Abstract
Background
To address the support needs of newly diagnosed patients with lung cancer with limited prognosis, the Milestone Communication Approach (MCA) was developed and implemented. The main elements of the MCA are situation‐specific conversations along the disease trajectory conducted by an interprofessional tandem of physician and nurse. The aim of the study was to evaluate the effects of MCA on addressing support needs, quality of life, and mood as compared with standard oncological care.
Patients and Methods
A randomized trial was conducted with baseline assessment and follow‐up assessments at 3, 6, and 9 months in outpatients with newly diagnosed lung cancer stage IV at a German thoracic oncology hospital. The primary outcome was the Health System and Information Needs subscale of the Short Form Supportive Care Needs Survey (SCNS‐SF34‐G) at 3‐month follow‐up. Secondary outcomes included the other subscales of the SCNS‐SF34‐G, the Schedule for the Evaluation of Individual Quality of Life, the Functional Assessment of Cancer Therapy lung module, the Patient Health Questionnaire for Depression and Anxiety, and the Distress Thermometer.
Results
At baseline, 174 patients were randomized, of whom 102 patients (MCA: n = 52; standard care: n = 50) provided data at 3‐month follow‐up. Patients of the MCA group reported lower information needs at 3‐month follow‐up (mean ± SD, 33.4 ± 27.5; standard care, 43.1 ± 29.9; p = .033). No effects were found for secondary outcomes.
Conclusion
MCA lowered patient‐reported information needs but did not have other effects. MCA contributed to tailored communication because an adequate level of information and orientation set the basis for patient‐centered care.
Implications for Practice
By addressing relevant issues at predefined times, the Milestone Communication Approach provides individual patient‐centered care facilitating the timely integration of palliative care for patients with a limited prognosis. The needs of patients with lung cancer must be assessed and addressed throughout the disease trajectory. Although specific topics may be relevant for all patients, such as information about the disease and associated health care, situations of individual patients and their families must be considered. Additionally, using the short form of the Supportive Care Needs Survey in clinical practice to identify patients’ problems might support individually targeted communication and preference‐sensitive care.
Keywords: Lung cancer, Communication, Support, Needs, Quality of life
Short abstract
Addressing patient information needs in a timely fashion is of paramount importance for preference‐sensitive decisions and patient‐centered care. This article evaluates the Milestone Communication Approach to oncological care.
Introduction
With the diagnosis of lung cancer, patients and family caregivers face challenges touching every part of their everyday lives, including physical symptoms like pain and fatigue, organization of therapies, and changes in social relationships. Challenges are increased by the nature of the disease with its limited prognosis, a median survival time of 12 months (stage IV) and a 5‐year survival rate of 14%–19% in Germany (all stages) because of its late detection [1]. Thus, palliative care issues are of paramount importance during the whole disease trajectory. The early integration of palliative care in lung cancer care has shown positive effects on symptom management, quality of life, and quality of care of patients [2, 3, 4].
Because of the complex care situation, patients (and family caregivers) need to be constantly informed about the current situation, care options, and consequences, especially with the rapid development of new therapeutic approaches in oncology care [5, 6]. Because therapeutic goals and preferences for care are also influenced by the burden of the disease [7, 8], health care professionals have a crucial role of eliciting patient preferences using communication skills appropriate to the individual patient's needs as they evolve along their disease trajectory [9].
At a thoracic oncology clinic, an interprofessional communication concept, the Milestone Communication Approach (MCA) [10], was developed and implemented, aiming both at strengthening communication skills of health care professionals and providing needs‐oriented and goal‐concordant care for patients. The MCA intends to achieve preference‐sensitive shared decisions, continuity of care, and early recognition, integration, and therapy of palliative care needs.
Addressing patient information needs in a timely fashion is of paramount importance for preference‐sensitive decisions and patient‐centered care. Thus, the aim of our study was to evaluate MCA care with respect to addressing information support needs compared with standard oncological care from the patients’ perspective (primary outcome). Additionally, effects on addressed physical and psychological support needs and on quality of life, distress, and mood (depression and anxiety) were examined.
Materials and Methods
Study Design
This randomized trial was part of a larger multiphase mixed‐method study focusing on the implementation and evaluation of the MCA concept in a thoracic oncology clinic [11]. Publications on the MCA concept development [10], implementation fidelity [12], and patient and caregiver experiences [13] are available. The present publication follows the recommendations of the Consolidated Standards of Reporting Trials (checklist available as supplemental online Table 1).
Table 1.
Baseline sociodemographic factors of patients (MCA randomized controlled trial)
| Sociodemographic factor | MCA intervention (n = 79), n (%) | Standard oncological care (n = 78), n (%) | p value |
|---|---|---|---|
| Age, mean ± SD, years | 67.2 ± 8.5 | 65.3 ± 9.2 | .194 a |
| Gender b | .831 c | ||
| Female | 34 (43.0) | 34 (43.6) | |
| Male | 45 (57.0) | 42 (53.8) | |
| Residence b | .381 c | ||
| City ≥100,000 inhabitants | 10 (12.7) | 6 (7.7) | |
| Town up to 100,000 inhabitants | 20 (25.3) | 26 (33.3) | |
| Rural up to 15,000 inhabitants | 48 (60.8) | 44 (56.4) | |
| Marital status b | .316 c | ||
| Single | 2 (2.5) | 4 (5.1) | |
| Married/ with partner | 61 (77.2) | 51 (65.4) | |
| Separated/divorced | 6 (7.6) | 12 (15.4) | |
| Widowed | 9 (11.4) | 9 (11.5) | |
| Living situation b | .170 c | ||
| Living alone | 13 (16.5) | 22 (28.2) | |
| Living with partner | 62 (78.5) | 50 (64.1) | |
| Other | 2 (2.5) | 2 (2.6) | |
| Children b | .035 c | ||
| Yes | 59 (74.7) | 66 (84.6) | |
| No | 20 (25.3) | 9 (11.5) | |
| Educational level b | .076 c | ||
| None | — | 1 (1.3) | |
| 8th/9th grade | 47 (59.5) | 38 (48.7) | |
| 10th grade | 18 (22.8) | 22 (28.2) | |
| 12th/13th grade | 10 (12.7) | 5 (6.4) | |
| Other | 2 (2.5) | 9 (11.5) | |
| Working status b | .574 c | ||
| Yes | 17 (21.5) | 19 (24.4) | |
| >40 hr/week | 7 (41.2) | 9 (47.4) | |
| 20–40 hr/week | 5 (29.4) | 6 (31.6) | |
| <20 hr/week | 5 (29.4) | 4 (21.1) | |
| No | 61 (77.2) | 55 (70.5) | |
| Homemaker | 3 (4.9) | 5 (9.1) | |
| Unemployed | 2 (3.3) | 1 (1.8) | |
| Retired | 49 (80.3) | 44 (80.0) | |
| Other | 5 (8.2) | 4 (7.3) | |
| Primary language b | .583 c | ||
| German | 77 (97.5) | 75 (96.2) | |
| Other | 2 (2.5) | 1 (1.3) | |
| Self‐reported smoking status b | .784 c | ||
| Yes | 10 (12.7) | 13 (16.7) | |
| No | 31 (39.2) | 32 (41.0) | |
| Occasionally | 9 (11.4) | 7 (9.0) | |
| Ex‐smoker | 29 (36.7) | 24 (30.8) | |
| Physical activity/sports b | .617 c | ||
| Never | 50 (63.3) | 42 (53.8) | |
| Once a week or less | 17 (21.5) | 19 (24.4) | |
| Several times a week | 8 (10.1) | 10 (12.8) | |
| Daily | 3 (3.8) | 1 (1.3) | |
| Health care insurance b | .575 c | ||
| Statutory | 75 (94.9) | 74 (94.9) | |
| Private | 2 (2.6) | 1 (1.3) | |
| Chronic diseases b | .181 c | ||
| No | 13 (16.5) | 21 (26.9) | |
| Yes | 54 (68.4) | 51 (65.4) | |
| Hypertension | 39 (72.2) | 37 (72.5) | |
| Diseases of the joints | 16 (29.6) | 13 (25.5) | |
| Chronic back pain | 15 (27.8) | 12 (23.5) | |
| Coronary heart disease | 13 (24.1) | 13 (25.5) | |
| Asthma/bronchitis | 14 (25.9) | 10 (19.6) | |
| Diabetes mellitus | 15 (27.8) | 7 (13.7) | |
| Allergies/ skin diseases | 11 (20.4) | 4 (7.8) | |
| Depression/anxiety | 3 (5.6) | 7 (13.7) | |
| Chronic gastrointestinal disease | 3 (5.6) | 3 (5.9) | |
| Other | 14 (25.9) | 10 (19.6) | |
| No. of current medications b | .913 c | ||
| None | 3 (3.8) | 2 (2.6) | |
| 1–2 | 15 (19.0) | 18 (23.1) | |
| 3–4 | 19 (24.1) | 19 (24.4) | |
| 5–6 | 16 (20.3) | 13 (16.7) | |
| ≥7 | 24 (30.4) | 20 (25.6) |
t test.
