Abstract
Somatic mutations in human epidermal growth factor receptor 2 (HER2) are present in approximately 3% of breast cancers. Some HER2 mutations are activating, and they represent a mechanism of resistance to conventional anti‐HER2 therapies such as trastuzumab and lapatinib. Consistently, in patients with HER2‐amplified breast cancer, these mutations are predominantly observed in metastatic tumors obtained after exposure to anti‐HER2 systemic therapies, possibly after clonal selection. Therefore, it is rare to find coexistent HER2 mutation and amplification in the early clinical course, and thus, the clinical relevance of HER2 mutation to the sensitivity to HER2‐targeted drugs, particularly antibody‐drug conjugates (ADCs) such as ado‐trastuzumab emtansine (T‐DM1) and the recently approved fam‐trastuzumab deruxtecan (T‐DXd), remains unclear. In this article, we describe a patient with de novo metastatic breast cancer who exhibited both HER2 amplification and the L755S mutation in the untreated primary breast tumor obtained at the initial diagnosis, and the lesion responded to T‐DM1 and T‐DXd after exhibiting clinical resistance to other HER2‐targeted drugs. Our current case findings suggested that anti‐HER2 ADCs should be prioritized over conventional trastuzumab‐ or lapatinib‐based therapies for patients with HER2‐amplified and comutated tumors.
Key Points
Although HER2 mutations were implicated in resistance to anti‐HER2 monoclonal antibodies or HER2 tyrosine kinase inhibitors in preclinical studies, their clinical impact on sensitivity to anti‐HER2 drugs is unclear owing to the rarity of concomitant HER2 mutation and HER2 amplification.
A case of de novo metastatic breast cancer harboring both HER2 amplification and the L755S mutation in an untreated breast primary tumor displayed clinical resistance to standard trastuzumab‐ or lapatinib‐based therapies but good responses to ado‐trastuzumab emtansine (T‐DM1) and fam‐trastuzumab deruxtecan (T‐DXd).
Anti‐HER2 antibody‐drug conjugates such as T‐DM1 and T‐DXd may be prioritized over conventional trastuzumab‐ or lapatinib‐containing therapies for patients with HER2‐amplified and comutated tumors.
Short abstract
This article reports the case of a patient with de novo metastatic breast cancer who harbored both HER2 amplification and the L755S mutation in the untreated primary breast tumor obtained at the initial diagnosis, detailing her response to treatment with HER2‐targeted drugs.
Introduction
Amplification of human epidermal growth factor receptor 2 (HER2) and/or overexpression of HER2 protein (HER2‐positive) are observed in approximately 20% of breast cancers, and these changes are associated with poor prognosis [1]. Since the late 1990s, the development of HER2‐targeted drugs, including anti‐HER2 monoclonal antibodies (mAbs), HER2 tyrosine kinase inhibitors (TKIs), and HER2‐directed antigen‐drug conjugates (ADCs), has significantly improved the prognosis of patients with HER2‐positive breast cancer [2].
Recent genome sequencing projects revealed that HER2 mutations also exist in breast cancer [3]. The incidence of HER2 mutations in breast cancer is generally low, including a reported rate of 3.5% (34/982) in The Cancer Genome Atlas (TCGA) database, which included untreated primary tumor samples. In a TCGA cohort of 169 patients with HER2 copy number gain, 10 patients (5.9%) also exhibited HER2 missense mutations. A recent study by Memorial Sloan Kettering‐Integrated Mutation Profiling of Actionable Cancer Targets also detected HER2 mutations in 13 of 184 patients (7.1%) with HER2‐positive breast cancer, but most mutations were observed in metastatic tumors obtained after treatment with systemic therapies [4].
One of the most frequently observed HER2 mutations is the L755S substitution [3]. Preclinical findings suggested that the HER2 L755S mutation is activating [5], and it may cause resistance to lapatinib and trastuzumab [3, 4, 6, 7]. Consistently, the mutation has been observed mainly in metastatic tumors after exposure to trastuzumab‐containing regimens [4, 8]. However, because of the rarity of the HER2 L755S mutation in the early clinical course, the clinical relevance of the mutation to the sensitivity to HER2‐targeted drugs, particularly HER2‐directed ADCs such as ado‐trastuzumab emtansine (T‐DM1) and fam‐trastuzumab deruxtecan (T‐DXd), is unclear.
