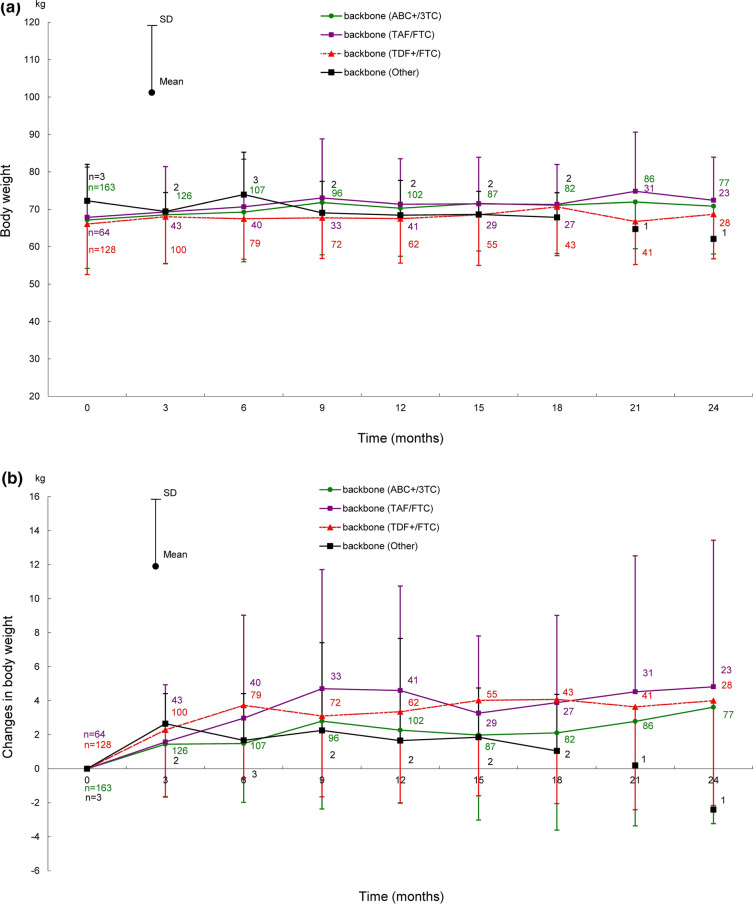
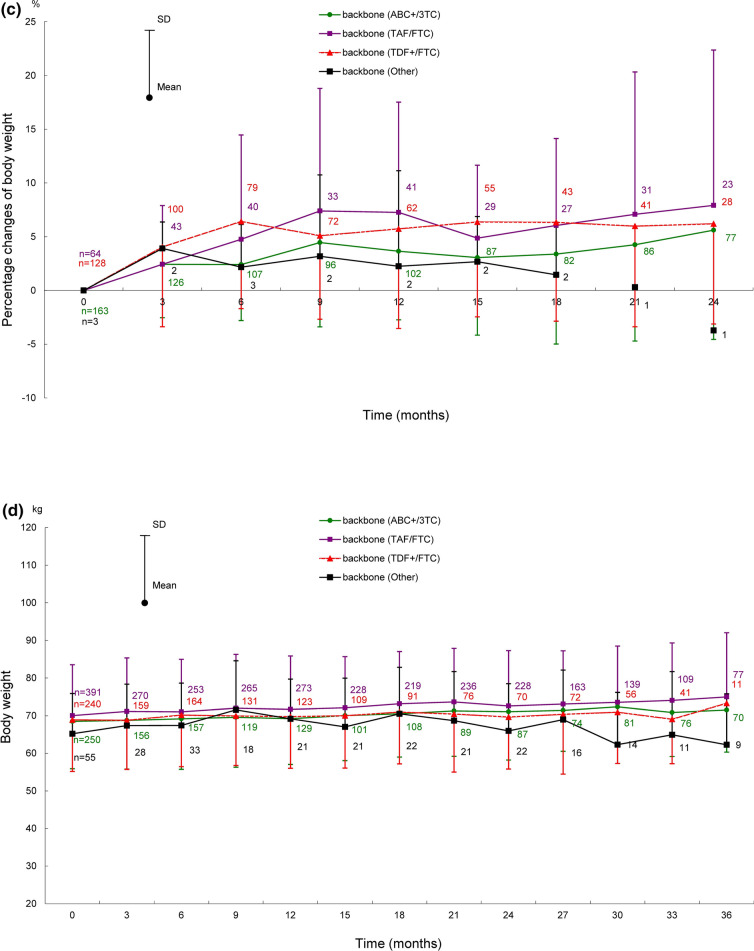
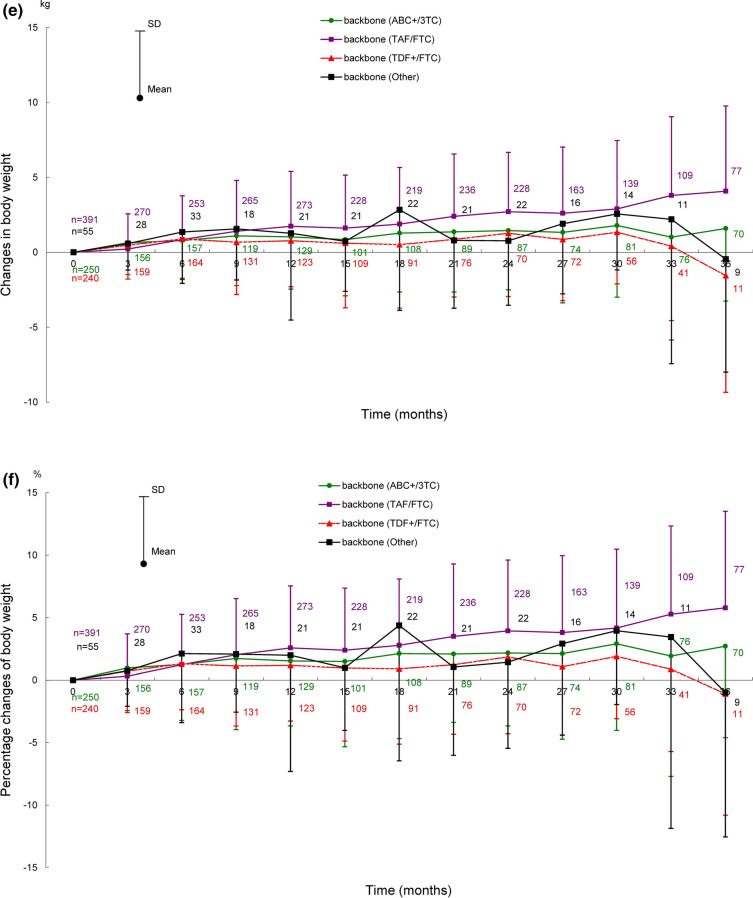
Shifts in body weight in ART-naïve and ART-experienced patients for 66 months after the start of DTG treatment are shown in Fig. 2, those for 72 months are provided in Fig. S1 in the supplementary material. Shifts in body weight by backbone (ABC+/3TC, TAF/FTC, and TDF+/FTC) for 24 months (ART-naïve patients) and 36 months (ART-experienced patients) after the start of DTG treatment are shown in Fig. 3, those for 72 months are provided in Fig. S2 in the supplementary material.
Fig. 2.
Shifts in body weight for 66 months after the start of DTG treatment in ART-naïve and ART-experienced patients. Shifts in a body weight, b change in body weight, c percentage change of body weight in ART-naïve and ART-experienced patients. Patients with no recorded baseline body weight were excluded because the amount and percentage of changes in body weight were calculated on the basis of the baseline body weight. The underlined numbers indicate the number of ART-experienced patients. ART antiretroviral therapy, DTG dolutegravir, SD standard deviation
Fig. 3.