Percentages do not add up to 100 because of missing values.
Chi‐square test.
Abbreviation: MCA, Milestone Communication Approach.
Primary outcome of the study was patient information needs on the Health System and Information Needs subscale of the German version of the Short Form Supportive Care Needs Survey (SCNS‐SF34‐G) measured 3 months after inclusion in the study. This was compared between patients receiving MCA and patients receiving standard oncological care. Secondary outcomes included physical and psychological supportive care needs (as measured by the SCNS‐SF34‐G), quality of life (assessed by the Schedule for the Evaluation of Individual Quality of Life [SEIQoL] and the Functional Assessment of Cancer Therapy–lung module [FACT‐L]), distress (assessed by the Distress Thermometer), and depression and anxiety (assessed by the Patient Health Questionnaire for Depression and Anxiety [PHQ‐4]).
Patients included in the study were randomized to either the MCA pathway or standard oncological care using blocked randomization with sealed opaque envelopes provided by the Institute of Medical Biometry and Informatics (which was not involved in the data collection or interventions; concealed allocation).
After inclusion in the study, participating patients were asked by a study nurse to fill in questionnaires at the time of recruitment (baseline [t0]), after 3 (t1), 6 (t2), and 9 months (t3). Data for t3 were assessed at 9 months instead of the planned 12 months (as described in the study protocol [11]) because of organizational aspects and a high dropout rate. Randomization took place after the baseline assessment and before the first appointment with the physician (and nurse for the MCA patients). Patients and health professionals were aware of the group assignment.
Selection and Description of Participants
To be eligible for inclusion in the study, patients had to be 18 years or older and being treated at the Department of Thoracic Oncology in Heidelberg with newly diagnosed metastatic lung cancer (stage IV). They needed to have a sufficient command of German and to be able to fill in the questionnaires. Patients were invited to participate while waiting for an outpatient appointment about diagnostic test results or after having received the diagnosis. Patients unwilling to participate and unable to give consent were excluded from the study.
The hospital is one of the largest thoracic oncology clinics in Germany, providing care for about 600 patients newly diagnosed with metastatic lung cancer per year (about 1% of incident yearly lung cancer cases in Germany).
Interventions
For an overview, the published study protocol (with supplements) includes a detailed description of the intervention following the template for intervention description and replication [14]. In summary, the MCA concept consists of three key features: structured, interprofessional (physician‐nurse tandem) milestone conversations, follow‐up phone calls by the nurse, and an interprofessional communication training [10].
For the conversations, milestones were defined at specific points in the disease trajectory (diagnosis, stable phase, progression, transition to best supportive care). Previously identified (information) needs specific to the milestones [15, 16, 17] were addressed in face‐to‐face conversations between patients (and family caregivers) and an interprofessional tandem of nurse and physician (with experience in oncology and palliative care). Particularly, milestone 1 focused on the disclosure of diagnosis and prognosis, and milestone 2 addressed the stable phase treatment with response under cancer‐specific treatment. At milestone 3, disease progression with reassessment of options and change or stop of disease‐modifying interventions were discussed, and milestone 4 introduced the transition to best supportive care leading to an end‐of‐life consensus [18]. Milestones 2 and 3 could be repeated or omitted depending on the individual development of the disease. A physician and a nurse conducted the milestone conversations together as a nurse‐physician tandem. The conversations were structured using SPIKES [19] and prepared and debriefed by the tandem. Physicians and nurses used a shared electronic documentation system that was also used for assessing implementation fidelity [12].
The nurse also conducted additional phone calls about a week after each conversation and at least once a month to follow up on the patients, to clarify questions occurring after the conversations, and to sustain communication. In detail, follow‐up calls included the assessment of symptoms and palliative care needs using the Integrated Palliative Outcome Scale [20], prognostic awareness [21], resuming main topics of the milestone conversations, and providing information about and contact to other health care professionals (e.g., general practitioner, psycho‐oncologist, social worker). For the nurses, contact to other physicians involved in the treatment was possible and even encouraged.
Physicians and nurses of the tandems received an interprofessional communication training before implementation of the MCA in practice. The training consisted of four training days (one day a month) with days 1 and 3 covering communication theories, MCA content, and communication training with standardized patients. Days 2 and 4 were trainings on the job: real‐life communications as a physician‐nurse tandem with patients were observed, and feedback was provided.
Standard oncological care was not explicitly oriented on the disease trajectory (i.e., general issues were addressed but not systematically trajectory‐specific), appointments were scheduled with patients and the physician alone (without involving a nurse), and there were no follow‐up calls. Patients receiving standard oncological care had contact with nurses in the ambulatory setting for the application of chemotherapy and on the ward during treatment of complications. Conversations were unplanned and unstructured, and there were no assessments of palliative needs on a regular basis. The standard procedure of communication among oncologists (e.g., in meetings) was not changed for the intervention. Patients not included in the study received standard oncological care.
The incremental resources contained additional working time of nurses and physicians of up to six MCA conversations (about 45–60 minutes) and up to 12 follow‐up conversations (about 20 minutes) per patient per year. At the same time, there were possibly working time savings through fewer unplanned, uncoordinated (between physicians and nurses), and unstructured conversations with multiple stakeholders.
Measures
Patient support needs were assessed by the German Version of the Short Form Supportive Care Needs Survey (SCNS‐SF34‐G) [22, 23]. The questionnaire includes 34 items on a response scale of 1 (no need), 2 (need sufficiently addressed), and 3–5 (low to high need), combined in five subscales: Physical and Daily Living Needs (five items, e.g., tiredness, pain), Psychological Needs (10 items, e.g., feelings of sadness, uncertainty about the future), Sexuality Needs (three items, e.g., changes in sexual feelings), Patient Care and Support Needs (five items, e.g., choice of hospital), and Health System and Information Needs (11 items, e.g., receiving timely information about test results, availability of one team member to discuss all aspects of the disease). The subscales are analyzed separately. Standardized Likert sum scores of each subscale lead to values between 0 (no need) and 100 (high need) [24]. The questionnaire has been validated and showed good psychometric properties (i.e., [22, 23, 25, 26]).
