Abstract
To analyze the relationship between the characteristics of burst suppression (BS) pattern and different etiologies in epilepsy. Patients with a BS pattern who were younger than 6 months old were screened from our electroencephalogram (EEG) database. The synchronized and symmetric BS patterns under different etiologies in epilepsy were analyzed. A total of 32 patients had a BS pattern on EEG. The etiologies included genetic disorders (37.5%), cortical malformations (28.1%), inborn errors of metabolism (12.5%), and unknown (21.9%). Twenty-five patients were diagnosed with Ohtahara syndrome, one as early myoclonic encephalopathy, and one as epilepsy of infancy with migrating focal seizure. Five cases could not be classified into any epileptic syndrome. Asynchronous BS pattern was identified in 18 cases, of which 13 (72%) patients had genetic and/or metabolic etiologies. Synchronous BS pattern was identified in 14 cases, of which 8 (57%) patients had structural etiologies. Twenty-three patients had symmetric BS patterns, of which 15 (65%) patients had genetic etiologies. Nine patients had asymmetric BS patterns, of which 8 (89%) patients had structural etiologies. Patients with genetic epilepsies tended to have asynchronous and symmetric BS patterns, whereas those with structural epilepsies were more likely to have synchronous and asymmetric BS patterns.
Subject terms: Diseases of the nervous system, Encephalopathy, Epilepsy
Introduction
Burst suppression (BS) is an abnormal electroencephalogram (EEG) pattern characterized by the alternating appearance of depressed background activity and bursts of mixed-frequency paroxysmal activity1. BS pattern is typically recorded in patients with early-onset epileptic encephalopathy (EOEE), such as Ohtahara syndrome (OS) and early myoclonic encephalopathy (EME)2–4. The BS pattern in OS is characterized by high-voltage bursts alternating with nearly flat periods at an approximately regular rate2. Bursts of 1- to 3-s duration comprise 150- to 350-microvolt high-voltage slow waves intermixed with multifocal spikes2. The duration of the suppression phase ranges from 2 to 5 s. Burst-to-burst interval usually ranges from 5 to10 seconds. The SB pattern of EME becomes more distinct during sleep, especially during deep sleep. The suppression phase is longer in the earlier stage of the disorder. It also tends to become longer in deeper sleep in the same tracing2. A few studies mentioned that the BS pattern could also be observed in patients with epilepsy of infancy with migrating focal seizures (EIMFS)5,6. Asymmetric BS pattern was reported in about two-thirds of cases, but no remarkable asynchronization was found except in the case of Aicardi’s syndrome7. Asynchronous and asymmetric BS pattern could be seen in patients with corpus callosum lesions8. Asymmetric BS pattern was usually observed in hemimegaloencephaly9,10. Studies showed that the synchronization and symmetry of BS pattern was related to different epileptic syndromes2,7,10. For example, the BS pattern in OS was bilaterally synchronous but some asymmetry between interhemispheres7,10. In EME, the BS pattern was often asynchronous and had irregular burst-to-burst intervals2,11. As for EIMFS, BS could be synchronous or asynchronous6. In this study, we mainly aimed to analyze the relationship between the characteristics of BS patterns and different etiologies in epileptic syndromes or epilepsy.
Patients and methods
Patients
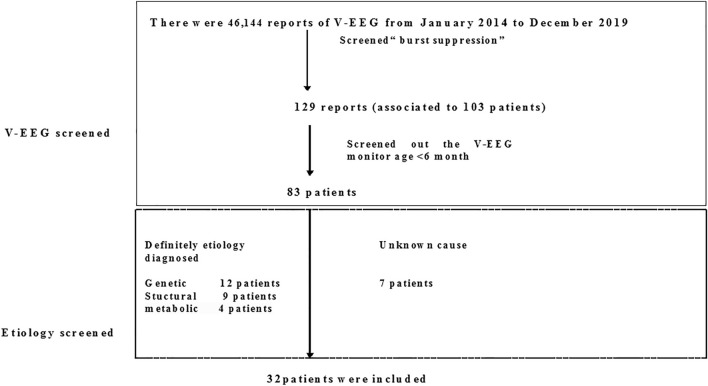
A total of 46,144 video-EEG (VEEG) recordings were screened from January 2014 to December 2019 at the Department of the Pediatrics of Peking University First Hospital. Firstly, a total of 129 EEG recordings were screened out from all the EEG data using the keywords “burst suppression pattern”. Secondly, these EEG recordings were associated with 103 patients (we will select the first EEG recording if one patient has more than one EEG recordings), among whom 83 patients younger than 6 months old at the time of the VEEG monitoring were chosen. Thirdly, patients who were diagnosed with genetic, structural and metabolic etiologies were selected. Patients with unknown causes were also included, provided that they must have completed genetic testing, brain magnetic resonance imaging (MRI) scanning, and metabolic workup with negative results. Finally, a total of 32 patients were included in this study (Fig. 1).
