Abstract
Background
Understanding the cost of delivering breast cancer (BC) care in low‐ and middle‐income countries (LMICs) is critical to guide effective care delivery strategies. This scoping review summarizes the scope of literature on the costs of BC care in LMICs and characterizes the methodological approaches of these economic evaluations.
Materials and Methods
A systematic literature search was performed in five databases and gray literature up to March 2020. Studies were screened to identify original articles that included a cost outcome for BC diagnosis or treatment in an LMIC. Two independent reviewers assessed articles for eligibility. Data related to study characteristics and methodology were extracted. Study quality was assessed using the Drummond et al. checklist.
Results
Ninety‐one articles across 38 countries were included. The majority (73%) of studies were published between 2013 and 2020. Low‐income countries (2%) and countries in Sub‐Saharan Africa (9%) were grossly underrepresented. The majority of studies (60%) used a health care system perspective. Time horizon was not reported in 30 studies (33%). Of the 33 studies that estimated the cost of multiple steps in the BC care pathway, the majority (73%) were of high quality, but studies varied in their inclusion of nonmedical direct and indirect costs.
Conclusion
There has been substantial growth in the number of BC economic evaluations in LMICs in the past decade, but there remain limited data from low‐income countries, especially those in Sub‐Saharan Africa. BC economic evaluations should be prioritized in these countries. Use of existing frameworks for economic evaluations may help achieve comparable, transparent costing analyses.
Implications for Practice
There has been substantial growth in the number of breast cancer economic evaluations in low‐ and middle‐income countries (LMICs) in the past decade, but there remain limited data from low‐income countries. Breast cancer economic evaluations should be prioritized in low‐income countries and in Sub‐Saharan Africa. Researchers should strive to use and report a costing perspective and time horizon that captures all costs relevant to the study objective, including those such as direct nonmedical and indirect costs. Use of existing frameworks for economic evaluations in LMICs may help achieve comparable, transparent costing analyses in order to guide breast cancer control strategies.
Keywords: Breast cancer, Costs, Developing countries, Health economics, Review
Short abstract
This review summarizes the literature on the cost of breast cancer care in low‐ and middle‐income countries.
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer among women worldwide and the leading cause of cancer death in more than 100 countries [1]. In 2020, there were about 2.3 million new BC cases and about 685,000 BC deaths [1, 2]. These deaths disproportionately occur in low‐ and middle‐income countries (LMICs), where BC mortality rates are rapidly rising [3]. Regions with the highest mortality to incidence ratio include Africa (0.47), South‐Central Asia (0.48), and Melanesia (0.48) [3]. This is in contrast to North America, Australia/New Zealand, and Western Europe, where the BC mortality to incidence ratio is 0.16, 0.17, and 0.18, respectively. Poor outcomes in LMICs reflect the large proportion of women with BC who present with advanced disease and have limited access to diagnosis and treatment [4].
Effective therapeutic options to treat early BC are becoming more widely available at low cost [5, 6, 7]. More widespread deployment of BC diagnostics and treatments could salvage many life years for women in LMICs. Although cancer programs established in low‐resource settings have demonstrated the feasibility of cancer care delivery in LMICs, concerns and misconceptions about affordability of cancer care continue to impede efforts to establish and expand care [8]. Understanding the cost of delivering high‐quality cancer care in LMICs is integral for strategic policy planning and investment in cancer control.
Prior systematic reviews of BC cost in LMICs have largely focused on screening programs and have noted a lack of strong evidence to provide specific recommendations [9, 10, 11]. Reviews that capture the cost of BC diagnosis or treatment in LMICs are limited, as they often consider the cost‐effectiveness of a specific chemotherapeutic or biologic therapy or are largely descriptive [5, 7, 12, 13, 14]. One prior systematic review of the cost of BC care in LMICs, published in 2013, concluded that the evidence base to guide strategies for BC control in LMICs was limited and of poor quality [15]. The majority of economic analyses captured by this review estimated the incremental cost or cost‐effectiveness of a singular diagnostic or therapeutic step, rather than the total cost of the various steps in the breast cancer care pathway. The focus on incremental cost may limit these studies’ applicability given that the breast cancer care pathway is complex and involves multiple diagnostic and therapeutic steps.
Since 2013, many LMICs have made major strides in building capacity to diagnose and treat BC, including the development of cancer centers in low‐resource settings as well as expanded access to inexpensive drugs and novel diagnostic technologies [16, 17, 18, 19]. In 2015, the United Nations Sustainable Development Goals included reducing premature deaths from noncommunicable diseases, of which breast cancer is a considerable part [7]. In order to support governments of LMICs in their commitment to developing and implementing locally appropriate cancer control strategies, researchers have developed frameworks and tools to analyze interventions for effectiveness and affordability in LMICs [7, 20, 21, 22]. Given these strides over the past decade, an updated assessment of the literature is needed to reflect the changing landscape of BC care in LMICs and guide research priorities. This review aims to summarize the scope of literature on the costs of BC care in LMICs, characterize the methodological approaches used in these economic evaluations, and evaluate their methodological rigor.
Materials and Methods
The scoping review was conducted in accordance with the Joanna Briggs Institute methodology for scoping reviews, and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses‐Scoping Review was referenced to ensure that all suggested reporting items were included [23, 24]. An a priori protocol was used [25].
Search Strategy
Five databases—including MEDLINE (Ovid), Embase (Elsevier), Web of Science (Clarivate Analytics), Global Health (EBSCO), and World Health Organization (WHO) Global Index Medicus—were searched up to March 19, 2020. The search strategy was developed in consultation with a medical librarian and used Medical Subject Headings related to breast neoplasms, costing, and LMICs (supplemental online Appendix 1). Sources of unpublished studies and gray literature—including the Breast Global Health Initiative, Disease Control Priorities 3rd edition, and the World Health Organization—were also searched for relevant articles. The reference lists of articles included in the scoping review were also screened for additional studies.
Eligibility Criteria
Studies included in the review were required to report a cost outcome for BC diagnosis or treatment in LMICs, as defined by 2020 World Bank classifications [26]. We excluded studies that assessed only BC screening, palliative care, or mortality costs as well as studies that did not include any original cost analysis but used previously published cost analyses. Studies that presented aggregate costs for multiple cancers or an entire world region but did not stratify costs for BC or LMICs, respectively, were excluded. Reviews, editorials, and meeting abstracts were also excluded. This scoping review was limited to studies for which manuscripts were available in English.
Screening and Data Extraction
Following the search, all identified records were collated and uploaded to Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) and duplicates were removed [27]. Studies underwent a primary and secondary screen as described in supplemental online Appendix 2A [28]. Final studies included in the scoping review were divided into two categories: studies that estimated the cost of a singular step in the BC care pathway (e.g., cost of chemotherapy alone, or cost of second‐line treatment for metastatic breast cancer) versus studies that estimated the cost of multiple steps in the BC care pathway (e.g., cost of multiple treatment modalities across breast cancer stages). We created this dichotomy in order to perform a more in‐depth characterization of studies that capture multiple steps in the breast cancer care pathway, as these studies offer an opportunity for more detailed cross‐study comparisons of cost comprehensiveness.
One reviewer (P.E.) extracted data from all studies using a data extraction tool programmed in Research Electronic Data Capture (REDCap) [29]. The data extraction tool was iteratively modified by the research team after pilot data extraction from 5 studies (supplemental online Appendix 2B, 2C) [5, 15, 30]. All studies underwent data extraction for variables related to study characteristics: world region, economic status, study design, BC stage, BC subtype, costing perspective, and time horizon [26]. Studies that estimated the cost of multiple steps in the BC care pathway underwent additional data extraction for variables related to cost estimation, in order to provide a more detailed characterization of cost categories and cost analysis approaches used in these studies. The additional variables related to cost estimation include costing approach, cost categories, cost inputs for each cost category, data sources for cost estimation, cost disaggregation by stage, cost disaggregation by cost input, currency details, cost discounting, inflation adjustments, uncertainty estimation, sensitivity analysis, and quality assessment.