Shifts in body weight after the start of DTG treatment in ART-naïve (for 24 months) and ART-experienced patients (for 36 months) by backbone. Shifts in a body weight, b change in body weight, c percentage change of body weight in ART-naïve patients by backbone. Shifts in d body weight, e change in body weight, f percentage change of body weight in ART-experienced patients by backbone. Patients with no recorded baseline body weight were excluded because the amounts and percentages of change in body weight were calculated on the basis of the baseline body weight. ABC+/3TC abacavir sulfate/lamivudine, ART antiretroviral therapy, DTG dolutegravir, SD standard deviation, TAF/FTC tenofovir alafenamide fumarate/emtricitabine, TDF+/FTC tenofovir disoproxil fumarate/emtricitabine
In these analyses, the evaluated duration was up to the time when 10 or more patients had available body weight in both ART-naïve and ART-experienced patients. A total of 825 patients with available baseline body weight were evaluated among 2292 patients in the safety analysis set.
Body weights (mean ± standard deviation [SD]) in ART-naïve and ART-experienced patients were 66.60 ± 13.52 kg (393 patients) and 68.32 ± 13.36 kg (432 patients) at baseline, and 70.44 ± 7.17 kg (11 patients) and 74.69 ± 13.24 kg (55 patients) at 66 months after the start of DTG treatment (Fig. 2a). The changes in body weight at 66 months after the start of DTG treatment from baseline in ART-naïve and ART-experienced patients were 8.93 ± 8.80 kg and 3.61 ± 4.92 kg (Fig. 2b); the percentage changes were 16.26% ± 16.12% and 5.38% ± 7.32% (Fig. 2c).
In ART-naïve patients, body weights by backbone (ABC+/3TC, TAF/FTC, and TDF+/FTC, the same order hereafter) were 67.15 ± 12.95 kg (163 patients), 67.85 ± 13.44 kg (64 patients), and 66.07 ± 13.53 kg (128 patients) at baseline; 70.84 ± 12.79 kg (77 patients), 72.41 ± 11.54 kg (23 patients), and 68.69 ± 11.92 kg (28 patients) at 24 months after the start of DTG treatment (Fig. 3a). The changes in body weight at 24 months after the start of DTG treatment from baseline were 3.62 ± 6.85 kg, 4.82 ± 8.62 kg, and 4.00 ± 6.16 kg (Fig. 3b); the percentage changes were 5.61% ± 10.17%, 7.92% ± 14.45%, and 6.20% ± 9.32% (Fig. 3c).
In ART-experienced patients, body weights by backbone were 68.48 ± 12.60 kg (250 patients), 70.02 ± 13.51 kg (391 patients), and 68.87 ± 13.71 kg (240 patients) at baseline; 71.50 ± 11.20 kg (70 patients), 74.97 ± 17.09 kg (77 patients), and 73.28 ± 10.78 kg (11 patients) at 36 months after the start of DTG treatment (Fig. 3d). The changes in body weight at 36 months after the start of DTG treatment from baseline were 1.59 ± 4.84 kg, 4.08 ± 5.69 kg, and − 1.55 ± 7.79 kg (Fig. 3e); the percentage changes were 2.71% ± 7.31%, 5.79% ± 7.72%, and − 1.09% ± 9.72% (Fig. 3f).
