Abstract
Introduction
Pembrolizumab, an immune checkpoint inhibitor (ICI), has become an integral part of front‐line treatment of metastatic non‐small cell lung cancer (NSCLC). However, pivotal trials had significant underrepresentation of Black patients (pts). Lack of sufficient evidence regarding safety and efficacy of ICIs among minority racial groups poses a challenge in delivery of optimal cancer directed care.
Methods
We retrospectively reviewed pts with stage IV NSCLC treated with first‐line pembrolizumab across three MedStar facilities between January 1, 2014, and May 3, 2019. Progression‐free survival (PFS) and overall survival (OS) were primary endpoints and were calculated using the Kaplan‐Meier method. Immune‐related adverse events (irAEs) were assessed according to Common Terminology Criteria for Adverse Events Version 5.0 (CTCAE v5.0).
Results
In total, 136 pts were identified, with 74 (54.4%) White, 53 (39%) Black, 2 (1.5%) Asian, and 7 (5.1%) other racial groups. Median age was 70 years in White pts and 65 years in Black pts (p < .01). There was no difference in median PFS (5.7 vs. 5.9 months; p = .651) or OS (11.8 vs. 12.4 months; p = .949) between White and Black pts. In the subset of patients whose tumors had high programmed death‐ligand 1 (PD‐L1) expression (≥50%), there was still no difference in efficacy by race. Median PFS (8.7 vs. 3.9 months; p = .843) and OS (14.7 vs. 11.3 months; p = .581) in White versus Black pts were not different. Incidence of irAEs in White versus Black pts was 24.3% and 22.6%, respectively (p = .83).
Conclusion
We found no major differences in either safety or efficacy of first‐line pembrolizumab between White and Black pts. Use of first‐line pembrolizumab‐based treatment in Black pts with stage IV NSCLC is safe and efficacious, based on these real‐world data.
Implications for Practice
Immunotherapy has revolutionized treatment of solid and hematological malignancies. There are certain populations of patients underrepresented in the original trials including minority racial groups, patients with autoimmune diseases, and those with chronic viral illnesses. Our study focuses on Black patients with metastatic lung cancer who received pembrolizumab and concludes similar safety and response to treatment when compared with White patients. Black patients are an important demographic group in clinical practice often facing systemic health care disparities. This study paves a path for future studies in underrepresented populations receiving immunotherapy across various malignancies.
Keywords: NSCLC, Pembrolizumab, Race, Survival, Safety
Short abstract
This article assesses differences in clinical outcomes across racially diverse patients with metastatic non‐small cell lung cancer who received treatment with a pembrolizumab‐based regimen in the front‐line setting.
Introduction
Lung cancer is the leading cause of cancer related mortality in the U.S., with a dismal 5‐year survival rate of only 6% in patients with metastatic non‐small cell lung cancer (NSCLC) [1]. The advent of immune checkpoint inhibitor (ICI) therapy has changed the treatment landscape for front‐line treatment of metastatic NSCLC with pembrolizumab, an anti‐programmed death‐1 monoclonal antibody, approved as both monotherapy and in combination with platinum‐based chemotherapy [2, 3, 4, 5, 6]. Most immunotherapy (IO) trials across all cancer types, including lung cancer, had significant underrepresentation of minority patients, especially Black patients, who composed less than 5% of the trial populations [2, 7, 8, 9, 10, 11]. For instance, KEYNOTE‐010 comparing two different doses of pembrolizumab to second‐line docetaxel chemotherapy in advanced NSCLC included 1,033 patients; however, only 28 (2.7%) were Black [9]. Exact representation of minority populations was not reported in pivotal first‐line pembrolizumab trials [2, 3, 6]. Several barriers exist for enrollment in clinical trials amongst minority groups, including lack of accessibility, stringent eligibility criteria, and/or patient misconceptions regarding clinical research [7].
Race is a social construct, not a genetic manifestation, and racial differences in cancer outcomes likely reflect socioeconomic disparities in health care delivery. Although previous studies identified genomic differences among patients with cancer of different ancestry, it is difficult to attribute any difference in outcome to these genomic traits; socioeconomic factors and access to specific therapies are significant confounders and likely explain many of the observed differences in outcome [12]. In NSCLC specifically, cancer stage and treatment were better predictors of survival than ancestry [13]. Still, there is value in identifying disparities in outcome to facilitate an understanding of potential barriers so that they can be removed. The lack of representation of Black patients on the landmark immunotherapy trials in NSCLC could limit generalizability of trial data in clinical practice. In this study we conducted a retrospective analysis to assess differences in clinical outcomes across racially diverse patients with metastatic NSCLC who received treatment with pembrolizumab‐based regimen in the front‐line setting.
