Abstract
Introduction
Optimal surveillance paradigms for survivors of early stage human papillomavirus (HPV)‐related oropharyngeal cancer are not well defined. This study aimed to characterize patient interest in and factors associated with an altered surveillance paradigm.
Materials and Methods
We surveyed patients with Stage I or II HPV‐related oropharyngeal cancer treated at a tertiary care institution from 2016 to 2019. Primary outcomes were descriptive assessment of patient knowledge, interest in altered surveillance, burdens of in‐person appointments, and priorities for surveillance visits. Ordinal regression was used to identify correlates of interest in altered surveillance.
Results
Sixty‐seven patients completed surveys from February to April 2020 at a median of 21 months since completing definitive treatment. A majority (61%) of patients were interested in a surveillance approach that decreased in‐person clinic visits. Patients who self‐identified as medical maximizers, had higher worry of cancer recurrence, or were in long‐term relationships were less likely to be interested. Patients reported significant burdens associated with surveillance visits, including driving distance, time off work, and nonmedical costs. Patients were most concerned with discussing cancer recurrence (76%), physical quality of life (70%), mortality (61%), and mental quality of life (52%) with their providers at follow‐up visits.
Conclusion
Patients with early stage HPV‐related oropharyngeal cancers are interested in altered surveillance approaches, experience significant burdens related to surveillance visits, and have concerns that are not well addressed with current surveillance approaches, including physical and mental quality of life. Optimized surveillance approaches should incorporate patient priorities and minimize associated burdens.
Implications for Practice
The number of patients with HPV‐related oropharyngeal cancers is increasing, and numerous clinical trials are investigating novel approaches to treating these good‐prognosis patients. There has been limited work assessing optimal surveillance paradigms in these patients. Patients experience significant appointment‐related burdens and have concerns such as physical and mental quality of life. Additionally, patients with early stage HPV‐related oropharyngeal cancers express interest in altered surveillance approaches that decrease in‐person clinic visits. Optimization of surveillance paradigms to promote broader survivorship care in clinical practice is needed.
Keywords: Surveillance, Oropharyngeal cancer, Human papillomavirus, Survivorship
Short abstract
Patient input is critical to improve surveillance options for human papillomavirus (HPV)‐related oropharyngeal cancer. This article assesses patient interest in and factors associated with alternative surveillance approaches, including patient knowledge, burdens of in‐person appointments, and priorities for follow‐up visits.
Introduction
Early stage human papillomavirus (HPV)‐related oropharyngeal cancer represents a distinct entity among head and neck malignancies given better outcomes [1] and differing demographics [2] compared with HPV‐negative head and neck cancers. Five‐year overall survival rates are in excess of 85% in patients with HPV‐related cancers [3, 4], whereas patients with HPV‐negative cancers have a 5‐year overall survival of less than 50% [1]. In HPV‐related cancers, stage I–III patients with a negative positron emission tomography scan at 3 months post‐treatment have a 5‐year disease‐free survival of 91% and 5‐year overall survival of 89% [5].
Much contemporary clinical research in these patients currently focuses on treatment deintensification strategies, including alterations in systemic therapies [6] and radiation approaches [7, 8, 9], aiming to reduce the known long‐term toxicities of treatment (80% peripheral neuropathy [10], 40% ototoxicity [11], 30% nephrotoxicity [12], 15%–20% late dysphagia [13, 14], 15% late xerostomia [15, 16]) and their subsequent impact of quality of life [17, 18]. HPV‐related oropharynx cancer‐specific surveillance after standard therapy, however, has not been well studied. Current surveillance paradigms offer the same surveillance schedule for all squamous cell carcinomas of the head and neck regardless of HPV status [19], ignoring vastly different outcomes, recurrence patterns, demographics, and comorbidities [20]. Recognizing the lower rates of recurrences in HPV‐related cancers as above, revising current surveillance guidelines to deintensify surveillance in good‐prognosis patients warrants further evaluation.
Observational epidemiology studies in patients with HPV‐related oropharynx cancer suggest that recurrences are frequently detected in the setting of new symptoms, rather than during surveillance visits. Moreover, adherence to standard surveillance paradigms offers limited tangible benefits [21]. Additionally, current surveillance options do not offer a means to detect distant recurrences, which is proportionally more common in this patient population [22].
As we seek ways to improve surveillance options for HPV‐related oropharyngeal cancer, patient input is critical. Patient burdens related to appointments and patient desires to address specific concerns during surveillance may illuminate methods to optimize broader survivorship care in addition to standard cancer recurrence surveillance. We aimed to assess patient interest in and factors associated with alternative surveillance approaches, including patient knowledge, burdens of in‐person appointments, and priorities for follow‐up visits.
Materials and Methods
Study Population and Data Collection
Patients who completed treatment for American Joint Commission on Cancer (AJCC) 8 stage I or II HPV‐related (assessed by p16 positivity [23]) oropharyngeal cancers without evidence of cancer recurrence and who had been seen at the University of Michigan within the past 6 months were eligible. All patients were followed with standard surveillance recommendations, which include clinic visits and nasopharyngolaryngoscopy every 3 months for the first 2 years after treatment and every 4–6 months years 3–5. The survey instrument was approved by University of Michigan's Institutional Review Board as part of a larger study following head and neck cancer patients (HUM00042189). Patients were enrolled and consented either while in clinic for a scheduled surveillance visit or via phone and completed the survey electronically. Study data were collected and managed using research electronic data capture tools [24, 25].