Quality assessment was performed using an established 35‐point economic evaluation checklist by Drummond et al. [31]. Similar to previous reviews of economic evaluation, checklist items that did not apply to any of the reviewed studies were removed [5, 15]. A three‐point response scale was used to grade the quality of each checklist item, including 0 (not considered), 1 (partially considered), 2 (fully considered), and not applicable [32]. The sum of scores for each study was compared with the maximum attainable score for each study. A study was considered to be of high, medium, or poor quality if it scored ≥70%, 51%–69%, or ≤ 50% of its maximum score, respectively [32]. The extracted data were tabulated and summated for all reviewed studies. A second reviewer (C.S.) independently performed data extraction for 15% of studies that estimated the cost of multiple steps in the BC care pathway (n = 5). The percent of agreement score for data extraction was calculated.
Results
Search Results
The search strategy resulted in a total of 6,340 studies: 1,670 from MEDLINE, 1,499 from Embase, 1,570 from Web of Science, 813 from Global Health, and 788 from WHO Global Index Medicus (Fig. 1). After merging the results from all sources and removing duplicates, 3,866 studies remained. After the primary screen of titles and abstracts, 227 studies remained. After a secondary screen with full text review, 91 studies met all eligibility criteria and were included in the scoping review [33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123]. The full texts of 13/227 articles (6%) were excluded because their full texts were not successfully retrieved. Thirty‐three of 227 (15%) articles were excluded because their English full texts were not available (Fig. 1; supplemental online Appendix 3; supplemental online Table 4). Cohen's Kappa score for the primary and secondary screen were 0.62 and 0.82, respectively. Of the 91 reviewed studies, 58 (64%) estimated the cost of a singular step in the BC care pathway [33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90] whereas 33 (36%) estimated the cost of multiple steps in the BC care pathway [91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123]. The percent of agreement for data extraction and the Drummond et al. checklist were 97% and 93%, respectively. Specific characteristics of the studies summarized below are outlined in supplemental online Appendix 3 and supplemental online Tables 1 and 2.
Figure 1.
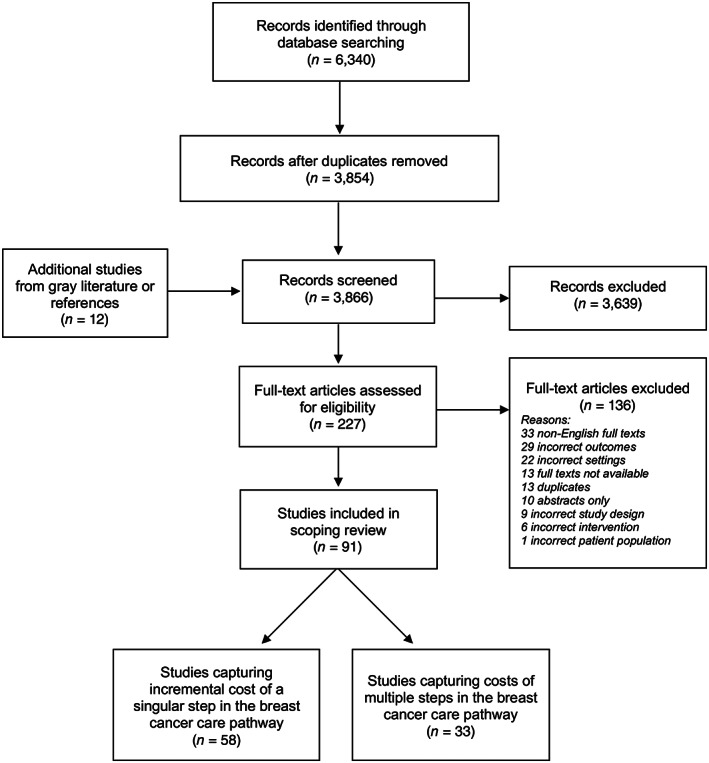
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram.
Scope of Literature and Study Characteristics
Table 1 outlines the scope of literature on the cost of BC care in LMICs. Of the 91 reviewed studies, 73% were published after 2012 (n = 66). Studies spanned across 38 countries (Fig. 2) [124]. Latin America & Caribbean (n = 26, 29%), East Asia & Pacific (n = 25, 27%), and Middle East & North Africa (n = 19, 21%) had the greatest number of studies, whereas South Asia (n = 8, 9%), Sub‐Saharan Africa (SSA; n = 8, 9%), and Europe & Central Asia (n = 5, 5%) had the fewest number of studies. Specific countries with the highest number of studies were China (n = 16), Iran (n = 14), Brazil (n = 12), and Mexico (n = 8). Of the reviewed studies, 70 (77%) were conducted in upper‐middle‐income countries, 22 (24%) in lower‐middle‐income countries, and 2 (2%) in low‐income countries. Forty‐four studies (48%) were cost analyses or cost of illness studies, whereas 45 studies (49%) were cost‐effectiveness or cost‐utility analyses.
Table 1.
Characteristics of studies in scoping review
| Characteristics | Reviewed studies (n = 91) |
|---|---|
| Publication year (1995–2020) | |
| Before 2013 | 25 (27) |
| 2013–2020 | 66 (73) |
| Number of countries | 38 |
| World region | |
| Latin America & Caribbean | 26 (29) |
| Sub‐Saharan Africa | 8 (9) |
| Middle East & North Africa | 19 (21) |
| Europe & Central Asia | 5 (5) |
| South Asia | 8 (9) |
| East Asia & Pacific | 25 (27) |
| Economic status a | |
| Upper‐middle income | 70 (77) |
| Lower‐middle income | 22 (24) |
| Low income | 2 (2) |
| Type of economic evaluation | |
| Cost analysis/Cost of illness | 44 (48) |
| Cost‐effectiveness/Utility analysis | 45 (49) |
| Cost minimization analysis | 2 (2) |
| Study design | |
| Observational | 41 (45) |
| Model‐based | 42 (46) |
| Experimental | 2 (2) |
| Other | 6 (7) |
| Evaluated intervention for cost estimation | |
| Diagnosis | 5 (5) |
| Treatment | 55 (60) |
| Diagnosis and treatment | 31 (34) |
| BC stages | |
| Early | 26 (29) |
| Advanced | 15 (16) |
| All | 44 (48) |
| Unknown | 1 (1) |
| Other (e.g., operable, node+) | 5 (5) |
| BC types | |
| Hormone receptor+ only | 13 (14) |
| HER2+ only | 19 (21) |
| Study perspective a | |
| Health care provider | 6 (7) |
| Health care payer | 20 (22) |
| Health care (not specified) | 29 (32) |
| Patient | 11 (12) |
| Societal | 23 (25) |
| Unknown | 10 (11) |
| Time horizon | |
| <1 year | 5 (5) |
| 1–9 years | 25 (27) |
| 10–19 years | 6 (7) |
| 20–39 years | 6 (7) |
| Lifetime or ≥ 40 | 19 (21) |
| Unknown | 30 (33) |
Data are presented as n (%).
Categories are not mutually exclusive, and percentages may sum up to more than 100%.
Abbreviations: BC, breast cancer; HER2, human epidermal growth factor receptor 2.
Figure 2.
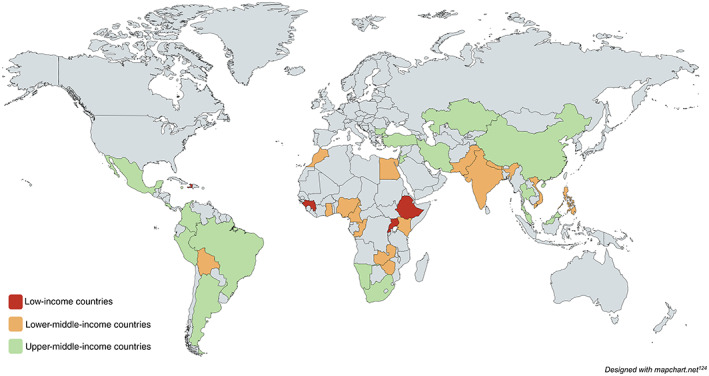
Countries represented in reviewed studies.