Effectiveness Analysis
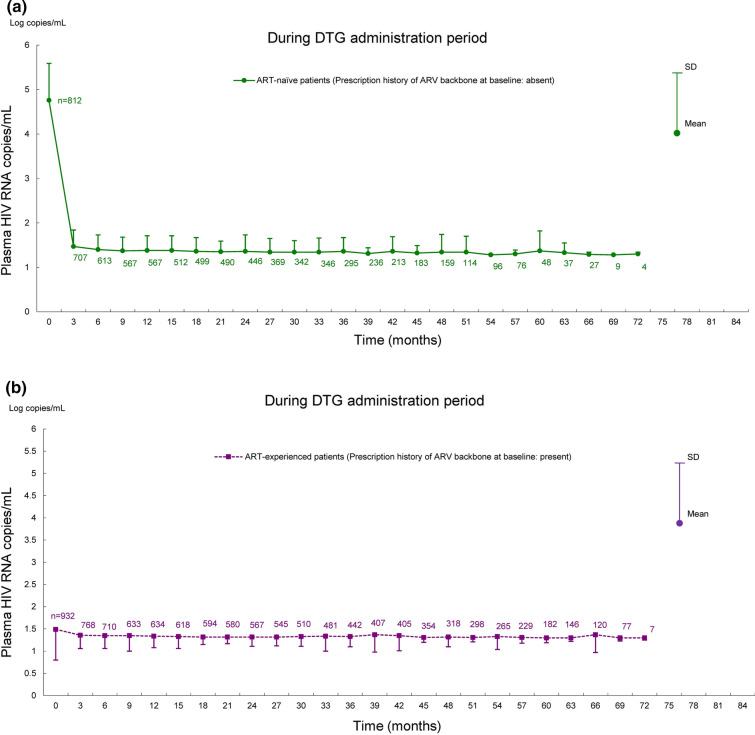
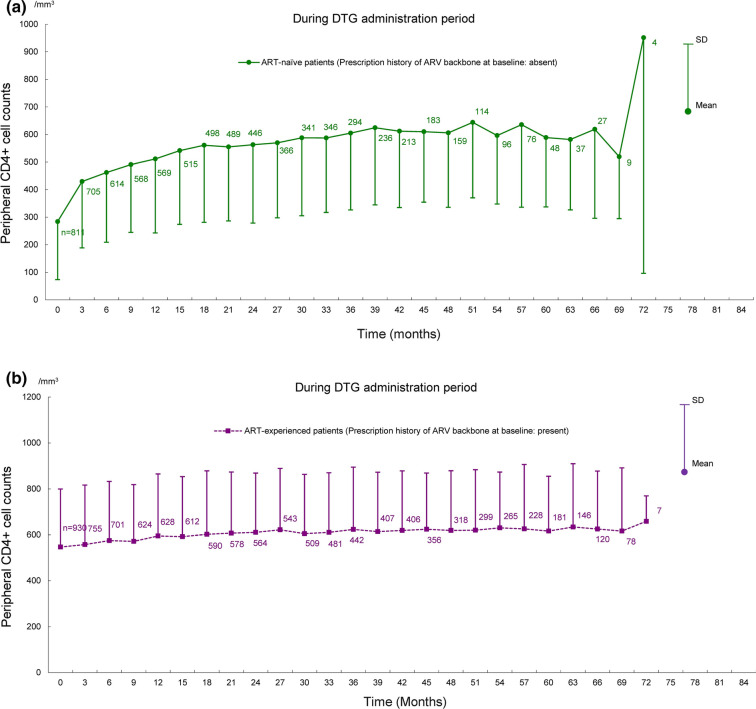
Shifts in HIV RNA copies/mL and CD4+ cell counts in ART-naïve and ART-experienced patients are shown in Figs. 4 and 5. In these analyses, the patients treated with DTG for at least 60 days and available HIV RNA copies/mL and CD4+ cell counts at pre and post DTG treatment were evaluated among 2235 patients in the effectiveness analysis set. The analysis included 812 ART-naïve patients and 932 ART-experienced patients for HIV RNA copies/mL, and 811 ART-naïve patients and 930 ART-experienced patients for CD4+ cell counts. In ART-naïve patients, HIV RNA copies/mL decreased at 3 months after the start of DTG treatment and remained up to 72 months; CD4+ cell counts increased after the start of DTG treatment. In ART-experienced patients, HIV RNA copies/mL and CD4+ cell counts remained almost unchanged from baseline to 72 months after the start of DTG treatment.
Fig. 4.
Shifts in plasma HIV RNA copies/mL for 72 months after the start of DTG treatment in ART-naïve and ART-experienced patients. Shifts in HIV RNA copies/mL in a ART-naïve patients and b ART-experienced patients. Mean ± SD of HIV RNA copies/mL was calculated after log transformation. ART antiretroviral therapy, ARV antiretroviral, DTG dolutegravir, HIV human immunodeficiency virus, RNA ribonucleic acid, SD standard deviation
Fig. 5.