Materials and Methods
This is a retrospective analysis of patients with metastatic NSCLC receiving first‐line pembrolizumab across three different MedStar facilities from January 1, 2014, to May 31, 2019. This study was approved by the MedStar institutional review board and a waiver of Health Insurance Portability and Accountability Act (HIPAA) authorization was obtained to perform this study given its retrospective nature.
Patients
Pharmacy records from MedStar Georgetown University Hospital (GUH), MedStar Washington Hospital Center (WHC), and MedStar Franklin Square Hospital (FSH) were used to identify patients treated with pembrolizumab between January 1, 2014, and May 31, 2019. These charts were manually reviewed to identify patients with stage IV NSCLC treated with pembrolizumab in the first‐line setting as either monotherapy or in combination with chemotherapy. Pembrolizumab was chosen given its status as the approved standard of care during the time period analyzed. Patients who received prior systemic treatment in the nonmetastatic setting were included. Patients treated with other ICIs or who received pembrolizumab as second‐line or beyond were excluded to limit heterogeneity of results.
Pertinent baseline demographic data collected for analysis included age, gender, race, Eastern Cooperative Oncology Group performance status, programmed death‐ligand‐1 (PD‐L1) expression per immunohistochemical staining using the Dako 22C3 clone, tumor histology, date of pembrolizumab initiation, combination versus monotherapy, and institution.
Age is reflective of the time at which pembrolizumab was initiated. Race was self‐identified by patients. Progression‐free survival (PFS) was defined as the time from initiation of pembrolizumab to radiographic progression or death. Overall survival (OS) was defined as the time from initiation of pembrolizumab to date of death. Information regarding immune‐related adverse events (irAEs) was collected and the severity of irAEs was graded based on Common Terminology Criteria for Adverse Events Version 5.0.
Endpoints
Primary endpoints were differences in PFS and OS among different racial groups treated with first‐line pembrolizumab for metastatic NSCLC. Secondary endpoints included differences in incidence of irAEs and median PFS and OS between White and Black patients stratified by high PD‐L1 expression defined as greater than or equal to 50%. Differences in median PFS and OS across institutions were also reported as an exploratory outcome.
Statistical Analysis
Baseline demographic characteristics by races were summarized using median and range for age, frequencies, and percentages for categorical characteristics. Characteristics of irAEs between White and Black patients were summarized descriptively. The Kaplan‐Meier method was used to estimate the median OS and PFS stratified by race, institutions, and three PD‐L1 expression categories (<1%, 1–49%, ≥50%), respectively, and log‐rank tests were conducted for each comparison. Subset survival analyses with PD‐L1 expression greater than or equal to 50% were conducted to compare median OS and PFS between White and Black patients, respectively. Statistical significance was determined if a two‐sided p < .05.
Results
Patient Characteristics
One hundred and thirty‐six eligible patients were identified. Patient characteristics are described in detail in Table 1. More than half of the patients were White at 54.4% (n = 74), whereas 39% (n = 53) were Black. Six patients (4.4%) identified as other; whereas only two (1.5%) patients identified as Asian. One patient declined to answer. Median age in White patients was higher at 70 years compared with Black patients at 65 years (95% confidence interval [CI], 1.176–9.511; p < .01). The sample proportion of females in Black patients was 45.3%, whereas it was 55.4% in White patients (95% CI, −29.8% to 8.4%, p = .31). Adenocarcinoma was the most common histology at 72.8% (n = 99), followed by squamous cell carcinoma at 22.8% (n = 31); 4.4% (n = 6) of the cases were not otherwise specified. Patients received a median of six cycles; 50 (36.8%) patients received pembrolizumab monotherapy and 86 (63.2%) patients received combination chemotherapy with pembrolizumab.
Table 1.