Quality of life was assessed by the SEIQoL [27] and the FACT‐L [28, 29]. The SEIQoL covers 12 areas of quality of life, which patients rate in importance and satisfaction on five‐point percentage scales (0, 25, 50, 75, 100). A quality‐of‐life index score with values between 0 (lowest quality of life) and 100 (highest quality of life) is calculated from satisfaction scores weighted by the respective importance. The FACT‐L comprises the basic module, FACT–General (FACT‐G), with 27 items covering four domains (physical well‐being, social well‐being, emotional well‐being, functional well‐being) and seven additional questions on lung cancer–specific symptoms on five‐point Likert scales. Domain sum scores take values between 0 designating low well‐being and 24 (emotional well‐being) and 28 (other domains and additional questions) designating high well‐being, leading to a FACT‐G total score of 0 to 108 and a FACT‐L total score of 0 to 136. Additionally, the FACT trial outcome index can be combined from the physical, functional, and additional questions subscales (0–84).
Mood (depression and anxiety) was assessed by the PHQ‐4, [30] consisting of two questions addressing depression (PHQ‐2) and two questions addressing anxiety (Generalized Anxiety Disorder two‐item questionnaire [GAD‐2]), with four‐point response scales leading to a sum score of 0–12 for the PHQ‐4 and transformed sum scores of 0–6 for the subscales. Values ≥3 in the subscales are considered elevated [30].
Furthermore, psychosocial distress was assessed with the German version of the National Comprehensive Cancer Network (NCCN) Distress Thermometer [31] taking values between 0 (no distress) and 10 (extreme distress) on an 11‐point scale with values ≥5 considered needing attention. On the accompanying problem list, 36 distress‐related issues can be identified (physical, emotional, practical, family, spiritual problems, e.g., fatigue, worry, living situation).
As the patients in our study completed the baseline questionnaire before or up to 1 week after their first appointment (and thus in some cases without knowing the diagnosis), baseline assessment focused on physical (five items of the SCNS‐SF34‐G), psychological (six items of the SCNS‐SF34‐G), and information needs (10 items of the SCNS‐SF34‐G), on quality of life (SEIQoL), distress (Distress Thermometer), and depression/anxiety (PHQ‐4). Follow‐up assessments included all questionnaires in full. Because of the limited prognosis of the disease and the palliative situation, only item 28 of the SCNS‐SF34‐G (to be informed about cancer that is under control or diminishing, i.e., remission) was removed from all assessments. Sociodemographic characteristics were also assessed from patients at baseline (age, gender, residence, marital status, living situation, children, educational level, working status, primary language, smoking status, physical activity/sports, health care insurance, chronic diseases, number of medications).
Data Analysis
SCNS‐SF34‐G subscales, SEIQoL index score, FACT subscales and total scores, PHQ‐4 sum score, and Distress Thermometer were described as means ± SD for both groups at all assessments. Categorical data (sociodemographics, PHQ‐2, GAD‐2, distress) were described as absolute and relative frequencies.
Between‐group differences in addressed information needs between MCA and standard care at t1 (primary outcome), at t2 and t3, for psychological and physical support needs, for quality of life (SEIQoL), patient health (PHQ‐4), and distress (secondary outcomes) were analyzed for the intention‐to‐treat population including all randomized patients using linear models including the baseline (t0) value as independent variable. Missing values of the primary outcome were replaced by multiple imputation (fully conditional specification method [32]). A complete case analysis was done for sensitivity analysis. Between‐group differences at t0 and for follow‐up scores without t0 values were analyzed descriptively using the nonparametric Mann‐Whitney U test. Categorical data were analyzed using chi‐square independence tests for t0 data and logistic regression with an adjustment for the baseline value if assessed. Values of p < .05 were considered statistically significant.
A sample size of 82 patients was planned to detect a relevant difference of five points on the SCNS‐SF34‐G information subscale in the linear model between groups at t1 with an SD of σ = 7.4 [33] (effect size of d = 0.676). The initially proposed dropout rate of 18.5% (based on prognoses of metastatic lung cancer and the experiences at the Department of Thoracic Oncology, University Hospital Heidelberg) had to be adjusted 6 months after starting patient recruitment because of a higher dropout rate (including mortality) at t1 of 57%; we therefore aimed at including 190 patients in the study.
For analyses, SAS version 9.4 (SAS Institute, Cary, NC) and IBM SPSS Statistics 25 were used.
Ethics Approval
The study was approved by the Ethics Committee of the University Hospital Heidelberg, Germany (protocol no. S‐561/2017) and performed in accordance with the ethical standards as laid down in the Declaration of Helsinki as revised in 2013. All patients gave written informed consent before enrolling in the study. The trial was prospectively registered at the German Clinical Trials Register (Deutsches Register Klinischer Studien, trial registration no. DRKS00013469).
Results
Between May 2018 and July 2019, 171 patients were included in the study and randomized (Fig. 1). Fourteen patients did not provide data; thus, baseline data of 157 patients were analyzed (MCA: n = 79, standard care: n = 78). Patients were on average aged 66.3 ± 8.9 years and predominantly male (n = 87, 55.4%). There were no differences between MCA and standard care group in sociodemographic factors, except that in the MCA group fewer patients had children (Table 1). For 154 patients, baseline information on support needs was available, with 102 patients also providing data at 3‐month follow‐up (t1; Fig. 1).
Figure 1.
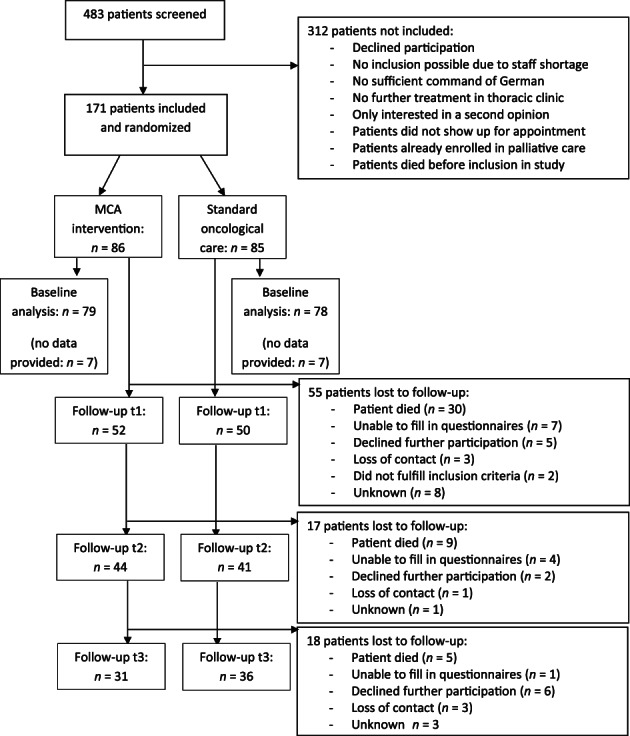
Flowchart of MCA randomized controlled trial.
Abbreviations: MCA, Milestone Communication Approach; t1, 3‐month follow‐up; t2, 6‐month follow‐up; t3, 9‐month follow‐up.
Health System and Information Needs
Patients in both MCA and standard care group had similar information needs at baseline (MCA: n = 78; mean ± SD, 53.8 ± 33.3; standard care: n = 76; 54.5 ± 31.3). At t1, patients receiving MCA care reported lower information needs (n = 52, 33.4 ± 27.5) than patients receiving standard oncological care (n = 50, 43.1 ± 29.9; p = .033; effect size: Cohen's d = −.0.37). The sensitivity analysis using complete cases (n = 102) showed similar results (p = .043). At t2 and t3, no differences in addressed information needs were observed between the groups (Table 2).