Five studies (5%) evaluated the cost of diagnosis, 55 studies (60%) evaluated the cost of treatment, and 31 studies (34%) evaluated the cost of both diagnosis and treatment. Forty‐four studies (48%) included all BC stages, whereas 26 studies (29%) and 15 studies (16%) included early or advanced stages, respectively. Thirteen studies (14%) focused on hormone receptor–positive BC, whereas 19 studies (21%) focused on HER2‐positive BC. Fifty‐five studies (60%) used a health care perspective for estimating costs, whereas 23 studies (25%) and 11 studies (12%) used a societal or patient perspective, respectively. Eight studies (9%) presented costing data from two perspectives. The perspectives of 10 studies (11%) were not reported. The time horizons of the studies ranged from 3 months to the lifetime of the study population. Thirty studies (33%) had a time horizon less than 10 years, 12 studies (13%) between 10 and 39 years, and 19 studies (21%) greater than 39 years or lifetime. The time horizons of 30 studies (33%) were not reported (Table 1).
Cost Estimation Characteristics
Table 2 outlines the cost estimation characteristics for the 33 studies that estimated the cost of multiple steps in the BC care pathway. The large majority of these studies (n = 27, 82%) were cost analyses or cost of illness studies (supplemental online Appendix 4). Of these 33 studies, 22 (67%) used a micro‐costing approach (in which the cost of each input was estimated separately to calculate total cost), 5 (15%) used a gross‐costing approach, and 6 (18%) used both. Studies used various data sources to estimate cost and resource use, including information from patients (e.g., interviews, questionnaires, shadowing), medical records, hospital data, government data, insurance data, literature, and expert opinion. The most commonly used sources included government data (n = 16, 48%), hospital data (n = 16, 48%), patients (n = 14, 42%), and medical records (n = 13, 39%; Table 2). Twenty‐three studies (70%) used more than one data source to estimate cost and resource use.
Table 2.
Cost estimation approach and inputs for studies estimating the cost of multiple steps in the breast cancer care pathway
| Costing characteristics | Reviewed studies (n = 33) a |
|---|---|
| Costing approach | |
| Micro | 22 (67) |
| Gross | 5 (15) |
| Micro + gross | 6 (18) |
| Sources for costs and resource use | |
| Patients (e.g., interviews, questionnaires) | 14 (42) |
| Medical records | 13 (39) |
| Hospital finance/administrative data | 16 (48) |
| Government data | 16 (48) |
| Insurance data | 4 (12) |
| Literature | 8 (24) |
| Expert opinion | 10 (30) |
| Cost categories included | |
| Direct medical | 33 (100) |
| Direct nonmedical | 9 (27) |
| Indirect | 7 (21) |
| Inputs into direct medical costs b | 33 (100) |
| Medical visits | 25 (76) |
| Diagnostic studies/pathology | 23 (70) |
| Tumor‐directed medications | 30 (91) |
| Supportive medications | 13 (39) |
| Surgery | 27 (82) |
| Radiotherapy | 22 (67) |
| Hospitalization | 26 (79) |
| Imaging | 14 (42) |
| Laboratory tests and blood services (electrolytes, urinalysis, complete blood count) | 22 (67) |
| Palliative care | 10 (30) |
| Training | 4 (12) |
| Administrative/overhead costs | 9 (27) |
| Unspecified medical costs | 4 (12) |
| Inputs into direct nonmedical costs b , c | 9 (100) |
| Food | 7 (78) |
| Travel/Transportation | 9 (100) |
| Accommodation | 5 (56) |
| Food, transportation, or accommodation for companion | 2 (22) |
| Other (e.g., child tutoring, home help) | 1 (11) |
| Inputs into indirect costs b , c | 7 (100) |
| Lost wages from cancer care | 7 (100) |
| Lost wages from disability/premature mortality | 6 (86) |
| Lost wages of companion | 5 (71) |
Data are presented as n (%).
Data from the 33 studies that estimate the cost of multiple steps in the breast cancer care pathway.
Categories are not mutually exclusive, and percentages may sum up to more than 100%.
Inputs into direct medical costs, n = 33; inputs into direct nonmedical costs, n = 9; inputs into indirect costs, n = 7.
Studies also varied greatly in the cost categories included in their cost estimation. All 33 (100%) studies included direct medical costs, but only 9 (27%) included direct nonmedical costs and 7 (21%) included indirect costs. Twenty‐three studies (70%) included only one cost category in their cost estimation (e.g., direct medical only), 4 (12%) included two cost categories (e.g., direct medical and direct nonmedical), and 6 (18%) included all three cost categories (Table 2).
The inputs into each cost category varied across studies. For the 33 studies that included direct medical costs, inputs included tumor‐directed medications (n = 30, 91%), surgery (n = 27, 82%), hospitalization (n = 26, 79%), medical visits (n = 25, 76%), diagnostic studies and pathology (n = 23, 70%), radiotherapy (n = 22, 67%), laboratory tests and blood services such as complete blood count, electrolytes, and urinalysis (n = 22, 67%), imaging (n = 14, 42%), and supportive medications (n = 13, 39%). For the nine studies that included direct nonmedical costs, inputs included travel (n = 9, 100%), food (n = 7, 78%), accommodations (n = 5, 56%), and companions’ food, transportation, or accommodation (n = 2, 22%). For the seven studies that included indirect costs, inputs included lost wages from cancer care (n = 7, 100%), lost wages from disability or premature mortality (n = 6, 86%), and lost wages of companion from cancer care (n = 5, 71%; Table 2).
In addition to variations in cost categories and inputs, studies also varied in their cost estimation analysis and reporting (Table 3). Of the studies in which costs were estimated beyond 1 year (n = 23), 13 studies (57%) did not discount costs. Of the studies in which costs were collected from various calendar years (n = 18), 10 studies (56%) did not adjust for inflation. Twenty‐six studies (79%) presented the costs in USD or International Dollars, whereas 7 studies (21%) used other currencies. Seven studies (21%) did not report the currency year. Eighteen studies (55%) did not report any uncertainty with their cost estimation and 24 studies (73%) did not perform a sensitivity analysis. The granularity of the presented cost outcome also varied across studies. Sixteen studies (48%) did not disaggregate costs by BC stage (n = 16). Of the studies that used micro‐costing (n = 28), 6 (21%) did not disaggregate costs by cost inputs (Table 3).
Table 3.
Cost estimation reporting and analysis for studies estimating the cost of multiple steps in the breast cancer care pathway
| Cost reporting and analysis characteristics | Reviewed studies (n = 33)a |
|---|---|
| Cost discounting b | |
| Yes | 10 (43) |
| No | 13 (57) |
| Not applicable | 10 |
| Inflation adjustment b | |
| Yes | 8 (44) |
| No | 10 (56) |
| Not applicable | 15 |
| Currency | |
| USD or International Dollars | 26 (79) |
| Other currencies | 7 (21) |
| Currency year reported | |
| Yes | 26 (79) |
| No | 7 (21) |
| Cost estimation uncertainty reported | |
| Yes | 15 (45) |
| No | 18 (55) |
| Sensitivity analysis performed | |
| Yes | 9 (27) |
| No | 24 (73) |
| Cost disaggregation by BC stage | |
| Yes | 17 (52) |
| No | 16 (48) |
| Costs disaggregation by inputs b | |
| Yes | 22 (79) |
| No | 6 (21) |
| Not applicable | 5 |
Data are presented as n (%).
Data from the 33 studies that estimate the cost of multiple steps in the breast cancer care pathway.
Percentage scores do not include studies for which the variable was not applicable. Cost discounting was applicable to studies in which costs were estimated beyond 1 year; inflation adjustment was applicable to studies in which costs were collected from various calendar years; cost disaggregation by inputs was applicable to studies in which a micro‐costing approach was used.
Abbreviation: BC, breast cancer.