Figure 1.
The flow diagram of patient's screening result.
The following demographic data were analyzed: gender, age at seizure onset, perinatal and personal history, and relevant data. Genetic testing was performed by whole-exome sequencing (WES) or gene panel. For patients who had undergone gene panel test, targeted sequencing was performed on genomic DNA samples from the proband and his/her parents for 535 epilepsy genes (supplementary table 1) by MyGenostics Technology, Inc. (Beijing, China). The etiologies were classified according to the etiological classification guidelines revised by the International League Against Epilepsy (ILAE) in 2017, and the epileptic syndromes were classified according to the classification revised by ILAE in 201012,13.
VEEG monitoring
EEG was recorded using the standard international 10–20 system, with a sampling frequency of 500 Hz (Neurofax; Nihon-Kohden, Tokyo, Japan). A low-cut filter at 1.6 Hz was used before digital sampling. A 4-h VEEG monitoring was performed. All cases must complete 4-h surveillance, including at least one complete wake-sleep cycle. All EEGs were analyzed by one EEG technician and at least two certified epileptologists in order to reach consensus conclusions. According to Andre M, et al.14, asynchrony implied a temporal delay longer than 1.5 to 2 s between the bursts of identical waveforms on both hemispheres, and the amplitude difference exceeding 50% over one hemisphere was considered as an abnormal EEG. We, therefore, defined interhemispheric synchrony as that a temporal delay was no longer than 1.5 to 2 s between the bursts of identical waveforms between hemispheres, and bilateral hemispheric asymmetry as that the amplitude of BS pattern in one hemisphere was 50% higher than the other.
Statistical analysis
For comparing the VEEG monitor age between the synchronous and the asynchronous group, an independent-sample t-test was performed using SPSS 16. The chi-square test was used to analyze the relationship between different patterns and etiologies. Results were considered statistically significant at p < 0.05.
This study was approved by the Ethical Committee of Peking University First Hospital, approval number: 2016-1135. Written informed consent was obtained from the legal guardians (parents) of the patient. All methods were carried out in accordance with relevant guidelines and regulations..
Results
General information
A total of 32 patients (20 boys and 12 girls) were involved in this study. One was born at pre-term (34 weeks) and the remainders were born at term. Six patients had abnormal birth history, including fetal heart rate monitor abnormality in two, premature rupture of membranes and amniotic fluid pollution in one, amniotic fluid pollution in one, malposition of the fetus and anoxia in one, threatened abortion in one. The median age of seizure onset was 3 days (range from 1 to 90 days). The median age of the patients who underwent EEG examination was 58.5 days (range from 19 to 168 days).
The relationship between etiologies and epileptic syndromes or epilepsy (Table 1)
Table 1.
The etiologies of patients.
| Etiology | Gene test | Brain MRI | Metabolism screening | Diagnosed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | Not done | Normal | Cortical malformations | Nonspecific abnormal | Normal | Abnormal | Not done | OS | EME | EIMFS | EP | |
| Genetic (n) | 0 | 12 | 0 | 10 | 0 | 2 | 9 | 0 | 3 | 11 | 0 | 1 | 0 |
| Structural (n) | 1 | 0 | 8 | 0 | 9 | 0 | 6 | 0 | 3 | 8 | 0 | 0 | 1 |
| Metabolic (n) | 0 | 2 | 2 | 0 | 0 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 4 |
| Unknown (n) | 7 | 0 | 0 | 3 | 0 | 4 | 7 | 0 | 0 | 6 | 1 | 0 | 0 |
| % | 25.0% | 43.8% | 31.2% | 40.6% | 28.1% | 31.3% | 68.8% | 12.5% | 18.7% | 78.1% | 3.1% | 3.1% | 15.6% |
OS Ohtahara syndrome, EME Early myoclonic encephalopathy, EIMFS Epilepsy of infancy with migrating focal seizure, EP Epilepsy, MRI Magnetic resonance imaging.
