Abstract
According to WHO, 2019 witnessed 229 million cases of malaria globally, of which Africa accounted for 94% of cases. Early diagnosis and treatment are the basis of malaria management, and the need for good chemoprophylaxis especially for people travelling to endemic areas is vital. There are a number of drug options available for the prophylaxis of malaria, mefloquine being one of the drugs used. Mefloquine has been around from the 1970s, and was developed in the United States keeping in mind the soldiers that were being deployed to areas where chloroquine resistant strains of Plasmodium were discovered. Mefloquine was preferred for its once a week dosage. Within a decade of its introduction, reports of the side effects associated with its long-term use surfaced. Mefloquine is now reported to cause a myriad of neuropsychiatric side effects including anxiety, sleep disturbance, depression, dizziness and frank psychosis, especially in patients with pre-existing psychiatric disorders. Many countries like the United States and the United Kingdom have updated their drug boxes to include the warning of these potential neuropsychiatric effects. This paper reviews the side effects of mefloquine and why there is a need to revisit its use in Indian drug policy.
Keywords: Malaria, Mefloquine, Neuropsychiatric side effects
Graphical abstract
Malaria continues to be a public health problem in India. Estimates by World Health Organization in 2019 (WHO) reported 229 million cases in comparison to 251 million in 2010 and 231 million in 2017 (World Malaria Report 2020). The African Region shouldered the largest burden with estimated 213 million cases (94%) whereas the South-East Asia Region reported 3% and the rest of the world accounted for 3% (World Malaria Report 2020). Almost 95% of all malaria cases globally in 2019 were in 29 countries, of which India reported the largest absolute reductions in cases over a decade i.e., 20 million cases in 2000 to 5.6 million in 2019 (World Malaria Report 2020).
Early diagnosis and timely treatment are the mainstay of malaria management and the chemoprevention of malaria is an important aspect. The need of chemoprophylaxis arises when people travel to malarious areas and desire protection against this potentially life-threatening infection, more so in susceptible populations. People travel for various reasons and travel has exponentially increased in recent times. Hence antimalarial prophylaxis is needed. International bodies like the World Health Organization (WHO: International Travel and Health, Chapter 7) and the Centre of Disease Control and Prevention (CDC Yellow Book 2020) recommend atovaquone–proguanil, doxycycline, chloroquine, mefloquine or tafenoquine (details listed in Table 1) as prophylactics for both short and long-term travelers. The drug list remains the same but special attention has to be paid to side effects if drug is taken for more than 6 months (WHO: International Travel and Health, Chapter 7).
Table 1.
Available drugs for malaria chemoprophylaxis for a non-immune traveler.
| Drug | Frequency | When to start before travel | When to stop after return | Contraindications |
|---|---|---|---|---|
| Atovaquone Proguanil | Daily | 1–2 days | 1 week (Unless any dose is missed during travel, 4 weeks) |
|
| Chloroquine | Weekly | 1–2 weeks | 4 weeks |
|
| Doxycycline | Daily | 1–2 days | 4 weeks |
|
| Mefloquine | Weekly | >2 weeks | 4 weeks |
|
| Primaquine | Daily | 1–2 days | 1 week |
|
Malaria prevention in armed forces, especially those deployed to endemic areas and forest areas (Ranjha and Sharma, 2021) is especially crucial. According to CDC guidelines (CDC Yellow Book 2020) in military population of USA, atovaquone-proguanil is the choice of prophylaxis for a short- and long-term deployments in high-transmission geographical areas. Those who are unable to take atovaquone-proguanil due to intolerance or contraindication, the second line prophylactic is doxycycline, followed by mefloquine. Prior to prescribing mefloquine for prophylaxis, absolute and relative contraindications are taken into consideration (HA Policy 2015). On the other hand, as per National Drug Policy on Malaria (2013) of India chemoprophylaxis is recommended in specific groups in P. falciparum malaria endemic areas. For short term travelers with stay of less than 6 weeks, daily dose of 100 mg of doxycycline is the drug of choice in adults, starting 2 days before travel to 4 weeks after departure. For a duration of more than 6 weeks, mefloquine chemoprophylaxis should be administered weekly, starting 2 weeks before travel to 4 weeks after departure (National Drug Policy on Malaria (2013) of India).