Quality Assessment
Table 4 outlines the quality assessment of the 33 studies that estimated the costs of multiple steps in the BC care pathway using the economic evaluation checklist by Drummond et al. [31]. Of the 33 studies, 24 (73%) scored ≥70% of the maximum possible score (high quality) whereas 9 (27%) scored 51%–69% of the maximum possible score (medium quality) [32]. In both the “Cost and Effect Estimation” and “Analysis and Interpretation” checklist categories, five studies (15%) scored ≤50% of the maximum score (poor quality). Total and disaggregated scoring for each study is outlined in supplemental online Appendix 3 and supplemental online Table 3.
Table 4.
Quality assessment of breast cancer costing studies
| Percentage of maximum score | Study design | Cost + effect estimation | Analysis + interpretation | Total |
|---|---|---|---|---|
| ≤50% | 0 (0) | 5 (15) | 5 (15) | 0 (0) |
| 50%–70% | 0 (0) | 7 (21) | 12 (36) | 9 (27) |
| ≥70% | 33 (100) | 21 (64) | 16 (48) | 24 (73) |
Data are presented as n (%).
Data from the 33 studies that estimate the cost of multiple steps in the breast cancer care pathway.
Quality assessment based on Drummond checklist used; studies scored based on applicable categories [30].
Discussion
This scoping review of literature on the cost of BC care in LMICs yielded 91 articles across 38 countries, with the majority of economic evaluations representing upper‐middle‐income countries in Latin America & Caribbean, East Asia & Pacific, and Middle East & North Africa. The most commonly used costing perspective was that of the health care system. The time horizon for costs ranged from 3 months to lifetime; however, about one third of studies did not report a time horizon. Of the studies that estimated the cost of multiple steps in the BC care pathway, the majority used a micro‐costing approach. All of these studies included direct medical costs, whereas a minority included direct nonmedical or indirect costs. Although the majority of these studies were of high quality, several studies lacked several important costing details.
Cost of BC care in LMICs remains an understudied area. A 2013 systematic review of BC control economic analyses in LMICs (including BC screening, diagnosis, and treatment) by Zelle et al. identified 24 studies and reported limited economic evidence on BC control [15]. Although our scoping review had a narrower focus (excluding studies only focused on BC screening), we identified 25 studies published before 2013, likely due to searching a greater number of databases. Since 2013, there has been a substantial increase in the number of BC costing studies in LMICs, suggesting a growing interest in BC costing data in low‐resource settings.
Despite the increasing number of studies, the majority of data have been largely limited to upper‐middle‐income countries. Low‐income countries make up 21% of LMICs (29/135) but represented only two (2%) of the reviewed studies [26]. One of the two studies in low‐income countries focused on out‐of‐pocket expenses incurred by patients obtaining free BC care in Haiti [106]. The other included minimal original data and relied heavily on costing studies from a middle‐income country (Bolivia) to evaluate the cost‐effectiveness of trastuzumab in SSA [81]. Specific world regions were also underrepresented. There were few studies from SSA—only eight (9%) from a region representing 34% of all LMICs (46/135). Similarly, there were only five studies (5%) from Europe & Central Asia, a region that represents 15% of all LMICs (20/135) [26]. These findings are consistent with a recent systematic review of BC treatments costs by stage worldwide by Sun et al., which only included five studies from LMICs, none of which were from low‐income countries or countries in SSA [5]. The underrepresentation of low‐income countries and countries in SSA may represent nascent cancer programs in these countries or limited research capacity to conduct such economic evaluations. However, BC costing data may be especially useful in these areas, as many of these countries are expanding national cancer care programs and making difficult decisions on resource allocation. Future costing studies for BC control should be prioritized in Sub‐Saharan Africa as well as in low‐income countries with developing BC programs.
One of the challenges in conducting costing studies in LMICs is the paucity of commonly accepted guidelines to design, conduct, and report economic evaluations. Several upper‐middle‐income countries (e.g., China, Brazil, Colombia) and lower‐middle‐income countries (e.g., Egypt, Bhutan, Philippines) have country‐specific guidelines [125]. Others use frameworks that have been designed for use in LMICs—such as the International Decision Supportive Initiative (iDSI) Reference Case or the WHO guide to cost‐effective analysis (WHO‐CHOICE) [21, 22]. The development of a unified framework, which can be adapted across different settings, may help promote transparency, consistency, and comparison.
The studies included in this scoping review showed mixed adherence to existing frameworks as it relates to transparency of cost perspective and time horizon. For example, 11% of studies did not report their costing perspectives, which limits the interpretability and utility of the presented data. Although a study's perspective depends on its purpose and audience, the current costing literature for BC care in LMICs is biased toward costs incurred by the health care sector—60% of reviewed studies used a health care perspective. A study's time horizon also depends on its purpose; for example, studies that estimate the cost of BC diagnosis may require a substantially shorter time horizon than those that estimate treatment costs [21, 22, 126]. However, in the reviewed literature, 33% of studies did not report their time horizon at all. One of the challenges of conducting costing studies with longer time horizons is the limited availability of empirical cost data in LMICs. For example, when studies in this review relied on patient interviews for costing data, they had particularly short time horizons, spanning 6 months to 2 years (likely due to limitations in patient recall of cost estimates) [94, 113, 118]. However, as previously described, time horizons do not necessarily need to be limited by the availability of empirical data, as economic evaluations may use validated imputation methods for missing data [22]. Researchers should strive to use and report a costing perspective and time horizon that comprehensively captures all costs relevant to the study objective.
For the 33 studies that estimated the cost of multiple steps in the BC care pathway, we examined the applied costing methodologies and included cost categories. The large majority of studies used a micro‐costing approach, which tends to be a more comprehensive form of costing that is less likely to underestimate costs compared with gross‐costing methods [127]. However, only 21% of studies explicitly reported the quantities of resources separately from their unit costs, suggesting suboptimal use or reporting of micro‐costing [68, 95, 98, 101, 103, 105, 112]. Micro‐costing may be very time intensive and onerous in low‐resource settings, as it requires disaggregation by input. Gross‐costing may provide some benefits such as ease of estimating overhead/administrative or training costs, which were captured by only a minority of reviewed studies [128]. Future BC costing studies in LMICs may consider using both micro‐ and gross‐ costing methods for different cost measures.
Furthermore, analysis of cost inputs for these 33 studies revealed that BC economic evaluations were often missing key costs categories. The large majority of studies were missing direct nonmedical and indirect costs. Exclusion of these costs inputs likely biases cost outcomes toward costs incurred by the health care sector, while missing potentially substantial costs incurred by patients or society [129]. Use of a societal or patient perspective may encourage the inclusion of direct nonmedical and indirect costs in future economic evaluations.
The overall quality of the 33 studies that estimated the cost of multiple steps in the BC care pathway ranged from medium to high quality based on the Drummond et al. checklist. In the 2013 Zelle et al. review of BC economic evaluations in LMICs (which included studies on BC screening), 11 out of the 24 studies had poor quality. In this scoping review, the three highest‐quality studies were all cost‐effectiveness studies published after 2013 and explicitly used the WHO‐CHOICE methodology (Zelle et al., Zelle et al., and Niens et al.) [98, 101, 103]. In contrast, the two lowest‐quality studies were the earliest studies in this review (Arredondo et al., Yazihan et al.) [91, 92]. Overall, the most commonly missed Drummond et al. checklist items included inclusion of time horizon, costing perspective, discounting, inflation adjustment, uncertainty estimation, consideration of productivity changes, and separate reporting of resource quantities and unit costs. The lack of uncertainty estimation (55% of studies) and sensitivity analyses (73% of studies) is especially notable. Given that costs vary within local settings and all costing analyses require some level of estimation, sensitivity analyses and uncertainty estimates are essential to strengthen confidence in the accuracy and generalizability of the results. Use of existing frameworks, such as WHO‐CHOICE or the iDSI Reference Case, may promote more consistent and transparent study designs, analyses, and reporting of future BC economic evaluations in LMICs [21, 22].