Shifts in peripheral CD4+ cell counts for 72 months after the start of DTG treatment in ART-naïve and ART-experienced patients. Shifts in CD4+ cell counts in a ART-naïve patients and b ART-experienced patients. ART antiretroviral therapy, ARV antiretroviral, DTG dolutegravir, SD standard deviation
Discussion
The overall incidence of ADRs was 24.65% (565 of 2292 patients). Many ADRs reported in this survey were the known events listed in the precautions of package inserts for Tivicay tablets or Triumeq combination tablets. In addition, these ADRs were consistent with ADRs or AEs reported in overseas clinical trials of DTG [2–9]. The commonly reported ADRs or AEs in the overseas clinical trials, such as nausea, headache, insomnia, and diarrhea, were also frequently reported in this survey. The most common ADR reported in this survey was blood creatinine increased. DTG inhibits organic cation transporter 2 (OCT2) on the basolateral surface of the proximal tubules in the kidneys, which results in increased serum creatinine levels without decreased true renal function [18]. In HIV-infected patients receiving DTG, an alternative test for creatinine measurement has been required to assess correct renal function. A real-world clinical value of cystatin C, which is not a substrate for OCT2, as a marker of renal function in those patients is suggested [19–22].
Neuropsychiatric ADRs, including psychiatric conditions, have been reported in patients receiving INSTIs [23]. In this survey, there were no differences in incidence of the most common neuropsychiatric ADR, insomnia (1.48%; 34 of 2292 patients), compared with five overseas clinical trials of DTG (SPRING-2, FLAMINGO, SINGLE, ARIA, SAILING) [24]. The incidence of the second most common neuropsychiatric ADR, headache (1.13%; 26 of 2292 patients), was lower than that of an AE in overseas clinical trials [25, 26]. The median time to onset was 10.0 days for the event contained in the headache-related group and 239.0 days for the event contained in the insomnia-related group. This suggested that there are two types of neuropsychiatric ADRs: events that should be monitored in the short term and in the long term after the start of DTG treatment.
The DHHS guidelines stated that depression and suicidality are rare, occurring primarily in patients with pre-existing psychiatric conditions when INSTIs are administered [16]. An analysis of psychiatric disorders reported in the World Health Organization’s Global Pharmacovigilance Database (Vigibase) suggests that there is an increased risk of depression, suicide, and self-injurious behaviors in patients receiving DTG or raltegravir potassium (RAL) compared with elvitegravir (EVG) [27]. In a pooled analysis from five overseas clinical trials of DTG (SPRING-2, FLAMINGO, SINGLE, ARIA, and SAILING), the incidence of ADRs related to depression or suicide was 0.90% (15 of 1672 patients) and 0.12% (2 of 1672 patients) in patients receiving DTG [24]. In this survey, ADRs related to depression or suicide were reported from 0.57% (13 of 2292 patients) and 0.22% (5 of 2292 patients) of patients receiving DTG, showing no notable differences from the pooled analysis mentioned above. The incidence of ADRs related to suicide or self-injurious behavior was statistically significantly higher in patients with psychiatric disorder (concurrent psychiatric disorders included depression, panic disorder, insomnia in three patients each) compared with those without psychiatric disorders. These results suggest that pre-existing psychiatric conditions are a risk factor for ADRs related to suicide or self-injurious behavior in patients receiving DTG.