Table 2.
Differences in supportive care needs (German version of the Short Form Supportive Care Needs Survey) between MCA and standard oncological care
| Supportive Care Needs subscale | MCA intervention | Standard oncological care | p value |
|---|---|---|---|
| Health System and Information Needs | |||
| t0 | |||
| n | 78 | 76 | |
| Mean ± SD | 53.8 ± 33.3 | 54.5 ± 31.3 | .899 a |
| t1 | |||
| n | 52 | 50 | |
| Mean ± SD | 33.4 ± 27.5 | 43.1 ± 29.9 |
.033 b .043 c |
| t2 | |||
| n | 44 | 41 | |
| Mean ± SD | 34.5 ± 30.9 | 34.7 ± 30.7 | .620 d |
| t3 | |||
| n | 31 | 35 | |
| Mean ± SD | 34.3 ± 33.7 | 25.6 ± 25.4 | .378 d |
| Physical and Daily Living Needs | |||
| t0 | |||
| n | 74 | 71 | |
| Mean ± SD | 32.5 ± 28.2 | 36.4 ± 25.7 | .396 a |
| t1 | |||
| n | 52 | 50 | |
| Mean ± SD | 33.1 ± 23.7 | 36.1 ± 25.2 | .840 d |
| t2 | |||
| n | 44 | 41 | |
| Mean ± SD | 28.8 ± 22.3 | 34.5 ± 28.1 | .409 d |
| t3 | |||
| n | 31 | 35 | |
| Mean ± SD | 26.4 ± 27.1 | 30.3 ± 28.1 | .824 d |
| Psychological Needs | |||
| t0 | |||
| n | 74 | 70 | |
| Mean ± SD | 39.2 ± 31.2 | 45.9 ± 29.6 | .190 a |
| t1 | |||
| n | 52 | 50 | |
| Mean ± SD | 37.2 ± 29.7 | 42.2 ± 29.2 | .662 d |
| t2 | |||
| n | 44 | 41 | |
| Mean ± SD | 43.4 ± 27.3 | 34.8 ± 26.8 | .063 d |
| t3 | |||
| n | 31 | 35 | |
| Mean ± SD | 40.9 ± 32.7 | 29.1 ± 23.6 | .053 d |
| Patient Care and Support Needs | |||
| t1 | |||
| n | 52 | 50 | |
| Mean ± SD | 25.5 ± 27.7 | 30.2 ± 28.1 | .489 a |
| t2 | |||
| n | 44 | 41 | |
| Mean ± SD | 24.3 ± 29.6 | 22.8 ± 28.0 | .785 a |
| t3 | |||
| n | 31 | 35 | |
| Mean ± SD | 26.5 ± 32.2 | 16.4 ± 23.2 | .190 a |
| Sexuality Needs | |||
| t1 | |||
| n | 47 | 44 | |
| Mean ± SD | 17.1 ± 22.9 | 15.4 ± 18.4 | .608 a |
| t2 | |||
| n | 42 | 39 | |
| Mean ± SD | 17.0 ± 23.1 | 14.4 ± 19.1 | .975 a |
| t3 | |||
| n | 29 | 34 | |
| Mean ± SD | 14.0 ± 20.7 | 11.2 ± 20.0 | .302 a |
Mann‐Whitney U test.
Linear model with multiple imputation and adjustment for baseline value.
Complete case linear model (sensitivity analysis).
Linear model with adjustment for baseline value.
Abbreviations: MCA, Milestone Communication Approach; t0, baseline assessment; t1, 3‐month follow‐up; t2, 6‐month follow‐up; t3 9‐month follow‐up.
Other Support Needs
At all assessment points, patients in both groups did not differ in their mean physical and daily living care needs and in their psychological care needs (Table 2). Patient care and support needs and sexuality needs were not assessed at baseline; group differences were only analyzed descriptively (Table 2).
Quality of Life
The SEIQoL quality‐of‐life index score showed a similar quality of life in both patient groups at baseline and follow‐up assessments (Table 3). The various FACT scores were not assessed at baseline; they showed no differences between MCA and standard oncological care group at follow‐up assessments (Table 3).
Table 3.
Differences in quality of life (SEIQoL, FACT) between MCA and standard oncological care
| Quality of life (sub‐)scale | MCA intervention | Standard oncological care | p value |
|---|---|---|---|
| SEIQoL quality of life index | |||
| t0 | |||
| n | 56 | 50 | |
| Mean ± SD | 61.6 ± 17.6 | 61.0 ± 17.7 | .922 a |
| t1 | |||
| n | 34 | 33 | |
| Mean ± SD | 59.6 ± 17.7 | 56.8 ± 17.3 | .832 b |
| t2 | |||
| n | 29 | 32 | |
| Mean ± SD | 62.4 ± 17.6 | 65.0 ± 16.8 | .238 b |
| t3 | |||
| n | 25 | 29 | |
| Mean ± SD | 59.2 ± 17.8 | 62.5 ± 15.8 | .473 b |
| FACT‐G total score | |||
| t1 | |||
| n | 50 | 48 | |
| Mean ± SD | 73.5 ± 16.7 | 69.1 ± 16.8 | .361 a |
| t2 | |||
| n | 41 | 39 | |
| Mean ± SD | 72.5 ± 16.3 | 72.6 ± 16.1 | .765 a |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 70.2 ± 20.0 | 75.4 ± 16.7 | .230 a |
| FACT‐L total score | |||
| t1 | |||
| n | 50 | 48 | |
| Mean ± SD | 93.9 ± 18.9 | 88.7 ± 20.3 | .301 a |
| t2 | |||
| n | 41 | 39 | |
| Mean ± SD | 93.1 ± 19.2 | 93.1 ± 19.8 | .904 a |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 89.9 ± 23.7 | 96.1 ± 20.4 | .266 a |
| FACT physical well‐being | |||
| t1 | |||
| n | 52 | 50 | |
| Mean ± SD | 20.0 ± 5.8 | 19.4 ± 6.1 | .673 a |
| t2 | |||
| n | 43 | 41 | |
| Mean ± SD | 20.7 ± 5.5 | 19.8 ± 6.5 | .723 a |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 19.3 ± 7.4 | 20.3 ± 6.3 | .619 a |
| FACT social/family well‐being | |||
| t1 | |||
| n | 50 | 49 | |
| Mean ± SD | 22.4 ± 4.7 | 20.4 ± 5.8 | .064 a |
| t2 | |||
| n | 42 | 41 | |
| Mean ± SD | 22.0 ± 4.4 | 21.4 ± 4.8 | .661 a |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 21.0 ± 5.4 | 21.8 ± 4.0 | .739 a |
| FACT emotional well‐being | |||
| t1 | |||
| n | 52 | 50 | |
| Mean ± SD | 17.1 ± 4.8 | 15.8 ± 5.5 | .280 a |
| t2 | |||
| n | 42 | 40 | |
| Mean ± SD | 16.1 ± 5.3 | 16.8 ± 4.8 | .707 a |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 15.5 ± 6.4 | 17.1 ± 5.1 | .341 a |
| FACT functional well‐being | |||
| t1 | |||
| n | 52 | 50 | |
| Mean ± SD | 13.4 ± 6.6 | 14.0 ± 6.0 | .555 a |
| t2 | |||
| n | 42 | 41 | |
| Mean ± SD | 13.2 ± 5.9 | 14.7 ± 5.7 | .301 a |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 14.4 ± 6.7 | 16.2 ± 6.5 | .287 a |
| FACT additional concerns | |||
| t1 | |||
| n | 52 | 50 | |
| Mean ± SD | 20.4 ± 4.0 | 19.7 ± 4.7 | .540 a |
| t2 | |||
| n | 43 | 41 | |
| Mean ± SD | 20.5 ± 4.4 | 20.4 ± 4.5 | .816 a |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 19.7 ± 5.2 | 20.7 ± 4.7 | .610 a |
| FACT trial outcome index | |||
| t1 | |||
| n | 52 | 50 | |
| Mean ± SD | 53.8 ± 14.0 | 53.1 ± 14.4 | .963 a |
| t2 | |||
| n | 42 | 41 | |
| Mean ± SD | 54.2 ± 13.5 | 54.9 ± 15.0 | .820 a |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 53.3 ± 16.9 | 57.2 ± 15.5 | .443 a |
Mann‐Whitney U test.