Measures
Study participants completed a 38‐item survey (see supplemental online Dataset 1). The instrument was based on Andersen's health service utilization model [26], developed using standardized approaches to questionnaire design [27], and based on systematic review of the literature, prior research in patients with head and neck cancer, input from a multidisciplinary team, and survey design experts. Information on patient sex, age, education, race/ethnicity, employment, and income was obtained from the survey. Details on cancer staging and treatment received were obtained from the medical record.
Questions regarding HPV etiology [28], cancer worry [29], self‐assessment of health status [30], trust in health care providers [31], shared decision‐making preferences [32], health literacy [33], and medical maximizer/minimizer preferences [34, 35] were adapted from previous reports in the literature. Medical minimizer/maximizer preferences distinguish patients who tend to prefer aggressive versus more passive approaches to health care [36]. Concerns related to treatment were adapted from the literature [37] and consisted of 11 topics rated on a three‐point scale (not at all, somewhat, and very much); these were used as proxies of items to be addressed during surveillance visits. Surveillance‐related burdens were assessed through self‐report of time allotted for surveillance visits, method for taking time off work, method of and difficulty of travel to appointments, and money spent on copays and other associated costs (including food, gas, lodging).
The survey presented scenarios to address options for altered surveillance patterns that included decreased clinic visits and assessed interest in nonclinic methods of surveillance. In scenario 1, patients were offered a vignette and asked to rate interest in returning to clinic for fewer routine surveillance visits, from every 3 months in the first 2 years after treatment to every 6–12 months; in scenario 2, patients were then offered a similar vignette with additional education on the expected low risk of recurrence, and again asked to rate interest in altered surveillance. For exploratory analyses, responses for scenario 2 were used as this scenario represents discussions that occur in routine clinical practice. Patients were also asked about potential adjuncts to standard surveillance including blood samples, urine samples, electronic symptom surveys, or expedited symptom‐directed survivorship visits (for example, speech‐language pathology).
Statistical Analysis
The primary aim of this analysis was to descriptively assess interest in altered surveillance, patient burdens of appointments, and patient priorities for follow‐up. Interest in altered surveillance was defined as responses of 4 or 5 on a Likert‐type scale from 0 to 5, where 0 represented “not at all interested” and 5 represented “definitely interested.” Wilcoxon signed‐rank test was used to compare means between the two scenarios. Treatment‐related concerns were analyzed as binary, with “not at all” compared with “somewhat” and “very much.” Exploratory analyses included associating five prespecified variables to avoid overfitting the data (relationship status, worry of cancer recurrence, self‐perception of physical health, shared decision‐making preferences, minimizer‐maximizer preferences) with interest in altered surveillance using ordinal regression. The χ2 test was used to assess changes in concern with median follow‐up time. The data were analyzed using SPSS, version 26 (SPSS, Chicago, IL). Two‐sided p values ≤0.05 were considered statistically significant.
Results
Sample
Of 90 patients invited to participate, 67 completed surveys, for a 74.4% response rate. Patients received treatment from October 2016 to December 2019, and surveys were completed February to April 2020 at a median of 21.2 months since completing treatment (range, 2.8 to 41 months). Patients had 71.6% AJCC 8 stage I disease, and 73.1% underwent definitive chemoradiation for treatment. Patients had a median age of 60 years (range, 41–83), 92.5% were male, 97% of patients were non‐Hispanic White, 53.7% had a bachelor's degree or higher, and 86.6% were in a long‐term relationship. Most patients were working at time of survey (61.1%), and nearly half made ≥$100,000 per year (Table 1).
Table 1.
Sample characteristics
| Characteristics | n (%) |
|---|---|
| Patient characteristics | |
| Age, yr | |
| Median | 60 |
| Min | 41 |
| Max | 83 |
| Gender | |
| Male | 62 (92.5) |
| Female | 5 (7.5) |
| Smoking status (at time of treatment) | |
| Never smoker | 40 (59.7) |
| Former smoker | 23 (34.3) |
| Current smoker | 4 (6.0) |
| Race/ethnicity | |
| Non‐Hispanic White | 65 (97.0) |
| Black | 1 (1.5) |
| Prefer not to answer | 1 (1.5) |
| Tumor and treatment characteristics | |
| AJCC 8 group stage | |
| I | 48 (71.6) |
| II | 19 (28.4) |
| Primary therapy | |
| Chemoradiation | 49 (73.1) |
| Radiation | 5 (7.5) |
| Surgery | 13 (19.4) |
| Median time since end of treatment, mo | 21.2 |
| Min | 2.8 |
| Max | 41.0 |
| Socioeconomic characteristics | |
| Education | |
| High school or less | 8 (11.9) |
| Some college or trade school | 23 (34.3) |
| Bachelor's degree or higher | 53.7) |
| In long‐term relationship | |
| Yes | 58 (86.6) |
| No | 9 (13.4) |
| Employment status | |
| Working full‐time | 34 (50.7) |
| Working part‐time | 7 (10.4) |
| Not working | 26 (38.8) |
| Health insurance | |
| Yes | 66 (98.5) |
| No | 1 (1.5) |
| Financial dependents | |
| 0–1 | 46 (68.7) |
| 2+ | 21 (31.3) |
| Household income | |
| Less than $50,000 | 9 (13.4) |
| $50,000–$99,999 | 16 (23.9) |
| $100,000 or more | 30 (44.8) |
| I prefer not to answer | 12 (17.9) |
Abbreviation: AJCC, American Joint Commission on Cancer.