In this study, we reported the case of a patient with de novo metastatic breast cancer who harbored both HER2 amplification and the L755S mutation in the untreated primary breast tumor obtained at the initial diagnosis, and the lesion responded to T‐DM1 and T‐DXd after exhibiting clinical resistance to trastuzumab‐ and lapatinib‐based therapies.
Patient Story
A 47‐year‐old premenopausal woman visited a local hospital with complaints of a rapidly enlarging left breast mass. The left breast was occupied by a tumor, and core needle biopsy of the tumor was performed (Fig. 1). Pathological analysis revealed estrogen receptor‐positive, progesterone receptor‐positive, and HER2 3+ (HER2‐positive) invasive ductal carcinoma. The patient had contralateral cervical lymph node metastasis (stage IV). Doxorubicin and cyclophosphamide chemotherapy was initiated, but the left breast primary tumor progressed according to inspection after three cycles (Fig. 1). The treatment was then switched to the combination of pertuzumab, trastuzumab, and docetaxel. Although the tumors partially responded to the combination therapy, the primary tumor progressed after nine cycles administered over 6 months (Fig. 1). The patient was referred to our hospital, and treatment with T‐DM1 was initiated. The tumors well responded to T‐DM1, and the response lasted for 1 year (Fig. 1). Because the left primary tumor slowly progressed during T‐DM1 therapy, the treatment was switched to trastuzumab and vinorelbine. The patient exhibited progression at the first disease evaluation after four cycles of this therapy, after which the treatment was switched to lapatinib and capecitabine. At that time, we submitted a core needle biopsy specimen of the primary tumor obtained at the initial diagnosis to multigene panel testing using FoundationOne CDx (Chugai Pharmaceuticals, Tokyo, Japan). Rapid progression was observed after four cycles of the combination of lapatinib and capecitabine (Fig. 1). Because T‐DXd then became available in Japan, we decided to treat the patient with this drug. The left primary tumor and contralateral cervical lymph nodes began to shrink and redness of the left breast was alleviated after the first dose of T‐DXd (Fig. 1).
Figure 1.
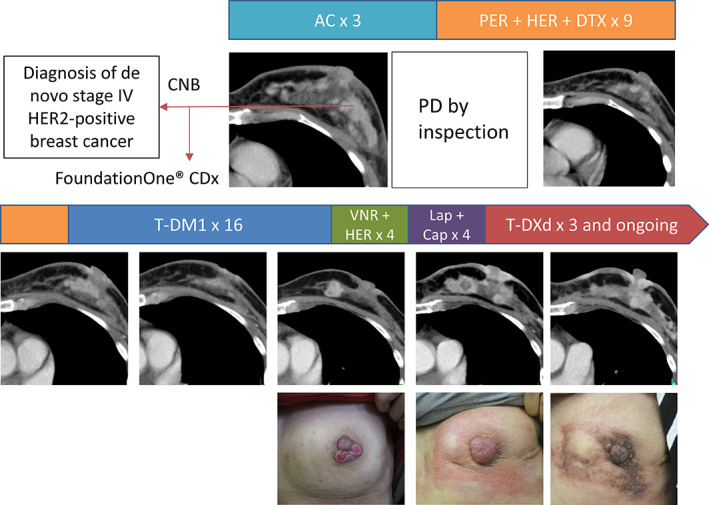
Computed tomography images and photographs of the left breast over the treatment course. Abbreviations: AC, doxorubicin + cyclophosphamide; Cap, capecitabine; CNB, core needle biopsy; Lap, lapatinib; PD, progressive disease; PER + HER + DTX, pertuzumab + trastuzumab + docetaxel; T‐DM1, ado‐trastuzumab emtansine; T‐DXd, fam‐trastuzumab deruxtecan; VNR, vinorelbine.
Molecular Tumor Board
Genotyping Results and Interpretation of the Molecular Results
The percentage of tumor nuclei content of the specimen submitted to FoundationOne CDx was 20%. The genomic findings of FoundationOne CDx revealed the HER2 L755S mutation along with amplification of the gene. The HER2 copy number and the variant allele frequency of L755S were 16% and 52%, respectively. Only TP53 H179R was identified as a pathogenic comutation.
