Abstract
Platypuses (Ornithorhynchus anatinus) inhabit the permanent rivers and creeks of eastern Australia, from north Queensland to Tasmania, but are experiencing multiple and synergistic anthropogenic threats. Baseline information of health is vital for effective monitoring of populations but is currently sparse for mainland platypuses. Focusing on seven hematology and serum chemistry metrics as indicators of health and nutrition (packed cell volume (PCV), total protein (TP), albumin, globulin, urea, creatinine, and triglycerides), we investigated their variation across the species’ range and across seasons. We analyzed 249 unique samples collected from platypuses in three river catchments in New South Wales and Victoria. Health metrics significantly varied across the populations’ range, with platypuses from the most northerly catchment, having lower PCV, and concentrations of albumin and triglycerides and higher levels of globulin, potentially reflecting geographic variation or thermal stress. The Snowy River showed significant seasonal patterns which varied between the sexes and coincided with differential reproductive stressors. Male creatinine and triglyceride levels were significantly lower than females, suggesting that reproduction is energetically more taxing on males. Age specific differences were also found, with juvenile PCV and TP levels significantly lower than adults. Additionally, the commonly used body condition index (tail volume index) was only negatively correlated with urea, and triglyceride levels. A meta-analysis of available literature revealed a significant latitudinal relationship with PCV, TP, albumin, and triglycerides but this was confounded by variation in sampling times and restraint methods. We expand understanding of mainland platypuses, providing reference intervals for PCV and six blood chemistry, while highlighting the importance of considering seasonal variation, to guide future assessments of individual and population condition.
Subject terms: Biochemistry, Ecology, Zoology, Ecology, Environmental sciences, Biomarkers, Signs and symptoms
Introduction
Body condition, morphology1, body mass2,3, skeletal size4,5, pelage6,7, and behaviour of many animal species vary across their ranges8,9. Mechanisms causing these variations are complex and differ among species10–12 but often reflect responses to climatic drivers, such as temperature and seasonality, altering niche quality13–17. Threatening processes, particularly those that alter the environment, can also impact a species’ realized niche18, affecting morphology19, breeding cycles20, and sometimes body condition of animals, resulting from poor nutrition or increased parasite load21–23.
The viability of a wild population is dependent on a range of life history characteristics such as life expectancy and reproductive rate. These life history characteristics can be adversely affected by poor individual health within the population. Level of nutrition is an important determinant of individual health24–26. Information on health or nutrition levels often relies on measuring surrogates of body condition such as mass or other morphological indices on wild animals, but these may not adequately measure health of individuals27,28. Morphological indices are useful field measurements, but many are subjective and often lack standardisation for assessment. Body condition is directly linked to nutritional status, with animals storing surplus energy in tissues and organs (e.g., fat deposits, kidney and liver) at times of high food availability and/or low metabolic need, mobilizing these stores at times of low food availability and/or high metabolic need. Hematology and serum chemistry analytes are useful to measure body condition and organ function29,30, and can detect more subtle variations in health that morphological indices might not detect. They are particularly valuable for endangered and difficult to study species. They can be used to investigate effects of environmental stressors31,32, underlying disease and parasite burden33,34, and reproductive cycles35,36. For example, packed cell volume (PCV), red blood count, white blood count, hemoglobin, urea, creatinine, triglycerides and albumin were used to identify a malnourished sub-population of Tammar wallaby (Macropus eugenii), in poorer condition than neighboring sub-populations37. Further, hematocrit, hemoglobin, red blood cell count, creatine, and triglycerides were used to effectively measure condition and effects of stressors over time in relocated eastern bettongs38. These cases demonstrate how hematology and serum chemistry can provide valuable population information not otherwise detected with physical examination alone.
PCV is the percentage of the blood volume that is made up of red blood cells, which carry oxygen and drive metabolism39. PCV has a complex relationship to body condition and stress responses. Commonly in wildlife, elevations may indicate dehydration or acute stress while decreases are often associated with anaemia. Alone its interpretation can be difficult, but it can be used with other variables to estimate response to stressors such as food availability40, seasons41,42, and breeding43. Serum chemistry analytes, including total protein, albumin, globulin, urea, creatinine, and triglycerides, measure proteins and fats, and are associated with energy and protein balance29,44,45. Proteins are critical for muscle mass and organ function. Relatively lower levels can reflect poor nutrition, and reduced muscle mass29,44. Further, low levels of albumin can reflect an acute phase protein response46, while high levels of globulin can reflect inflammation47. Urea and creatinine primarily indicate kidney dysfunction when elevated48,49. Protein intake can be measured with total protein, albumin, and urea, useful for measuring short term nutrition, while creatinine levels indicate long-term nutrition44,50–53. Triglycerides correlate to fat stores, generally used first before protein stores, indicating energy available for metabolic demands, with low levels reflecting recent metabolic stress47. For free-ranging wildlife their value for providing richer information on population health and its potential correlations with environmental stress which is otherwise difficult measure. Importantly, establishing species and population specific Reference Intervals (RI) for health data provides a quantifiable method to assess population specific stressors and understanding of seasonal variation54,55. This was highlighted in a recent study of eastern grey kangaroos (Macropus giganteus)56 which found a significant number of animals from a high density population were outside of the RI’s for the species, indicating the health impact of the increased population density which can then be used to inform management of the population. The international standard for reliable representation of a population requires 120 individuals57,58, which can be difficult to achieve. Consequently, few species have established baseline data.
There are a range of factors that might cause variation in hematology and serum chemistry analytes between platypus populations. Platypuses have four genetically distinct populations across their distribution in Australia: Tasmania, Victoria and New South Wales combined, Queensland, and King Island59. They also vary in morphology across their range including: body mass (610–1500 g in Queensland to 1200–3000 g in Tasmania60) and body length (Tasmanian individuals 21–29% longer than on the mainland61). These differences reflect Bergmann’s and Allen’s rules 62,63 of adaptation to decreasing temperature with latitude (20°–32 °C Queensland, 9°–25 °C Tasmania). Climatic influence on breeding seasons can be seen across their range also, with courtship followed by nesting behaviour beginning in August in New South Wales while in Tasmania it is starts in October64–66. Habitats also vary across the platypuses’ range, with differences in their food of aquatic macroinvertebrates67–71. Platypus habitat quality and health are under increasing pressure from anthropogenically driven stressors, including water resource development lowering water availability and altering the natural flow regime, land clearing destroying riparian vegetation, bank erosion and sedimentation, as well as pollution, all of which vary in largely unquantified severity across their range72–78. The health of the Tasmanian population is increasingly understood in relation to sex, disease, and seasonal variation79–81. However all three studies on the New South Wales and Victorian population have had insufficient sample sizes82–84, n = < 50 (except Booth and Connolly82with n = 87 for PCV), to meet the accepted international standard of 120 for reliable representation of a population.
We aimed to add to the sparse health data and establish reference intervals for mainland platypuses and for the species in general. Using pathology parameters that have been associated with body condition and nutrition in other wildlife species, we evaluated parameters of platypus health related to energy and protein. We investigated hematology and serum chemistry analytes from three studied areas in New South Wales and Victoria, examining variation among seasons, sexes, age, and elevation. Specifically, we tested for differences in PCV, TP, albumin, globulin, urea, creatinine, and triglycerides levels. We predicted that hematology and serum chemistry analytes from mainland platypuses would follow a similar pattern to that observed for PCV and albumin in Tasmanian individuals previously studied, increasing in winter and decreasing in summer85, and varying between river catchments80, sex, and age85. We also examined the association between hematology and serum chemistry analytes and Tail Volume Index (TVI)85, the most commonly used field body condition metric for platypuses. Finally, we reviewed available literature and evaluated for any biogeographic variation in examined serum chemistry analytes and PCV across the species’ range.
Methods
Field samples
Between January 2016 and May 2018, 259 platypuses were trapped using fyke and gill nets, and anaesthetized using isoflurane gas prior to inspection and collection of blood86,87. Platypuses were surveyed in three river catchments across seven rivers in New South Wales; Border Rivers Catchment (Tenterfield Creek n = 42, and Severn River n = 41, Jan–May 2016), Snowy Rivers Catchment (Eucumbene n = 26, Snowy n = 90, Thredbo Rivers n = 22, Dec 2016-Nov 2017); and Victoria, Upper Murray Rivers Catchment (Mitta Mitta n = 18, and Ovens Rivers n = 20, Jan–May 2018, Fig. 1). Elevation was calculated using a one second Digital Elevation Models88 for each sample (Severn 393 m-819 m, Tenterfield 373 m-816 m, Eucumbene 939 m-1336 m Snowy 745 m-847 m, Thredbo 921 m-1352 m, Mitta Mitta 282-m541m, Ovens 244 m-405 m). Body condition of each platypus was evaluated, inspecting for external injuries and fat content using tail volume index (TVI, 1–5 with 1 indicating high levels of fat stored in the tail and 5 indicating low levels), a commonly used qualitative measure of fat reserves85. No individuals presented with apparent diseases or injuries. Sex and age (Juvenile or Adult) were also determined, based on presence and shape of spurs89,90. Blood samples of 2 mL were collected from the bill sinus83. Packed cell volume (PCV, %) and total protein (TP, g/L) were determined using a microhematocrit heparinized capillary tube and refractometer, following centrifugation (75 mL, 10,000 g, 3 min), with the remaining blood centrifuged (2000 g) for 10 min to separate serum, which was then stored at -80° C. Chilled serum was analyzed in 2019 by Vetnostics Laboratory, using a Cobas 8000 (Roche diagnostic systems), providing blood serum chemistry data for albumin (g/L), urea (mmol/L), creatinine (umol/L), and triglycerides (mmol/L). Globulin (g/L) was determined by subtracting albumin from the TP.
Figure 1.