Baseline demographic characteristics of all patients
| Characteristics | All patients, n = 136 | White, n = 74 | Black, n = 53 | Asian, n = 2 | Other/unknown, n = 7 |
|---|---|---|---|---|---|
| Median age (range), yr | 68 (25–88) | 70 (42–87) | 65 (25–88) | 65 (60–70) | 61 (54–64) |
| Sex, n (%) | |||||
| Female | 69 (50.7) | 41 (55.4) | 24 (45.3) | 1 (50) | 3 (42.9) |
| Male | 67 (49.3) | 33 (44.6) | 29 (54.7) | 1 (50) | 4 (57.1) |
| ECOG, n (%) a | |||||
| 0 | 22 (16.2) | 8 (10.8) | 10 (18.9) | 1 (50) | 3 (42.9) |
| 1 | 84 (61.8) | 51 (68.9) | 28 (52.8) | 1 (50) | 4 (57.1) |
| 2 | 26 (19.1) | 13 (17.6) | 13 (24.5) | 0 | 0 |
| 3 | 3 (2.2) | 2 (2.7) | 1 (1.9) | 0 | 0 |
| Histology, n (%) | |||||
| Adenocarcinoma | 99 (72.8) | 53 (71.6) | 40 (75.5) | 1 (50) | 5 (71.4) |
| Squamous | 31 (22.8) | 16 (21.6) | 13 (24.5) | 0 | 2 (28.6) |
| NOS | 6 (4.4) | 5 (6.8) | 0 | 1 (50) | 0 |
| % PDL1, n (%) b | |||||
| <1 | 40 (29.4) | 20 (27.0) | 19 (35.8) | 1 (50) | 1 (14.3) |
| 1–49 | 27 (19.9) | 14 (18.9) | 10 (18.9) | 0 | 2 (28.6) |
| ≥50 | 66 (48.5) | 39 (52.7) | 22 (41.5) | 1 (50) | 4 (57.1) |
| Pembrolizumab, n (%) | |||||
| Monotherapy | 50 (36.8) | 30 (40.5) | 14 (26.4) | 1 (50) | 5 (71.4) |
| Combination | 86 (63.2) | 44 (59.5) | 39 (73.6) | 1 (50) | 2 (28.6) |
| Institution, n (%) | |||||
| GUH | 51 (37.5) | 30 (40.5) | 17 (32.1) | 1 (50) | 3 (42.9) |
| MWHC | 23 (16.9) | 2 (2.7) | 20 (37.7) | 1 (50) | 0 |
| FSH | 62 (45.6) | 42 (56.8) | 16 (30.2) | 0 | 4 (57.1) |
One patient in Black group did not have ECOG status listed.
Three patients (1 White and 2 Black) did not have PDL1 status available.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FSH, Franklin Square Hospital; GUH, Georgetown University Hospital; MWHC, MedStar Washington Health Center; NOS, not otherwise specified; PD‐L1, programmed death‐ligand 1.
Primary Efficacy Outcomes
White and Black patients were the two most commonly represented races in the analysis. Fifty‐one out of 74 (68.9%) White, 40 out of 53 Black (75.5%), and 1 out of 2 (50%) Asian patients had disease progression. At the time of data cutoff on May 31, 2019, 37 out of 74 (50.0%) White, 27 out of 53 (50.9%) Black, and 1 out of 2 Asian patients were deceased. The median PFS was 5.7 months in White patients (95% CI, 3.877–9.068), 5.9 months in Black patients (95% CI, 3.22–8.509), 4.1 months in Asian patients (95% CI, 4.107–not available [NA]; p = .651). Median OS was not significantly different among these three racial groups at 11.8 (95% CI, 7.261–NA), 12.4 (95% CI, 7.392‐NA), and 4.5 (95% CI, 4.501–NA) months (p = .949), respectively (Fig. 1).
Figure 1.