Like other drugs, antimalarial drugs also are contraindicated in certain health conditions. Most of the side effects experienced with chemoprophylactic anti-malarial drugs are minor. However, there can be serious adverse events, which can be life threatening, requiring hospitalization or prolonging it, and/or resulting in significant incapacity. These adverse effects are usually identified in post-marketing surveillance. In people experiencing serious adverse effects, immediate withdrawal of the drug and medical attention is recommended. There are a number of drugs available for chemoprophylaxis of malaria one of which is mefloquine. In this paper, we focus on prophylaxis by mefloquine, its related side effects and an appropriate alternative.
In the 1970s, the development of mefloquine was initiated by Walter Reed Army Institute of Research (WRAIR), USA, owing to emergence of chloroquine resistance in P.falciparum malaria in Southeast Asia (WHO and F. Hoffmann-La Roche, 1991). The drug was tested in clinical trials on prisoners and soldiers, and people in developing countries. In an extensive review by WHO, in late 1980s and early 1990s, after the licensing and introduction of mefloquine, it became extensively used for chemoprophylaxis. Over 20 million people consumed mefloquine as it was preferred for its weekly-single dose (WHO and F. Hoffmann-La Roche, 1991). Despite the lack of clinical safety and tolerability phase III data in civilian volunteers, initial license was granted (Croft, 2007) and numerous trials have been conducted since then.
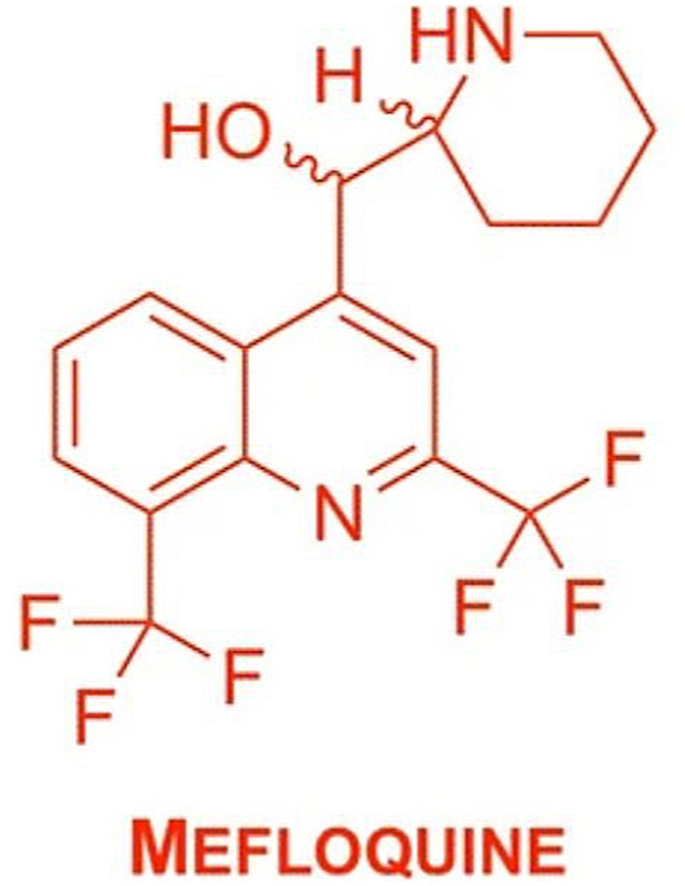
Mefloquine, [2,8-bis(trifluoromethyl)quinolin-4-yl]-piperidin-2-ylmethanol is a 4-quinolinemethanol antimalarial and antiparasitic which acts as a blood schizonticide, structurally related to quinine. Since its development it is indicated for both prophylaxis and treatment of malaria, despite the inadequately understood mechanism of action (Taylor and White, 2004). A long half-life of 13–30 days provides an edge over other prophylactics as it can be used in a once-a-week format in lower doses (WHO and F. Hoffmann-La Roche, 1991).