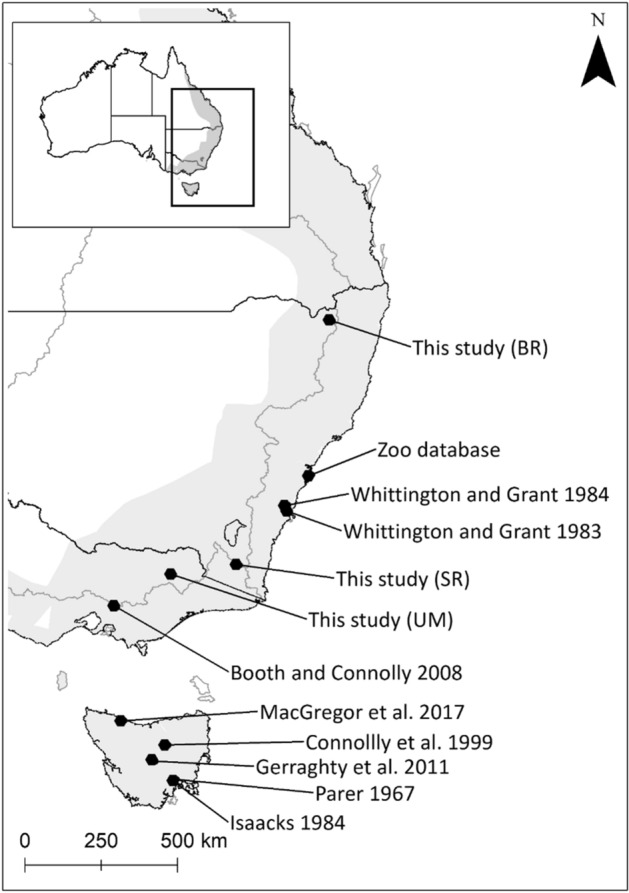
IUCN distribution of platypuses (grey shading in insert and main figure) and sampling locations for this study (BR Border Rivers, SR Snowy River, UM Upper Murray River) and other published or unpublished studies (see Table 1 for numbers related to publications and other available sources).
Analytical approach
We predicted that hematology and serum chemistry analytes from platypus in New South Wales and Victoria would follow a similar pattern to that observed for PCV and albumin in Tasmanian platypuses, increasing in winter and decreasing in summer81. We examined associations between seven dependent hematology and serum chemistry response variables (PCV, TP, albumin, globulin, urea, creatinine, and triglycerides) and six independent explanatory variables: month (Jan-May, continuous 1 to 5), catchment (three levels), elevation of sample, sex (female/male), age (adult/juvenile), and tail volume index. We evaluated a linear response to month, assuming a general trend between Jan and May81 while accounting for non-linear responses in addition to an interaction term with sex. We fitted a Generalized Linear Model (GLM) assuming a Gaussian error distribution, examining all possible predictor combinations of the seven response variables (n = 64), and ranked model performance, based on the corrected Akaike Information Criterion (AICc)91. All dependent variables were initially checked for normality using the Shapiro–Wilk Normality Test before analysis. Appropriateness of linear models were also visually evaluated using the residuals vs fitted plots to check homogeneity and linear relation, along with normal q-q plots to check normal distribution of residuals. No data required transformation. To address model uncertainty, we considered the full model space, examining all possible explanatory variable combinations to identify the best fit models. As a measure of model fit, we used the corrected Akaike’s Information Criterion (AICc) and considered those having AICc scores not greater than four relative to the lowest score92. We then applied a model averaging approach, weighting predictor effect sizes by model AICc scores, to quantify the average strength of association between response variables and examined predictors. Associations were considered significant if P ≤ 0.05. We used the ‘dredge’ and ‘model.avg’ functions in the ‘MuMIn’ package within the R environment93. The ‘ggplot2′94 package was used to represent the data.
Additional surveys undertaken on the Snowy River between Jindabyne Dam and the town of Dalgety (~ 20 km)87, carried out of between December 2016 to November 2017, allowed us to investigate seasonal variation in adult hematology and serum chemistry parameters, along with TVI, between sexes over a full annual cycle. We used a Generalized Additive Model (GAM), assuming a Gaussian error distribution fitted, with a cyclical relationship (cyclic cubic spline) with month as a continuous variable (in concordance with sampling sequence) and an interaction term for sex (n = 82). To allow for seasonal variation with a single maxima and minima and avoid over-fitting, we limited the number of knots (i.e. polynomial level) to three. Associations were considered significant if P ≤ 0.05. We used the ‘gam’ function in the ‘mgcv’ package95,96 in the R93. The ‘visreg’97 package was used to represent the data.
We used samples collected in this study to establish reference intervals for New South Wales and Victorian platypuses in our three study catchments using Reference Value Advisor v2.198, and the standards set by the American Society for Veterinary Clinical Practice99. Using non-parametric methods, as recommended for sample sizes over 12098, we calculated the 95th percentile confidence range and used the 2.5 and 97.5 percentile as the lower and upper limits, and the 90th percentile confidence range as the upper and lower ranges within the reference interval. For reference intervals calculated for individual catchments, sex, and age where sample sizes were between 40 and 120, we estimated reference interval using a non-parametric bootstrapping method98,99. In the few cases where sample size was less than 40 a robust method with a Box-Cox transformation of the data was used to provide reference interval98.
Review of published hematology and serum chemistry
We performed a literature search for publications relating to platypus hematology and serum chemistry, using search engines and databases100, including: in Google Scholar, Ovid, Scopus and Web of Science. We searched for publications with the terms: ‘platypus hematology’, ‘platypus serum chemistry’, ‘platypus biochemistry’. We identified 11 sources, including eight peer reviewed papers and four book chapters but removed duplicated results, leaving a total of eight sources with hematology and serum chemistry data from Tasmania, Victoria, and New South Wales. In addition, we sourced unpublished data from the ZIMS species360 database (www.species360.org), a global online database used by zoos for captive animals. In total, we used 12 different platypus studies and locations, including the three catchments that we sampled in this study (Fig. 1 and Table 1). To investigate whether hematological or blood chemistry analytes varied non-linearly across the species’ latitudinal range, we used the mean for each analyte and a Generalized Linear Model weighted by sample size101, fitted with a second order polynomial relationship, to allow a non-linear association, with latitude as a predictor variable, implemented in R93. Associations were considered significant if P ≤ 0.05. The ‘ggplot2′94 package was used to represent the data. To further future research into population health of platypus, our data is made open source via Dryad (https://doi.org/10.5061/dryad.brv15dv9d).
Table 1.
Identified studies with reported platypus hematology and serum chemistry values (location number refers to location on Fig. 1).
| #Location | Source | Season\Month | PCV | TP | Albumin | Globulin | Urea | Creatinine | Triglycerides | Lat |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Inglis River (TAS) | Macgregor, et al.81 | Annual | 0.46 ± 0.04 (0.36–0.57) n = 126 | 65 ± 4.2 (55.3–77) n = 109 | 31.1 ± 2.2 (24.9–34.9) n = 109 | 34 ± 3 (27–44) n = 108 | 28.7 ± 3.1 (18.1–37.6) n = 108 | 34 ± 8 (15–59) n = 113 | N/A | − 41.05 |
| 2. Tasmania | Geraghty, et al.80 | Jan-Jun | N/A | 80.2 ± 28.4 n = 116 | 21.5 ± 30.4 n = 31 | 65.3 ± 42.7 n = 29 | 33.9 ± 31.3 n = 32 | 30.1 ± 40 n = 33 | N/A | − 43.05 |
| 3. Brumbys Creek (TAS) | Connolly, et al.79 | N/A | 0.48 ± 0.04 (0.40–0.59) n = 27 | N/A | 27.2 ± 2.6 (22.5–33.8) N = 27 | 47 ± 5.4 (34.3–56) n = 27 | 31.6 ± 2.9 (27–36.4) n = 27 | 35.2 ± 5 (25–44) n = 27 | N/A | − 41.75 |
| 4. Kangaroo River (NSW) | Whittington and Grant84 | Nov | 0.49 ± 0.05 n = 9 | 72 ± 2 n = 4 | 32 ± 1 n = 4 | N/A | 31.5 ± 1.7 n = 6 | 26 ± 1.4 n = 2 | 2.89 ± 0.55 (1.79–3.99) n = 4 | − 34.71 |
| 5. Upper Shoalhaven River (NSW) | Whittington and Grant83 | Jan/Mar | 0.52 ± 0.01 n = 29 | 68 ± 0.8 n = 24 | 32 ± 0.4 n = 24 | N/A | 28.9 ± 0.08 n = 24 | 35 ± 2.2 n = 20 | 1.58 ± 0.13 (1.32–1.84) n = 24 | − 34.86 |
| 6. Healesville (VIC) | Booth and Connolly82 | N/A | 0.51 (0.35–0.62) n = 87 | 66 (57–75) n = 40 | 28 (22–33) n = 39 | 37.5 ± 5.25 (25–46) n = 40 | N/A | 30 (10–40) n = 38 | 0.87 (0.50–1.5) n = 28 | − 37.65 |
| 7. Tasmania | Parer and Metcalfe165 | N/A | 0.52 (0.50–0.54) n = 3 | N/A | N/A | N/A | N/A | N/A | N/A | − 42.87 |
| 8. Tasmania | Isaacks, et al.166 | N/A | 0.43 ± 0.07 (0.29–0.57) n = 10 | N/A | N/A | N/A | N/A | N/A | N/A | − 42.47 |
| 9. Taronga Zoo (NSW) | Zims 2020 | N/A | 0.50 (0.38–0.60) n = 22 | 72 (57–90) n = 22 | N/A | N/A | 26.8 (16.3–39.6) n = 22 | 79 (4–124) n = 20 | N/A | − 33.84 |
| 10. Border Rivers (NSW) | This paper | Dec–May | 0.44 ± 0.04 (0.31–0.54) n = 63 | 68.3 ± 0.6 (44–84) n = 63 | 31 ± 8 (11–42) n = 57 | 37.4 ± 14 (11–67) n = 57 | 30.6 ± 4.2 (15.9–38.7) n = 57 | 49.1 ± 16.8 (15–90) n = 47 | 0.67 ± 0.41 (0.2–2.1) n = 43 | − 29.24 |
| 11. Snowy River (NSW) | This paper | Annual | 0.50 ± 0.05 (0.30–0.60) n = 106 | 65.74 ± 4.2 (57–82) n = 104 | 34 ± 4 (24–44) n = 106 | 31.1 ± 6.3 (23–48) n = 97 | 29.7 ± 3.7 (20.4–36.7) n = 106 | 35.10 ± 14.4 (15–75) n = 97 | 1.25 ± 0.55 (0.50–3.10) n = 105 | − 36.44 |
| 12. Upper Murray River (VIC) | This paper | Jan–May | 0.53 ± 0.04 (0.43–0.60) n = 22 | 66.7 ± 5.4 (58–78) n = 21 | 36 ± 12 (30–41) n = 21 | 33.6 ± 13.3 (17–70) n = 21 | 30.7 ± 3.1 (26.6–37.2) n = 21 | 35.9 ± 12.0 (20–70) n = 17 | 1.49 ± 1.23 (0.7–5.0) n = 21 | − 36.72 |
Values are listed as mean ± SD (min to max), with sample size for particular analyte listed. N/A – unavailable data.