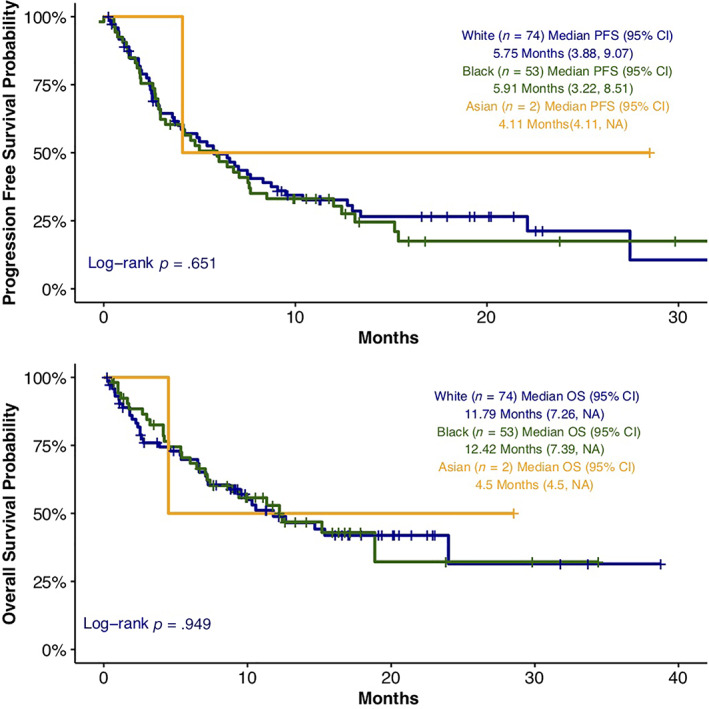
Progression‐free survival and overall survival among racial groups. Abbreviations: CI, confidence interval; OS, overall survival; NA, not available; PFS, progression‐free survival.
Secondary Efficacy Outcomes
There were 66 patients (48.5%) with high PD‐L1 expression defined as expression in ≥50% of cells. Among the PD‐L1–high patients, 52.7% (n = 39) were White and 41.5% (n = 22) were Black. There was no significant difference in PD‐L1 expression, categorized as <1%, 1–49%, and ≥ 50%, noted between the two races (Pearson's χ2 p = .37). No statistically significant difference was observed in median PFS 8.7 months (95% CI, 3.581–NA) versus 3.9 months (95% CI, 2.694–NA; p = .843). There was also no difference in median OS 14.7 months (95% CI, 9.495–NA) versus 11.3 months (95% CI, 4.501–NA; p = .581) between White and Black patients with high PD‐L1 expression (Fig. 2).
Figure 2.
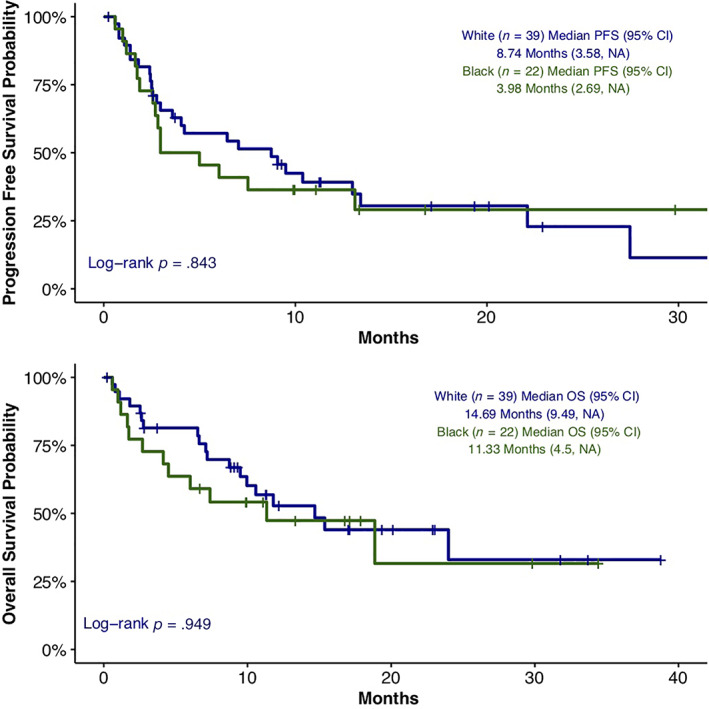
Progression‐free survival and overall survival among White and Black patients with PD‐L1 expression ≥50%.
There was also no difference in median PFS based on the institution at which treatment was given with 5.9 months, 7.6 months, and 4.7 months at GUH, WHC, and FSH, respectively (p = .723). The median OS among the three institutions was also not significant (p = .714) at 15.4, 12.2, and 10.6 months, respectively (supplemental online Fig. 1).
Safety Outcomes
The incidence of all‐grade irAEs in White versus Black patients was 24.3% and 22.6%, respectively (p = .83; Table 2). In White patients, rash was the most common irAE (n = 7; 9.4%), followed by pneumonitis (n = 3; 4%) and hypothyroidism (n = 3; 4%). In Black patients, pneumonitis (n = 3; 5.7%) and hepatitis (n = 3; 5.7%) were the most common irAEs, followed by rash (n = 2; 3.8%) and colitis (n = 2; 3.8%). The incidence of any grade 3–4 irAEs was similar between the two groups (10.8% White vs. 9.4% Black). The incidence of grade 3 or 4 rash was the most common at 5.4% in White patients, whereas 3.8% of Black patients had grade 3 or 4 pneumonitis.