Of the 32 patients, 25 patients were diagnosed as OS, one as EME, and one as EIMFS. Five cases could not be classified into any epileptic syndrome.
Thirty-two patients were divided into the following categories based on their etiologies: 12 patients (12/32, 37.5%) had genetic disorders, including eight patients who underwent WES and four patients who underwent the gene panel. The pathogenic variants were identified in the following genes (Table 2): KCNQ2 in five cases, SCN2A in four, KCNT1 in one, STXBP1 in one, and GNAO1 in one. Two out of the 12 patients had nonspecifically abnormal findings on brain MRI, including the abnormal signal of the patchy center of left hemioval and dysplasia of the corpus callosum. Eleven patients were diagnosed with OS and one with EIMFS (caused by a mutation in KCNT1). Cortical malformations were found in nine patients (9/32, 28.1%), including focal cortical dysplasia in four and hemimegalencephaly in five. In these nine patients, eight were diagnosed with OS; one could not be diagnosed with any epileptic syndrome. Four patients (4/32, 12.5%) had metabolic abnormalities, including methylmalonicacidemia (MMA) in three, and carnitine-acylcarnitine translocase deficiency (CACTD) in one. All these four patients had brain MRI abnormalities, including the ventricular system expansion and hydrocephalus in three MMA patients, and bilateral semioval center, lateral ventricle, an abnormal signal of white matter in one CACTD patient. One out of three patients with MMA was confirmed carrying homozygous mutations in the metabolism of cobalamin-associated C (MMACHC). The CACTD patient was confirmed carrying compound heterozygous mutations in SLC25A20. Seven cases (7 /32, 21.9%) were classified into the group with unknown causes. Four out of them had abnormal brain MRI findings, including a small number of abnormal signals in the posterior ventricle angle in one patient, delayed myelination of the white matter and widened subarachnoid space of the bilateral front temporal lobes in one, corpus callosum thin in one and extra encephalic space widened in one case. In the seven patients, six were diagnosed with OS, one was categorized into EME.
Table 2.
The gene mutation types of patients.
| ID | Epilepsy gene | Suspected or known pathogenic mutation | Inheritance pattern | Parental origin | Inheritance type | Phenotype |
|---|---|---|---|---|---|---|
| 1 | KCNQ2 | c.736G > C | Heterozygous | De novo | AD | OS |
| 2 | KCNQ2 | c.647C > T | Heterozygous | De novo | AD | OS |
| 3 | KCNQ2 | c.740C > T | Heterozygous | De novo | AD | OS |
| 4 | KCNQ2 | c.829A > G | Heterozygous | De novo | AD | OS |
| 5 | KCNQ2 | c.794C > T | Heterozygous | De novo | AD | OS |
| 6 | SCN2A | c.1108 T > C | Heterozygous | De novo | AD | OS |
| 7 | SCN2A | c.655 T > G | Heterozygous | De novo | AD | OS |
| 8 | SCN2A | c.468G > T | Heterozygous | De novo | AD | OS |
| 9 | SCN2A | c.2604 T > A | Heterozygous | De novo | AD | OS |
| 10 | STXBP1 | c.143-144insA | Heterozygous | De novo | AD | OS |
| 11 | GNAO1 | c.810C > A | Heterozygous | De novo | AD | OS |
| 12 | KCNT1 | c.1420C > T | Heterozygous | De novo | AD | EIMFS |
| 13 | SLC25A20 | c.842C > T;c.199-10 T > C | Compound heterozygous | Paternal; Maternal | AR | CACTD |
| 14 | MMACHC | c.609G > A; c.609G > A | Homozygous | Paternal; Maternal | AR | MMA |
OS Ohtahara syndrome, EIMFS Epilepsy of infancy with migrating focal seizure, CACTD Carnitine-acylcarnitine translocase deficiency, MMA Methylmalonic academia, AD Autosomal dominant, AR Autosomal recessive.