Linear model with adjustment for baseline value.
Abbreviations: FACT, Functional Assessment of Cancer Therapy; FACT‐G, FACT general module; FACT‐L, FACT lung module; MCA, Milestone Communication Approach; SEIQoL, Schedule for the Evaluation of Individual Quality of Life; t0, baseline assessment; t1, 3‐month follow‐up; t2, 6‐month follow‐up; t3, 12‐month follow‐up.
Mood (Depression/Anxiety)
Generally, depression and anxiety as measured with the four‐item PHQ‐4 did not show differences between the treatment groups at any time (Table 4). For depression measured with the two‐item PHQ‐2, patients in standard oncological care reported higher scores at baseline (MCA: mean ± SD, 2.0 ± 1.5; standard oncological care: 2.6 ± 1.6; p < .05). There were no differences at other assessment times adjusted for baseline. Neither did patients report elevated scores for anxiety (GAD‐2) at any point in time.
Table 4.
Differences in depression, anxiety, and distress between MCA and standard oncological care
| Mood scale | MCA intervention | Standard oncological care | p value |
|---|---|---|---|
| PHQ‐4 sum score | |||
| t0 | |||
| n | 79 | 74 | |
| Mean ± SD | 4.1 ± 3.1 | 5.0 ± 3.4 | .129 a |
| t1 | |||
| n | 50 | 50 | |
| Mean ± SD | 3.5 ± 2.9 | 3.9 ± 2.9 | .565 b |
| t2 | |||
| n | 43 | 40 | |
| Mean ± SD | 3.4 ± 2.9 | 4.1 ± 3.1 | .724 b |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 4.2 ± 3.6 | 3.6 ± 3.1 | .284 b |
| PHQ‐2 sum score | |||
| t0 | |||
| n | 79 | 74 | |
| Mean ± SD | 2.0 ± 1.5 | 2.6 ± 1.6 | .041 a |
| ≥3, n (%) | 26 (32.9) | 31 (41.9) | .251 c |
| t1 | |||
| n | 50 | 50 | |
| Mean ± SD | 1.8 ± 1.6 | 1.9 ± 1.4 | .421 b |
| ≥3, n (%) | 12 (24.0) | 13 (26.0) | .446 d |
| t2 | |||
| n | 43 | 40 | |
| Mean ± SD | 1.7 ± 1.4 | 2.1 ± 1.6 | .555 b |
| ≥3 n (%) | 7 (16.3) | 15 (37.5) | .236 d |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 2.1 ± 1.8 | 2.0 ± 1.6 | .256 b |
| ≥3, n (%) | 10 (32.3) | 12 (33.3) | .867 d |
| GAD‐2 sum score | |||
| t0 | |||
| n | 79 | 74 | |
| Mean ± SD | 2.1 ± 1.8 | 2.4 ± 2.0 | .361 a |
| ≥3 n (%) | 28 (35.4) | 28 (37.8) | .759 c |
| t1 | |||
| n | 50 | 50 | |
| Mean ± SD | 1.7 ± 1.5 | 2.1 ± 1.8 | .814 b |
| ≥3, n (%) | 9 (18.0) | 15 (30.0) | .833 d |
| t2 | |||
| n | 43 | 41 | |
| Mean ± SD | 1.7 ± 1.7 | 1.9 ± 1.7 | .889 b |
| ≥3, n (%) | 7 (16.3) | 11 (26.8) | .964 d |
| t3 | |||
| n | 31 | 36 | |
| Mean ± SD | 2.1 ± 1.9 | 1.6 ± 1.6 | .300 b |
| ≥3, n (%) | 10 (32.3) | 8 (22.2) | .120 d |
| Distress Thermometer | |||
| t0 | |||
| n | 72 | 74 | |
| Mean ± SD | 5.9 ± 2.4 | 6.3 ± 2.6 | .416 a |
| ≥5, n (%) | 55 (76.4) | 50 (67.6) | .236 c |
| t1 | |||
| n | 51 | 46 | |
| Mean ± SD | 4.9 ± 2.5 | 5.5 ± 2.4 | .146 b |
| ≥5, n (%) | 33 (64.7) | 31 (67.4) | .357 d |
| t2 | |||
| n | 38 | 38 | |
| Mean ± SD | 5.2 ± 2.7 | 4.7 ± 2.6 | .309 b |
| ≥5, n (%) | 26 (68.4) | 21 (55.3) | .551 d |
| t3 | |||
| n | 27 | 36 | |
| Mean ± SD | 5.8 ± 3.2 | 5.2 ± 2.6 | .473 b |
| ≥5, n (%) | 17 (63.0) | 23 (63.9) | .837 d |
Mann‐Whitney U test.
Linear model with adjustment for baseline value.
Chi‐square independence test.
Logistic regression with adjustment for baseline value.
Abbreviations: GAD‐2, Generalized Anxiety Disorder two‐item questionnaire; MCA, Milestone Communication Approach; PHQ‐2, Patient Health Questionnaire (two depression items); PHQ‐4, Patient Health Questionnaire for Depression and Anxiety (PHQ‐2 and GAD‐2); t0, baseline assessment; t1, 3‐month follow‐up; t2, 6‐month follow‐up; t3, 12‐month follow‐up.
Distress
More than 50% of patients in both groups reported increased distress across assessments. There were no differences between groups in the proportion of patients reporting critical distress (at least five points on the Distress Thermometer; Table 4). At baseline, patients identified a median of 8.0 (interquartile range [IQR], 4.0–12.0) issues (MCA: median, 8.0; IQR, 5.0–12.0; standard oncological care: median, 9.0; IQR, 5.0–12.3) and at 3‐month follow‐up a median of 4.0 (IQR, 0.0–10.0) issues (MCA: median, 4.0; IQR, 0.0–10.0; standard oncological care: median, 5.0; IQR, 0.0–11.0) on the problem list. The most frequently marked issues referred to physical and emotional problems. Fatigue remained a problem for more than 60% of the patients throughout the study; however, emotional problems like worry and fear became less important (Table 5).
Table 5.