This scoping review has several strengths. The review utilizes a systematic search strategy developed in consultation with a medical librarian that spans across five databases and gray literature. In addition, the review takes an exhaustive approach with evaluating the methodology of the economic evaluations, detailing the included cost categories and the comprehensiveness of inputs included in these categories. Nonetheless, this review also has some limitations. First, the review excluded studies with non‐English full texts, which may have introduced language bias as 33 articles were excluded because of language restrictions. Despite these exclusions, the review included studies from all relevant regions, resulting in more studies than any of the prior reviews. Second, only one reviewer performed data extraction from all reviewed studies. An independent, second reviewer performed data extraction for a fraction of studies and the percent of agreement for data extraction and the Drummond et al. checklist were 97% and 93%, respectively. Third, our quality assessment of reviewed articles was based on a checklist that grants the highest scores for full reporting of all domains. However, several checklist items were not applicable for many studies, and thus, each remaining checked item carried a disproportionate weight. Therefore, the quality scores for these studies should be interpreted with caution. Other assessment tools for economic evaluations share similar limitations [130].
Conclusion
This scoping review highlights a substantial increase in the number of BC economic evaluations in LMICs in the past decade. Despite this growing body of literature, there remain limited data from low‐income countries, especially those in Sub‐Saharan Africa. Future BC economic evaluations should be prioritized in these countries. The current literature was biased toward costs incurred by the health care sector, and as such, direct nonmedical costs and indirect costs were often not included. Although most studies assessing multiple steps in the BC pathway were high quality, notable gaps included missing specification of time horizon, cost estimation uncertainty, and sensitivity analyses. Researchers should strive to use and report a costing perspective and time horizon that captures all costs relevant to the study objective. Use of existing frameworks for economic evaluations in LMICs (such as WHO‐CHOICE or the iDSI Reference Case) may help achieve transparent, comparable costing analyses that can be used to guide BC control strategies.
Author Contributions
Conception/design: Parsa Erfani, Kayleigh Bhangdia, Catherine Stauber, Jean Claude Mugunga, Lydia E. Pace, Temidayo Fadelu
Provision of study material or patients: Parsa Erfani, Kayleigh Bhangdia, Temidayo Fadelu
Collection and/or assembly of data: Parsa Erfani, Kayleigh Bhangdia, Catherine Stauber
Data analysis and interpretation: Parsa Erfani, Kayleigh Bhangdia, Catherine Stauber, Jean Claude Mugunga, Lydia E. Pace, Temidayo Fadelu
Manuscript writing: Parsa Erfani, Kayleigh Bhangdia, Catherine Stauber, Jean Claude Mugunga, Lydia E. Pace, Temidayo Fadelu
Final approval of manuscript: Parsa Erfani, Kayleigh Bhangdia, Catherine Stauber, Jean Claude Mugunga, Lydia E. Pace, Temidayo Fadelu
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Appendix S2. Tables.
Acknowledgments
We acknowledge Paul Bain, Ph.D., M.LI.S., from the Harvard Countway Library for guidance in the search strategy. This study was supported by the Breast Cancer Research Foundation (BCRF‐20‐149), the Conquer Cancer Foundation Young Investigator Award, the Fogarty International Center (D43TW010543), and the National Cancer Institute K07 Career Development Award (1K07CA215819‐01A1).
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Cancer today . World Health Organization: International Agency for Research on Cancer, 2020. Available at https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0. Accessed December 15, 2020.
- 3. DeSantis CE, Bray F, Ferlay J et al. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev 2015;24:1495–1506. [DOI] [PubMed] [Google Scholar]
- 4. El Saghir NS, Adebamowo CA, Anderson BO et al. Breast cancer management in low resource countries (LRCs): Consensus statement from the Breast Health Global Initiative. Breast 2011;20(suppl 2):S3–S11. [DOI] [PubMed] [Google Scholar]
- 5. Sun L, Legood R, Dos‐Santos‐Silva I et al. Global treatment costs of breast cancer by stage: A systematic review. PLoS One 2018;13:e0207993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sullivan R, Alatise OI, Anderson BO et al. Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol 2015;16:1193–1224. [DOI] [PubMed] [Google Scholar]
- 7. Gelband H, Jha P, Sankaranarayanan R et al., eds. Cancer: Disease control priorities. 3rd ed. Vol. 3. Washington DC: The International Bank for Reconstruction and Development/The World Bank, 2015. [PubMed] [Google Scholar]
- 8. Wagner CM, Antillón F, Uwinkindi F et al. Establishing cancer treatment programs in resource‐limited settings: Lessons learned from Guatemala, Rwanda, and Vietnam. J Glob Oncol 2018;4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rashidian A, Barfar E, Hosseini H et al. Cost effectiveness of breast cancer screening using mammography; a systematic review. Iran J Public Health 2013;42:347–357. [PMC free article] [PubMed] [Google Scholar]
- 10. Wang L, Shi JF, Huang HY et al. Economic evaluation on breast cancer screening in mainland china: A systematic review [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 2016;37:1662–1669. [DOI] [PubMed] [Google Scholar]
- 11. Yoo KB, Kwon JA, Cho E et al. Is mammography for breast cancer screening cost‐effective in both Western and Asian countries?: Results of a systematic review. Asian Pac J Cancer Prev 2013;14:4141–4149. [DOI] [PubMed] [Google Scholar]
- 12. Abdul Rafar NR, Hong YH, Wu DB et al. Cost‐effectiveness of adjuvant trastuzumab therapy for early breast cancer in Asia: A systematic review. Value Health Reg Issues 2019;18:151–158. [DOI] [PubMed] [Google Scholar]
- 13. Reiazi R, Norozi A, Etedadialiabadi M. A literature survey on cost‐effectiveness of proton beam therapy in the management of breast cancer patients. Iran J Cancer Prev 2015;8:e4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al‐Ziftawi NH, Shafie AA, Mohamed Ibrahim MI. Cost‐effectiveness analyses of breast cancer medications use in developing countries: A systematic review. Expert Rev Pharmacoecon Outcomes Res 2020:1–11. [DOI] [PubMed] [Google Scholar]
- 15. Zelle SG, Baltussen RM. Economic analyses of breast cancer control in low‐ and middle‐income countries: A systematic review. Syst Rev 2013;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Binagwaho A, Wagner CM, Farmer PE. A vision for global cancer medicine: Pursuing the equity of chance. J Clin Oncol 2016;34:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atun R, Jaffray DA, Barton MB et al. Expanding global access to radiotherapy. Lancet Oncol 2015;16:1153–1186. [DOI] [PubMed] [Google Scholar]
- 18. Stulac S, Binagwaho A, Tapela NM et al. Capacity building for oncology programmes in Sub‐Saharan Africa: The Rwanda experience. Lancet Oncol 2015;16:e405–e413. [DOI] [PubMed] [Google Scholar]
- 19. Li N, Huang HY, Wu DW et al. Changes in clinical trials of cancer drugs in mainland China over the decade 2009–18: A systematic review. Lancet Oncol 2019;20:e619–e626. [DOI] [PubMed] [Google Scholar]
- 20. Global Health Cost Consortium . Reference case for estimating the costs of global health services and interventions. Available at https://ghcosting.org/pages/standards/reference_case. Accessed November 6, 2020,
- 21. World Health Organization . Making choices in health: WHO guide to cost‐effectiveness analysis: Cost effectiveness and strategic planning (WHO‐CHOICE). Geneva: World Health Organization, 2003. [Google Scholar]
- 22. Wilkinson T, Sculpher MJ, Claxton K et al. The international decision support initiative reference case for economic evaluation: An aid to thought. Value Health 2016;19:921–928. [DOI] [PubMed] [Google Scholar]
- 23. Peters M, Godfrey C, McInerney P et al. Chapter 11: Scoping reviews (2020 version). In: JBI Manual for Evidence Synthesis. Adelaide, Australia: JBI, 2020. https://synthesismanual.jbi.global. 10.46658/JBIMES-20-12. [DOI] [Google Scholar]
- 24. Tricco AC, Lillie E, Zarin W et al. PRISMA extension for scoping reviews (PRISMA‐ScR): Checklist and explanation. Ann Intern Med 2018;169:467–473. [DOI] [PubMed] [Google Scholar]
- 25. Erfani P, Bhangdia K, Stauber C et al. Cost of breast cancer care in low and middle‐income countries: A scoping review protocol. JBI Evid Synth 2021. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The World Bank . World bank country and lending groups. 2020. Available at https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed April 1, 2020.