Previous studies consistently illustrated that in HER2‐amplified breast cancer, HER2 mutations are found predominantly in metastatic tumors obtained after exposure to anti‐HER2 systemic therapies [4, 8], and this finding is speculated to be a consequence of clonal selection. If this hypothesis is correct, then mutant HER2 alleles may not be detected in primary tumors because only a tiny fraction of cells have mutant alleles and/or because mutant alleles are diluted in amplified predominantly wild‐type HER2 alleles. Assuming that a part of the tumor cell population has 16 HER2 alleles carrying the L755S mutation and the remaining cells have 16 wild‐type HER2 alleles, L755S‐positive cells are estimated to occupy 78% of the tumor cell population. Conversely, assuming that L755S alleles are distributed equally among tumor cells with 16 HER2 alleles, each cell is estimated to have approximately 12.5 mutant alleles. In either case, the L755S mutation in this particular case was prominent at the cellular and allelic levels despite being present in the primary tumor before systemic therapy, which provided us an ideal opportunity to gain insight into the therapeutic impact of the mutation. Conversely, it should be noted that although the genotypes of HER2 and other related genes could have changed in response to systemic therapies, we do not have genomic data from serial biopsies or circulating tumor DNA (ctDNA) to confirm this supposition.
Functional and Clinical Significance of the Specific Mutation in the Particular Cancer
The L755S substitution is one of the most frequently observed HER2 mutations [3]. This mutation was reported to be an activating mutation [5] that induced resistance to the HER2 TKI lapatinib when transfected into HER2 nonamplified cells [3, 6, 7]. A protein structure simulation study suggested that the L755 side chain is in close proximity to the binding site for HER2 TKIs, and mutation of this residue to serine may impair the binding and activity of lapatinib [3]. A recent study demonstrated that ectopic expression of the HER2 L755S mutation in HER2‐amplified SKBR3 and BT474 cells induced biochemical and/or biological resistance to the anti‐HER2 mAbs trastuzumab and lapatinib (Fig. 2) [4]. Another study revealed that the only common somatic mutation gained in BT474 cells chronically exposed to lapatinib alone or together with trastuzumab was the HER2 L755S substitution [9]. Another study discussed three HER2‐positive breast cancer cases in which the HER2 L755S mutation was found in recurrent metastatic tumors obtained after adjuvant therapy with trastuzumab but not in primary tumors obtained before trastuzumab exposure [8]. These preclinical and clinical findings strongly suggest that the HER2 L755S mutation may cause resistance to lapatinib and trastuzumab.
Figure 2.
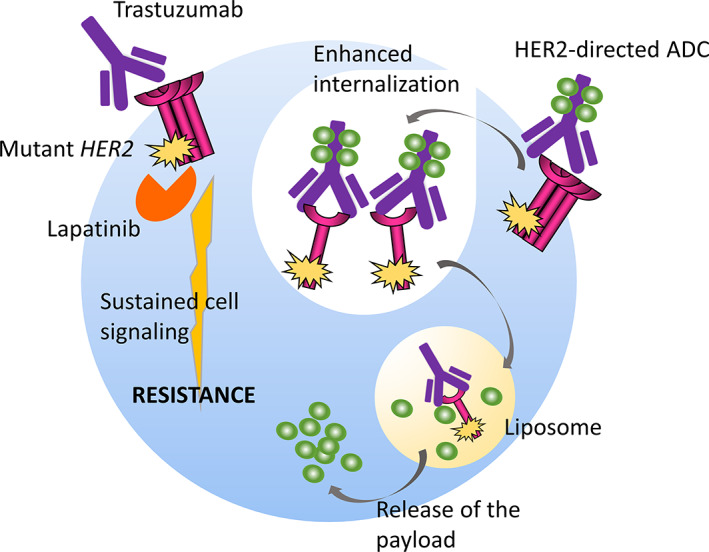
The schematic reveals that although HER2 mutations induce biochemical resistance to conventional anti‐HER2 drugs such as trastuzumab and lapatinib by activating downstream cell signaling pathways, they may also enhance internalization of the HER2 protein and the consequent endocytosis of the HER2–ADC complex and release of the ADC payload in both HER2‐mutant and HER2‐amplified tumors.