In randomized clinical trials of first-line treatment, INSTI regimens were associated with greater body weight gain than protease inhibitors (PIs) or non-nucleoside reverse transcriptase inhibitor (NNRTI) regimens. In comparison among INSTI regimens, the mean body weight gains were similar between DTG and bictegravir sodium (BIC) and both were greater than EVG/cobicistat (cobi) [28]. In this survey, the mean body weight also tended to increase over time from the start of DTG treatment. The mean changes and percentage changes from baseline in body weight tended to be greater in ART-naïve patients compared with ART-experienced patients. On the basis of the report that high pretreatment plasma HIV RNA copies/mL and low peripheral CD4+ cell counts are risk factors for body weight gain [28], less weight gain in ART-experienced patients might be caused by improvements of plasma HIV RNA copies/mL and peripheral CD4+ cell counts by another ARV treatment before the start of DTG treatment. On the other hand, a cohort study in patients with HIV aged 65 years or older did not show a significant weight gain associated with switch to DTG from the other INSTIs on ART [29, 30]. In this survey, the analysis for change in body weight was not performed in patients aged 65 years or older. However, the proportion of patients in this age category was only 4.54% (104 of 2292 patients); taking this into account, body weight gain in this survey may have largely reflected data in non-elderly patients. In a 96-week, investigator-led, open-label, randomized phase III trial conducted in South Africa, the mean body weight gain was statistically significantly greater in TAF than TDF among NRTIs in combination with DTG [31, 32]. In addition, a pooled analysis in eight randomized active-controlled clinical trials in ART-naïve patients showed greater mean body weight gain in TAF compared with ABC, TDF, and zidovudine (ZDV) among NRTI-containing regimens [28]. In this survey, body weight gain by backbone tended to be greater in TAF/FTC compared with ABC+/3TC and TDF+/FTC, which was consistent with the previous reports mentioned above. In contrast, another report suggests that body weight gain by ARV treatments is the “return-to-health” phenomenon after body weight loss due to HIV infection [33]. Of 2292 patients in the safety analysis set in this survey, only three patients had ADRs of body weight gain out of 825 patients with available baseline body weight. This indicated that most events of body weight gain were not determined to be ADRs or not considered to be related with DTG treatment under daily clinical practice. The clinical significance of body weight gain caused by ART including DTG has not been clear. Therefore, the event has to be monitored carefully in long-term treatment of the ART.
In this survey, the mean plasma HIV RNA copies/mL and peripheral CD4+ cell counts showed favorable shifts after treatment with DTG in both ART-naïve and ART-experienced patients. This demonstrated effectiveness of DTG in Japanese patients, more specifically, CD4+ cell count increase associated with suppression of viral replication. Note that there were two events of suspected drug resistance reported in two patients, and counted as drug resistance; however, neither of them showed resistance that would affect the effectiveness of DTG.
The safety of ABC and 3TC, the other active ingredients of Triumeq combination tablets, had been confirmed in Japanese patients with HIV by the final results of the 10-year post-marketing surveillances of Ziagen tablets (ABC) and Epzicom combination tablets (ABC/3TC) [34, 35].
This survey has the following four limitations: there are certain limitations regarding the patient population because the survey utilized anonymized data collected from 30 sites participating in the HRD cooperative survey; in addition to the fact that a post-marketing surveillance usually employs no control groups, assessment of safety and effectiveness of DTG alone is impossible because DTG must be used in combination with other ARVs. Some ADRs might not be directly related to DTG but be caused by concomitant use of various NRTIs; some patients had missing data because this survey used data obtained in daily practice, where laboratory results were not always available at all time points such as pretreatment, during and post-treatment (in particular, the patients with no available baseline body weight were excluded from the analysis of change in body weight); and it was difficult to compare the company’s special interest ADRs in this survey with the ADRs in previous clinical trials because many published data did not refer to ADRs but AEs which did not mention the causality.
Conclusions
No new clinical issues regarding safety and effectiveness of DTG were identified in this interim results of an approximately 6-year post-marketing surveillance conducted under daily clinical practice in Japan. ViiV Healthcare K.K. will continue to have strict pharmacovigilance to ensure safety of DTG use in people living with HIV/AIDS and publish the results in medical journals.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge all the physicians, associated healthcare professionals, and patients who took part in this post-marketing surveillance.