- 27. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation. [Google Scholar]
- 28. Google . Google translate. Available at https://translate.google.com/. Accessed September 9, 2020.
- 29. Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)–A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fung A, Horton S, Zabih V et al. Cost and cost‐effectiveness of childhood cancer treatment in low‐income and middle‐income countries: A systematic review. BMJ Glob Health 2019;4:e001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerard K, Seymour J, Smoker I. A tool to improve quality of reporting published economic analyses. Int J Technol Assess Health Care 2000;16:100–110. [DOI] [PubMed] [Google Scholar]
- 33. Thomas JO, Amanguno AU, Adeyi OA et al. Fine needle aspiration (FNA) in the management of palpable masses in Ibadan: Impact on the cost of care. Cytopathology 1999;10:206–210. [DOI] [PubMed] [Google Scholar]
- 34. Love RR, Duc NB, Allred DC et al. Oophorectomy and tamoxifen adjuvant therapy in premenopausal Vietnamese and Chinese women with operable breast cancer. J Clin Oncol 2002;20:2559–2566. [DOI] [PubMed] [Google Scholar]
- 35. Limwattananon S, Limwattananon C, Maoleekulpairoj S et al. Cost‐effectiveness analysis of sequential paclitaxel adjuvant chemotherapy for patients with node positive primary breast cancer. J Med Assoc Thai 2006;89:690–698. [PubMed] [Google Scholar]
- 36. Supakul S, Sooksriwong C, Chiersilpa A et al. Cost of breast cancer treatment with adjuvant therapy in Thai women. Mahidol University Journal of Pharmaceutical Sciences 2006;33:9–14. [Google Scholar]
- 37. Chen W, Jiang Z, Shao Z et al. An economic evaluation of adjuvant trastuzumab therapy in HER2‐positive early breast cancer. Value Health 2009;12(suppl 3):S82–S84. [DOI] [PubMed] [Google Scholar]
- 38. Fonseca M, Araújo GT, Saad ED. Cost‐effectiveness of anastrozole, in comparison with tamoxifen, in the adjuvant treatment of early breast cancer in Brazil. Rev Assoc Med Bras (1992) 2009;55:410–415. [DOI] [PubMed] [Google Scholar]
- 39. Liubao P, Xiaomin W, Chongqing T et al. Cost‐effectiveness analysis of adjuvant therapy for operable breast cancer from a Chinese perspective: Doxorubicin plus cyclophosphamide versus docetaxel plus cyclophosphamide. Pharmacoeconomics 2009;27:873–886. [DOI] [PubMed] [Google Scholar]
- 40. Sasse AD, Sasse EC. Cost‐effectiveness analysis of adjuvant anastrozol in post‐menopausal women with breast cancer [in Portuguese]. Rev Assoc Med Bras (1992) 2009;55:535–540. [DOI] [PubMed] [Google Scholar]
- 41. Bacchi CE, Prisco F, Carvalho FM et al. Potential economic impact of the 21‐gene expression assay on the treatment of breast cancer in Brazil. Rev Assoc Med Bras (1992) 2010;56:186–191. [DOI] [PubMed] [Google Scholar]
- 42. Boutayeb S, Boutayeb A, Ahbeddou N et al. Estimation of the cost of treatment by chemotherapy for early breast cancer in Morocco. Cost Eff Resour Alloc 2010;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guggisberg K, Okorie C, Khalil M. Cytopathology including fine‐needle aspiration in Sub‐Saharan Africa: A Cameroon experience. Arch Pathol Lab Med 2011;135:200–206. [DOI] [PubMed] [Google Scholar]
- 44. Nasrinossadat A, Ladan F, Fereshte E et al. Marking non‐palpable breast masses with injected methylene blue dye, an easy, safe and low cost method for developing countries and resource‐limited areas. Asian Pac J Cancer Prev 2011;12:1189–1192. [PubMed] [Google Scholar]
- 45. Bai Y, Ye M, Cao H et al. Economic evaluation of radiotherapy for early breast cancer after breast‐conserving surgery in a health resource‐limited setting. Breast Cancer Res Treat 2012;136:547–557. [DOI] [PubMed] [Google Scholar]
- 46. Bastani P, Kiadaliri AA. Cost‐utility analysis of adjuvant therapies for breast cancer in Iran. Int J Technol Assess Health Care 2012;28:110–114. [DOI] [PubMed] [Google Scholar]
- 47. Lima DE, Veiga Filho J, Ribeiro LM et al. Oncoplastic approach in the conservative treatment of breast cancer: Analysis of costs. Acta Cir Bras 2012;27:311–314. [DOI] [PubMed] [Google Scholar]
- 48. Machado M, Einarson TR. Lapatinib in patients with metastatic breast cancer following initial treatment with trastuzumab: An economic analysis from the Brazilian public health care perspective. Breast Cancer (Dove Med Press) 2012;4:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sookprasert A, Chindaprasirt J, Wirasorn K et al. Patterns of chemotherapy usage in hospitalized breast cancer patients. J Med Assoc Thai 2012;95(suppl 7):S206–S210. [PubMed] [Google Scholar]
- 50. Buendía JA, Vallejos C, Pichón‐Rivière A. An economic evaluation of trastuzumab as adjuvant treatment of early HER2‐positive breast cancer patients in Colombia. Biomedica 2013;33:411–417. [DOI] [PubMed] [Google Scholar]
- 51. Chicaíza‐Becerra L, García‐Molina M, Gamboa O et al. ErbB2+ metastatic breast cancer treatment after progression on trastuzumab: A cost‐effectiveness analysis for a developing country. Rev Salud Publica (Bogota) 2014;16:270–280. [DOI] [PubMed] [Google Scholar]
- 52. Dranitsaris G, Yu B, Wang L et al. Abraxane® versus Taxol® for patients with advanced breast cancer: A prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract 2016;22:205–211. [DOI] [PubMed] [Google Scholar]
- 53. Nobrega CR, Lima AF. Procedures' costs related to outpatient chemotherapy treatment of women suffering from breast cancer [in Portuguese]. Rev Esc Enferm USP 2014;48:698–705. [PubMed] [Google Scholar]
- 54. Songtish D, Praditsitthikorn N, Teerawattananon Y. A cost‐utility analysis comparing standard axillary lymph node dissection with sentinel lymph node biopsy in patients with early stage breast cancer in Thailand. Value Health Reg Issues 2014;3:59–66. [DOI] [PubMed] [Google Scholar]
- 55. Aboutorabi A, Hadian M, Ghaderi H et al. Cost‐effectiveness analysis of trastuzumab in the adjuvant treatment for early breast cancer. Glob J Health Sci 2014;7:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bargalló‐Rocha JE, Lara‐Medina F, Pérez‐Sánchez V et al. Cost‐effectiveness of the 21‐gene breast cancer assay in Mexico. Adv Ther 2015;32:239–253. [DOI] [PubMed] [Google Scholar]
- 57. Bandeira T, Mosegui G, Vianna C et al. Cost‐effectiveness analysis of trastuzumab in the treatment of metastatic breast cancer. Int J Pharm Pharm Sci 2015;7. [Google Scholar]
- 58. Hatam N, Askarian M, Javan‐Noghabi J et al. Cost‐utility of "doxorubicin and cyclophosphamide" versus "gemcitabine and paclitaxel" for treatment of patients with breast cancer in Iran. Asian Pac J Cancer Prev 2015;16:8265–8270. [DOI] [PubMed] [Google Scholar]
- 59. Lewis L, Taylor M, Suriya Ertugyrovna Y et al. Budget impact analysis of everolimus for the treatment of hormone receptor positive, human epidermal growth factor receptor‐2 negative (HER2‐) advanced breast cancer in Kazakhstan. J Med Econ 2015;18:189–199. [DOI] [PubMed] [Google Scholar]
- 60. Madubogwu C, Ukah C, Onyiaorah I et al. Cost effectiveness of fine needle aspiration cytology for breast masses. Orient J Med 2015;27. [Google Scholar]
- 61. Pichon‐Riviere A, Garay OU, Augustovski F et al. Implications of global pricing policies on access to innovative drugs: The case of trastuzumab in seven Latin American countries. Int J Technol Assess Health Care 2015;31:2–11. [DOI] [PubMed] [Google Scholar]