Guidelines and regulations
Guidelines and Regulations This study was performed in accordance with the guidelines set out and approved by the by the NSW Office of Environmental Heritage (SL101655), NSW Department of Primary Industries (P15/0096=1.0 & OUT15/26392), and UNSW's Animal Care and Ethics Committee (16/14A). This study complies with ARRIVE guidelines.
Results
Tail volume index (TVI) was not significantly associated to PCV or any of the tested serum chemistry analytes (Appendix S1), although there was some suggestive negative association with levels of triglycerides (P = 0.074) and urea (P = 0.064). Across the three catchments, TVI was not associated with month (Jan-May), sex, age, or elevation (Fig. 2, Appendix S1). On the Snowy River, TVI did not differ between the sexes, but did significantly fluctuate in male platypuses (P = 0.001) and marginally in females (P = 0.086), increasing towards the onset of the breeding season (October) and reaching a minimum by April (Fig. 3, Appendix S2).
Figure 2.
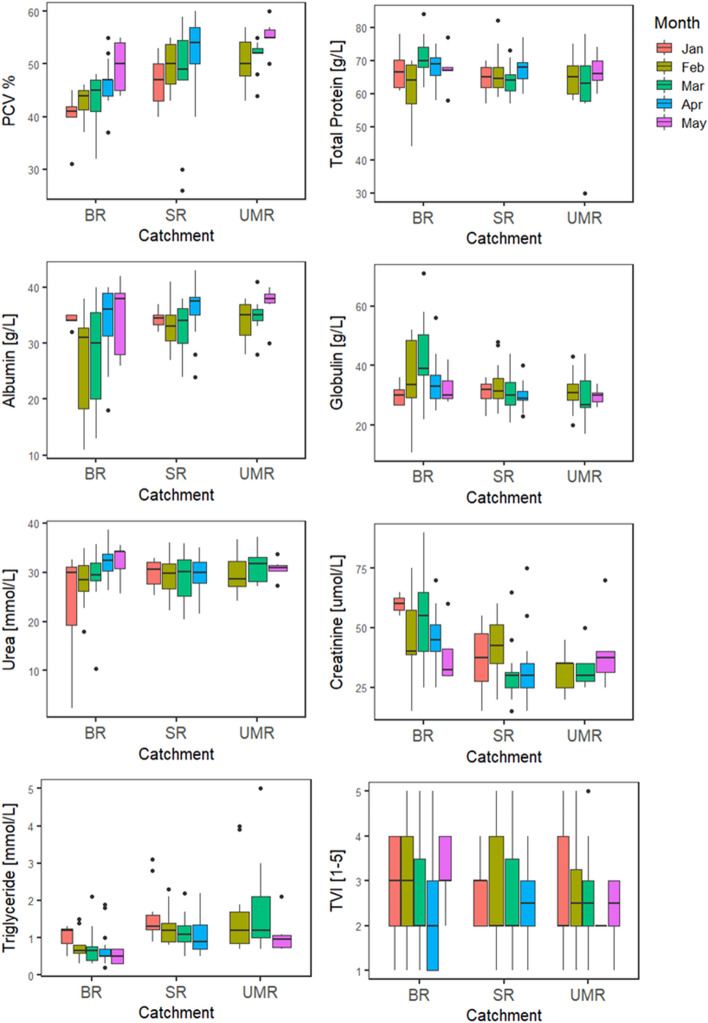
Box plots of seven hematology and serum chemistry analytes of platypuses from all rivers (BR Border Rivers n = 83, SR Snowy Rivers n = 88, UM Upper Murray Rivers n = 38, January to May), collected between January 2016 and May 2018. Boxes represent the 2nd and 3rd quartiles with median represented by the line and whiskers extend to the upper and lower quartiles no further than 1.5 IQR.
Figure 3.
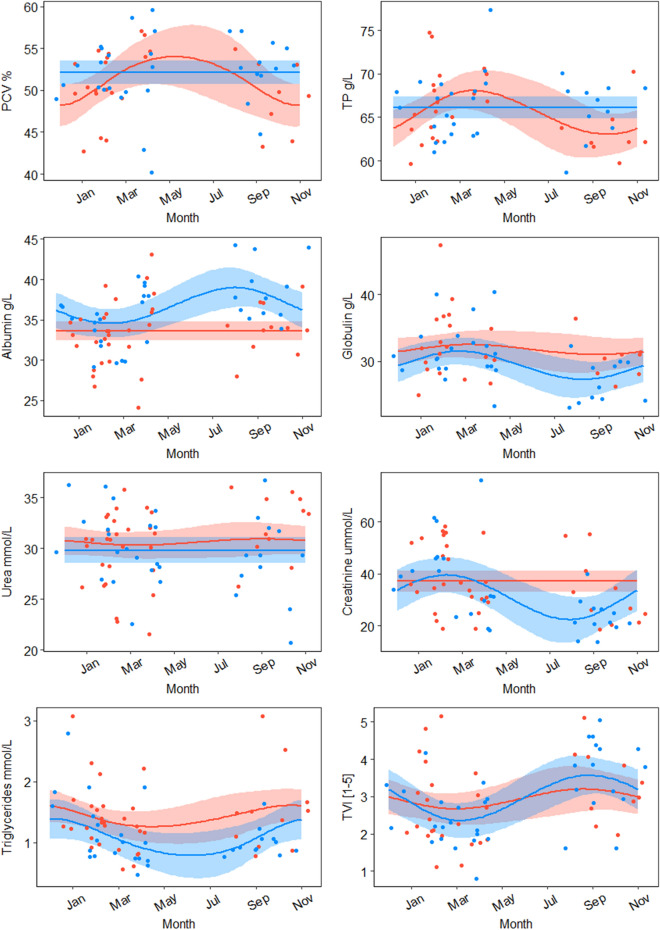
Predicted responses from Generalized Additive Models in seven hematology and serum chemistry analytes and body condition (Tail Volume Index) of female (red) and male (blue) platypuses between December 2016 and November 2017 in the Snowy River Catchment.
All hematology and serum chemistry analytes, apart from urea, were significantly related to catchment (Fig. 2, Appendix S1). Platypuses from the Border Rivers catchment had lower PCV (P = 0.001), and lower concentrations of albumin (P = 0.006), and triglycerides (P = 0.001), compared to those from the Snowy and Upper Murray Rivers catchments (Fig. 2, Appendix S1). Also, TP (P = 0.02), globulin (P = 0.001), and creatinine (P = 0.005) concentrations were significantly higher in platypuses from the Border Rivers catchment, compared to the Snowy and Upper Murray Rivers catchments (Fig. 2, Appendix S1). Urea levels did not vary significantly among rivers, although there was some evidence for lower concentrations in platypuses from the Snowy Rivers catchment, compared to individuals from the other two catchments (P = 0.087), (Appendix S1). Platypuses in the geographically proximal Snowy and Upper Murray River catchments (Fig. 1) had similar levels for all hematology and serum chemistry analytes (Table S1). Further, no hematology nor serum chemistry analytes were related to elevation (Appendix S1).
PCV, and concentrations of TP, albumin, and urea were positively (max P = 0.02) associated with month (Jan to May), while triglycerides concentrations were negatively associated (P = 0.001), and creatinine concentrations were not related to month (P = 0.226) (Fig. 2, Appendix S1). Globulin had a slight negative association with month (P = 0.07). Over a period of a year in the Snowy River catchment, there were no significant fluctuations in concentrations of albumin, urea or triglycerides (Appendix S2). In contrast, PCV (P = 0.03), TP (P = 0.01), and globulin (P = 0.01) concentrations of females in the Snowy Rivers catchment varied significantly over a year (Fig. 3, Appendix S2): all lowest at the end of winter but PCV peaked mid-winter and TP and globulin were highest at the end of summer (Fig. 3). Male globulin also varied significantly (P = 0.03), but had highest globulin levels in autumn and decreasing until their lowest levels in spring (Fig. 3). Male creatinine also fluctuated over the year (P = 0.07) following a similar pattern that of globulin (Fig. 3).
Triglyceride concentrations were lower in males than females (P = 0.001), across all rivers between January to May (Table S1), but there were no other significant differences between the sexes across all rivers. PCV (P = 0.001), TP (P = 0.006) and globulin (P = 0.02) concentrations were higher in males than females in Snowy River platypuses, throughout the year (Fig. 3, Appendix S2). There were no significant differences between the sexes for concentrations of creatinine, albumin, urea, and triglycerides in the Snowy River throughout the year (Appendix S2). Juvenile platypuses had significantly lower PCV (P = 0.002) and lower concentrations of TP (P ≤ 0.001), than adults across all rivers. Globulin was also lower in juveniles but only slightly associated (P = 0.08), (Appendix S1), but there were no significant differences between juveniles and adults for any of the other hematology and serum chemistry analytes.
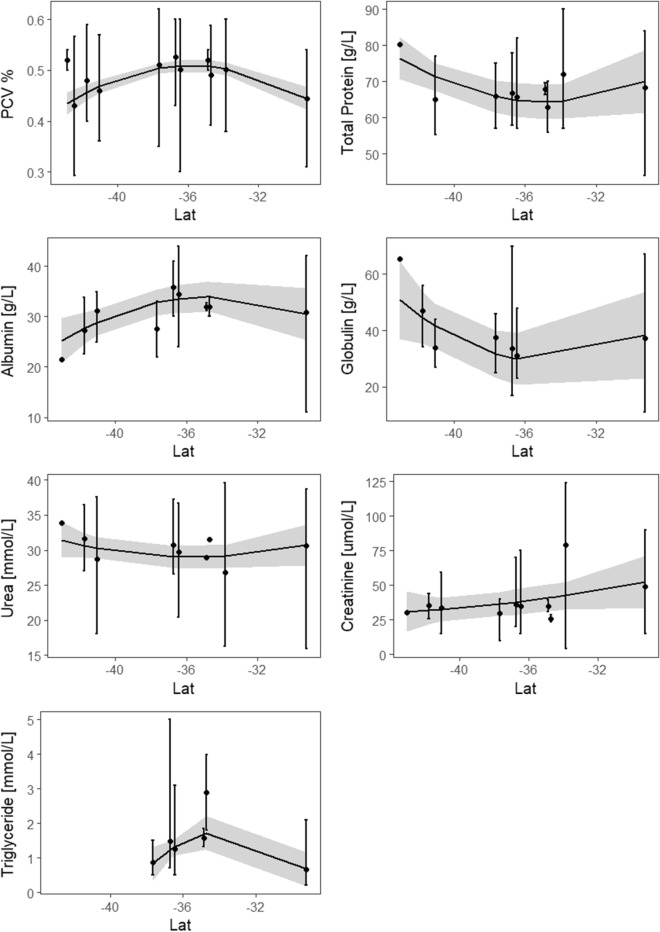
When all available data from northern New South Wales (Lat -29.24) to Tasmania (Lat -43.05) were analyzed (Figs. 1 & 4, Table 1, Appendix S3), ranges of hematology and serum chemistry analytes in platypus from this study were slightly higher than those of platypus from Tasmania (Table 2). Albumin and PCV decreased with latitude (P = 0.03 and P = 0.001), TP and Triglycerides also showed a slight association (P = 0.06 and P = 0.08), triglycerides however increased with latitude. There were no other significant associations between latitude and the hematology and serum chemistry analytes.