The relationship between EEG features and epileptic characteristics (Table 3)
Table 3.
The relationship between EEG features and epileptic characteristics.
| BS pattern | Etiology | Chi-square test | Epileptic syndrome and Epilepsy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Genetic | metabolic | Structural | Unknown | OS | EME | EIMFS | EP | ||
| Synchronous | 3 | 0 | 8 | 3 | P = 0.006 | 12 | 1 | 0 | 1 |
| Asynchronous | 9 | 4 | 1 | 4 | 13 | 0 | 1 | 4 | |
| Symmetrical | 11 | 4 | 1 | 7 | P = 0.000 | 17 | 1 | 1 | 4 |
| Asymmetrical | 1 | 0 | 8 | 0 | 8 | 0 | 0 | 1 | |
BS Burst suppression, OS Ohtahara syndrome, EME Early myoclonic encephalopathy, EIMFS Epilepsy of infancy with migrating focal seizure, EP Epilepsy.
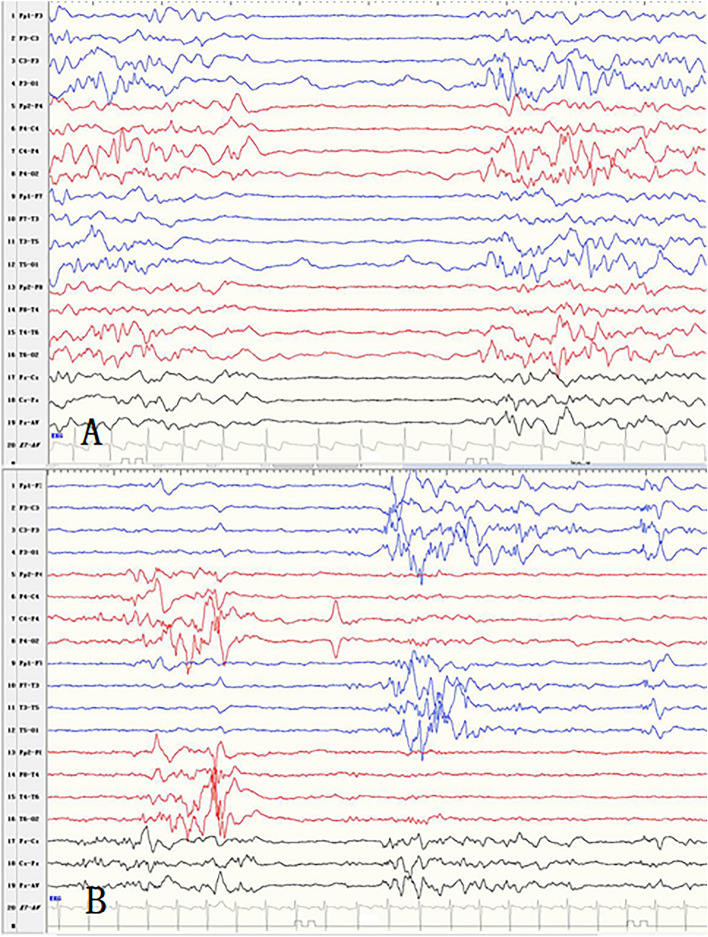
Interhemispheric synchrony in BS pattern was observed in 14 patients, including three cases of genetic epilepsy, eight of structural epilepsy, and three of epilepsy with unknown causes (Fig. 2). Twelve patients were diagnosed with OS, one with EME, and one could not be diagnosed with any epileptic syndrome. Interhemispheric asynchrony in BS pattern was observed in 18 patients, including nine cases of genetic epilepsy, one of structural epilepsy, four of metabolic epilepsy, and four of epilepsy with unknown causes. Of these 18 patients, 13 patients were diagnosed with OS, one with EIMFS, and four patients could not be diagnosed with any epileptic syndrome. The relationship between synchronism and the etiologies was analyzed by the Chi-square test. The results showed significant differences among different etiologies with synchronous and asynchronous BS patterns (p = 0.006). The median ages of EEG detection in the synchronous group and asynchronous group were 58.5 days and 55 days , respectively. The difference was not statistically significant by independent t-test analysis using SPSS (p = 0.40).