1. Adverse effects of mefloquine
Gastrointestinal adverse effects like vomiting, nausea, anorexia, abdominal pain and diarrhea were noted. Neurological disorders such as balance disorder, convulsions, encephalopathy, headache, hearing impairment, memory impairment, neuropathy (including paraesthesia, tremor, and ataxia), syncope, tremors and vestibular disorders were also experienced. Other side effects like fatigue, fever, muscle pain, palpitations, rashes and weakness were also noted (Toovey, 2009; Lee et al., 2017).
Nearly a decade after mefloquine's use, neurotoxicity was reported in a French paper in 1987 (Bernard et al., 1987) and subsequently, after drawing attention of the media over this, it was further evaluated by the WHO in 1991. This report concluded that at doses of 500 mg and above, adverse effects have been seen with mefloquine intake but the data collected was not enough to change the treatment guideline pertaining to mefloquine (WHO and F. Hoffmann-La Roche, 1991).
In 2006, direct evidence was reported that mefloquine is neurotoxic at dosages used for malaria treatment, and animal models showed permanent brainstem lesions. Subsequent studies showed neurotoxicity in animal and human neurons (Mccarthy, 2015). Cognitive impairment similar to neurotoxic brainstem lesions were observed. Mefloquine neurotoxicity has been described as chronic sequelae of nervous system toxicity syndrome and possibly permanent neuronal degeneration of the brainstem (Mccarthy, 2015) and neurotoxic vestibulopathy (Nevin, 2014). The product information also mentions that if any sign such as acute anxiety, depression, confusion or restlessness occur, it is to be considered prodromal to a more serious event. In such a situation the drug should be discontinued (Nevin 2014).
2. Possible mechanisms of neurotoxicity
The gastrointestinal side effects of mefloquine are known to be caused by pancreatic β-cell type-KATP channel Kir6.2/SUR1 inhibition. The mechanism behind these neurological and psychiatric effects is not completely known but the mechanisms implicated include: cholinesterases inhibition, non-receptor tyrosine kinase 2 (Pyk2 and/or interaction with adenosine A (2A) receptors (Lee et al., 2017). Some studies have also shown mefloquine to cause GABAergic interneuron dysfunction, inhibition of cellular transport and depression of cortical activity (Martins et al., 2021).
Studies have focused on the role of mefloquine in causing hallucinations, nightmares and a flare up of symptoms of Post-Traumatic Stress Disorder (PTSD) (Janowsky et al., 2014). The detrimental effects mefloquine can produce, have the potential to continue even after drug is stopped leading to long-term neurotoxic effects (Quinn, 2015). Experiences in Armed Forces and civilian populations in different countries (summarized in Table 2) have highlighted the significance of taking mefloquine's adverse effects in cognizance.
Table 2.
Results of studies pertaining to mefloquine prophylaxis safety.
| Year | Study | Country | Participants | Result: % of participants suffering adverse effects | Result: % of participants suffering neuropsychiatric adverse effects |
|---|---|---|---|---|---|
| 1993 | Boudreau et al. | US | 203 | 43% | Not determined |
| 1996 | Phillips and Kass | Australia | 285 | 51.2% | 6.3% |
| 1996 | Buma et al. | Netherlands | 2289 | 22.8% | Not determined |
| 1996 | Schlagenhauf et al. | Switzerland | 420 | 11.2% | 7.9% |
| 1996 | Croft and World | UK | 317 | 29% | Not determined |
| 1999 | Peragallo et al. | Italy | 1386 | 17% | Not determined |
| 2001 | Overbosch et al. | Netherland, Germany, UK, Canada and SA | 483 | 42% | 29% |
| 2005 | Kitchener et al. | Australia | 1157 | 57% | 29% |
| 2005 | Vaillancourt et al. | Canada | 1413 | 74.7% | Not determined |
| 2007 | Fujii et al. | Japan | 1876 | 24.4% | 18.2% |
| 2008 | Andersson et al. | Sweden | 488 | 57% | 21.7% |
| 2010 | Nasveld et al. | Australia | 162 | 11.7% | 5% |
| 2014 | Ringqvist et al. | Denmark | 73 | Not determined | 55% |
| 2014 | Peragallo et al. | Italy | 4123 | 21.2% | Not determined |
| 2016 | Ministry of defence | UK | 116704 | Not determined | 10% |
| 2017 | Eick-Cost et al. | US | 367840 | Not determined | 10.8% |
On the 29th of July 2013, the FDA issued a Drug Safety Communication, reinforcing the updated warnings regarding these side effects of mefloquine and adding a black boxed warning to the drug label. The medication guide has also been revised by the FDA to include these side effects and that these may be persistent or become permanent. Since the FDA warning, drug regulatory agencies in various countries, including the UK, Ireland (Nevin and Byrd, 2016), and Canada (Nevin, 2017) have made it mandatory to add a warnings update in their mefloquine drug labels.