Funding
In this survey, the study design, collection, analysis, and interpretation of the data were funded by ViiV Healthcare K.K. which all authors belong to. ViiV Healthcare K.K. also funded the journal’s rapid service and open access fees.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance was provided by A2 Healthcare Corporation. This assistance was funded by ViiV Healthcare K.K.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Harayuki Hongo and Takako Nagao led conception and design of the study, and data analysis and interpretation. Harayuki Hongo also drafted the manuscript and Takako Nagao, Kyoko Nakamura, Tomomi Kitaichi, Yuko Maeno, Teruhisa Tokunaga, Akiko Fukuda, and Ichiro Koga contributed to writing and reviewing the work.
Prior Presentation
This survey was presented at the 31st Annual Meeting of the Japanese Society for AIDS Research in 2017.
Disclosures
Haruyuki Hongo, Takako Nagao, Kyoko Nakamura, Tomomi Kitaichi, Yuko Maeno, Teruhisa Tokunaga, Akiko Fukuda, and Ichiro Koga are employees of ViiV Healthcare K.K. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Compliance with Ethics Guidelines
This post-marketing surveillance has been performed with the participation of 30 sites by CMIC HOLDINGS Co., Ltd. on behalf of ViiV Healthcare K.K. in compliance with the Japanese ministry directive on GPSP. The study protocol was reviewed and approved by the ethics committee at each study site. In compliance with GPSP, consent for participation is not required in post-marketing surveillances; however, we defined the process of obtaining verbal consent in the study protocol. In line with the study protocol, use of DTG in treatment of HIV infection was explained to each potential subject, and verbal consent for the use was obtained prior to study initiation from all patients who would receive DTG.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Joint United Nations Programme on HIV/AIDS. UNAIDS data 2020. 2020. https://www.unaids.org/en/resources/documents/2020/unaids-data. Accessed 2 Apr 2021.
- 2.Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013;27(11):1771–1778. doi: 10.1097/QAD.0b013e3283612419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927–935. doi: 10.1016/S1473-3099(13)70257-3. [DOI] [PubMed] [Google Scholar]
- 4.Molina JM, Clotet B, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV. 2015;2(4):e127–e136. doi: 10.1016/S2352-3018(15)00027-2. [DOI] [PubMed] [Google Scholar]
- 5.Walmsley S, Baumgarten A, Berenguer J, et al. Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr. 2015;70(5):515–519. doi: 10.1097/QAI.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–708. doi: 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 7.Eron JJ, Clotet B, Durant J, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207(5):740–748. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castagna A, Maggiolo F, Penco G, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014;210(3):354–362. doi: 10.1093/infdis/jiu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, phase IIIb study. Antivir Ther. 2017;22(4):295–305. doi: 10.3851/IMP3166. [DOI] [PubMed] [Google Scholar]
- 10.Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839–849. doi: 10.1016/S0140-6736(17)33095-7. [DOI] [PubMed] [Google Scholar]
- 11.Aboud M, Orkin C, Podzamczer D, et al. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV. 2019;6(9):e576–e587. doi: 10.1016/S2352-3018(19)30149-3. [DOI] [PubMed] [Google Scholar]
- 12.Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143–155. doi: 10.1016/S0140-6736(18)32462-0. [DOI] [PubMed] [Google Scholar]
- 13.Cahn P, Madero JS, Arribas JR, et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with hiv-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr. 2020;83(3):310–318. doi: 10.1097/QAI.0000000000002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis. 2020;71(8):1920–1929. doi: 10.1093/cid/ciz1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Health and Labour Sciences Research Grants, Research on HIV/AIDS, Ministry of Health, Labour and Welfare, Japan. Anti-HIV treatment guidelines. 2021. https://www.haart-support.jp/guideline.htm. Accessed 2 Apr 2021.
- 16.U.S. Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2019. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/459/cost-considerations-and-antiretroviral-therapy. Accessed 2 Apr 2021.
- 17.European AIDS Clinical Society. EACS Guidelines Version 10.1. 2020. https://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. Accessed 2 Apr 2021.