Patients exhibited high levels of self‐assessment of physical health, with 64.2% assessing health as excellent or very good. Most patients exhibited preferences for a spectrum of shared decision‐making, with only 3% preferring to leave decision‐making up to the physician and no patients wanting to make the decision themselves. All exhibited trust in their health care provider (100% yes or definitely yes to some extent). Most patients self‐identified as medical maximizers (71.6%), most had low levels of cancer worry (mean, 5.48; SD, 3.56 on 20‐point scale), and most reported high levels of health literacy (86.6%, Table 2).
Table 2.
Health‐related preferences
| n (%) | |
|---|---|
| Self‐assessment of physical health | |
| Excellent | 14 (20.9) |
| Very good | 29 (43.3) |
| Good | 18 (26.9) |
| Fair | 5 (7.5) |
| Trust in health care provider | |
| Yes, definitely | 66 (98.5) |
| Yes, to some extent | 1 (1.5) |
| No, not at all | 0 (0.0) |
| Shared decision‐making preferences | |
| I prefer to make the final treatment decision | 0 (0.0) |
| I prefer to make the final treatment decision after seriously considering my doctor's opinion | 24 (35.8) |
| I prefer that my doctor and I share responsibility for deciding which treatment is best | 33 (49.3) |
| I prefer that my doctor makes the final treatment decision, but seriously considers my opinion | 8 (11.9) |
| I prefer to leave all treatment decisions to my doctor | 2 (3.0) |
| Medical minimizer‐maximizer scale | |
| Maximizer | 48 (71.6) |
| Minimizer | 19 (28.4) |
| Health literacy: confidence in filling out forms | |
| All of the time | 58 (86.6) |
| Most of the time | 6 (9.0) |
| Some of the time | 1 (1.5) |
| A little of the time | 2 (3.0) |
| None of the time | 0 (0.0) |
| Worry of cancer recurrence | |
| Mean | 5.48 |
| SD | 3.56 |
Knowledge
Almost all patients knew that HPV caused their cancer (94.0%), and most agreed that their particular cancer was unlikely to recur (74.6%). Most patients characterized risk of recurrence in either a local or distant location as low or very low (70.1%). Slightly more patients expected a recurrence to be local, although the proportion was near 50% in both: 58% of patients agreed with the statement “if my cancer comes back, it is likely to come back in my throat”; 53% of patients agreed with the statement “if my cancer comes back, it is likely to come back elsewhere in my body.”
Altered Surveillance
Patients were asked to report interest in decreasing in‐person post‐treatment surveillance clinic visits. In scenario 1, asking about patient interest in decreased in‐clinic surveillance visits, 55.2% of patients were interested in altered surveillance (rated 4–5 on Likert scale), and only 8.9% were not interested at all (rated 0–1 on Likert scale). When offered additional information regarding low risk of recurrence before asking again about interest in decreased in‐clinic surveillance visits in scenario 2, 61.2% were interested in altered surveillance (rated 4–5 on Likert scale), and only 7.4% were not interested at all (rated 0–1 on Likert scale). Comparison of responses to both scenarios showed there was no significant difference in responses to scenario 1 (mean, 3.46; SD, 1.50) versus scenario 2 (mean, 3.58; SD, 1.36; p = .203).
An exploratory analysis of factors associated with interest in altered surveillance were assessed with an ordinal regression model incorporating prespecified variables of relationship status, worry of cancer recurrence, self‐perception of physical health, shared decision‐making preferences, and minimizer‐maximizer preferences. On multivariable regression, being a medical maximizer (higher on the minimizer‐maximizer scale; odds ratio [OR], 0.64; 95% confidence interval [CI], 0.45–0.89, p = .008), being in a long‐term relationship (OR, 0.12; 95% CI, 0.03–0.56; p = .007), and having higher worry of cancer recurrence (OR, 0.86; 95% CI, 0.75–0.99; p = .041) were all associated with decreased interest in altered surveillance, whereas physical health (p = .36) and shared decision‐making (p = .60) were not associated with interest in altered surveillance. Although time from end of treatment was not prespecified as a variable of interest, in a separate model, time from the end of treatment was assessed and was not significantly associated with interest in altered surveillance.
Patients were asked to select from four nonclinic based surveillance options as a means to supplement surveillance. When asked to select only one option, 61% selected blood samples as the preferred nonclinic way to follow cancer, 19% selected surveys, 10% selected symptom‐directed survivorship visits, and 9% selected urine samples. When allowed to select multiple options as a nonclinic based surveillance option, 94% selected blood samples, 63% selected urine samples, 58% selected surveys, and 48% selected symptom‐management visits.
Surveillance Burden
Patients were asked to assess varying burdens related to surveillance appointments. Most patients felt it was easy to get to follow‐up appointments, with 59.7% stating “very easy” and 32.8% stating “somewhat easy.” A minority of patients (9.0%) felt that it was somewhat difficult to come to appointments. Patients drove a median of 57 miles to reach appointments (range, 2–346), and 22.4% of patients drove more than 100 miles to come to appointments.