Most frequently reported problems (Distress Thermometer)
| Problem (whole sample) | t0 | n (%) | t1 | n (%) | t2 | n (%) | t3 | n (%) |
|---|---|---|---|---|---|---|---|---|
| Physical problem: fatigue | 1 | 109 (72.7) | 1 | 65 (65.0) | 1 | 51 (63.7) | 1 | 40 (62.5) |
| Emotional problem: fears | 2 | 92 (61.3) | 2 | 54 (54.0) | 4 | 38 (46.3 | 14 | 22 (33.8) |
| Physical problem: getting around | 3 | 91 (60.3) | 3 | 53 (53.5) | 3 | 41 (49.4) | 2 | 33 (50.0) |
| Emotional problem: worry | 4 | 87 (59.6) | 4 | 49 (49.5) | 2 | 44 (52.4) | 10 | 25 (39.1) |
| Physical problem: pain | 5 | 82 (54.3) | 5 | 49 (49.5) | 7 | 33 (40.7) | 4 | 28 (43.1) |
| Physical problem: skin dry/itchy | 12 | 47 (31.3) | 9 | 39 (39.4) | 10 | 30 (37.0) | 3 | 33 (49.3) |
| Physical problem: memory/concentration | 13 | 44 (29.3) | 16 | 32 (31.4) | 5 | 35 (41.7) | 6 | 28 (42.4) |
Abbreviations: t0, baseline assessment; t1, 3‐month follow‐up; t2, 6‐month follow‐up; t3, 12‐month follow‐up.
Discussion
The structured Milestone Communication Approach more effectively managed patients’ health system and information needs compared with standard oncological care. Needs were high in the patient population related to the predefined primary outcome of the randomized trial. Following the milestone structure, patients received necessary information about diagnosis and prognosis when they needed it. Patients with standard oncological care also experienced decreasing information needs over time but with a delay of at least 3 months behind the MCA group. Addressing information needs in a timely patient‐centered way is one important aspect of facilitating prognostic awareness and advance care planning.
With the primary focus on meeting information needs after receiving the diagnosis of stage IV lung cancer, the MCA addressed the most pressing questions determined by patients at time. Nevertheless, other issues were not neglected but were only touched upon (so as not to overwhelm patients unless they voiced the need to discuss those as well). The MCA provides a method to find a balance between sufficiently meeting information needs and inundating patients with information that they cannot process in due time [34].
Information needs assessed by the SCNS‐SF34 covered issues like receiving timely information about test results, having access to professional counseling if needed, being informed about benefits and side effects of treatments, and the availability of one team member to discuss all aspects of the disease. The MCA addressed those needs, not only by structured conversations but also by providing continuity of care.
A stronger role was given to oncology nurses in the MCA. They were in closer contact with the patients, kept the contact up through regular conversations (not only within the milestone conversations) and phone calls, and built a trusting relationship to the patients, often being the first health care professional to whom patients reported their concerns. Nurses responded to individual needs of patients, including giving information about treatment and symptom management and sharing information with the interprofessional team by documentation. There was significantly increased contribution to patient‐centered care within the MCA by the nursing team members.
Health system and informational needs have been reported as key issues for patients with cancer in various studies [35, 36, 37, 38, 39, 40, 41], including the German validation study of the SCNS‐SF34 [23]. In this study, the decision to choose information needs as the primary outcome related to the problem of uncertainty in the decision‐making process. Cancer therapeutics develop rapidly and the constantly changing treatment algorithms challenge the clinician‐patient communication. In addition, the trend for shared decision‐making and the need for timely information by patients were strong influences. Being informed constitutes the necessary basis for realistic prognostic awareness and patient‐centered shared decisions during treatment and at the end of life.
For the SCNS‐SF34, no minimally important difference indicating a clinically relevant change or difference between treatment groups has been published. For planning our study, we considered a mean difference of five points on the 0–100 scale as sufficient for a relevant difference between MCA and standard care groups. The observed mean difference of 10 points in our study was in line with the results reported for known‐groups validity in the original validation study (mean difference: 11.5 points [22]) and international translations [42, 43, 44].
Although the average reported needs in other studies are similar to our findings, our patient sample reported a larger deviation in individual needs. Thus, at first glance this homogeneous patient group (only patients with stage IV lung cancer) had heterogeneous needs, which must be considered when planning individualized patient care. New treatment regimens, such as targeted therapy and immunotherapy, may further trigger individualized approaches to distinct disease trajectories with varying needs. Communication interventions aiming at patient values and goals, such as the Serious Illness Care Program [45], could be combined with the longitudinal perspective. The Milestone Communication Approach may be a concept to assist in navigating the complex balance between individual needs and trajectory‐specific challenges.
Patients who are newly diagnosed with metastatic lung cancer are confronted with a very challenging situation. Getting timely and tailored information about the disease and what to expect in the future supports them in maintaining a sense of control [46]. Structured communication and having a responsive member of the health care team following up on patients assist in patient coping [13] and reducing uncertainty in a highly uncertain situation [47]. Interprofessional collaboration (notably physicians and nurses) and a longitudinal communication approach (including aspects of illness understanding, prognostic awareness, coping, and advance care planning) are key features for effectiveness in early palliative care according to cancer care guidelines [48]. MCA integrated these aspects into patient‐centered oncological care pathways. Trials evaluating early palliative care focused on health‐related quality of life, depression, symptom intensity, and survival [49].
Similar to other trials, the communication approach showed no effects on patient‐reported quality of life. Generally, quality‐of‐life scores were comparable to those of other study samples [50]. Other studies addressing patients with advanced cancer did not report change in quality of life either [51, 52], although the integration of early palliative care has led to improved quality‐of‐life scores [2, 49]. In our study, potential changes in quality of life were analyzed using the SEIQoL, which considers shifts in relevance of areas of life. In a life‐changing situation, patients adapt their priorities of what is important for them in life to cope with the challenges of the situation and thus to maintain quality of life.
Symptoms, anxiety, and depression are relevant patient‐reported outcomes for which no differences were found for patients in MCA care and patients in standard oncological care. Other studies fostering the early integration of palliative care could also not detect advantages of early palliative care for physical functioning and symptom management [49] but reported a higher satisfaction of patients, especially with physicians’ attention and information given [53]. With our study, the MCA highlighted the importance of communication between health care professionals and patients using an individualized approach. However, patients in both groups of our study reported elevated scores of depression and distress that call for further attention. Other studies reported similar levels of distress and depression in patients with lung cancer [50]. The regular use of short screening tools like the PHQ‐4 and the NCCN Distress Thermometer should be considered in clinical practice to identify patients in need of specific support [54].
Limitations
Patients in the intervention and the control group were cared for by the same oncologists. Thus, it cannot be excluded that the communication training possibly had effects on their interactions with both patient groups. Still, the main intervention—the conversation with a physician‐nurse tandem and continuity of care by the nurse—was only given to patients in the intervention group. The reported difference between the patient groups in addressing information needs was observed despite possible contamination and might thus be underestimated.
Group allocation could not be blinded for patients and health care providers. The information given to all patients for informed consent to participate in the study concentrated on the structure of the intervention (additional contacts for patients, training for physicians and nurses to promote interprofessional communication) and did not reveal actual contents of the intervention conversations. Higher expectations in the group of patients receiving standard oncological care might have led to reporting bias. Patient‐reported topics addressed in conversations could have been compared with topics in the documentation file filled in by the physicians. For communication with patients, it is more important, however, which information the patient remembers from the conversation than what was objectively addressed [55]. An underreporting caused by poor recollection of the conversation would also provide further aspects for improvement in patient communication.
Differences between the groups observed in secondary outcomes have to be interpreted with caution. The study was powered to detect a difference in information needs—which could be shown—but other observed differences would need to be confirmed in future studies. For further evaluation and improvement of the concept, other aims, such as prognostic awareness and advance care planning, should be focused on in addition to quality‐of‐life and palliative care outcomes.
An additional limitation is the high dropout rate. The large amount of loss to follow‐up was largely due to high mortality, which was higher than expected. The median survival in our study was lower than in the general population of patients with lung cancer with a median survival of 12 months. All included patients had stage IV lung cancer. Other clinical characteristics were not assessed in the study, so these patients may have had a more severe type of cancer.