Figure 4.
Data from meta-analysis comparing variation (point plot with generalized linear model fitted to data) in levels of seven hematology and serum chemistry variables by latitude.
Table 2.
The seven hematology and serum chemistry variables (sample size), Reference Intervals (RI) (where N ≥ 120) using non-parametric methods98,99, mean, SD, and lower and upper 90% confidence intervals, partitioned by age and sex, for platypus captured from New South Wales and Victorian catchments (Snowy, Upper Murray, and Border Rivers) (see Fig. 1).
| Group | Variable (n) | RI | Mean | SD | 90% CI lower limit | 90% CI upper limit |
|---|---|---|---|---|---|---|
| Adults | PCV (191) | 33.7–61.8 | 49 | 6.6 | 30–40 | 58–78 |
| TP (188) | 46.5–78 | 66.2 | 6.6 | 30–55 | 75–84 | |
| Albumin (184) | 14.7–42.4 | 33.3 | 6 | 11–18 | 40–44 | |
| Urea (184) | 19.62–36.73 | 29.98 | 4.52 | 2.3–22.8 | 36.1–38.7 | |
| Creatinine (161) | 15–75 | 38.8 | 16.2 | 15–20 | 70–90 | |
| Triglycerides (169) | 0.3–3.1 | 1.12 | 0.69 | 0.2–0.3 | 2.3–5 | |
| Globulin (175) | 23–57.5 | 33.8 | 8.5 | 11–24 | 51–71 | |
| Juveniles | PCV (44) | 27.4–55 | 44 | 5.6 | 26–40 | 54.8–55 |
| TP (44) | 33–74.9 | 63.1 | 7.1 | 30–57 | 72–75 | |
| Albumin (42) | 18.3–40.9 | 33.6 | 5.1 | 18–24.2 | 38.9–41 | |
| Urea (42) | 21.74–34.94 | 29.38 | 3.74 | 21.7–22.78 | 33.8–35 | |
| Creatinine* (37) | 15.4–62 | 37.6 | 11.1 | 12–20.5 | 55–66.5 | |
| Triglycerides* (37) | 0.32–2.52 | 1.06 | 0.57 | 0.28–0.41 | 1.95–3.04 | |
| Males | PCV (97) | 30.9–62.8 | 49 | 7.1 | 30–37.5 | 59–70 |
| TP (94) | 53.1–76.6 | 66.4 | 5.1 | 47–57.7 | 74.6–78 | |
| Albumin (91) | 15.3–44 | 33.5 | 6.5 | 13–18 | 40–44 | |
| Urea (92) | 17.46–37.07 | 289.74 | 4.86 | 2.3–24.07 | 36.38–38.7 | |
| Creatinine (78) | 15–75 | 38.8 | 16.4 | 15–19.9 | 70–75 | |
| Triglycerides (85) | 0.3–2.66 | 0.96 | 0.54 | 0.2–0.3 | 1.77–4 | |
| Globulin (89) | 20.8–57.5 | 33.1 | 8.2 | 17–23 | 51–58 | |
| Females | PCV (84) | 40–63.1 | 49 | 6.1 | 31–42 | 57–78 |
| TP (85) | 44.2–81.4 | 66.1 | 8 | 30–48.9 | 76.7–84 | |
| Albumin (94) | 13.4–41.3 | 33 | 5.5 | 11–24 | 40–43 | |
| Urea (94) | 18.9–36.19 | 30.22 | 4.18 | 10.4–23.59 | 35.46–38.7 | |
| Creatinine (83) | 15–74.5 | 38.9 | 16.1 | 15–20 | 65–90 | |
| Triglycerides (84) | 0.3–3.8 | 1.28 | 0.78 | 0.3–0.5 | 2.5–5 | |
| Globulin (78) | 22.7–51.5 | 34.2 | 7.9 | 11–26 | 46.6–71 |
*Robust method with Cox-Box transformation used due to sample size < 40.
Discussion
We evaluate several parameters relating to platypus health associated with energy and protein intake by analyzing variation in seven condition-related hematology and serum chemistry analytes. Our study is the largest scale investigation to date of platypus health for mainland platypuses, spanning three different river catchments in New South Wales and Victoria. Measurement of platypus tail volume index, often used to measure condition85 was not particularly correlated to the body condition variables in this study. Significant differences occurred across river catchments, between sexes and ages, and among months. Understanding natural variation and impacts of stressors is important for individual and population health assessments55 and identifying vulnerabilities in species33,42,56,102–104.
All hematology and serum chemistry analytes, except urea, varied significantly between river catchments, with the Border Rivers being significantly different to the other two catchments (Appendix S1, Fig. 2). While our original intent was to create reference intervals for mainland platypus populations, requiring at least 120 samples57,58, clear differences in levels in platypuses within the Border Rivers catchment suggested developing a single reference interval across all three studied areas was not appropriate. Consequently, we also calculated confidence intervals for each catchment separately (Table 1), providing geographic comparisons. The relatively lower levels of PCV, albumin and triglycerides, in addition to higher creatinine and globulin levels in platypuses from the Border Rivers catchment, compared to the other two catchments in the species’ southern extent, (Fig. 2, Table 1), may indicate poorer health44,47,50, possibly a result of lower food availability, but may also reflect other environmental effects. The significant differences in evaluated hematology and serum chemistry analytes in platypuses in the Border Rivers catchment, the most northerly river catchment surveyed (Fig. 1) may reflect naturally lower levels associated with the climate, indicative of possible habitat-related stressors or some combination of both105–107. The sensitivity of platypuses to high temperatures, with limited cooling options108–111 is likely a key factor limiting their distribution into the lowland parts of the rivers of the Murray-Darling Basin, despite being widely distributed in connected upper parts (Fig. 1)78,112–114. Thermal stress can directly impact health, and lead to changes in hematology and serum chemistry analytes105,106,115–117. In addition, indirect effects on health may be caused by changes in temperature shifting food sources and availability18. Platypuses rely on aquatic macroinvertebrates for food and are particularly sensitive to changes in temperature regimes118–121. Ephemeroptera and Trichoptera have been found to be some of the more sensitive prey to habitat changes122 and are also two of the three most common consumed orders by platypus123 Understanding the potential risks associated with thermal stress will increase in importance in coming decades, with the northern range of the platypus projected to decrease in size by 31% by 2070 in response to global warming75. Thermal stress on the northern populations should be tested with more data from platypuses in Queensland, which is currently poorly sampled78,124. Using all available data, latitudinal effects were detected in albumin and PCV, and to some extent with TP and triglycerides (Fig. 4, Table 1). However, variability and reporting deficiencies likely confounded our ability to draw broad conclusions on geographic variation. Collecting more information on platypuses in the species’ northern range (northern New South Wales and Queensland) is needed to evaluate whether observed differences in the Border Rivers were due to geographic variation, habitat stressors, or thermal stress.
Beyond the notable seasonal variation (i.e., month), other potential confounders were present. Methods of restraint varied across studies with some using no anaesthetic and others using different types of anaesthetic (e.g. ether, isoflurane). Anaesthetics can decrease PCV in some species125–127, while the stress of handling non-anaesthetised animals can increase PCV128–131. In addition, studies differed in blood collection methods and whether PCV or hematocrit was reported132. Small sample sets and the inclusion of juveniles in some studies likely further reduced statistical power. This highlights the need for consistent health data collection methods across the species range required to develop population relevant reference intervals. The hematology and serum chemistry analytes for the New South Wales and Victorian populations were similar to those in Tasmania (Table 1, Table 2). Hematology and serum chemistry analytes did not vary across examined elevation gradients, but most varied between the months of Jan and May (Table S2), as well as throughout the year on the Snowy River (Table S3), with some differentiation between the sexes.
There were also seasonal patterns in hematology and serum chemistry, not previously reported for the mainland, such as low levels of triglycerides in winter which may be due to reduced food resources in winter, coinciding with relatively low weight levels133,134, and when glucocorticoids are at their highest as animals mobilize energy stores135,136. Levels of other hematology and serum chemistry analytes also changed with reproductive status and sex, during the breeding season from August- March64,66. Packed Cell Volume in Tasmanian platypuses81 were significantly correlated to water and ambient temperature and time of year, increasing from autumn through winter, then decreasing from spring to summer. However, this study found PCV was consistent throughout the year for males (Fig. 3), but females varied with highest levels at the start of winter then decreasing through autumn to the lowest levels throughout summer (Fig. 3). This reflected the considerable metabolic demand on breeding females from construction of nesting burrows in September to October, then egg laying and incubation, followed by four months of lactation before young emerge between January to late February64,137–139. The metabolic demand of supporting up to three young is considerable60, requiring dramatically increased daily food consumption, during lactation, of 90–100% of their bodyweight140–142. Increased PCV in females during breeding times is also evident in other species143,144. A similar pattern was observed for TP and globulin (Fig. 3), indicating that variances in blood protein concentrations may be associated with egg production as seen in other egg-laying species45,145,146. PCV and serum chemistry concentrations of males were relatively more stable than those in females in the winter months. Patterns of variance were similar between the sexes, however males slightly lagged behind females; lowest values for males were observed early in the breeding season around September for males, while values for females were lowest around December to February (Fig. 3). During the breeding season males may engage in territorial and aggressive behaviour, potentially utilizing their venomous spurs89,147, coinciding with increased testosterone production89,148,149. Testosterone production negatively affects fat stores and metabolism in other species150. Male platypuses in captivity were more active than females in the pre-mating season (May to August) and the post-mating season (October to January)151,152, with most of their time spent defending territory from other males or seeking out females rather than foraging. This may explain the lower concentrations observed in males during the breeding season. Detection of monthly patterns in hematology and serum chemistry were weakened because of the absence of sampling in May–July, although these monthly patterns were still evident in the data (Fig. 3).