Figure 2.
(A) The synchronous and symmetric burst suppression pattern in a patient with genetic disorder. (B) The asynchronous but symmetric burst suppression pattern in a patient with genetic disorder.
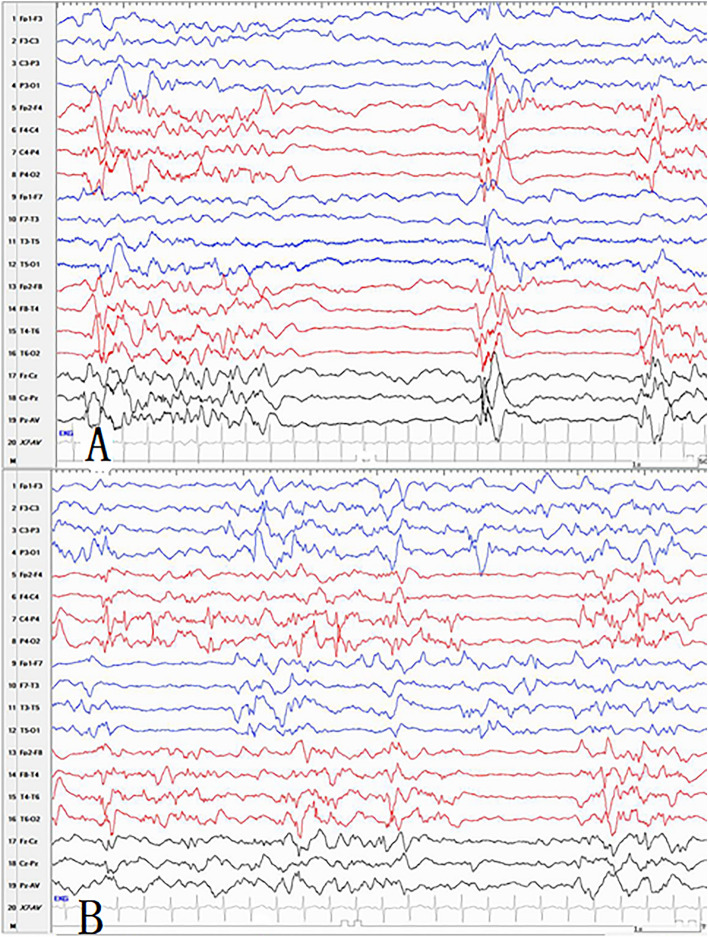
Twenty-three out of the 32 patients (71.9%) had interhemispheric symmetry BS patterns, including 11 patients of genetic epilepsy, one of structural epilepsy, four of metabolic epilepsy, and seven of epilepsy with unknown causes (Fig. 3). In the 23 patients, 17 were diagnosed as OS, one as EME, one as EIMFS, and the remaining four patients could not be diagnosed with any epileptic syndrome. Nine out of all patients (28.1%) showed asymmetry BS pattern, including one patient with genetic epilepsy and eight with structural epilepsy. Further, eight patients were diagnosed as OS, and one patient could not be diagnosed with any epileptic syndrome. There were significant differences between different etiologies with symmetric and asymmetric BS pattern analyzed by the Chi-square test. (p = 0.000).
Figure 3.
(A) The synchronous and asymmetric burst suppression pattern in a patient with structural etiology. (B) The asynchronous but asymmetric burst suppression pattern in a patient with genetic disorder.
Discussion
In the present study, 37.5% of patients had genetic disorders, 28.1% patients had cortical malformations, 12.5% patients had metabolism abnormalities, and 21.9% patients had unknown causes. Previous studies showed that the proportion of genetic etiologies in BS pattern children was 20–30%15–17. Besides 12 patients with genetic disorders, the present study also involved four patients with metabolism abnormalities with definite or possible genetic etiologies. Therefore, the proportion of genetic disorders in our study was about 50% (16/32), which was higher than the previous reports15–17. In the study of Olsen et al.18, they identified pathogenic variants in 61% of patients excluding those with cortical malformations, and the most commonly identified gene was KCNQ2. In our study, after excluding the nine patients with hemimegalencephaly or focal cortical dysplasia, the percentage of patients with genetic disorders was further up to 69.6% (16/23). Consistent with the previous study, KCNQ2 was also the most common gene18.