3. The Indian context
National Drug Policy on Malaria (2013) of India entails mefloquine use as chemoprophylaxis when stay in a malarious region is for more than 6 weeks. This policy has implications for the general public but especially so for armed forces personnel who are posted in malarious areas where they are exposed to risks of contracting malaria. Generally, chemoprophylaxis for malaria is prescribed only in certain groups and in areas with high prevalence of P. falciparum. In addition to vector control products, for longer stays chemoprophylaxis is required. Armed personnel who are posted in malaria endemic regions for long-term are at high risk of infection and as per guidelines mefloquine is recommended for periods above 6 weeks. However, to our knowledge there are no reports pertaining to neuropsychiatric side effects till date in context of Indian troops, this may be due to lack of systematic studies. Further, mefloquine is still used in India in civilian populations as well albeit with warnings.
4. Conclusions
After years of use in international military, many of the risks of using mefloquine have been recognized and now more informed policies are being formulated in many countries. The change in policies is based on evidence generated by scientific studies and clinical experience of adverse side effects especially those of neuropsychiatric nature. Mefloquine still remains indicated for malaria prophylaxis for long-term use. Chemoprophylaxis of malaria can be achieved, especially in chloroquine-resistant P. falciparum regions by other drugs available like doxycycline and atovaquone/proguanil as both are well tolerated for longer prophylactic use. India has been considering tafenoquine for P. vivax treatment (Ahmad SS et al., 2021) and similarly there is a need in India to invest in research and evaluation of safer alternative drugs for chemoprophylaxis for various groups that have a high malaria burden like pregnant women (Pandey et al., 2021). In light of the scientific literature now available on the adverse effects of mefloquine there is a need to revisit the Indian national guidelines and consider adoption of alternative drugs.
Authors contributions
AS conceived the idea, VR and SSA did extensive literature reviews. SSA and MR drafted the manuscript. MR made important additions. All authors wrote the paper. All authors read and approved the final manuscript.
Disclaimers
The views expressed in the submitted article are authors’ and not an official position of the institution.
Sources of support
None.
Funding
None.
Data sharing
No additional data.
Ethical approval
Not required.
Declaration of competing interest
None of the authors have any conflicts of interest to declare.
Acknowledgments
We thank Department of Science and Technology (DST) for the JC Bose fellowship to AS.