Patients allotted a significant portion of their day to be able to attend surveillance visits. More than 80% of patients allotted a half day or more to attend a single surveillance visit, with nearly half of patients allotting at least a half day (49.3%) and 26.9% allotting a full working day for one appointment. A minority of patients, 6.0%, allotted more than one full day (this presumably includes an overnight stay for those who drove from long distances). To obtain time off from work to attend these visits, 19.4% used unpaid time off, and 16.4% used sick days, vacation days, or the Family and Medical Leave Act; 41.8% of patients did not work.
Almost all patients, 98.5%, had health insurance. Patients were asked to estimate direct cost to them associated with surveillance visits. Patients were first asked about copays, or immediate out‐of‐pocket costs associated with the visit itself. Nearly half of patients (46.3%) were unable to identify an amount. In free text, 21 patients stated that they did not know the amount; 2 patients stated that it varies; 1 person gave a percent based on insurance; and 2 patients stated “up to” a certain dollar amount ($1,000 and $3,500 for these 2 patients, presumably reflecting insurance deductible amounts). Two patients explicitly stated that they had a very high deductible plan; 19.4% of patients did not pay any copay. The remaining 34.3% reported a range of costs, with 17.9% reporting $26–$50 and 7.5% reporting >$50.
Patients were also asked to estimate out‐of‐pocket costs related to gas, food, or lodging for each visit; 17.9% of patients were unsure or reported no costs. The remaining 82.1% reported some cost, with 40.3% reporting $1–$25 in cost, 19.4% reporting $26–$50, and 22.4% reporting >$50 (Table 3). For all patients who reported a dollar amount for copay or nonmedical out‐of‐pocket cost, 22.4% of patients reported spending more than $100. Of note, the costs assessed here did not include medical bills or additional subsequent costs from the health care system or insurers.
Table 3.
Costs associated with surveillance visits
| Dollar amount | Copay, n (%) | Out‐of‐pocket costs, n (%) | Total costs, n (%) |
|---|---|---|---|
| $101+ | 3 (4.5) | 6 (9.0) | 15 (22.4) |
| $51–100 | 2 (3.0) | 9 (13.4) | 7 (10.4) |
| $26–50 | 12 (17.9) | 13 (19.4) | 13 (19.4) |
| $1–25 | 6 (9.0) | 27 (40.3) | 27 (40.3) |
| $0 | 13 (19.4) | 5 (7.5) | 5 (7.5) |
| N/A | 31 (46.3) | 7 (10.4) | 7 (10.4) |
Copay costs indicate money due by the patient at the time of appointment. Out‐of‐pocket costs reflect gas, food, lodging, or other nonmedical costs. Total costs are the sum of these two for all patients that reported at least one of these costs; not available in this latter column indicates participants who did not mark any costs down for either prior column. Of note, costs assessed here did not include medical bills or additional subsequent costs from the health care system or insurers.
Priorities for Surveillance
Treatment‐related concerns were elicited from each patient to allow for outlining concerns that could be addressed during surveillance care. Cancer recurrence remained the most important concern, with 76% of patients noting this as somewhat or very important. Mortality was important to 61% of patients. Physical quality of life was important to 70% of patients, and mental quality of life was important to 52% of patients. The rest of the 11 concerns were noted as important by fewer than half of patients (Fig. 1); notably, despite the costs noted above, 79% of patients reported that financial issues were not a concern.
Figure 1.
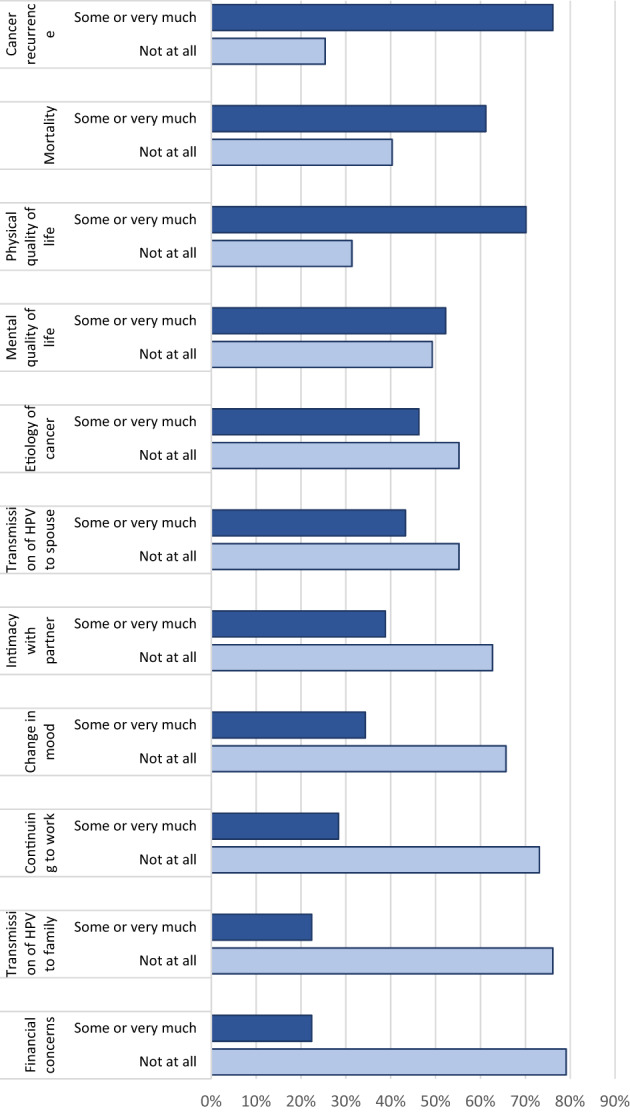
Treatment‐related concerns listed as important by patients.