In the present study, 14 patients had synchronous BS patterns, eight of whom had cortical malformations, suggesting that the BS pattern in patients with structural etiologies tended to be synchronous. Eighteen patients had asynchronous BS patterns, and 13 out of them had genetic disorders (including four patients with metabolic abnormality), which suggested that the BS patterns in patients with genetic etiologies tended to be asynchronous. The statistical analysis also confirmed that there was a significant difference between different etiologies under synchronous and asynchronous BS patterns. Twenty-three patients had symmetric BS patterns, of whom 15 patients had genetic disorders (including four patients with metabolic abnormality), indicating that the BS pattern in patients with genetic etiologies tended to be symmetric. Asymmetric BS pattern predominantly appeared in the malformed hemisphere in hemimegalencephaly, suggesting that the brain lesion extended diffusely from the subcortical layer to cortex19,20. In our cohort, nine patients had asymmetric BS patterns, and 88.9% of patients had cortical malformations. In the asymmetric BS pattern, the location of prominent discharges was in accordant with the cortical malformations side, which was consistent with the previous reports19,20. The statistical analysis also confirmed that there was a significant difference between different etiologies under symmetric and asymmetric BS patterns.
OS was usually caused by obvious static brain lesions, such as brain malformations even in the early stages7,19,21. The BS pattern in OS was reported typically and bilaterally synchronous between hemispheres10, but might show some asymmetry7. Recently, many genes have been found in patients with OS, such as KCNQ2, SCN2A, STXBP1, and so on22. In the present study, 44% of OS patients had genetic abnormalities, while 32% of OS patients had brain MRI abnormalities. For the 25 OS patients, the synchronization and symmetry analysis showed that it presented with asynchronous and symmetrical BS pattern in 48% of patients, synchronous and asymmetrical BS pattern in 28%, synchronous and symmetrical BS pattern in 20%, and asynchronous and asymmetrical BS pattern in 4%. Therefore, patients with OS could have different forms of BS pattern, which might be related to the different etiologies with prominent genetic etiology.
Previous literature showed that EME was most often caused by inherited metabolic disorders, such as nonketotic hyperglycinemia, organic acidemias, or mitochondrial cytopathies23. The BS pattern often showed some asynchrony, irregular or longer burst-to-burst intervals, or more multifocal spikes in the suppression phase2. As for the present study, the etiology of one case with EME was unknown. The BS pattern of this patient was synchronous and symmetrical, which was different from the previous report2. Thus, the BS pattern of EME should also be considered to be closely related to different etiologies.
Genetic causes were found in 69% of patients with EIMFS, and the most commonly involved gene was KCNT124. BS was divided into two patterns in patients with EIMFS, one was an early-onset asynchronous pattern, and the other was a late-onset synchronous pattern6. In our study, six patients were diagnosed with EIMFS, of whom four patients had KCNT1 mutations, two patients did not undergo genetic tests6. In the four patients with KCNT1 mutation, BS in one patient belonged to the early-onset pattern, and BS in three patients belonged to the late-onset pattern6. The age at EEG recording of the early-onset pattern was within one month after birth, and the asynchronous BS pattern might be associated with premature brain development6, because the degree of synchrony might approach 100% in normal-term neonates1. Inconsistent with previous studies, our patient with EIMFS caused by KCNT1 mutation had an asynchronous BS pattern, and the age at EEG recording was 2 months. In order to observe the influence of the developing brain maturity on the BS pattern, the EEG examination age of the synchronous group and asynchronous group was compared, and there was no significant difference in the EEG examination age between the two groups. Hence, the premature EEG could not fully explain the asynchronous BS pattern, and the etiologies might also play a role.