Table 2.
Characteristics of irAE between White and Black patients
| irAE | White (n = 74) | Black (n = 53) | ||||
|---|---|---|---|---|---|---|
| Any grade | Grade 1–2 | Grade 3–4 | Any grade | Grade 1–2 | Grade 3–4 | |
| Any irAE | 18 (24.3) | 10 (13.5) | 8 (10.8) | 12 (22.6) | 7 (13.2) | 5 (9.4) |
| Rash | 7 (9.4) | 3 (4.1) | 4 (5.4) | 2 (3.8) a | 1 (1.9) | 1 (1.9) |
| Pneumonitis | 3 (4) | 1 (1.4) | 2 (2.7) | 3 (5.7) | 1 (1.9) | 2 (3.8) |
| Hepatitis | 1 (1.4) | 1 (1.4) | 0 | 3 (5.7) a | 2 (3.8) | 1 (1.9) |
| Hypothyroidism | 3 (4) | 3 (4) | 0 | 1 (1.9) | 1 (1.9) | 0 |
| Adrenal insufficiency | 0 | 0 | 0 | 1 (1.9) | 1 (1.9) | 0 |
| Diabetes | 1 (1.4) | 0 | 1 (1.4) | 0 | 0 | 0 |
| Colitis | 1 (1.4) | 1 (1.4) | 0 | 2 (3.8) | 1 (1.9) | 1 (1.9) |
| Arthritis | 1 (1.4) | 0 | 1 (1.4) | 0 | 0 | 0 |
| Myocarditis | 0 | 0 | 0 | 1 (1.9) | 0 | 1 (1.9) |
| Neuropathy | 1 (1.4) | 1 (1.4) | 0 | 0 | 0 | 0 |
All data are presented as n (%) unless otherwise indicated.
One patient had both rash and hepatitis.
Abbreviation: irAE, immune‐related adverse event.
Discussion
ICIs are established as the backbone of first‐line treatment for patients with metastatic NSCLC. Minority populations, specifically Black patients, have historically been underrepresented or not well studied in pivotal immunotherapy trials, limiting generalizability. Safety and efficacy of ICIs in minority populations is not well studied, which poses a challenge in treatment of these patients. This is the first study, to our knowledge, to assess differences in efficacy and safety of pembrolizumab in Black patients compared with White patients with metastatic NSCLC treated in the first‐line setting.
Our study demonstrates that there are no significant differences in PFS or OS in Black patients when compared with White patients with similar baseline characteristics. These results were replicated in a subgroup analysis of those patients with high PD‐L1 expression. In contrast, Tiu et al. conducted a small retrospective study of 38 patients on nivolumab treatment for advanced or metastatic NSCLC out of which 29 (76.3%) patients were Black. Median PFS and OS in the Black patients were significantly higher when compared with two landmark clinical trials (CheckMate 017 and 057) in which superiority of nivolumab over docetaxel in the second‐line setting was shown [10, 11, 14]. However, there are several limitations to the study including a small sample size, lack of a control arm with non‐Black patients, and short follow up time compared with the prospective trials, possibly contributing to discordantly higher PFS and OS. Moreover, PD‐L1 expression of these patients was not reported, and two patients were treated with nivolumab as first‐line, increasing the heterogeneity in the patient population [14]. Butaney et al. conducted a meta‐analysis of immunotherapy trials across different cancer types and concluded no significant difference in OS (efficacy) among White versus non‐White patients, although only two NSCLC trials that were included in the study had patients that were substratified as White versus non‐White [15].
The majority of patients in our study received pembrolizumab in combination with chemotherapy (63.2%). This combination was approved as first‐line therapy based on results from KEYNOTE‐189 and KEYNOTE‐407 [3, 5, 6]. The incidences of irAEs in KEYNOTE‐189 and KEYNOTE‐407 were 22.7% and 28.8%, respectively, which is similar to what was seen in our analysis. The incidence of irAEs in KEYNOTE‐024, a phase III, randomized trial of pembrolizumab monotherapy in patients with high PD‐L1 expression, was also similar at 29.2%. Although hypothyroidism was the most common irAE reported in all three seminal KEYNOTE trials, this was not seen in our study, potentially because of less regular thyroid stimulating hormone (TSH) testing outside of the clinical trial setting. Pneumonitis is the second most common irAE in those clinical trials and incidence is similar to our study at appropriately 4%–6% between the two groups. Although the rate of irAEs between White and Black patients are similar (24.3% vs. 22.6%), including similar rates of grade 3 or higher toxicities (5.4% vs. 3.8%), different patterns of irAEs were seen between the two races, with rash being the most commonly occurring event in White patients. It is unclear if this is due to a true difference in incidence or due to easier recognition of a rash by patients and physicians on the skin of White patients.