Discussion
Patients with early stage HPV‐related oropharyngeal cancer offer insights into ways to optimize surveillance and survivorship care. As the treatment paradigm for HPV‐related cancers continues to evolve, efforts must also focus on individualizing follow‐up care based upon the outcomes of the disease itself and patient preferences.
Substantial work in careful treatment deintensification in these excellent‐prognosis patients is ongoing. Although RTOG 1016 [6] and De‐ESCALaTE [38] have shown that de‐escalating systemic therapy with cetuximab failed, other approaches are investigating numerous promising approaches to de‐escalation such as the use of induction chemotherapy [39], decreasing radiation dose [40] or volumes [41], or modifying indications for postoperative treatment. As these investigations cautiously continue, standard of care therapy should remain unchanged, as standard of care is associated with excellent outcomes. In contrast to treatment studies, current surveillance paradigms do not differentiate follow‐up recommendations by HPV status and do not adapt to the excellent outcomes in HPV‐related populations. The current study aimed to obtain the patient perspective on altered surveillance to inform strategies to optimize surveillance in the HPV‐related population.
A substantial portion of patients (61.2%) would be interested in a post‐treatment surveillance option that included fewer clinic visits. There was no significant increase in interest with additional information provided in the vignette for scenario 2, possibly reflecting the high knowledge of this population regarding HPV‐related cancer outcomes and etiology. Cancer‐related knowledge was higher in this study as compared with previous reports, which have previously suggested that the proportion of patients with HPV‐related oropharyngeal cancer understanding the viral etiology of their cancer may be as low as 35% [28, 42]. The exploratory analysis of factors that correlated with interest in altered surveillance is in line with what many physicians intuitively know to be true: some patients are intrinsically more likely to seek out health care (medical maximizers) or are more worried about cancer recurrence [43]. In this article, we see that these intuitive perceptions about patients may play out as interest in novel surveillance or treatment paradigms.
It is possible that the proportion of patients who are interested in remote approaches or decreased surveillance may have increased because of the ongoing COVID‐19 pandemic and current interest in fewer surveillance visits may be higher under current circumstances. Additionally, telemedicine capabilities have increased as a result of COVID‐19 in tandem with favorable reimbursement modifications, likely making remote monitoring more accessible at head and neck cancer centers [44]. A recent telephone‐based quality of life survey in patients with oral cavity cancer showed that remote monitoring via patient‐reported outcomes may offer an excellent means to detect cancer recurrences while managing appointment‐related burdens [45].
In this study, patients reported significant burdens associated with surveillance visits at a tertiary care facility, including a substantial distance driven for each appointment, allotting a significant portion of a working day for a single visit, and costs including copays and other nonmedical costs associated with each visit. Interestingly, a substantial portion of patients in this sample reported uncertainty with identifying their typical copay costs (46.3%) and other nonmedical out‐of‐pocket costs (10.4%), with the former possibly reflecting poor transparency in medical billing and suggesting that these financial burdens may be higher than reported in this study. Despite this, 22.4% of patients reported spending >$100 per visit on these costs.
Importantly, almost all patients in this study were insured, and the costs reported in this article likely underestimate these burdens in the wider population. Additionally, these costs did not include direct medical bills related to surveillance. We have previously shown that 33% of patients with head and neck cancer going through radiation treatment reported at least a moderate financial burden from treatment, and this was associated with increased treatment noncompliance [46]. Although 79% of participants in this study reported that financial issues were not a concern, possibly reflecting the relatively affluent population studied here, options that incorporate fewer in‐person surveillance visits and more remote monitoring may help offset these patient‐borne burdens without compromising ability to detect recurrences.
Patients reported concerns that they wished to address during surveillance visits. Consistent with prior reports [37], patients rated cancer recurrence as the most important concern (76%); the next most important concern was physical quality of life (70%). Current surveillance approaches focus on the risk of local recurrence, with frequent nasopharyngoscopy to directly assess for recurrence. Despite clinical practice guidelines that have endorsed comprehensive survivorship care [47, 48], it remains challenging to implement surveillance approaches that cover all of these comprehensive facets and new approaches are needed. For example, incorporation of quality of life and patient‐reported outcomes into optimized surveillance approaches may offer a means to meet goals outlined for survivorship care.
Because of recent publications investigating the use of circulating tumor DNA in surveillance of patients with HPV‐related cancers [49], this topic was included in the survey. Patients were most interested in including blood tests as a nonclinic‐based surveillance option to incorporate into care. This may be reflective of standard association of blood tests with clinical care; it may also be reflective of publicity surrounding the use of circulating tumor DNA to follow patients with HPV‐related cancers [49]. Further studies validating the role of circulating tumor DNA into surveillance are needed. When allowed to select multiple options for adjuncts to surveillance, more than half of patients selected urine tests or surveys, offering two additional tools to add to surveillance that could potentially be administered remotely and subsequently minimize surveillance‐related burdens. Additionally, patient‐reported quality‐of‐life metrics may offer a means to tailor survivorship symptom‐directed visits and better incorporate patient preferences. They may also allow for detection of recurrence, with changes in quality‐of‐life score potentially predicting for both local and distant recurrence [45, 50]. Systematic administration of quality of life instruments in the metastatic setting has been shown to improve quality of life [51] and increase overall survival [52]. Patients in this study were least interested in symptom‐directed visits such as speech‐language pathology visits for dysphagia, suggesting that engaging patients in identifying optimal methods to incorporate survivorship care is needed.