Corpus callosum immature or dysfunction was reported to be associated with the asynchronous and symmetry of BS pattern8,25. The association of asynchronous BS with Aicardi's syndrome supported the role of the corpus callosum in interhemispheric synchrony26. Two out of our patients with asynchronous BS patterns had corpus callosum abnormal, which was consistent with the previous report26. However, another literature reported that children with normal corpus callosum also had asynchrony EEG pattern5. The persistence of bilaterally synchronous interictal EEG discharges following partial or complete corpus callosum section suggested that corpus callosum was not the sole pathway for hemisphere synchronization27. Experimental evidence suggested that a pathway between ipsilateral and contralateral cortex relaying via thalamic and mesencephalic subcortical structures might contribute to secondary bilateral synchrony. Abnormal thalamic loop or abnormal synaptic development was associated with bilateral hemispheric asynchrony as well27,28. Indeed, 16 out of our patients with asynchronous BS patterns had normal corpus callosum, and of whom 11 patients had genetic disorders. Therefore, the asynchronous BS pattern might be associated with genetic etiologies.
Conclusion
As this was a retrospective study and the patients were retrieved from the EEG database, we could only analyze the limited and available EEG data. BS pattern could be observed in a variety of epilepsy syndromes. The most common epileptic syndrome was OS in our study. Genetic etiology was commonly identified. Asynchronous and symmetrical BS patterns were mostly observed in patients with genetic etiologies. Whereas, synchronized but asymmetrical BS patterns were found in patients with structural etiologies. Our findings suggested that characteristics of the BS patterns could help to predict the etiology of the patient with epilepsy.
Supplementary Information
Acknowledgements
This work was financed by National Nature Science Foundation of China (81771393), Beijing Municipal Science & Technology Commission (Z171100001017125), Beijing Natural Science Foundation (7202210), and Capital’s Funds for Health Improvement and Research (2020-2-4077).
Author contributions
Haipo Yang and Zhixian Yang designed the study, drafted the initial manuscript, and revised the manuscript; Pan Gong, Xianru Jiao, Qiujun Zhou, Yuehua Zhang and Yuwu Jiang helped to collect and summarize data and revised the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95040-4.
References
- 1.Tsuchida TN, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: Report of the American Clinical Neurophysiology Society critical care monitoring committee. J. Clin. Neurophysiol. 2013;30:161–173. doi: 10.1097/WNP.0b013e3182872b24. [DOI] [PubMed] [Google Scholar]
- 2.Ohtahara S, Yamatogi Y. Epileptic encephalopathies in early infancy with suppression-burst. J. Clin. Neurophysiol. 2003;20:398–407. doi: 10.1097/00004691-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Murakami N, Ohtsuka Y, Ohtahara S. Early infantile epileptic syndromes with suppression-bursts: Early myoclonic encephalopathy vs ohtahara syndrome. Jpn. J. Psychiatry Neurol. 1993;47:197–200. doi: 10.1111/j.1440-1819.1993.tb02050.x. [DOI] [PubMed] [Google Scholar]
- 4.Pavone P, et al. Benign and severe early-life seizures: a round in the first year of life. Ital. J. Pediatr. 2018;44:54. doi: 10.1186/s13052-018-0491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selioutski O, Seltzer LE, Burchfiel J, Paciorkowski AR, Erba G. Characteristic features of the interictal EEG background in 2 patients with malignant migrating partial epilepsy in infancy. J. Clin. Neurophysiol. 2015;32:23–29. doi: 10.1097/WNP.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshitomi S, et al. Different types of suppression-burst patterns in patients with epilepsy of infancy with migrating focal seizures (EIMFS) Seizure. 2019;65:118–123. doi: 10.1016/j.seizure.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Yamatogi Y, Ohtahara S. Early-infantile epileptic encephalopathy with suppression-bursts, Ohtahara syndrome; its overview referring to our 16 cases. Brain Dev. 2002;24:13–23. doi: 10.1016/S0387-7604(01)00392-8. [DOI] [PubMed] [Google Scholar]
- 8.Lambrakis CC, Lancman ME, Romano C. Asynchronous and asymmetric burst-suppression in a patient with a corpus callosum lesion. Clin. Neurophysiol. 1999;110:103–105. doi: 10.1016/S0013-4694(98)00102-3. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsuka Y, Ohno S, Oka E. Electroclinical characteristics of hemimegalencephaly. Pediatr. Neurol. 1999;20:390–393. doi: 10.1016/S0887-8994(98)00165-9. [DOI] [PubMed] [Google Scholar]
- 10.Hmaimess G, et al. Impact of early hemispherotomy in a case of Ohtahara syndrome with left parieto-occipital megalencephaly. Seizure. 2005;14:439–442. doi: 10.1016/j.seizure.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Panayiotopoulos, C. P. Atlas of epilepsies. Springer, London. (Panayiotopoulos, 2010).