Implications for Future Research
Involving patients in their care, including making decisions about treatments, especially for patients with life‐threatening diseases and a limited prognosis (e.g., stage IV lung cancer), is increasingly important. Patients should have the ability to determine their care and treatment according to their individual wishes. Therefore, identifying support needs of patients and ways to address them early in the disease trajectory must have a focus in oncology care and research [4]. Future studies should also analyze if and how the concept of structured communication along the disease trajectory addresses individual advance care planning for the benefit of patients and their families.
Conclusion
Patients reported decreased (unmet) information needs after having received MCA care in comparison with patients offered standard oncological care. Balancing information flow and occasions for communication in the disease trajectory according to patient's own self‐determined readiness led to well‐informed patients better able to understand their situation and the limited prognosis. The MCA intervention is a worthwhile tool for improving the communication processes at key stages of the patient journey for newly diagnosed patients with cancer with limited prognosis.
Author Contributions
Conception/design: Katja Krug, Jasmin Bossert, Johannes Krisam, Matthias Villalobos, Anja Siegle, Jana Jünger, Michael Thomas, Michel Wensing
Provision of study material or patients: Matthias Villalobos, Michael Thomas
Collection and/or assembly of data: Nicole Deis, Matthias Villalobos, Anja Siegle, Corinna Jung, Laura Hagelskamp, Laura Unsöld, Michael Thomas
Data analysis and interpretation: Katja Krug, Jasmin Bossert, Nicole Deis, Johannes Krisam, Matthias Villalobos, Anja Siegle, Corinna Jung, Laura Hagelskamp, Laura Unsöld, Jana Jünger, Michael Thomas, Michel Wensing
Manuscript writing: Katja Krug, Jasmin Bossert, Nicole Deis, Johannes Krisam, Matthias Villalobos, Anja Siegle, Corinna Jung, Laura Hagelskamp, Laura Unsöld, Jana Jünger, Michael Thomas, Michel Wensing
Final approval of manuscript: Katja Krug, Jasmin Bossert, Nicole Deis, Johannes Krisam, Matthias Villalobos, Anja Siegle, Corinna Jung, Laura Hagelskamp, Laura Unsöld, Jana Jünger, Michael Thomas, Michel Wensing
Disclosures
The authors indicated no financial relationships.
Acknowledgments
We would like to thank the patients, physicians, and nurses for their participation in this study. We are obliged to Marianne Förderer for patient recruitment. We sincerely thank Sarah Jane Berger for reviewing language and expression. The study was funded by the German Federal Ministry of Health (ZMV I1–2517 FSB 001) and the National Center for Tumor Diseases (NCT 3.0, G835). L.H. is currently affiliated with University Hospital Tuebingen, Institute of Health Sciences, Department of Nursing Science, Hoppe‐Seyler‐Str. 9, 72076 Tuebingen, Germany. Open access funding was enabled and organized by Projekt DEAL.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Zentrum für Krebsregisterdaten . Datenbankabfrage. Robert Koch Institut, 2020. Available at https://www.krebsdaten.de/Krebs/SiteGlobals/Forms/Datenbankabfrage/datenbankabfrage_stufe2_form.html. Accessed June 15, 2020.
- 2. Temel JS, Greer JA, Muzikansky A et al. Early palliative care for patients with metastatic non–small‐cell lung cancer. N Engl J Med 2010;363:733–742. [DOI] [PubMed] [Google Scholar]
- 3. Irwin KE, Greer JA, Khatib J et al. Early palliative care and metastatic non‐small cell lung cancer: Potential mechanisms of prolonged survival. Chron Respir Dis 2013;10:35–47. [DOI] [PubMed] [Google Scholar]
- 4. Temel JS, Greer JA, El‐Jawahri A et al. Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J Clin Oncol 2017;35:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishijima TF, Shachar SS, Nyrop KA et al. Safety and tolerability of PD‐1/PD‐L1 inhibitors compared with chemotherapy in patients with advanced cancer: A meta‐analysis. The Oncologist 2017;22:470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sung MR, Patel MV, Djalalov S et al. Evolution of symptom burden of advanced lung cancer over a decade. Clin Lung Cancer 2017;18:274–280.e276. [DOI] [PubMed] [Google Scholar]
- 7. Muhlbacher AC, Bethge S. Patients’ preferences: A discrete‐choice experiment for treatment of non‐small‐cell lung cancer. Eur J Health Econ 2015;16:657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mosher CE, Ott MA, Hanna N et al. Development of a symptom management intervention: Qualitative feedback from advanced lung cancer patients and their family caregivers. Cancer Nurs 2017;40:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peppercorn JM, Smith TJ, Helft PR et al. American Society of Clinical Oncology statement: Toward individualized care for patients with advanced cancer. J Clin Oncol 2011;29:755–760. [DOI] [PubMed] [Google Scholar]
- 10. Villalobos M, Siegle A, Hagelskamp L et al. HeiMeKOM (Heidelberg Milestones Communication): Development of an interprofessional intervention for improvement of communication in patients with limited prognosis [in German]. Z Evid Fortbild Qual Gesundhwes 2019;147–148:28–33. [DOI] [PubMed] [Google Scholar]
- 11. Siegle A, Villalobos M, Bossert J et al. The Heidelberg Milestones Communication Approach (MCA) for patients with prognosis <12 months: Protocol for a mixed‐methods study including a randomized controlled trial. Trials 2018;19:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bossert J, Wensing M, Thomas M et al. Implementation of the Milestones Communication Approach for patients with limited prognosis: Evaluation of intervention fidelity. BMC Palliat Care 2020;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krug K, Bossert J, Stooss L et al. Consideration of sense of coherence in a structured communication approach with stage IV lung cancer patients and their informal caregivers: A qualitative interview study. Support Care Cancer 2021;29:2153–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmann TC, Glasziou PP, Boutron I et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 15. Sparla A, Flach‐Vorgang S, Villalobos M et al. Reflection of illness and strategies for handling advanced lung cancer ‐ a qualitative analysis in patients and their relatives. BMC Health Serv Res 2017;17:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sparla A, Flach‐Vorgang S, Villalobos M et al. Individual difficulties and resources ‐ a qualitative analysis in patients with advanced lung cancer and their relatives. Patient Prefer Adherence 2016;10:2021–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Villalobos M, Coulibaly K, Krug K et al. A longitudinal communication approach in advanced lung cancer: A qualitative study of patients’, relatives’ and staff's perspectives. Eur J Cancer Care (Engl) 2018;27:e12794. [DOI] [PubMed] [Google Scholar]
- 18. Villalobos M, Siegle A, Hagelskamp L et al. Communication along milestones in lung cancer patients with advanced disease. Oncol Res Treat 2019;42:41–46. [DOI] [PubMed] [Google Scholar]
- 19. Baile WF, Buckman R, Lenzi R et al. SPIKES—A six‐step protocol for delivering bad news: Application to the patient with cancer. The Oncologist 2000;5:302–311. [DOI] [PubMed] [Google Scholar]
- 20. Bausewein C, Fegg M, Radbruch L et al. Validation and clinical application of the German version of the palliative care outcome scale. J Pain Symptom Manage 2005;30:51–62. [DOI] [PubMed] [Google Scholar]
- 21. Jackson VA, Jacobsen J, Greer JA et al. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: A communication guide. J Palliat Med 2013;16:894–900. [DOI] [PubMed] [Google Scholar]