Current surveillance recommendations do little to address long‐term quality of life or survivorship issues in patients with head and neck cancer. Survivorship care after cancer treatment has several components: the detection of recurrences and new cancers, which is well addressed by current surveillance recommendations, but also physical effects of treatment, psychosocial effects of treatment, health promotion, and management of chronic conditions [53], the latter four of which are poorly addressed by current surveillance paradigms despite publication of survivorship guidelines [47, 48]. With patient interest in decreased clinic visits as demonstrated in the current survey study and need to address these additional domains of survivorship care, there is opportunity to improve surveillance paradigms especially in this good‐prognosis group of patients with HPV‐related oropharynx cancer.
Strengths of this study include its high survey response rate, indicating that these responses likely represent the wider early stage HPV population seen at this tertiary academic center. The older white male population is reflective of the population predicted to hold the largest burden of HPV‐related cancers in the U.S. in the coming decades [54]. Limitations of this study include its small sample size, single institution, and lack of diversity. Minorities have worse outcomes in this cancer subtype [55], and it may be expected that the characteristics and perspectives described in this study may differ for a more diverse population, such as one that is more diverse in race, income, and insurance status. Additionally, all patients surveyed here were patients at a tertiary care institution, and it is unclear how priorities and burdens may change for patients treated in smaller community centers. Finally, this study did not include items specific to telemedicine or radiologic surveillance for distant disease. Current head and neck cancer guidelines do not incorporate routine imaging, but recent publications suggest this may be of interest in developing future altered surveillance methods [56, 57].
Conclusion
Patients with early stage HPV‐related oropharyngeal cancers are interested in altered survivorship paradigms, experience significant time and cost burdens related to surveillance visits, and have concerns that are not well addressed in the current paradigm including physical and mental quality of life. Optimized surveillance approaches should incorporate these patient priorities and minimize associated burdens.
Author Contributions
Conception/design: Laila A. Gharzai, Nicholas Burger, Keith Casper, Michelle L. Mierzwa
Provision of study material or patients: Laila A. Gharzai, Nicholas Burger, Elizabeth M. Jaworski, Caitlin Henderson, Matthew Spector, Andy Rosko, Michelle M. Chen, Mark E. Prince, Carol R. Bradford, Kelly M. Malloy, Chaz L. Stucken, Paul Swiecicki, Francis Worden, Caitlin A. Schonewolf, Jennifer Shah, Reshma Jagsi, Steve Chinn, Andrew Shuman, Keith Casper, Michelle L. Mierzwa
Collection and/or assembly of data: Laila A. Gharzai, Nicholas Burger, Pin Li, Matthew J. Schipper, Catilin A. Schonewolf, Jennifer Shah, Reshma Jagsi, Steve Chinn, Andrew Shuman, Keith Casper, Michelle L. Mierzwa
Data analysis and interpretation: Laila A. Gharzai, Nicholas Burger, Pin Li, Elizabeth M. Jaworski, Steve Chinn, Andrew Shuman, Keith Casper, Michelle L. Mierzwa
Manuscript writing: Laila A. Gharzai, Nicholas Burger, Pin Li, Elizabeth M. Jaworski, Catilin Henderson, Matthew Spector, Andy Rosko, Michelle M. Chen, Mark E. Prince, Carol R. Bradford, Kelly M. Malloy, Chaz L. Stucken, Paul Swiecicki, Francis Worden, Matthew J. Schipper, Caitlin A. Schonewolf, Jennifer Shah, Reshma Jagsi, Steve Chinn, Andrew Shuman, Keith Casper, Michelle L. Mierzwa
Final approval of manuscript: Laila A. Gharzai, Nicholas Burger, Pin Li, Elizabeth M. Jaworski, Caitlin Henderson, Matthew Spector, Andy Rosko, Michelle M. Chen, Mark E. Prince, Carol R. Bradford, Kelly M. Malloy, Chaz L. Stucken, Paul Swiecicki, Francis Worden, Matthew J. Schipper, Caitlin A. Schonewolf, Jennifer Shah, Reshma Jagsi, Steve Chinn, Andrew Shuman, Keith Casper, Michelle L. Mierzwa
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Acknowledgments
Michigan Institute for Clinical & Health Research grant support (CTSA: UL1TR002240).