- 62. Vekov T, Lebanova H, Grigorov E. Pharmacotherapeutic recommendations for application of target oncological drug therapies for treatment of breast cancer in Bulgaria ‐ Therapeutic efficacy and cost effectiveness. J BUON 2015;20:1420–1425. [PubMed] [Google Scholar]
- 63. Wan X, Peng L, Ma J et al. Subgroup economic evaluation of radiotherapy for breast cancer after mastectomy. Clin Ther 2015;37:2515–2526.e2515. [DOI] [PubMed] [Google Scholar]
- 64. Mosalanezhad H, Kavosi Z, Keshavarz K et al. Cost‐effectiveness of radiotherapy during surgery compared with external radiation therapy in the treatment of women with breast cancer. J Health Manag Informatics 2016;3. [Google Scholar]
- 65. Elias F, Khuri FR, Adib SM et al. Financial burden of cancer drug treatment in Lebanon. Asian Pac J Cancer Prev 2016;17:3173–3177. [PubMed] [Google Scholar]
- 66. Hatam N, Ahmadloo N, Vazirzadeh M, et al. Cost‐effectiveness of intensive vs. standard follow‐up models for patients with breast cancer in Shiraz, Iran. Asian Pac J Cancer Prev 2016;17:5309–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee W, Haron M, Kong Y et al. Economic analysis of intravenous vs. subcutaneously administered trastuzumab for the treatment of HER2+ early breast cancer in Malaysia. Adv Breast Cancer Res 2016;5:1–13. [Google Scholar]
- 68. Ansaripour A, Uyl‐de Groot CA, Redekop WK. Adjuvant trastuzumab therapy for early HER2‐positive breast cancer in Iran: A cost‐effectiveness and scenario analysis for an optimal treatment strategy. Pharmacoeconomics 2018;36:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mosegui G, Vianna C, Rodrigues M et al. Cost‐effectiveness analysis of trastuzumab emtansine in the treatment of metastatic breast cancer. Int J Pharm Pharm Sci 2017;9. [Google Scholar]
- 70. Bargallo‐Rocha JE, Soto‐Perez‐de‐Celis E, Picó‐Guzmán FJ et al. The impact of the use of intraoperative radiotherapy on costs, travel time and distance for women with breast cancer in the Mexico city metropolitan area. J Surg Oncol 2017;116:683–689. [DOI] [PubMed] [Google Scholar]
- 71. Diaby V, Ali AA, Williams KJ et al. Economic evaluation of sequencing strategies in HER2‐positive metastatic breast cancer in Mexico: A contrast between public and private payer perspectives. Breast Cancer Res Treat 2017;166:951–963. [DOI] [PubMed] [Google Scholar]
- 72. Ding H, Fang L, Xin W et al. Cost‐effectiveness analysis of fulvestrant versus anastrozole as first‐line treatment for hormone receptor‐positive advanced breast cancer. Eur J Cancer Care (Engl) 2017;26. [DOI] [PubMed] [Google Scholar]
- 73. Li R, Zhang L, Yang J et al. Analysis of inpatient payments of breast cancer patients with different medical insurance coverages in China (mainland) in 2011‐2015. Chin J Cancer Res 2017;29:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Javan‐Noughabi J, Rezapour A, Kassani A et al. The cost‐effectiveness of neoadjuvant chemotherapy in women with locally advanced breast cancer: Adriamycin and cyclophosphamide in comparison with paclitaxel and gemcitabine. J Res Med Sci 2018;23:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ye M, Lu J, Yang F et al. Economic evaluation of letrozole for early breast cancer in a health resource‐limited setting. Biomed Res Int 2018;2018:9282646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kongsakon R, Lochid‐Amnuay S, Kapol N et al. From research to policy implementation: Trastuzumab in early‐stage breast cancer treatment in Thailand. Value Health Reg Issues 2019;18:47–53. [DOI] [PubMed] [Google Scholar]
- 77. Akbari M, Nabatzade M, Azargashb E et al. Evaluation of clinical assessment, mammography, and ultrasonography in diagnosis of benign and malignant breast lesions and determining their cost‐effectiveness. Int J Cancer Manag 2019;12. [Google Scholar]
- 78. Alshreef A, MacQuilkan K, Dawkins B et al. Cost‐effectiveness of docetaxel and paclitaxel for adjuvant treatment of early breast cancer: Adaptation of a model‐based economic evaluation from the United Kingdom to South Africa. Value Health Reg Issues 2019;19:65–74. [DOI] [PubMed] [Google Scholar]
- 79. Askarzade E, Adel A, Ebrahimipour H et al. Epidemiology and cost of patients with cancer in Iran: 2018. Middle East J Cancer 2019;10:362–371. [Google Scholar]
- 80. Genuino AJ, Chaikledkaew U, Guerrero AM et al. Cost‐utility analysis of adjuvant trastuzumab therapy for HER2‐positive early‐stage breast cancer in the Philippines. BMC Health Serv Res 2019;19:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gershon N, Berchenko Y, Hall PS et al. Cost effectiveness and affordability of trastuzumab in Sub‐Saharan Africa for early stage. Cost Eff Resour Alloc 2019;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kashyap A, Balaji MN, Chhabra M et al. Cost analysis of various branded versus generic chemotherapeutic agents used for the treatment of early breast cancer‐ A deep insight from India. Expert Rev Pharmacoecon Outcomes Res 2020;20:355–361. [DOI] [PubMed] [Google Scholar]
- 83. Liao M, Jiang Q, Hu H et al. Cost‐effectiveness analysis of utidelone plus capecitabine for metastatic breast cancer in China. J Med Econ 2019;22:584–592. [DOI] [PubMed] [Google Scholar]
- 84. Özmen V, Çakar B, Gökmen E et al. Cost effectiveness of gene expression profiling in patients with early‐stage breast cancer in a middle‐income country, Turkey: Results of a prospective multicenter study. Eur J Breast Health 2019;15:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wan X, Zhang Y, Ma J et al. Ribociclib in hormone‐receptor‐positive advanced breast cancer: Establishing a value‐based cost in China. Breast 2019;43:1–6. [DOI] [PubMed] [Google Scholar]
- 86. Zhang Y, Zeng X, Deng H et al. Cost‐effectiveness analysis of adding palbociclib as a second‐line endocrine therapy for HR+/HER2‐ metastatic breast cancer from the US and Chinese Perspectives. Clin Ther 2019;41:1175–1185. [DOI] [PubMed] [Google Scholar]
- 87. Gupta N, Verma RK, Gupta S et al. Cost effectiveness of trastuzumab for management of breast cancer in India. JCO Glob Oncol 2020;6:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sánchez‐Calderón D, Pedraza A, Mancera Urrego C et al. Analysis of the cost‐effectiveness of liquid biopsy to determine treatment change in patients with HER2‐positive advanced breast cancer in Colombia. Clinicoecon Outcomes Res 2020;12:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wei X, Sun H, Zhuang J et al. Cost‐effectiveness analysis of cyp2d6*10 pharmacogenetic testing to guide the adjuvant endocrine therapy for postmenopausal women with estrogen receptor positive early breast cancer in China. Clin Drug Investig 2020;40:25–32. [DOI] [PubMed] [Google Scholar]
- 90. Wu Q, Wang X, Zhang M et al. Cost‐effectiveness analysis of bevacizumab plus paclitaxel versus bevacizumab plus capecitabine for HER2‐negative locally recurrent or metastatic breast cancer. Oncol Res Treat 2020;43:153–159. [DOI] [PubMed] [Google Scholar]
- 91. Arredondo A, Lockett LY, de Icaza E. Cost of diseases in Brazil: Breast cancer, enteritis, cardiac valve disease and bronchopneumonia. Rev Saude Publica 1995;29:349–354. [DOI] [PubMed] [Google Scholar]
- 92. Yazihan N, Yilmaz HH. Breast cancer in Turkey: Economic efficiency and cost effectiveness. Cancer Control in Turkey, Cancer Control in Turkey Health Ministry Publication 2006.