The effect of age on hematology and serum chemistry can be seen in many marsupials17,153–159 and has been linked to changes in body mass, ontogenesis of the immune system160, energy requirements for growth, and changes in behaviour. For example, juvenile platypuses often disperse further than adults, particularly males133. Packed Cell Volume and TP concentrations generally increase with age, as seen with juveniles in this study having significantly lower PCV, TP and globulin compared to adults (Appendix S1).
In conclusion, we investigated PCV and six serum chemistry analytes and found a number of seasonal changes and associations with river catchment, sex and age. The causes of spatial differences remain largely unknown, a reflection of natural variability and the range of environmental stressors affecting platypus populations72,73,75–78,161. Further investigations incorporating direct measures of health provided by hematology and serum chemistry analytes in conjunction with indirect measures of body condition are warranted with expansion of the number of analytes measured (Table 2). Measurement of hematology and serum chemistry analytes may provide more comprehensive information on an animal’s health than indirect measures alone55,107. While we acknowledge that this study used a subset of hematology and serum chemistry analytes, given the association of these analytes to energy, protein intake, body mass and organ function, we suspect TVI may not provide an accurate measure of health but nonetheless reflective of fat storages which fluctuate seasonally85,162. Although a slight negative association was seen in urea and triglycerides, warranting further investigation as an indicator of body condition28,162,163. There is also a need to better understand spatial relationships between hematology and serum chemistry analytes and the range of threatening processes, varying across the distribution of the platypus72,124,164. There are still considerable knowledge gaps in understanding of platypus ecology and biology across their range, with this compilation of data providing the first large-scale assessment of condition for mainland platypuses, including reference intervals for selected hematology and serum chemistry analytes for the most northern population to date. Measurement of these variables in platypus can continue to improve current understanding of the ecology of this scientifically valuable and irreplaceable part of Australian and global biodiversity, contributing to its future survival.
Supplementary Information
Acknowledgements
Our sincere thanks to all volunteers, land holders, and National Parks and Wildlife Service employees who helped make this research possible. Our thanks to the pathologists at Vetnostics Laboratory: Bruce Duff, Susan Jaensch, and Doug Hayward.
Author contributions
G.B. and T.H. collected the data, J.S. and G.B. analyzed the data, J.S. led the writing and all authors contributed to writing of the manuscript and gave final approval for publication.
Funding
This study was funded by ARC Linkage LP150100093 and the Taronga Conservation Society.
Data availability
Data is available through Dryad (10.5061/dryad.brv15dv9d).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95544-z.
References
- 1.Mayr E. Geographical character gradients and climatic adaptation. Evolution. 1956;10:105–108. doi: 10.1111/j.1558-5646.1956.tb02836.x. [DOI] [Google Scholar]
- 2.Sand H, Cederlund G, Danell K. Geographical and latitudinal variation in growth patterns and adult body size of Swedish moose (Alces alces) Oecologia. 1995;102:433–442. doi: 10.1007/BF00341355. [DOI] [PubMed] [Google Scholar]
- 3.Gigliotti LC, et al. Latitudinal variation in snowshoe hare (Lepus americanus) body mass: a test of Bergmann’s rule. Can. J. Zool. 2020;98:88–95. doi: 10.1139/cjz-2019-0184. [DOI] [Google Scholar]
- 4.Best TL. Intraspecific Variation in the Agile Kangaroo Rat (Dipodomys agilis) J. Mammal. 1983;64:426–436. doi: 10.2307/1380355. [DOI] [Google Scholar]
- 5.Terada C, Tatsuzawa S, Saitoh T. Ecological correlates and determinants in the geographical variation of deer morphology. Oecologia. 2012;169:981–994. doi: 10.1007/s00442-012-2270-7. [DOI] [PubMed] [Google Scholar]
- 6.Gigliotti LC, Diefenbach DR, Sheriff MJ. Geographic variation in winter adaptations of snowshoe hares (Lepus americanus) Can. J. Zool. 2017;95:539–545. doi: 10.1139/cjz-2016-0165. [DOI] [Google Scholar]
- 7.Singaravelan N, et al. Adaptation of pelage color and pigment variations in Israeli subterranean blind mole rats, Spalax ehrenbergi. PloS ONE. 2013;8:119. doi: 10.1371/annotation/27bebc65-09c5-4c58-be6c-4f22c4fe0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price T, Ndiaye O, Hammerschmidt K, Fischer J. Limited geographic variation in the acoustic structure of and responses to adult male alarm barks of African green monkeys. Behav. Ecol. Sociobiol. 2014;68:815–825. doi: 10.1007/s00265-014-1694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagos L, Bárcena F. Spatial variability in wolf diet and prey selection in Galicia (NW Spain) Mammal Res. 2018;63:125–139. doi: 10.1007/s13364-018-0352-6. [DOI] [Google Scholar]
- 10.Ashton KG, Tracy MC, Queiroz AD. Is Bergmann’s rule valid for mammals? Am. Nat. 2000;156:390–415. doi: 10.1086/303400. [DOI] [PubMed] [Google Scholar]
- 11.Watt C, Mitchell S, Salewski V. Bergmann's rule; a concept cluster? Oikos. 2010;119:89–100. doi: 10.1111/j.1600-0706.2009.17959.x. [DOI] [Google Scholar]
- 12.Yom-Tov Y, Geffen E. Recent spatial and temporal changes in body size of terrestrial vertebrates: probable causes and pitfalls. Biol. Rev. 2011;86:531–541. doi: 10.1111/j.1469-185X.2010.00168.x. [DOI] [PubMed] [Google Scholar]
- 13.Basuony M, Mohamed W, Shalabi M. Food and feeding ecology of the Egyptian Mongoose, Herpestes ichneumon (Linnaeus, 1758) in Egypt. J. Appl. Sci. Res. 2013;9:5811–5816. [Google Scholar]
- 14.McNab BK. Geographic and temporal correlations of mammalian size reconsidered: a resource rule. Oecologia. 2010;164:13–23. doi: 10.1007/s00442-010-1621-5. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, et al. Ambient temperature correlates with geographic variation in body size of least horseshoe bats. Curr. Zool. 2020;2:19. doi: 10.1093/cz/zoaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taggart DA, et al. Environmental factors influencing hairy-nosed wombat abundance in semi-arid rangelands. J. Wildl. Manag. 2020;84:921–929. doi: 10.1002/jwmg.21858. [DOI] [Google Scholar]
- 17.Brandimarti, M. E. et al. Reference intervals for parameters of health of eastern grey kangaroos Macropus giganteus and management implications across their geographic range. Wildl. Biol.2020 (2020).
- 18.Fancourt BA, Hawkins CE, Nicol SC. Mechanisms of climate-change-induced species decline: spatial, temporal and long-term variation in the diet of an endangered marsupial carnivore, the eastern quoll. Wildl. Res. 2019;45:737–750. doi: 10.1071/WR18063. [DOI] [Google Scholar]
- 19.Phillips BL, Shine R. Adapting to an invasive species: toxic cane toads induce morphological change in Australian snakes. Proc. Natl. Acad. Sci. 2004;101:17150–17155. doi: 10.1073/pnas.0406440101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Both C, Visser ME. The effect of climate change on the correlation between avian life-history traits. Global Change Biol. 2005;11:1606–1613. doi: 10.1111/j.1365-2486.2005.01038.x. [DOI] [Google Scholar]
- 21.Borg C, Majolo B, Qarro M, Semple S. A comparison of body size, coat condition and endoparasite diversity of wild Barbary macaques exposed to different levels of tourism. Anthrozoös. 2014;27:49–63. doi: 10.2752/175303714X13837396326378. [DOI] [Google Scholar]
- 22.Maceda-Veiga A, Green AJ, De Sostoa A. Scaled body-mass index shows how habitat quality influences the condition of four fish taxa in north-eastern Spain and provides a novel indicator of ecosystem health. Freshwat. Biol. 2014;59:1145–1160. doi: 10.1111/fwb.12336. [DOI] [Google Scholar]
- 23.Thatcher HR, Downs CT, Koyama NF. Using parasitic load to measure the effect of anthropogenic disturbance on vervet monkeys. EcoHealth. 2018;15:676–681. doi: 10.1007/s10393-018-1349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyce MS. Population viability analysis. Annu. Rev. Ecol. Syst. 1992;23:481–497. doi: 10.1146/annurev.es.23.110192.002405. [DOI] [Google Scholar]
- 25.Gaillard J-M, Festa-Bianchet M, Yoccoz N, Loison A, Toigo C. Temporal variation in fitness components and population dynamics of large herbivores. Annu. Rev. Ecol. Syst. 2000;31:367–393. doi: 10.1146/annurev.ecolsys.31.1.367. [DOI] [Google Scholar]
- 26.Reed DH, O'Grady JJ, Brook BW, Ballou JD, Frankham R. Estimates of minimum viable population sizes for vertebrates and factors influencing those estimates. Biol. Conserv. 2003;113:23–34. doi: 10.1016/S0006-3207(02)00346-4. [DOI] [Google Scholar]
- 27.Stevenson R, Woods WA., Jr Condition indices for conservation: new uses for evolving tools. Integr. Comp. Biol. 2006;46:1169–1190. doi: 10.1093/icb/icl052. [DOI] [PubMed] [Google Scholar]
- 28.Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ. Restitution of mass–size residuals: validating body condition indices. Ecology. 2005;86:155–163. doi: 10.1890/04-0232. [DOI] [Google Scholar]
- 29.Weiss DJ, Wardrop KJ. Schalm's Veterinary Hematology. Wiley; 2011. [Google Scholar]
- 30.Hanks J, Fowler C, Smith T. Dynamics of large mammal populations. Dyn. Large Mamm. Popul. 1981;2:47–73. [Google Scholar]
- 31.Mapfumo L, Muchenje V, Mupangwa JF, Scholtz MM. Changes in biochemical proxy indicators for nutritional stress resilience from Boran and Nguni cows reared in dry arid rangeland. Trop. Anim. Health Prod. 2017;49:1383–1392. doi: 10.1007/s11250-017-1338-0. [DOI] [PubMed] [Google Scholar]
- 32.Miller DS, et al. Biomedical evaluation of free-ranging ring-tailed lemurs (Lemur catta) in three habitats at the Beza Mahafaly Special Reserve, Madagascar. J. Zoo Wildl. Med. 2007;38:201–216. doi: 10.1638/1042-7260(2007)038[0201:BEOFRL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Pérez JM, et al. Distinguishing disease effects from environmental effects in a mountain ungulate: seasonal variation in body weight, hematology, and serum chemistry among Iberian ibex (Capra pyrenaica) affected by sarcoptic mange. J. Wildl. Dis. 2015;51:148–156. doi: 10.7589/2014-01-008. [DOI] [PubMed] [Google Scholar]