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Ang KK, Harris J, Wheeler R et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dahlstrom KR, Bell D, Hanby D et al. Socioeconomic characteristics of patients with oropharyngeal carcinoma according to tumor HPV status, patient smoking status, and sexual behavior. Oral Oncol 2015;51:832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Gysen K, Stevens M, Guo L et al. Validation of the 8(th) edition UICC/AJCC TNM staging system for HPV associated oropharyngeal cancer patients managed with contemporary chemo‐radiotherapy. BMC Cancer 2019;19:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malm IJ, Fan CJ, Yin LX et al. Evaluation of proposed staging systems for human papillomavirus‐related oropharyngeal squamous cell carcinoma. Cancer 2017;123:1768–1777. [DOI] [PubMed] [Google Scholar]
- 5. Ng SP, Johnson JM, Gunn GB et al. Significance of negative posttreatment 18‐FDG PET/CT imaging in patients with p16/HPV‐positive oropharyngeal cancer. Int J Radiat Oncol Biol Phys 2018;102:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gillison ML, Trotti AM, Harris J et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus‐positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non‐inferiority trial. Lancet 2019;393:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yom SS, Torres‐Saavedra P, Caudell JJ et al. NRG‐HN002: A randomized phase II trial for patients with p16‐positive, non‐smoking‐associated, locoregionally advanced oropharyngeal cancer. Int J Radiat Oncol Biol Phys 2019;105:684–685. [Google Scholar]
- 8. Chera BS, Amdur RJ, Green R et al. Phase II trial of de‐intensified chemoradiotherapy for human papillomavirus‐associated oropharyngeal squamous cell carcinoma. J Clin Oncol 2019;37:2661–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swisher‐McClure S, Lukens JN, Aggarwal C et al. A phase 2 trial of alternative volumes of oropharyngeal irradiation for de‐intensification (AVOID): Omission of the resected primary tumor bed after transoral robotic surgery for human papilloma virus‐related squamous cell carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys 2020;106:725–732. [DOI] [PubMed] [Google Scholar]
- 10. Alberts DS, Noel JK. Cisplatin‐associated neurotoxicity: Can it be prevented? Anticancer Drugs 1995;6:369–383. [DOI] [PubMed] [Google Scholar]
- 11. Zuur CL, Simis YJ, Lansdaal PE et al. Ototoxicity in a randomized phase III trial of intra‐arterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J Clin Oncol 2007;25:3759–3765. [DOI] [PubMed] [Google Scholar]
- 12. Dobyan DC, Levi J, Jacobs C, Kosek J et al. Mechanism of cis‐platinum nephrotoxicity: II. Morphologic observations. J Pharmacol Exp Ther 1980;213:551–556. [PubMed] [Google Scholar]
- 13. Dong Y, Ridge JA, Li T et al. Long‐term toxicities in 10‐year survivors of radiation treatment for head and neck cancer. Oral Oncol 2017;71:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gharzai LA, Li P, Schipper MJ et al. Characterization of very late dysphagia after chemoradiation for oropharyngeal squamous cell carcinoma. Oral Oncol 2020;111:104853. [DOI] [PubMed] [Google Scholar]
- 15. Eisbruch A, Harris J, Garden AS et al. Multi‐institutional trial of accelerated hypofractionated intensity‐modulated radiation therapy for early‐stage oropharyngeal cancer (RTOG 00‐22). Int J Radiat Oncol Biol Phys 2010;76:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen AM, Daly ME, Farwell DG et al. Quality of life among long‐term survivors of head and neck cancer treated by intensity‐modulated radiotherapy. JAMA Otolaryngol Head Neck Surg 2014;140:129–133. [DOI] [PubMed] [Google Scholar]
- 17. Hawkins PG, Kadam AS, Jackson WC et al. Organ‐sparing in radiotherapy for head‐and‐neck cancer: Improving quality of life. Semin Radiat Oncol 2018;28:46–52. [DOI] [PubMed] [Google Scholar]
- 18. Nayak SG, Pai MS, George LS. Quality of life of patients with head and neck cancer: A mixed method study. J Cancer Res Ther 2019;15:638–644. [DOI] [PubMed] [Google Scholar]
- 19. National Comprehensive Cancer Network . Head and Neck Cancers (Version 1.2020). Available at https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed August 01, 2020.
- 20. Guo T, Rettig E, Fakhry C. Understanding the impact of survival and human papillomavirus tumor status on timing of recurrence in oropharyngeal squamous cell carcinoma. Oral Oncol 2016;52:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masroor F, Corpman D, Carpenter DM et al. Association of NCCN‐recommended posttreatment surveillance with outcomes in patients with HPV‐associated oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg 2019;145:903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imbimbo M, Alfieri S, Botta L et al. Surveillance of patients with head and neck cancer with an intensive clinical and radiologic follow‐up. Otolaryngol Head Neck Surg 2019;161:635–642. [DOI] [PubMed] [Google Scholar]
- 23. El‐Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV‐related oropharyngeal carcinoma: A guide for interpretative relevance and consistency. Head Neck 2012;34:459–461. [DOI] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)–A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harris PA, Taylor R, Minor BL et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersen RM. Revisiting the behavioral model and access to medical care: Does it matter? J Health Soc Behav 1995;36:1–10. [PubMed] [Google Scholar]
- 27. Fowler FJ Jr. Survey Research Methods (Applied Social Research Methods). Fifth ed. Thousand Oaks, CA: SAGE Publications; 2013. [Google Scholar]
- 28. Milbury K, Rosenthal DI, El‐Naggar A et al. An exploratory study of the informational and psychosocial needs of patients with human papillomavirus‐associated oropharyngeal cancer. Oral Oncol 2013;49:1067–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hodges LJ, Humphris GM. Fear of recurrence and psychological distress in head and neck cancer patients and their carers. Psychooncology 2009;18:841–848. [DOI] [PubMed] [Google Scholar]