- 93. Gómez‐Rico JA, Altagracia‐Martínez M, Kravzov‐Jinich J et al. The costs of breast cancer in a Mexican public health institution. Risk Manag Healthc Policy 2008;1:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pakseresht S, Ingle GK, Garg S et al. Expenditure audit of women with breast cancer in a tertiary care hospital of Delhi. Indian J Cancer 2011;48:428–437. [DOI] [PubMed] [Google Scholar]
- 95. Salomon JA, Carvalho N, Gutiérrez‐Delgado C et al. Intervention strategies to reduce the burden of non‐communicable diseases in Mexico: Cost effectiveness analysis. BMJ 2012;344:e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hoang Lan N, Laohasiriwong W, Stewart JF et al. Cost of treatment for breast cancer in central Vietnam. Glob Health Action 2013;6:18872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Meneses‐García A, Ramírez T, Ruiz‐Godoy L et al. Costs of breast cancer treatment prior to the introduction of immune‐based therapy in Mexico. Rev Med Inst Mex Seguro Soc 2012;50:19–24. [PubMed] [Google Scholar]
- 98. Zelle SG, Nyarko KM, Bosu WK et al. Costs, effects and cost‐effectiveness of breast cancer control in Ghana. Trop Med Int Health 2012;17:1031–1043. [DOI] [PubMed] [Google Scholar]
- 99. Davari M, Yazdanpanah F, Aslani A et al. The direct medical costs of breast cancer in Iran: Analyzing the patient's level data from a cancer specific hospital in Isfahan. Int J Prev Med 2013;4:748–754. [PMC free article] [PubMed] [Google Scholar]
- 100. Kaliks RA, Pontes LdB, Bognar CL et al. Treatment of breast cancer patients from a public healthcare system in a private center: Costs of care for a pilot public‐private partnership in oncology. Einstein (Sao Paulo) 2013;11:216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zelle SG, Vidaurre T, Abugattas JE et al. Cost‐effectiveness analysis of breast cancer control interventions in Peru. PLoS One 2013;8:e82575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hatam N, Keshtkar V, Salehi A et al. The financial cost of preventive and curative programs for breast cancer: A case study of women in Shiraz‐Iran. Int J Health Policy Manag 2014;2:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Niëns LM, Zelle SG, Gutiérrez‐Delgado C et al. Cost‐effectiveness of breast cancer control strategies in Central America: The cases of Costa Rica and Mexico. PLoS One 2014;9:e95836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Daroudi R, Akbari Sari A, Nahvijou A et al. The economic burden of breast cancer in Iran. Iran J Public Health 2015;44:1225–1233. [PMC free article] [PubMed] [Google Scholar]
- 105. González‐Robledo MC, Wong R, Ornelas HA et al. Costs of breast cancer care in Mexico: Analysis of two insurance coverage scenarios. Ecancermedicalscience 2015;9:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. O'Neill KM, Mandigo M, Pyda J et al. Out‐of‐pocket expenses incurred by patients obtaining free breast cancer care in Haiti: A pilot study. Surgery 2015;158:747–755. [DOI] [PubMed] [Google Scholar]
- 107. Fray‐Aiken C, Wilks R, Abdulkadri A et al. Cost of care of chronic non‐communicable diseases in Jamaican patients: The role of obesity. Farmeconomia. Health economics and therapeutic pathways 2016;17. [Google Scholar]
- 108. Jain M, Mukherjee K. Economic burden of breast cancer to the households in Punjab, India. Int J Med Public Health 2016;6. [Google Scholar]
- 109. Cai Y, Xue M, Chen W et al. Expenditure of hospital care on cancer in China, from 2011 to 2015. Chin J Cancer Res 2017;29:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liao XZ, Shi JF, Liu JS et al. Medical and non‐medical expenditure for breast cancer diagnosis and treatment in china: A multicenter cross‐sectional study. Asia Pac J Clin Oncol 2018;14:167–178. [DOI] [PubMed] [Google Scholar]
- 111. Ansaripour A, Zendehdel K, Tadayon N et al. Use of data‐mining to support real‐world cost analyses: An example using HER2‐positive breast cancer in Iran. PLoS One 2018;13:e0205079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Atieno OM, Opanga S, Martin A et al. Pilot study assessing the direct medical cost of treating patients with cancer in Kenya; findings and implications for the future. J Med Econ 2018;21:878–887. [DOI] [PubMed] [Google Scholar]
- 113. Hameed Khaliq I, Zahid Mahmood H, Akhter N et al. Comparison of two public sector tertiary care hospitals' management in reducing direct medical cost burden on breast carcinoma patients in Lahore, Pakistan. J BUON 2018;23:143–149. [PubMed] [Google Scholar]
- 114. Zahid Mahmood H, Khaliq IH, Bhatti ZI et al. Household costs of breast cancer morbidity: An empirical assessment from Pakistan. J BUON 2018;23:28–33. [PubMed] [Google Scholar]
- 115. Shankar S, Boyanagari M, Boyanagari V et al. Profile of breast cancer patients receiving government sponsored free treatment and the associated economic costs. Clin Epidemiol Glob Health 2018;6:203–207. [Google Scholar]
- 116. Skrundevskiy AN, Omar OS, Kim J et al. Return on investment analysis of breast cancer screening and downstaging in Egypt: Implications for developing countries. Value Health Reg Issues 2018;16:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Subramanian S, Gakunga R, Kibachio J et al. Cost and affordability of non‐communicable disease screening, diagnosis and treatment in Kenya: Patient payments in the private and public sectors. PLoS One 2018;13:e0190113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Khatiwoda SR, Dhungana RR, Sapkota VP et al. Estimating the direct cost of cancer in Nepal: A cross‐sectional study in a tertiary cancer hospital. Front Public Health 2019;7:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Seroiska M, Lenzi L, Wiens A. Cost of the disease in patients with breast cancer treated with tamoxifen. Revista Brasileira de Cancerologia 2019;65. [Google Scholar]
- 120. Tekin RN, Saygılı M. Determining breast cancer treatment costs using the top down cost approach. Eur J Breast Health 2019;15:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yin X, Xu Y, Man X et al. Direct costs of both inpatient and outpatient care for all type cancers: The evidence from Beijing, China. Cancer Med 2019;8:3250–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Alefan Q, Saadeh, A , Yaghan, R. Direct medical costs for stage‐specific breast cancer: A retrospective analysis. Breast Cancer Manag 2020;9. [Google Scholar]
- 123. Elsisi GH, Nada Y, Rashad N et al. Cost‐effectiveness of six months versus 1‐year adjuvant trastuzumab in HER2 positive early breast cancer in Egypt. J Med Econ 2020;23:575–580. [DOI] [PubMed] [Google Scholar]
- 124. Map chart . Available at https://mapchart.net. Accessed November 6, 2020.
- 125. Guide to Economic Analysis and Research (GEAR) . Guidelines comparison. Available at http://www.gear4health.com/gear/health-economic-evaluation-guidelines. Accessed November 6, 2020.
- 126. Sanders GD, Neumann PJ, Basu A et al. Recommendations for conduct, methodological practices, and reporting of cost‐effectiveness analyses: Second panel on cost‐effectiveness in health and medicine. JAMA 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 127. Helen D, Giselle A, Sarah W. What is the value of collecting detailed costing data in clinical trials? Trials 2011;12:A42. [Google Scholar]
- 128. Global Health Cost Consortium . Resource use measurement: Principles 6‐10. Available at https://ghcosting.org/pages/standards/principles/resource_use_measurement. Accessed November 6, 2020.
- 129. Broekx S, Den Hond E, Torfs R et al. The costs of breast cancer prior to and following diagnosis. Eur J Health Econ 2011;12:311–317. [DOI] [PubMed] [Google Scholar]
- 130. Husereau D, Drummond M, Petrou S et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Appendix S2. Tables.