- 34.Webster KN, Hill NJ, Burnett L, Deane EM. Ectoparasite infestation patterns, haematology and serum biochemistry of urban-dwelling common brushtail possums. Wildl. Biol. 2014;20:206–216. doi: 10.2981/wlb.00027. [DOI] [Google Scholar]
- 35.Perrault JR, Stacy NI. Note on the unique physiologic state of loggerhead sea turtles (Caretta caretta) during nesting season as evidenced by a suite of health variables. Mar. Biol. 2018;165:71. doi: 10.1007/s00227-018-3331-1. [DOI] [Google Scholar]
- 36.O’Brien J, Schmitt T, Nollens H, Dubach J, Robeck T. Reproductive physiology of the female Magellanic penguin (Spheniscus magellanicus): insights from the study of a zoological colony. Gen. Comp. Endocrinol. 2016;225:81–94. doi: 10.1016/j.ygcen.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Robert KA, Schwanz LE. Monitoring the health status of free-ranging tammar wallabies using hematology, serum biochemistry, and parasite loads. J. Wildl. Manag. 2013;77:1232–1243. doi: 10.1002/jwmg.561. [DOI] [Google Scholar]
- 38.Portas TJ, et al. Beyond morbidity and mortality in reintroduction programmes: changing health parameters in reintroduced eastern bettongs Bettongia gaimardi. Oryx. 2016;50:674–683. doi: 10.1017/S0030605315001283. [DOI] [Google Scholar]
- 39.Lücker A, Secomb TW, Weber B, Jenny P. The relative influence of hematocrit and red blood cell velocity on oxygen transport from capillaries to tissue. Microcirculation. 2017;24:e12337. doi: 10.1111/micc.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shield J. A seasonal change in blood cell volume of the Rottnest Island quokka, Setonix brachyurus. J. Zool. 1971;165:343–354. doi: 10.1111/j.1469-7998.1971.tb02192.x. [DOI] [Google Scholar]
- 41.Sealander JA. Seasonal changes in blood values of deer mice and other small mammals. Ecology. 1962;12:107–119. doi: 10.2307/1932045. [DOI] [Google Scholar]
- 42.Trumble SJ, Castellini MA, Mau TL, Castellini JM. Dietary and seasonal influences on blood chemistry and hematology in captive harbor seals. Mar. Mamm. Sci. 2006;22:104–123. doi: 10.1111/j.1748-7692.2006.00008.x. [DOI] [Google Scholar]
- 43.Boonstra R, McColl CJ, Karels TJ. Reproduction at all costs: The adaptive stress response of male Arctic ground squirrels. Ecology. 2001;82:1930–1946. doi: 10.1890/0012-9658(2001)082[1930:RAACTA]2.0.CO;2. [DOI] [Google Scholar]
- 44.Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology. Wiley; 2013. [Google Scholar]
- 45.Thrall MA, Weiser G, Allison RW, Campbell TW. Veterinary Hematology and Clinical Chemistry. Wiley; 2012. [Google Scholar]
- 46.Gruys E, Toussaint M, Niewold T, Koopmans S. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B. 2005;6:1045. doi: 10.1631/jzus.2005.B1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano E, et al. The use of total serum proteins and triglycerides for monitoring body condition in the Iberian wild goat (Capra pyrenaica) J. Zoo Wildl. Med. 2008;39:646–649. doi: 10.1638/2007-0088.1. [DOI] [PubMed] [Google Scholar]
- 48.Stevens LA, Levey AS. Measurement of kidney function. Med. Clin. 2005;89:457–473. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Vanholder R, Glorieux G, De Smet R, Lameire N. New insights in uremic toxins. Kidney Int. 2003;63:S6–S10. doi: 10.1046/j.1523-1755.63.s84.43.x. [DOI] [PubMed] [Google Scholar]
- 50.Caldeira R, Belo A, Santos C, Vazques M, Portugal A. The effect of body condition score on blood metabolites and hormonal profiles in ewes. Small Rumin. Res. 2007;68:233–241. doi: 10.1016/j.smallrumres.2005.08.027. [DOI] [Google Scholar]
- 51.Schutte JE, Longhurst JC, Gaffney FA, Bastian BC, Blomqvist CG. Total plasma creatinine: an accurate measure of total striated muscle mass. J. Appl. Physiol. 1981;51:762–766. doi: 10.1152/jappl.1981.51.3.762. [DOI] [PubMed] [Google Scholar]
- 52.Kaneko, J. J., Harvey, J. W. & Bruss, M. L. Clinical Biochemistry of Domestic Animals. (Academic Press, 2008).
- 53.Stirrat SC. Body condition and blood chemistry of agile wallabies (Macropus agilis) in the wet–dry tropics. Wildl. Res. 2003;30:59–67. doi: 10.1071/WR01041. [DOI] [Google Scholar]
- 54.Lassen E. Perspectives in data interpretation. Vet. Hematol. Clini. Chem. 2004;5:45–49. [Google Scholar]
- 55.Maceda-Veiga A, et al. Inside the Redbox: applications of haematology in wildlife monitoring and ecosystem health assessment. Sci. Total Environ. 2015;514:322–332. doi: 10.1016/j.scitotenv.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Brandimarti ME, Gray R, Silva FR, Herbert CA. Kangaroos at maximum capacity: health assessment of free-ranging eastern grey kangaroos on a coastal headland. J. Mamm. 2021;2:96. doi: 10.1093/jmammal/gyab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark, P. Haematology of Australian Mammals. (CSIRO Publishing, 2004).
- 58.Solberg H. A guide to IFCC recommendations on reference values. J. Int. Fed. Clin. Chem. 1993;5:162–165. [PubMed] [Google Scholar]
- 59.Gongora J, et al. Genetic structure and phylogeography of platypuses revealed by mitochondrial DNA. J. Zool. 2012;286:110–119. doi: 10.1111/j.1469-7998.2011.00854.x. [DOI] [Google Scholar]
- 60.Grant, T. & Fanning, D. The Platypus: A Unique Mammal. (University of New South Wales Press, 1995).
- 61.Furlan E, et al. Is body size variation in the platypus (Ornithorhynchus anatinus) associated with environmental variables? Aust. J. Zool. 2012;59:201–215. doi: 10.1071/ZO11056. [DOI] [Google Scholar]
- 62.Allen A. Allens rule. The influence of Physical conditions in the genesis of species. Rad. Rev. 1877;1:108–140. [Google Scholar]
- 63.Bergmann C. Uber die Verhaltnisse der warmeokonomie der Thiere zu uber Grosso. Gottinger Studien. 1847;3:595–708. [Google Scholar]
- 64.Grant, T., Griffiths, M. & Temple-Smith, P. in Proc. Linn. Soc. N.S.W. 227 (Linnean Society of New South Wales).
- 65.Munks S, Otley H, Bethge P, Jackson J. Reproduction, diet and daily energy expenditure of the platypus in a sub-alpine Tasmanian lake. Aust. Mamm. 2000;21:260–261. [Google Scholar]
- 66.Temple-Smith P, Grant T. Uncertain breeding: a short history of reproduction in monotremes. Reprod. Fertil. Dev. 2001;13:487–497. doi: 10.1071/RD01110. [DOI] [PubMed] [Google Scholar]
- 67.Chessman BC, Williams SA. Biodiversity and conservation of river macroinvertebrates on an expanding urban fringe: western Sydney, New South Wales, Australia. Pac. Conserv. Biol. 1999;5:36–55. doi: 10.1071/PC990036. [DOI] [Google Scholar]
- 68.Magierowski RH, Davies PE, Read SM, Horrigan N. Impacts of land use on the structure of river macroinvertebrate communities across Tasmania, Australia: spatial scales and thresholds. Mar. Freshw. Res. 2012;63:762–776. doi: 10.1071/MF11267. [DOI] [Google Scholar]
- 69.Verkaik I, Prat N, Rieradevall M, Reich P, Lake PS. Effects of bushfire on macroinvertebrate communities in south-east Australian streams affected by a megadrought. Mar. Freshw. Res. 2014;65:359–369. doi: 10.1071/MF13039. [DOI] [Google Scholar]
- 70.Stitz L, Fabbro L, Kinnear S. Response of macroinvertebrate communities to seasonal hydrologic changes in three sub-tropical Australian streams. Environ. Monit. Assess. 2017;189:254. doi: 10.1007/s10661-017-5957-8. [DOI] [PubMed] [Google Scholar]
- 71.McLachlan-Troup T, Dickman C, Grant T. Diet and dietary selectivity of the platypus in relation to season, sex and macroinvertebrate assemblages. J. Zool. 2010;280:237–246. doi: 10.1111/j.1469-7998.2009.00645.x. [DOI] [Google Scholar]
- 72.Bino G, et al. The platypus: evolutionary history, biology, and an uncertain future. J. Mamm. 2019;100:308–327. doi: 10.1093/jmammal/gyz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grant T, Temple-Smith P. Conservation of the platypus, Ornithorhynchus anatinus: threats and challenges. Aquat. Ecosyst. Health Manag. 2003;6:5–18. doi: 10.1080/14634980301481. [DOI] [Google Scholar]
- 74.Gust N, et al. Distribution, prevalence and persistence of mucormycosis in Tasmanian platypuses (Ornithorhynchus anatinus) Aust. J. Zool. 2009;57:245–254. doi: 10.1071/ZO09034. [DOI] [Google Scholar]
- 75.Klamt M, Thompson R, Davis J. Early response of the platypus to climate warming. Global Change Biol. 2011;17:3011–3018. doi: 10.1111/j.1365-2486.2011.02472.x. [DOI] [Google Scholar]
- 76.Richmond EK, et al. A diverse suite of pharmaceuticals contaminates stream and riparian food webs. Nat. Commun. 2018;9:4491. doi: 10.1038/s41467-018-06822-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scheelings T. Morbidity and mortality of monotremes admitted to the Australian Wildlife Health Centre, Healesville Sanctuary, Australia, 2000–2014. Aust. Vet. J. 2016;94:121–124. doi: 10.1111/avj.12417. [DOI] [PubMed] [Google Scholar]
- 78.Hawke T, Bino G, Kingsford RT. A silent demise: historical insights into population changes of the iconic platypus (Ornithorhynchus anatinus) Global Ecol. Conserv. 2019;20:720. [Google Scholar]
- 79.Connolly J, Obendorf D, Whittington R. Haematological, serum biochemical and serological features of platypuses with and without mycotic granulomatous dermatitis. Aust. Vet. J. 1999;77:809–813. doi: 10.1111/j.1751-0813.1999.tb12952.x. [DOI] [PubMed] [Google Scholar]
- 80.Geraghty DP, Griffiths J, Stewart N, Robertson IK, Gust N. Hematologic, plasma biochemical, and other indicators of the health of Tasmanian platypuses (Ornithorhynchus anatinus): predictors of mucormycosis. J. Wildl. Dis. 2011;47:483–493. doi: 10.7589/0090-3558-47.3.483. [DOI] [PubMed] [Google Scholar]
- 81.Macgregor JW, et al. A need for dynamic hematology and serum biochemistry reference tools: Novel use of sine wave functions to produce seasonally varying reference curves in platypuses (Ornithorhynchus anatinus) J. Wildl. Dis. 2017;53:235–247. doi: 10.7589/2015-12-336. [DOI] [PubMed] [Google Scholar]
- 82.Booth, R. & Connolly, J. in Medicine in Australian Mammals 103–132 (CSIRO Publishing, 2008).