- 30. Hays RD, Spritzer KL, Thompson WW et al. U.S. general population estimate for "excellent" to "poor" self‐rated health item. J Gen Intern Med 2015;30:1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Campbell J, Smith P, Nissen S et al. The GP Patient Survey for use in primary care in the National Health Service in the UK – Development and psychometric characteristics. BMC Fam Pract 2009:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res 1997;29:21–43. [PubMed] [Google Scholar]
- 33. Chew LD, Griffin JM, Partin MR et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008;23:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scherer LD, Zikmund‐Fisher BJ. Eliciting medical maximizing‐minimizing preferences with a single question: Development and validation of the MM1. Med Decis Making 2020;40:545–550. [DOI] [PubMed] [Google Scholar]
- 35. Scherer LD, Shaffer VA, Caverly T et al. Medical maximizing‐minimizing predicts patient preferences for high‐ and low‐benefit care. Med Decis Making 2020;40:72–80. [DOI] [PubMed] [Google Scholar]
- 36. Scherer LD, Caverly TJ, Burke J et al. Development of the medical maximizer‐minimizer scale. Health Psychol 2016;35:1276–1287. [DOI] [PubMed] [Google Scholar]
- 37. Windon MJ, D'Souza G, Faraji F et al. Priorities, concerns, and regret among patients with head and neck cancer. Cancer 2019;125:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mehanna H, Robinson M, Hartley A et al. Radiotherapy plus cisplatin or cetuximab in low‐risk human papillomavirus‐positive oropharyngeal cancer (De‐ESCALaTE HPV): An open‐label randomised controlled phase 3 trial. Lancet 2019;393:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marur S, Li S, Cmelak AJ et al. E1308: Phase II trial of induction chemotherapy followed by reduced‐dose radiation and weekly cetuximab in patients with HPV‐associated resectable squamous cell carcinoma of the oropharynx‐ ECOG‐ACRIN Cancer Research Group. J Clin Oncol 2017;35:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yom SS, Torres‐Saavedra P, Caudell JJ et al. Reduced‐dose radiation therapy for HPV‐associated oropharyngeal carcinoma (NRG Oncology HN002). J Clin Oncol 2021;39:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seiwert TY, Foster CC, Blair EA et al. OPTIMA: A phase II dose and volume de‐escalation trial for human papillomavirus‐positive oropharyngeal cancer. Ann Oncol 2019;30:1673. [DOI] [PubMed] [Google Scholar]
- 42. Best AL, Logan RG, Vázquez‐Otero C et al. Application of a health literacy framework to explore patients’ knowledge of the link between HPV and cancer. J Health Commun 2018;23:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Llewellyn CD, Weinman J, McGurk M et al. Can we predict which head and neck cancer survivors develop fears of recurrence? J Psychosom Res 2008;65:525–532. [DOI] [PubMed] [Google Scholar]
- 44. Medicare telemedicine health care provider fact sheet . Centers for Medicare & Medicaid Services. Available at https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 31, 2020.
- 45. Malik A, Nair S, Sonawane K et al. Outcomes of a telephone‐based questionnaire for follow‐up of patients who have completed curative‐intent treatment for oral cancers. JAMA Otolaryngol Head Neck Surg 2020;146:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beeler WH, Bellile EL, Casper KA et al. Patient‐reported financial toxicity and adverse medical consequences in head and neck cancer. Oral Oncol 2020;101:104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cohen EE, LaMonte SJ, Erb NL et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J Clin 2016;66:203–239. [DOI] [PubMed] [Google Scholar]
- 48. Nekhlyudov L, Lacchetti C, Davis NB et al. Head and neck cancer survivorship care guideline: American Society of Clinical Oncology clinical practice guideline Endorsement of the American Cancer Society Guideline. J Clin Oncol 2017;35:1606–1621. [DOI] [PubMed] [Google Scholar]
- 49. Chera BS, Kumar S, Shen C et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV‐associated oropharyngeal cancer. J Clin Oncol 2020;38:1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gharzai LA, Li P, Jaworski E et al. Can patient reported quality of life predict locoregional recurrence in oropharyngeal cancer? Int J Radiat Oncol Biol Phys 2020;106:1199–1200. [Google Scholar]
- 51. Basch E, Deal AM, Kris MG et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 2016;34:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Basch E, Deal AM, Dueck AC et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nekhlyudov L, Mollica MA, Jacobsen PB et al. Developing a quality of cancer survivorship care framework: Implications for clinical care, research, and policy. J Natl Cancer Inst 2019;111:1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tota JE, Best AF, Zumsteg ZS et al. Evolution of the oropharynx cancer epidemic in the United States: Moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J Clin Oncol 2019;37:1538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pike LRG, Royce TJ, Mahal AR et al. Outcomes of HPV‐associated squamous cell carcinoma of the head and neck: Impact of race and socioeconomic status. J Natl Compr Canc Netw 2020;18:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Su W, Miles BA, Posner M et al. Surveillance Imaging in HPV‐related Oropharyngeal Cancer. Anticancer Res 2018;38:1525–1529. [DOI] [PubMed] [Google Scholar]
- 57. Canavan JF, Harr BA, Bodmann JW et al. Impact of routine surveillance imaging on detecting recurrence in human papillomavirus associated oropharyngeal cancer. Oral Oncol 2020;103:104585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.