- 18.Koteff J, Borland J, Chen S, et al. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br J Clin Pharmacol. 2013;75(4):990–996. doi: 10.1111/j.1365-2125.2012.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshino Y, Koga I, Seo K, Kitazawa T, Ota Y. Short communication: the clinical value of cystatin C as a marker of renal function in HIV patients receiving dolutegravir. AIDS Res Hum Retrovir. 2017;33(11):1080–1082. doi: 10.1089/aid.2017.0074. [DOI] [PubMed] [Google Scholar]
- 20.Hikasa S, Hideta K, Shimabukuro S, et al. Utility of estimated glomerular filtration rate based on cystatin C in patients receiving combination antiretroviral therapy including dolutegravir. Infect Dis (Lond) 2018;50(1):77–79. doi: 10.1080/23744235.2017.1370128. [DOI] [PubMed] [Google Scholar]
- 21.Yukawa S, Watanabe D, Uehira T, Shirasaka T. Clinical benefits of using inulin clearance and cystatin C for determining glomerular filtration rate in HIV-1-infected individuals treated with dolutegravir. J Infect Chemother. 2018;24(3):199–205. doi: 10.1016/j.jiac.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Palich R, Tubiana R, Abdi B, et al. Plasma cystatin C as a marker for estimated glomerular filtration rate assessment in HIV-1-infected patients treated with dolutegravir-based ART. J Antimicrob Chemother. 2018;73(7):1935–1939. doi: 10.1093/jac/dky112. [DOI] [PubMed] [Google Scholar]
- 23.Park TE, Mohamed A, Kalabalik J, Sharma R. Review of integrase strand transfer inhibitors for the treatment of human immunodeficiency virus infection. Expert Rev Anti Infect Ther. 2015;13(10):1195–1212. doi: 10.1586/14787210.2015.1075393. [DOI] [PubMed] [Google Scholar]
- 24.Fettiplace A, Stainsby C, Winston A, et al. Psychiatric symptoms in patients receiving dolutegravir. J Acquir Immune Defic Syndr. 2017;74(4):423–431. doi: 10.1097/QAI.0000000000001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–743. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- 26.Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–2231. doi: 10.1016/S0140-6736(14)60084-2. [DOI] [PubMed] [Google Scholar]
- 27.Kheloufi F, Boucherie Q, Blin O, Micallef J. Neuropsychiatric events and dolutegravir in HIV patients: a worldwide issue involving a class effect. AIDS. 2017;31(12):1775–1777. doi: 10.1097/QAD.0000000000001557. [DOI] [PubMed] [Google Scholar]
- 28.Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71(6):1379–1389. doi: 10.1093/cid/ciz999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guaraldi G, Calza S, Milic J, et al. Dolutegravir is not associated with weight gain in antiretroviral therapy experienced geriatric patients living with HIV. AIDS. 2021;35(6):939–945. doi: 10.1097/QAD.0000000000002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taramasso L, Stapleton JT, Siedner MJ. Weight gain and aging in people with HIV. AIDS. 2021;35(6):987–989. doi: 10.1097/QAD.0000000000002849. [DOI] [PubMed] [Google Scholar]
- 31.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803–815. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]
- 32.Venter WDF, Sokhela S, Simmons B, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020;7(10):e666–e676. doi: 10.1016/S2352-3018(20)30241-1. [DOI] [PubMed] [Google Scholar]
- 33.Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70(7):1267–1274. doi: 10.1093/cid/ciz407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurita T, Kitaichi T, Nagao T, Miura T, Kitazono Y. Safety analysis of Ziagen® (abacavir sulfate) in postmarketing surveillance in Japan. Pharmacoepidemiol Drug Saf. 2014;23(4):361–371. doi: 10.1002/pds.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurita T, Kitaichi T, Nagao T, Miura T, Kitazono Y. Safety analysis of Epzicom® (lamivudine/abacavir sulfate) in post-marketing surveillance in Japan. Pharmacoepidemiol Drug Saf. 2014;23(4):372–381. doi: 10.1002/pds.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.