- 83.Whittington R, Grant T. Haematology and blood chemistry of the free-living platypus, Ornithorhynchus anatinus (Shaw) (Monotremata: Ornithorhynchidae) Aust. J. Zool. 1983;31:475–482. doi: 10.1071/ZO9830475. [DOI] [Google Scholar]
- 84.Whittington R, Grant T. Haematology and Blood Chemistry of the Conscious Platypus, Ornithorhynchus anatinus (Shaw) (Monotremata: Ornithorhynchidae) Aust. J. Zool. 1984;32:631–635. doi: 10.1071/ZO9840631. [DOI] [Google Scholar]
- 85.Grant T, Carrick F. Some aspects of the ecology of the platypus, Ornithorhynchus anatinus, in the upper Shoalhaven River. New South Wales. Australian Zool. 1978;20:181–199. [Google Scholar]
- 86.Bino G, Kingsford RT, Grant T, Taylor MD, Vogelnest L. Use of implanted acoustic tags to assess platypus movement behaviour across spatial and temporal scales. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-23461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hawke, T., Bino, G. & Kingsford, R. T. Damming insights: impacts and implications of river regulation on platypus populations. Aquatic Conservationin press (2020).
- 88.Gallant J, Read A. A near-global bare-Earth DEM from SRTM. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016;41:B4. [Google Scholar]
- 89.Temple-Smith, P. D. M. Seasonal breeding biology of the platypus, Ornithorhynchus anatinus (Shaw, 1799), with special reference to the male. (1973).
- 90.Williams G, Serena M, Grant T. Age-related change in spurs and spur sheaths of the platypus (Ornithorhynchus anatinus) Australian Mammalogy. 2013;35:107–114. doi: 10.1071/AM12011. [DOI] [Google Scholar]
- 91.Grueber C, Nakagawa S, Laws R, Jamieson I. Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 2011;24:699–711. doi: 10.1111/j.1420-9101.2010.02210.x. [DOI] [PubMed] [Google Scholar]
- 92.Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol. Methods Res. 2004;33:261–304. doi: 10.1177/0049124104268644. [DOI] [Google Scholar]
- 93.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing., (R Foundation for Statistical Computing., 2020).
- 94.Wickham H. ggplot2-Elegant Graphics for Data Analysis. Springer International Publishing; 2016. [Google Scholar]
- 95.Wood, S. Mixed GAM computation vehicle with GCV/AIC/REML smoothness estimation and GAMMs by REML/PQL. R Package Version, 1.8–23 (2018).
- 96.Wood S, Wood MS. Package ‘mgcv’. R Package Ver. 2015;1:29. [Google Scholar]
- 97.Breheny P, Burchett W. Visualization of regression models using visreg. R J. 2017;9:56. doi: 10.32614/RJ-2017-046. [DOI] [Google Scholar]
- 98.Geffré A, Concordet D, Braun JP, Trumel C. Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet. Clin. Pathol. 2011;40:107–112. doi: 10.1111/j.1939-165X.2011.00287.x. [DOI] [PubMed] [Google Scholar]
- 99.Friedrichs KR, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012;41:441–453. doi: 10.1111/vcp.12006. [DOI] [PubMed] [Google Scholar]
- 100.Calver MC, Goldman B, Hutchings PA, Kingsford RT. Why discrepancies in searching the conservation biology literature matter. Biol. Conserv. 2017;213:19–26. doi: 10.1016/j.biocon.2017.06.028. [DOI] [Google Scholar]
- 101.Pfeffermann, D. The role of sampling weights when modeling survey data. International Statistical Review/Revue Internationale de Statistique, 317–337 (1993).
- 102.Deem SL, Karesh WB, Weisman W. Putting theory into practice: wildlife health in conservation. Conserv. Biol. 2001;15:1224–1233. doi: 10.1046/j.1523-1739.2001.00336.x. [DOI] [Google Scholar]
- 103.Isaksson C. Urbanization, oxidative stress and inflammation: a question of evolving, acclimatizing or coping with urban environmental stress. Funct. Ecol. 2015;29:913–923. doi: 10.1111/1365-2435.12477. [DOI] [Google Scholar]
- 104.Karesh WB, Cook RA. Applications of veterinary medicine to in situ conservation efforts. Oryx. 1995;29:244–252. doi: 10.1017/S0030605300021232. [DOI] [Google Scholar]
- 105.Cahill AE, et al. Causes of warm-edge range limits: systematic review, proximate factors and implications for climate change. J. Biogeogr. 2014;41:429–442. doi: 10.1111/jbi.12231. [DOI] [Google Scholar]
- 106.Elmore RD, et al. Implications of the thermal environment for terrestrial wildlife management. Wildl. Soc. Bull. 2017;41:183–193. doi: 10.1002/wsb.772. [DOI] [Google Scholar]
- 107.Todgham AE, Stillman JH. Physiological responses to shifts in multiple environmental stressors: relevance in a changing world. Integr. Comput. Biol. 2013;53:539–544. doi: 10.1093/icb/ict086. [DOI] [PubMed] [Google Scholar]
- 108.Brice PH. Thermoregulation in monotremes: riddles in a mosaic. Aust. J. Zool. 2009;57:255–263. doi: 10.1071/ZO09039. [DOI] [Google Scholar]
- 109.Grant T. Body temperatures of free-ranging platypuses, Ornithorhynchus anatinus (Monotremata), with observations on their use of burrows. Aust. J. Zool. 1983;31:117–122. doi: 10.1071/ZO9830117. [DOI] [Google Scholar]
- 110.Grant T, Dawson T. Temperature regulation in the platypus, Ornithorhynchus anatinus: maintenance of body temperature in air and water. Physiol. Zool. 1978;51:1–6. doi: 10.1086/physzool.51.1.30158659. [DOI] [Google Scholar]
- 111.Grant T, Dawson TJ. Temperature regulation in the platypus, Ornithorhynchus anatinus: production and loss of metabolic heat in air and water. Physiol. Zool. 1978;51:315–332. doi: 10.1086/physzool.51.4.30160956. [DOI] [Google Scholar]
- 112.Connolly JH, Claridge T, Cordell SM, Nielsen S, Dutton GJ. Distribution and characteristics of the platypus (Ornithorhynchus anatinus) in the Murrumbidgee catchment. Aust. Mamm. 2016;38:58–67. doi: 10.1071/AM14039. [DOI] [Google Scholar]
- 113.Grant, T. Historical and current distribution of the platypus, Ornithorhynchus anatinus. Australia. In Platypus and echidnas (ed. ML Augee), 232–254 (1992).
- 114.Grant T, Gehrke P, Harris J, Hartley S. Distribution of the platypus (Ornithorhynchus anatinus) in NSW: results of the 1994–96 NSW Rivers Survey. Aust. Mamm. 2000;21:177–184. doi: 10.1071/AM00177. [DOI] [Google Scholar]
- 115.Nazifi S, Gheisari H, Poorabbas H. The influences of thermal stress on serum biochemical parameters of dromedary camels and their correlation with thyroid activity. Comp. Haematol. Int. 1999;9:49–54. doi: 10.1007/PL00010007. [DOI] [Google Scholar]
- 116.Singh KM, et al. Evaluation of Indian sheep breeds of arid zone under heat stress condition. Small Rumin. Res. 2016;141:113–117. doi: 10.1016/j.smallrumres.2016.07.008. [DOI] [Google Scholar]
- 117.Zhang Y, Kieffer JD. Critical thermal maximum (CTmax) and hematology of shortnose sturgeons (Acipenser brevirostrum) acclimated to three temperatures. Can. J. Zool. 2014;92:215–221. doi: 10.1139/cjz-2013-0223. [DOI] [Google Scholar]
- 118.Burgmer T, Hillebrand H, Pfenninger M. Effects of climate-driven temperature changes on the diversity of freshwater macroinvertebrates. Oecologia. 2007;151:93–103. doi: 10.1007/s00442-006-0542-9. [DOI] [PubMed] [Google Scholar]
- 119.Carr M, Li L, Sadeghian A, Phillips ID, Lindenschmidt KE. Modelling the possible impacts of climate change on the thermal regime and macroinvertebrate species of a regulated prairie river. Ecohydrology. 2019;12:e2102. doi: 10.1002/eco.2102. [DOI] [Google Scholar]
- 120.Daufresne M, Bady P, Fruget J-F. Impacts of global changes and extreme hydroclimatic events on macroinvertebrate community structures in the French Rhône River. Oecologia. 2007;151:544–559. doi: 10.1007/s00442-006-0655-1. [DOI] [PubMed] [Google Scholar]
- 121.Durance I, Ormerod SJ. Climate change effects on upland stream macroinvertebrates over a 25-year period. Global Change Biol. 2007;13:942–957. doi: 10.1111/j.1365-2486.2007.01340.x. [DOI] [Google Scholar]
- 122.Walsh CJ. Biological indicators of stream health using macroinvertebrate assemblage composition: a comparison of sensitivity to an urban gradient. Mar. Freshw. Res. 2006;57:37–47. doi: 10.1071/MF05041. [DOI] [Google Scholar]
- 123.Marchant R, Grant T. The productivity of the macroinvertebrate prey of the platypus in the upper Shoalhaven River, New South Wales. Mar. Freshw. Res. 2015;66:1128–1137. doi: 10.1071/MF14301. [DOI] [Google Scholar]
- 124.Bino, G., Kingsford, R. T. & Wintle, B. A. A stitch in time–Synergistic impacts to platypus metapopulation extinction risk. Biol. Conserv.242, 108399 (2020).