- 22. Boyes A, Girgis A, Lecathelinais C. Brief assessment of adult cancer patients’ perceived needs: Development and validation of the 34‐item Supportive Care Needs Survey (SCNS‐SF34). J Eval Clin Pract 2009;15:602–606. [DOI] [PubMed] [Google Scholar]
- 23. Lehmann C, Koch U, Mehnert A. Psychometric properties of the German version of the Short‐Form Supportive Care Needs Survey Questionnaire (SCNS‐SF34‐G). Support Care Cancer 2012;20:2415–2424. [DOI] [PubMed] [Google Scholar]
- 24. McElduff P, Boyes A, Zucca A et al. The Supportive Care Needs Survey: A Guide to Administration, Scoring and Analysis. Newcastle, UK: Centre for Health Research & Psycho‐Oncology, 2004. [Google Scholar]
- 25. Jansen F, Witte BI, van Uden‐Kraan CF et al. The need for supportive care among head and neck cancer patients: Psychometric assessment of the Dutch version of the Supportive Care Needs Survey Short‐Form (SCNS‐SF34) and the newly developed head and neck cancer module (SCNS‐HNC). Support Care Cancer 2016;24:4639–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bredart A, Kop JL, Griesser AC et al. Validation of the 34‐item Supportive Care Needs Survey and 8‐item breast module French versions (SCNS‐SF34‐Fr and SCNS‐BR8‐Fr) in breast cancer patients. Eur J Cancer Care (Engl) 2012;21:450–459. [DOI] [PubMed] [Google Scholar]
- 27. Wettergren L, Kettis‐Lindblad A, Sprangers M et al. The use, feasibility and psychometric properties of an individualised quality‐of‐life instrument: A systematic review of the SEIQoL‐DW. Qual Life Res 2009;18:737–746. [DOI] [PubMed] [Google Scholar]
- 28. Bonomi AE, Cella DF, Hahn EA et al. Multilingual translation of the Functional Assessment of Cancer Therapy (FACT) quality of life measurement system. Qual Life Res 1996;5:309–320. [DOI] [PubMed] [Google Scholar]
- 29. Cella DF, Bonomi AE, Lloyd SR et al. Reliability and validity of the Functional Assessment of Cancer Therapy‐Lung (FACT‐L) quality of life instrument. Lung Cancer 1995;12:199–220. [DOI] [PubMed] [Google Scholar]
- 30. Lowe B, Wahl I, Rose M et al. A 4‐item measure of depression and anxiety: Validation and standardization of the Patient Health Questionnaire‐4 (PHQ‐4) in the general population. J Affect Disord 2010;122:86–95. [DOI] [PubMed] [Google Scholar]
- 31. Mehnert A, Müller D, Lehmann C et al. Die deutsche Version des NCCN Distress‐Thermometers ‐ empirische Prüfung eines Screening‐Instruments zur Erfassung psychosozialer Belastung bei Krebspatienten. Z Psychiatr Psychol Psychother 2006;54:213–223. [Google Scholar]
- 32. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- 33. Giuliani ME, Milne RA, Puts M et al. The prevalence and nature of supportive care needs in lung cancer patients. Curr Oncol 2016;23:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skalla KA, Bakitas M, Furstenberg CT et al. Patients’ need for information about cancer therapy. Oncol Nurs Forum 2004;31:313–319. [DOI] [PubMed] [Google Scholar]
- 35. Jie Y, Wang Y, Chen J et al. Unmet supportive care needs and its relation to quality of life among adult acute leukaemia patients in China: A cross‐sectional study. Health Qual Life Outcomes 2020;18:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gebresillassie BM, Ayele AA, Abegaz TM. Unmet supportive care needs and determinants among cancer patients treated at University of Gondar Specialized Hospital, Northwest Ethiopia: A prospective cross‐sectional study. J Oncol Pharm Pract 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37. Yu FF, Bai YN, He H et al. Identifying the unmet supportive care needs, with concomitant influencing factors, in adult acute leukemia patients in China. Eur J Oncol Nurs 2017;30:67–74. [DOI] [PubMed] [Google Scholar]
- 38. Pérez‐Fortis A, Fleer J, Schroevers MJ et al. Course and predictors of supportive care needs among Mexican breast cancer patients: A longitudinal study. Psychooncology 2018;27:2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang S, Li Y, Li C et al. Distribution and determinants of unmet need for supportive care among women with breast cancer in China. Med Sci Monit 2018;24:1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Williams N, Griffin G, Farrell V et al. The supportive care needs of women experiencing gynaecological cancer: A Western Australian cross‐sectional study. BMC Cancer 2018;18:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarkar S, Sautier L, Schilling G et al. Anxiety and fear of cancer recurrence and its association with supportive care needs and health‐care service utilization in cancer patients. J Cancer Surviv 2015;9:567–575. [DOI] [PubMed] [Google Scholar]
- 42. Choi EPH, Liao Q, Soong I et al. Measurement invariance across gender and age groups, validity and reliability of the Chinese version of the short‐form supportive care needs survey questionnaire (SCNS‐SF34). Health Qual Life Outcomes 2020;18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Han Y, Zhou Y, Wang J et al. Psychometric testing of the Mandarin version of the 34‐item Short‐Form Supportive Care Needs Survey in patients with cancer in mainland China. Support Care Cancer 2017;25:3329–3338. [DOI] [PubMed] [Google Scholar]
- 44. Au A, Lam WW, Kwong A et al. Validation of the Chinese version of the short‐form Supportive Care Needs Survey Questionnaire (SCNS‐SF34‐C). Psychooncology 2011;20:1292–1300. [DOI] [PubMed] [Google Scholar]
- 45. Bernacki R, Hutchings M, Vick J et al. Development of the Serious Illness Care Program: A randomised controlled trial of a palliative care communication intervention. BMJ Open 2015;5:e009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blödt S, Kaiser M, Adam Y et al. Understanding the role of health information in patients’ experiences: Secondary analysis of qualitative narrative interviews with people diagnosed with cancer in Germany. BMJ Open 2018;8:e019576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y. Uncertainty in illness: Theory review, application, and extension. Oncol Nurs Forum 2017;44:645–649. [DOI] [PubMed] [Google Scholar]
- 48. Gilligan T, Coyle N, Frankel RM et al. Patient‐clinician communication: American Society of Clinical Oncology consensus guideline. J Clin Oncol 2017;35:3618–3632. [DOI] [PubMed] [Google Scholar]
- 49. Haun MW, Estel S, Rucker G et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev 2017;(6):CD011129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuon J, Vogt J, Mehnert A et al. Symptoms and needs of patients with advanced lung cancer: Early prevalence assessment. Oncol Res Treat 2019;42:650–659. [DOI] [PubMed] [Google Scholar]
- 51. Bakitas MA, Tosteson TD, Li Z et al. Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zimmermann C, Swami N, Krzyzanowska M et al. Early palliative care for patients with advanced cancer: A cluster‐randomised controlled trial. Lancet 2014;383:1721–1730. [DOI] [PubMed] [Google Scholar]
- 53. Johnsen AT, Petersen MA, Sjogren P et al. Exploratory analyses of the Danish Palliative Care Trial (DanPaCT): A randomized trial of early specialized palliative care plus standard care versus standard care in advanced cancer patients. Support Care Cancer 2020;28:2145–2155. [DOI] [PubMed] [Google Scholar]
- 54. Alt‐Epping B, Seidel W, Vogt J et al. Symptoms and needs of head and neck cancer patients at diagnosis of incurability ‐ prevalences, clinical implications, and feasibility of a prospective longitudinal multicenter cohort study. Oncol Res Treat 2016;39:186–191. [DOI] [PubMed] [Google Scholar]
- 55. Kessels RP. Patients’ memory for medical information. J R Soc Med 2003;96:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]