References
- Ahmad S.S., Rahi M., Sharma A. Relapses of Plasmodium vivax malaria threaten disease elimination: time to deploy tafenoquine in India? BMJ Global Health. 2021;6(2) doi: 10.1136/bmjgh-2020-004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson H., Askling H.H., Falck B. Well-Tolerated chemoprophylaxis uniformly prevented Swedish soldiers from Plasmodium falciparum malaria in Liberia, 2004-2006. Mil. Med. 2008;173:1194–1198. doi: 10.7205/milmed.173.12.1194. [DOI] [PubMed] [Google Scholar]
- Bernard J., Le C.J., Sarrouy J., Renaudineau J., Geffray L., Dufour P. Toxic encephalopathy caused by mefloquine? Presse Med. 1987;16(33):1654e5. [PubMed] [Google Scholar]
- Boudreau E., Schuster B., Sanchez J. Tolerability of prophylactic Lariam regimens. Trop. Med. Parasitol. 1993;44(3):257–265. [PubMed] [Google Scholar]
- Buma A.P.H., van Thiel P.P., Lobel H.O. Long-term malaria chemoprophylaxis with mefloquine in Dutch marines in Cambodia. J. Infect. Dis. 1996;173(6):1506–1509. doi: 10.1093/infdis/173.6.1506. [DOI] [PubMed] [Google Scholar]
- Croft A.M. A lesson learnt: the rise and fall of Lariam and Halfan. J. R. Soc. Med. 2007;1000(4):170–174. doi: 10.1258/jrsm.100.4.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft A.M., World M.J. Neuropsychiatric reactions with mefloquine chemoprophylaxis. Lancet. 1996;347:326. doi: 10.1016/s0140-6736(96)90500-0. [DOI] [PubMed] [Google Scholar]
- Eick-Cost A.A., Hu Z., Rohrbeck P. Neuropsychiatric outcomes after mefloquine exposure among U.S. military service members. Am. J. Trop. Med. Hyg. 2017;96:159–166. doi: 10.4269/ajtmh.16-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Kaku K., Jelinek T., Kimura M. Malaria and mefloquine prophylaxis use among Japan ground self-defense force personnel deployed in East Timor. J. Trav. Med. 2007;14(4):226–232. doi: 10.1111/j.1708-8305.2007.00122.x. [DOI] [PubMed] [Google Scholar]
- International Travel and Health- Chapter 7, Malaria. Available from: https://www.who.int/ith/2017-ith-chapter7.pdf. Accessed on December 20, 2020.
- Janowsky A. Mefloquine and psychotomimetics share neurotransmitter receptor and transporter interactions in vitro. Psychopharmacology. 2014;231(14):2771–2783. doi: 10.1007/s00213-014-3446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchener S.J., Nasveld P.E., Gregory R.M., Edstein M.D. Mefloquine and doxycycline malaria prophylaxis in Australian soldiers in East Timor. Med. J. Aust. 2005;182(4):168–171. doi: 10.5694/j.1326-5377.2005.tb06647.x. [DOI] [PubMed] [Google Scholar]
- Lee S.J., ter Kuile F.O., Price R.N., Luxemburger C., Nosten F. Adverse effects of mefloquine for the treatment of uncomplicated malaria in Thailand: a pooled analysis of 19, 850 individual patients. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0168780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins A.C. Review of the mechanism underlying mefloquine-induced neurotoxicity. Crit. Rev. Toxicol. 2021 Mar;51(3):209–216. doi: 10.1080/10408444.2021.1901258. [DOI] [PubMed] [Google Scholar]
- Mccarthy, S. Malaria prevention, mefloquine neurotoxicity, neuropsychiatric illness, and risk-benefit analysis in the Australian defence force. J. Parasitol. Res. 2015:1-23. Doi:10.1155/2015/287651. M. A. Phillips and R. B. Kass.User acceptability patterns for mefloquine and doxycycline malaria chemoprophylaxis.Journal of Travel Medicine.1996;3(1):40–45. [DOI] [PubMed]
- Ministry of Defence . 2016. Uk Armed Forces Prescribed Mefloquine Hydrochloride and Subsequent Presentation to Mod Specialist Mental Health Services,1 April 2007 – 30 September 2015.https://www.gov.uk/government/statistics/modnational- and- official- statistics- by- topic Available from: [Google Scholar]
- Nasveld P.E., Edstein M.D., Reid M. Randomized,double-blind study of the safety, tolerability, and efficacy of tafenoquine versus mefloquine for malaria prophylaxis in nonimmune subjects. Antimicrob. Agents Chemother. 2010;54(2):792–798. doi: 10.1128/AAC.00354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Drug Policy on Malaria 2013. https://nvbdcp.gov.in/index.php Available from:
- Nevin R.L., Byrd A.M. Neuropsychiatric adverse reactions to mefloquine: a systematic comparison of prescribing and patient safety guidance in the US, UK, Ireland, Australia, New Zealand, and Canada. Neurol Ther. 2016;5(1):69–83. doi: 10.1007/s40120-016-0045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin R.L. Implications of changes to the mefloquine product monograph. Can. J. Hosp. Pharm. 2017;70(4):323–324. doi: 10.4212/cjhp.v70i4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin R.L. Vestibular Disorders Association; Portland, Ore, USA: 2014. Neurotoxic Vestibulopathy: Antimalarial Drugs that Can Cause Vestibular Dysfunction.http://vestibular.org/sites/default/files/pagefiles/Documents/Mefloquine Neurotoxicity.pdf Available from: [Google Scholar]
- Overbosch D., Schilthuis H., Bienzle U. Atovaquone/proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clin. Infect. Dis. 2001; Oct 1;33(7):1015–1021. doi: 10.1086/322694. [DOI] [PubMed] [Google Scholar]
- Pandey M., Rahi M., Sharma A. The Indian burden of malaria in pregnancy needs assessment. Cell Press Med. 2021;2(5):464–469. doi: 10.1016/j.medj.2021.04.017. [DOI] [PubMed] [Google Scholar]
- Peragallo M.S., Sabatinelli G., Sarnicola G. Compliance and tolerability of mefloquine and chloroquine plus proguanil for long-term malaria chemoprophylaxis in groups at particular risk (the military) Trans. R. Soc. Trop. Med. Hyg. 1999;93(1):73–77. doi: 10.1016/s0035-9203(99)90187-6. [DOI] [PubMed] [Google Scholar]
- Peragallo M.S., Sarnicola G., Boccolini D. Risk assessment and prevention of malaria among Italian troops in Afghanistan, 2002 to 2011. J. Trav. Med. 2014;21:24–32. doi: 10.1111/jtm.12046. [DOI] [PubMed] [Google Scholar]
- Phillips M.A., Kass R.B. User acceptability patterns for mefloquine and doxycycline malaria chemoprophylaxis. J. Trav. Med. 1996;3(1):40–45. doi: 10.1111/j.1708-8305.1996.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Quinn J.C. Complex membrane channel blockade: a unifying hypothesis for the prodromal and acute neuropsychiatric sequelae resulting from exposure to the antimalarial drug mefloquine. J Parasitol Res. 2015;368064(10):20. doi: 10.1155/2015/368064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjha R., Sharma A. Forest malaria: the prevailing obstacle for malaria control and elimination in India. BMJ Global Health. 2021;6(5) doi: 10.1136/bmjgh-2021-005391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringqvist A., Bech P., Glenthøj B., Petersen E. Acute and long-term psychiatric side effects of mefloquine: a follow-up on Danish adverse event reports. Trav. Med. Infect. Dis. 2014;13(1):80–88. doi: 10.1016/j.tmaid.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf P., Steffen R., Lobel H. Mefloquine tolerability during chemoprophylaxis: focus on adverse event assessments, stereochemistry and compliance. Trop. Med. Int. Health. 1996;1(4):485–494. doi: 10.1046/j.1365-3156.1996.d01-85.x. [DOI] [PubMed] [Google Scholar]
- Taylor W.R., White N.J. Antimalarial drug toxicity: a review. Drug Saf. 2004;27(1):25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- Toovey S. Mefloquine neurotoxicity: a literature review. Trav. Med. Infect. Dis. 2009;7(1):2–6. doi: 10.1016/j.tmaid.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Travel- Related Infectious Diseases- Chapter 4. Yellow Book 2020. CDC. Available from: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/malaria. Accessed on December 20, 2020.
- Vaillancourt R., Ma J., Sampalis J. Assessment of risks associated with short-term use of mefloquine in Canadian forces members: a descriptive cross-sectional study. Can. Pharm. J. 2005;138(7):42. [Google Scholar]
- World Malaria Report 2020 https://www.who.int/publications/i/item/9789240015791 Geneva. Available from:
- World Health Organization. F. Hoffmann-La Roche . World Health Organization; Geneva, Switzerland: 1991. Review of the Central Nervous System Adverse Events Related to the Anti-malarial Drug, Mefloquine (1985–1990)http://apps.who.int/iris/bitstream/10665/61327/1/WHO MAL 91.1063.pdf Tech. Rep. WHO/MAL/91.1063. Available from: [Google Scholar]