- 125.Ambrosio AM, et al. Significant hematocrit decrease in healthy horses during clinical anesthesia. Braz. j. vet. Res. Anim. Sci. 2012;49:139–145. doi: 10.11606/issn.2318-3659.v49i2p139-145. [DOI] [Google Scholar]
- 126.Dhumeaux MP, et al. Effects of a standardized anesthetic protocol on hematologic variables in healthy cats. J. Feline Med. Surg. 2012;14:701–705. doi: 10.1177/1098612X12448588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marini R, et al. Effect of isoflurane on hematologic variables in ferrets. Am. J. Vet. Res. 1994;55:1479–1483. [PubMed] [Google Scholar]
- 128.Bejaei M, Cheng K. Effects of pretransport handling stress on physiological and behavioral response of ostriches. Poult. Sci. 2014;93:1137–1148. doi: 10.3382/ps.2013-03478. [DOI] [PubMed] [Google Scholar]
- 129.Delgiudice GD, Kunkel KE, Mech LD, Seal US. Minimizing capture-related stress on white-tailed deer with a capture collar. J. Wildl. Manag. 1990;11:299–303. doi: 10.2307/3809046. [DOI] [Google Scholar]
- 130.Harvey, J. W. Veterinary Hematology-E-Book: A Diagnostic Guide and Color Atlas. (Elsevier Health Sciences, 2011).
- 131.Raskin, R. E. Hematologic disorders 6. Clinical medicine of the dog and cat, Schaer M, editor. Manson Publishing, London, UK, 227–288 (2009).
- 132.Mayer, J. & Donnelly, T. M. Clinical Veterinary Advisor-E-Book: Birds and Exotic Pets. (Elsevier Health Sciences, 2012).
- 133.Bino G, Grant TR, Kingsford RT. Life history and dynamics of a platypus (Ornithorhynchus anatinus) population: four decades of mark-recapture surveys. Sci. Rep. 2015;5:16073. doi: 10.1038/srep16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gust N, Handasyde K. Seasonal-variation in the ranging behavior of the platypus (Ornithorhynchus-anatinus) on the Goulburn River, Victoria. Aust. J. Zool. 1995;43:193–208. doi: 10.1071/ZO9950193. [DOI] [Google Scholar]
- 135.Handasyde K, McDonald I, Evans B. Plasma glucocorticoid concentrations in free-ranging platypuses (Ornithorhynchus anatinus): response to capture and patterns in relation to reproduction. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2003;136:895–902. doi: 10.1016/S1095-6433(03)00280-0. [DOI] [PubMed] [Google Scholar]
- 136.Wang J-C, Gray NE, Kuo T, Harris CA. Regulation of triglyceride metabolism by glucocorticoid receptor. Cell Biosci. 2012;2:19–19. doi: 10.1186/2045-3701-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Griffiths, M. Reproduction and embryology. Biol. Monotremes, 209–254 (1978).
- 138.Hawkins M, Battaglia A. Breeding behaviour of the platypus (Ornithorhynchus anatinus) in captivity. Aust. J. Zool. 2009;57:283–293. doi: 10.1071/ZO09090. [DOI] [Google Scholar]
- 139.Thomas J, Handasyde K, Parrott M, Temple-Smith P. The platypus nest: burrow structure and nesting behaviour in captivity. Aust. J. Zool. 2018;65:347–356. doi: 10.1071/ZO18007. [DOI] [Google Scholar]
- 140.Holland N, Jackson SM. Reproductive behaviour and food consumption associated with the captive breeding of platypus (Ornithorhynchus anatinus) J. Zool. 2002;256:279–288. doi: 10.1017/S0952836902000328. [DOI] [Google Scholar]
- 141.Thomas JL, Handasyde KA, Temple-Smith P, Parrott ML. Seasonal changes in food selection and nutrition of captive platypuses (Ornithorhynchus anatinus) Aust. J. Zool. 2017;65:319–327. doi: 10.1071/ZO18004. [DOI] [Google Scholar]
- 142.Kruger B, Hunter S, Serena M. Husbandry, diet and behaviour of platypus Ornithorhynchus anatinus at Healesville Sanctuary. International Zoo Yearbook. 1992;31:64–71. doi: 10.1111/j.1748-1090.1991.tb02364.x. [DOI] [Google Scholar]
- 143.El-Sherif M, Assad F. Changes in some blood constituents of Barki ewes during pregnancy and lactation under semi arid conditions. Small Rumin. Res. 2001;40:269–277. doi: 10.1016/S0921-4488(01)00174-2. [DOI] [PubMed] [Google Scholar]
- 144.Hõrak P, Jenni-Eiermann S, Ots I, Tegelmann L. Health and reproduction: the sex-specific clinical profile of great tits (Parus major) in relation to breeding. Can. J. Zool. 1998;76:2235–2244. doi: 10.1139/z98-154. [DOI] [Google Scholar]
- 145.dos Santos Schmidt, E. M. et al. Serum biochemical parameters of female bronze turkeys (Meleagris gallopavo) during egg-laying season. Int J Poult Sci9, 177–179 (2010).
- 146.Lumeij, J. in Clinical biochemistry of domestic animals 857–883 (Elsevier, 1997).
- 147.Whittington CM, Belov K. Tracing monotreme venom evolution in the genomics era. Toxins. 2014;6:1260–1273. doi: 10.3390/toxins6041260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grant, T. & Temple–Smith, P. Field biology of the platypus (Ornithorhynchus anatinus): historical and current perspectives. Philos. Trans. R. Soc. London. Ser. B Biol. Sci.353, 1081–1091 (1998). [DOI] [PMC free article] [PubMed]
- 149.Handasyde, K. & McDonald, I. Reproductive hormones and reproduction in the platypus. Progress Comp. Endocrinol., 184–185 (1993).
- 150.Wikelski M, Lynn S, Breuner J, Wingfield J, Kenagy G. Energy metabolism, testosterone and corticosterone in white-crowned sparrows. J. Comp. Physiol. A. 1999;185:463–470. doi: 10.1007/s003590050407. [DOI] [Google Scholar]
- 151.Thomas JL, Parrott ML, Handasyde KA, Temple-Smith P. Female control of reproductive behaviour in the platypus (Ornithorhynchus anatinus), with notes on female competition for mating. Behaviour. 2018;155:27–53. doi: 10.1163/1568539X-00003476. [DOI] [Google Scholar]
- 152.Hawke, T. et al. Long term movements and activity patterns of platypus on regulated rivers. Scientific Reportsin press (2020). [DOI] [PMC free article] [PubMed]
- 153.Andersen NA, Mesch U, Lovell DJ, Nicol SC. The effects of sex, season, and hibernation on haematology and blood viscosity of free-ranging echidnas (Tachyglossus aculeatus) Can. J. Zool. 2000;78:174–181. doi: 10.1139/z99-199. [DOI] [Google Scholar]
- 154.Barnett, J., How, R. & Humphreys, W. Blood parameters in natural populations of Trichosurus species (Marsupialia: Phalangeridae). I. Age, sex and seasonal variation in T. caninus and T. vulpecula. II. Influence of habitat and population strategies of T. caninus and T. vulpecula. Aust. J. Zool.27, 913–926 (1979).
- 155.Fancourt BA, Nicol SC. Hematologic and serum biochemical reference intervals for wild eastern quolls (Dasyurus viverrinus): variation by age, sex, and season. Vet. Clin. Pathol. 2019;48:114–124. doi: 10.1111/vcp.12703. [DOI] [PubMed] [Google Scholar]
- 156.McKenzie S, Deane E, Burnett L. Haematology and serum biochemistry of the tammar wallaby, Macropus eugenii. Comp. Clin. Pathol. 2002;11:229–237. doi: 10.1007/s005800200024. [DOI] [Google Scholar]
- 157.Schultz DJ, et al. Investigations into the health of brush-tailed rock-wallabies (Petrogale penicillata) before and after reintroduction. Aust. Mamm. 2011;33:235–244. doi: 10.1071/AM11010. [DOI] [Google Scholar]
- 158.Warren KS, Holyoake CS, Friend TJ, Yeap L, McConnell M. Hematologic and serum biochemical reference intervals for the bilby (Macrotis lagotis) J. Wildl. Dis. 2015;51:889–895. doi: 10.7589/2014-03-074. [DOI] [PubMed] [Google Scholar]
- 159.Woolford L, et al. Serum biochemistry of free-ranging southern hairy-nosed wombats (Lasiorhinus latifrons) J. Zool. Wildl. Med. 2020;50:937–946. doi: 10.1638/2019-0001. [DOI] [PubMed] [Google Scholar]
- 160.Sidman CL, et al. Increased expression of major histocompatibility complex antigens on lymphocytes from aged mice. Proc. Natl. Acad. Sci. 1987;84:7624–7628. doi: 10.1073/pnas.84.21.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gust N, Griffiths J. Platypus mucormycosis and its conservation implications. Australasian Mycol. 2009;28:1–8. [Google Scholar]
- 162.MacGregor JW, et al. Assessing body condition in the platypus (Ornithorhynchus anatinus): A comparison of new and old methods. Aust. J. Zool. 2016;64:421–429. doi: 10.1071/ZO16071. [DOI] [Google Scholar]
- 163.Peig J, Green AJ. The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct. Ecol. 2010;24:1323–1332. doi: 10.1111/j.1365-2435.2010.01751.x. [DOI] [Google Scholar]
- 164.Woinarski, J. C., Burbidge, A. A. & Harrison, P. L. The action plan for Australian mammals 2012. (2014).
- 165.Parer J, Metcalfe J. Respiratory studies of monotremes. I. Blood of the platypus (Ornithorynchus anatinus) Respir. Physiol. 1967;3:136–142. doi: 10.1016/0034-5687(67)90003-5. [DOI] [PubMed] [Google Scholar]
- 166.Isaacks R, Nicol S, Sallis J, Zeidler R, Kim HD. Erythrocyte phosphates and hemoglobin function in monotremes and some marsupials. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1984;246:R236–R241. doi: 10.1152/ajpregu.1984.246.2.R236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available through Dryad (10.5061/dryad.brv15dv9d).