Our study had only two patients of Asian origin; conclusions cannot be made in this patient population. Representation of Asian patients was higher in ICI trials for NSCLC ranging from 3%–21% [2, 7, 8, 9, 10, 11]. A similar retrospective analysis by Qian et al. assessed differences in efficacy between Asian and White patients with NSCLC who received second‐line atezolizumab in the POPLAR and OAK trials [16]. A total of 390 patients were evaluated, out of which 304 (77.9%) were White and 86 (22.1%) were Asian; inferior responses to atezolizumab were noted in the White cohort even after adjusting for confounding factors such as lower proportion of PD‐L1 expression and blood‐based tumor‐mutation burden and higher incidence of epidermal growth factor receptor mutations in Asians [16]. The authors concluded that racial differences in mutational analyses are a potential contributing factor. In that study, White patients were noted to have higher incidence of STK11 alterations, which, according to the authors, may have conferred inferior response to atezolizumab due to primary resistance to checkpoint inhibition [16]. However, it is very challenging to isolate genomic changes as a true determinant of these disparities, and differences are likely to reflect other, nonbiologic factors. Several other studies indirectly compared efficacy outcomes from a subgroup of Japanese or Chinese patients with the overall population and reported comparable or higher response rates in Asian patients [17, 18, 19].
Inferior survival outcomes in Black patients across certain cancer groups including lung cancer have previously been reported [20]. Although several factors contribute to these differences, a study by Albain et al. attempted to adjust for demographics, clinical variables, and socioeconomic status [21]. They included 19,457 adult patients with cancer from 35 phase III SWOG trials, out of which 2,699 patients had lung cancer. When adjusted for prognostic factors and socioeconomic status, no difference was noted in patients with advanced stage NSCLC [21]. In our study, when patients were further stratified based on the institution at which they received treatment, survival outcomes did not differ despite variances in socioeconomic status among the patients from each institution.
The retrospective nature of our study may be a potential limitation in drawing major conclusions. Race is a social construct. Differences in cancer outcomes reported by race likely reflect disparities in access rather than true biologic differences, yet it is still important to highlight these differences so that disparities can be properly addressed. The lack of minority representation on registrational clinical trials reflects these ongoing disparities. Until minority patient enrollment in clinical trials is improved, we rely heavily on such retrospective studies to highlight safety and efficacy of cancer drugs. This data set did not identify any meaningful differences in efficacy or safety of the front‐line immunotherapy by race.
Conclusion
There are very limited data on the survival outcomes of Black patients with lung cancer treated with ICIs. Our retrospective study showed no difference in safety or efficacy between White and Black patients when treated with pembrolizumab‐based regimens in the front‐line setting. We conclude that pembrolizumab is safe and efficacious in Black patients with metastatic NSCLC and results from the landmark trials should be generalized across racial subgroups.
Author Contributions
Conception/design: Monica Peravali, Stephen V. Liu, Chul Kim
Provision of study material or patients: Monica Peravali, Kevin Chen, Suman Rao, Irina Veytsman, Stephen V. Liu, Chul Kim
Collection and/or assembly of data: Monica Peravali, Jaeil Ahn
Data analysis and interpretation: Monica Peravali, Jaeil Ahn, Stephen V. Liu, Chul Kim
Manuscript writing: Monica Peravali, Jaeil Ahn, Kevin Chen, Suman Rao, Irina Veytsman, Stephen V. Liu, Chul Kim
Final approval of manuscript: Monica Peravali, Jaeil Ahn, Kevin Chen, Suman Rao, Irina Veytsman, Stephen V. Liu, Chul Kim
Disclosures
Suman Rao: AstraZeneca (C/A), Merck, AstraZeneca, Genentech (RF—Institution); Stephen V. Liu: AstraZeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Catalyst, Celgene, G1 Therapeutics, Genentech/Roche, Janssen, Eli Lilly & Co., Merck Sharp & Dohme, Pfizer, PharmaMar, Regeneron, Takeda (C/A), Alkermes, AstraZeneca, Blueprint, Bristol‐Myers Squibb, Corvus, Genentech, Eli Lilly & Co., Merck, Merus, Pfizer, Rain, RAPT, Spectrum, Turning Point Therapeutics (RF); Chul Kim: AstraZeneca, Bristol‐Myers Squibb, Novartis, Regeneron, Tesaro, Karyopharm, Debiopharm (RF—institution), Novartis (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
Supplementary Figure S1
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Contributor Information
Monica Peravali, Email: monica.peravali@medstar.net.