Consistently, in our current case, although the patient initially responded to the pertuzumab, trastuzumab, and docetaxel regimen, the observation of tumor progression after 6 months of therapy indicated that the tumor was refractory considering that the median progression‐free survival (PFS) in patients treated with the triplet regimen was 18.7 months in the CLEOPATRA registry trial [10]. Consistent with previous preclinical and clinical reports, this patient also exhibited clinical resistance to the trastuzumab plus vinorelbine and lapatinib plus capecitabine regimens (Fig. 1).
Potential Strategies to Target the Pathway and Implications for Clinical Practice
A recently published study using isogenic cell lines stably expressing wild‐type or mutant (S310F and L755S) HER2 demonstrated that HER2 mutations enhance the internalization of T‐DM1 [11] (Fig. 2). The study also revealed that T‐DXd was active in a patient with lung cancer whose tumor harbored both HER2 amplification and the S310F mutation and who experienced progression during T‐DM1 treatment [11]. The patient in our current report displayed tumor regression for 1 year during T‐DM1 therapy (Fig. 1). Considering that the median PFS of T‐DM1 observed in its registry trial (EMILIA study) was 9.6 months [12], T‐DM1 was apparently efficacious in the patient. Furthermore, this patient rapidly responded to the newly approved drug T‐DXd (Fig. 1). These findings support the hypothesis that HER2 mutations do not compromise the activity of HER2‐directed ADCs such as T‐DM1 and T‐DXd. However, additional clinical data are required to confirm whether the copresence of HER2 mutations enhance the activity of these drugs. Investigations assessing whether the mutant HER2 allele fraction decreases after T‐DXd exposure using serial ctDNA monitoring would help answer this question.
As another means to overcome L755S‐induced resistance to trastuzumab or lapatinib, irreversible pan‐HER family inhibitors such as neratinib and afatinib have been tested preclinically and clinically [4, 13, 14]. SKBR3 and BT474 HER2‐amplified cell lines acquired resistance to lapatinib when transduced with lentiviral vectors encoding L755S‐mutant HER2 but remained sensitive to neratinib [4]. Consistent with this finding, in the SUMMIT trial, a basket trial of neratinib, several patients with L755S HER2 breast (HER2‐negative) or lung cancer displayed partial responses [13]. In the SUMMIT trial, 15 of 86 patients (17%) had concurrent HER2 mutations and gene amplification, but the copresence of gene amplification was not reported to be correlated with clinical benefit [13]. Likewise, a recent study focusing on patients with breast cancer in the SUMMIT trial reported that two of three patients with both HER2 mutation and amplification did not experience a clinical benefit from neratinib therapy [14]. Another recently published study demonstrated that the irreversible kinase inhibitors neratinib and afatinib, but not the reversible inhibitors lapatinib and tucatinib, enhanced the internalization of T‐DM1 by promoting the ubiquitination of HER2 protein and therefore increased the antitumor activity of T‐DM1 [11], suggesting the utility of the combination of an irreversible HER2 TKI with a HER‐2–directed ADC.
Patient Update
At the time of this writing, the patient has completed nine cycles of T‐DXd, and her left breast tumors are stabilized at the approximate size presented in Figure 1. She has not experienced any major toxicity from T‐DXd.
Conclusion
Our current case provided evidence that the HER2 L755S mutation could coexist with HER2 amplification in a primary tumor before exposure to systemic therapy prominently at the cellular and allelic levels and suggested that anti‐HER2 ADCs such as T‐DM1 and T‐DXd should be prioritized over conventional trastuzumab‐ or lapatinib‐containing therapies for patients with HER2‐amplified and comutated tumors.