- 12.Scheffer IE, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg AT, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 14.Andre M, et al. Electroencephalography in premature and full-term infants. Developmental features and glossary. Neurophysiol. Clin. 2010;40:59–124. doi: 10.1016/j.neucli.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Allen NM, et al. Unexplained early onset epileptic encephalopathy: Exome screening and phenotype expansion. Epilepsia. 2016;57:12–17. doi: 10.1111/epi.13250. [DOI] [PubMed] [Google Scholar]
- 16.Veeramah KR, et al. Exome sequencing reveals new causal mutations in children with epileptic encephalopathies. Epilepsia. 2013;54:1270–1281. doi: 10.1111/epi.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epi4K Consortium et al. De novo mutations in epileptic encephalopathies. Nature. 501, 217–221 (2013). [DOI] [PMC free article] [PubMed]
- 18.Olson HE, et al. Genetics and genotype-phenotype correlations in early onset epileptic encephalopathy with burst suppression. Ann. Neurol. 2017;81:419–429. doi: 10.1002/ana.24883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtsuka Y, Sato M, Sanada S, Yoshinaga H, Oka E. Suppression-burst patterns in intractable epilepsy with focal cortical dysplasia. Brain Dev. 2000;22:135–138. doi: 10.1016/S0387-7604(00)00090-5. [DOI] [PubMed] [Google Scholar]
- 20.Steriade M, Amzica F, Contreras D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr. Clin. Neurophysiol. 1994;90:1–16. doi: 10.1016/0013-4694(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 21.Fusco L, Pachatz C, Capua DM, Vigevano F. Video/EEG aspects of early-infantile epileptic encephalopathy with suppression-bursts (Ohtahara syndrome) Brain Dev. 2001;23:708–714. doi: 10.1016/S0387-7604(01)00280-7. [DOI] [PubMed] [Google Scholar]
- 22.Cornet MC, Cilio MR. Genetics of neonatal-onset epilepsies. Handb. Clin. Neurol. 2019;162:415–433. doi: 10.1016/B978-0-444-64029-1.00020-5. [DOI] [PubMed] [Google Scholar]
- 23.Beal JC, Cherian K, Moshe SL. Early-onset epileptic encephalopathies: Ohtahara syndrome and early myoclonic encephalopathy. Pediatr. Neurol. 2012;47:317–323. doi: 10.1016/j.pediatrneurol.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Burgess R, et al. The genetic landscape of epilepsy of infancy with migrating focal seizures. Ann. Neurol. 2019;86:821–831. doi: 10.1002/ana.25619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazar LM, Milrod LM, Solomon GE, Labar DR. Asynchronous pentobarbital-induced burst suppression with corpus callosum hemorrhage. Clin. Neurophysiol. 1999;110:1036–1040. doi: 10.1016/S1388-2457(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 26.Fariello RG, Chun RW, Doro JM, Buncic JR, Prichard JS. EEG recognition of Aicardi's syndrome. Arch Neurol. 1977;34:563–566. doi: 10.1001/archneur.1977.00500210065012. [DOI] [PubMed] [Google Scholar]
- 27.Spencer SS, Spencer DD, Williamson PD, Mattson RH. Effects of corpus callosum section on secondary bilaterally synchronous interictal EEG discharges. Neurology. 1985;35:1689–1694. doi: 10.1212/WNL.35.12.1689. [DOI] [PubMed] [Google Scholar]
- 28.Silvestri-Hobson, R. C. & Benbadis, S. R. EMEDICINE. Abnormal neonatal EEG. http://emedicine.medscape.com/article/1139692-overview#showall (2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.