Chul Kim, Email: chul.kim@gunet.georgetown.edu.
References
- 1. American Cancer Society . Cancer Facts & Figures 2019. Atlanta: American Cancer Society, 2019. [Google Scholar]
- 2. Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 3. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non–small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 4. Reck M, Rodríguez‐Abreu D, Robinson AG et al. Updated analysis of KEYNOTE‐024: Pembrolizumab versus platinum‐based chemotherapy for advanced non–small‐cell lung cancer with PD‐L1 tumor proportion score of 50% or greater. J Clin Oncol 2019;37:537–546. [DOI] [PubMed] [Google Scholar]
- 5. Gadgeel SM, Garassino MC, Esteban E et al. KEYNOTE‐189: Updated OS and progression after the next line of therapy (PFS2) with pembrolizumab (pembro) plus chemo with pemetrexed and platinum vs placebo plus chemo for metastatic nonsquamous NSCLC. J Clin Oncol 2019;37(suppl 15):9013a. [Google Scholar]
- 6. Paz‐Ares L, Luft A, Vicente D et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med 2018;379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 7. Nazha B, Mishra M, Pentz R et al. Enrollment of racial minorities in clinical trials: Old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Educ Book 2019;39:3–10. [DOI] [PubMed] [Google Scholar]
- 8. Rittmeyer A, Barlesi F, Waterkamp D et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 10. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manojlovic Z, Christofferson A, Liang WS et al. Comprehensive molecular profiling of 718 multiple myelomas reveals significant differences in mutation frequencies between African and European descent cases. PLoS Genetics 2017;13:e1007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones CC, Mercaldo SF, Blume JD et al. Racial disparities in lung cancer survival: The contribution of stage, treatment, and ancestry. J Thorac Oncol 2018;13:1464–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tiu AC, Potdar R, Djibo DA et al. Clinical outcomes of African American patients with advanced or metastatic non‐small cell lung cancer on nivolumab in a single community‐based cancer center. Med Oncol 2018;35:109. [DOI] [PubMed] [Google Scholar]
- 15. Butaney M, Satkunasivam R, Goldberg H et al. Analysis of heterogeneity in survival benefit of immunotherapy in oncology according to patient demographics and performance status: A systematic review and meta‐analysis of overall survival data. Am J Clin Oncol 2020;43:193–202. [DOI] [PubMed] [Google Scholar]
- 16. Qian J, Nie W, Lu J et al. Racial differences in characteristics and prognoses between Asian and white patients with nonsmall cell lung cancer receiving atezolizumab: An ancillary analysis of the POPLAR and OAK studies. Int J Cancer 2020;146:3124–3133. [DOI] [PubMed] [Google Scholar]
- 17. Nishio M, Takahashi T, Yoshioka H et al. KEYNOTE‐025: Phase 1b study of pembrolizumab in Japanese patients with previously treated programmed death ligand 1‐positive advanced non‐small‐cell lung cancer. Cancer Sci 2019;110:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hida T, Kaji R, Satouchi M et al. Atezolizumab in Japanese patients with previously treated advanced non‐small‐cell lung cancer: A subgroup analysis of the phase 3 OAK study. Clin Lung Cancer 2018;19:e405–e415. [DOI] [PubMed] [Google Scholar]
- 19. Wu YL, Lu S, Cheng Y et al. Nivolumab Versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol 2019;14:867–875. [DOI] [PubMed] [Google Scholar]
- 20. Underwood SM. Reducing the burden of cancer borne by African Americans: if not now, when? Cancer Epidemiol Biomarkers Prev 2003;12:270s–276s. [PubMed] [Google Scholar]
- 21. Albain KS, Unger JM, Crowley JJ et al. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 2009;101:984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1