Author Contributions
Conception/design: Toru Mukohara
Provision of study material or patients: Ako Hosono
Collection and/or assembly of data: Akiko Nakayama, Sachiyo Mimaki
Data analysis and interpretation: Toru Mukohara, Sachiyo Mimaki, Katsuya Tsuchihara, Takeshi Kuwata
Manuscript writing: Toru Mukohara, Sachiyo Mimaki, Kenichi Harano, Nobuaki Matsubara, Takeshi Kuwata
Final manuscript approval: Toru Mukohara, Ako Hosono, Sachiyo Mimaki, Akiko Nakayama, Shota Kusuhara, Chikako Funasaka, Takehiro Nakao, Yoko Fukasawa, Chihiro Kondoh, Kenichi Harano, Yoichi Naito, Nobuaki Matsubara, Katsuya Tsuchihara, Takeshi Kuwata
Disclosures
Toru Mukohara: Daiichi‐Sankyo, Chugai Pharmaceuticals (RF); Akiko Nakayama: Chugai, Novartis (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank Joe Barber Jr., Ph.D., from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
For Further Reading: Richard S.P. Huang, Xinyan Li, James Haberberger et al. Biomarkers in Breast Cancer: An Integrated Analysis of Comprehensive Genomic Profiling and PD‐L1 Immunohistochemistry Biomarkers in 312 Patients with Breast Cancer. The Oncologist 2020;25;943–953.
Implications for Practice: This integrated programmed death‐ligand 1 immunohistochemistry and comprehensive genomic profiling methodology identified 32% of the tested patients as eligible for at least one of the two new Food and Drug Administration‐approved therapies, atezolizumab or alpelisib, and an additional 61.2% (191/312) had other biomarker‐guided potential therapeutic options. These findings suggest new research opportunities to evaluate the predictive utility of other commonly seen PIK3CA mutations in hormone receptor‐positive breast cancers and to standardize tumor mutation burden cutoffs to evaluate its potentially predictive role in triple‐negative breast cancer.
References
- 1. Singh K, Tantravahi U, Lomme MM et al. Updated 2013 College of American Pathologists/American Society of Clinical Oncology (CAP/ASCO) guideline recommendations for human epidermal growth factor receptor 2 (HER2) fluorescent in situ hybridization (FISH) testing increase HER2 positive and HER2 equivocal breast cancer cases; Retrospective study of HER2 FISH results of 836 invasive breast cancers. Breast Cancer Res Treat 2016;157:405–411. [DOI] [PubMed] [Google Scholar]
- 2. Wang J and Xu B. Targeted therapeutic options and future perspectives for Her2‐positive breast cancer. Signal Transduct Target Ther 2019;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bose R, Kavuri SM, Searleman AC et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 2013;3:224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cocco E, Javier Carmona F, Razavi P et al. Neratinib is effective in breast tumors bearing both amplification and mutation of ERBB2 (HER2). Sci Signal 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nagano M, Kohsaka S, Ueno T et al. High‐throughput functional evaluation of variants of unknown significance in ERBB2. Clin Cancer Res 2018;24:5112–5122. [DOI] [PubMed] [Google Scholar]
- 6. Trowe T, Boukouvala S, Calkins K et al. EXEL‐7647 inhibits mutant forms of ERBB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res 2008;14:2465–2475. [DOI] [PubMed] [Google Scholar]
- 7. Kancha RK, von Bubnoff N, Bartosch N et al. Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PLoS One 2011;6:e26760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zuo WJ, Jiang YZ, Wang YJ et al. Dual characteristics of novel HER2 kinase domain mutations in response to HER2‐targeted therapies in human breast cancer. Clin Cancer Res 2016;22:4859‐4869. [DOI] [PubMed] [Google Scholar]
- 9. Xu X, De Angelis C, Burke KA et al. HER2 reactivation through acquisition of the HER2 L755S mutation as a mechanism of acquired resistance to HER2‐targeted therapy in HER2(+) breast cancer. Clin Cancer Res 2017;23:5123–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baselga J, Cortes J, Kim SB et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li BT, Michelini F, Misale S et al. HER2‐mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov 2020;10:674–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verma S, Miles D, Gianni L et al. Trastuzumab emtansine for HER2‐positive advanced breast cancer. N Engl J Med 2012;367:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hyman DM, Piha‐Paul SA, Won H et al. HER kinase inhibition in patients with HER2‐ and HER3‐mutant cancers. Nature 2018;554:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smyth LM, Piha‐Paul SA, Won HH et al. Efficacy and determinants of response to HER kinase inhibition in HER2‐mutant metastatic breast cancer. Cancer Discov 2020;10:198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]


