Abstract
Background
This analysis investigated whether baseline characteristics affect the survival benefit derived from palbociclib‐fulvestrant and the optimal timing of cyclin‐dependent kinase 4/6 inhibitor therapy for advanced breast cancer (ABC) in patients from PALOMA‐3.
Patients and Methods
In total, 521 patients were randomized 2:1 to receive palbociclib (125 mg/day, 3/1 schedule)–fulvestrant (500 mg, intramuscular injection, on days 1 and 15 of cycle 1, and then day 1 of each subsequent cycle) or matching placebo‐fulvestrant. Median overall survival (OS) and progression‐free survival were estimated using the Kaplan‐Meier method.
Results
Multivariable analysis identified endocrine sensitivity, nonvisceral disease, no prior chemotherapy for ABC, and Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 as significant prognostic factors for OS. Patients without chemotherapy for ABC had fewer prior lines of treatment in any setting and in the ABC setting versus patients with prior chemotherapy for ABC (two or fewer prior systemic therapies: 69% vs. 42%; no more than one prior line for ABC: 82% vs. 33%, respectively). Median OS was prolonged with palbociclib‐fulvestrant in patients without prior chemotherapy for ABC (39.7 vs. 29.5 months; hazard ratio, 0.75; 95% confidence interval [CI]: 0.56–1.01) and was similar in patients with prior chemotherapy for ABC (25.6 vs. 26.2 months; hazard ratio, 0.91 [95% CI: 0.63–1.32]) versus placebo‐fulvestrant.
Conclusion
Prognostic factors for OS included endocrine sensitivity, nonvisceral disease, ECOG PS of 0, and no prior chemotherapy for ABC. Exploratory analyses suggest improved OS with palbociclib‐fulvestrant versus placebo‐fulvestrant in patients with no prior chemotherapy for ABC, prior endocrine sensitivity, and fewer prior regimens of systemic therapy. (Clinical trial identification number: NCT01942135).
Implications for Practice
Prognostic factors for overall survival in HR+/HER2− advanced breast cancer (ABC) include the absence of prior chemotherapy in the advanced setting, endocrine sensitivity, nonvisceral disease, and an ECOG performance status of 0. Improved overall survival benefit was observed with palbociclib‐fulvestrant versus placebo‐fulvestrant in patients (regardless of menopausal status or visceral involvement) with no prior chemotherapy for ABC, with prior endocrine sensitivity, and fewer prior regimens of systemic therapy. Progression‐free survival was prolonged with palbociclib across subgroups (regardless of chemotherapy exposure in ABC). These exploratory findings suggest that patients may receive greater clinical benefit from palbociclib‐fulvestrant if they receive the combination before chemotherapy in the advanced setting.
Keywords: Palbociclib, Overall survival, HR+/HER2− advanced breast cancer, Prior chemotherapy, Visceral
Short abstract
This article focuses on whether baseline characteristics affect the survival benefit derived from palbociclib‐fulvestrant and the optimal timing of cyclin‐dependent kinase 4/6 inhibitor therapy for advanced breast cancer in patients from the PALOMA‐3 clinical trial.
Introduction
Cyclin‐dependent kinase 4/6 (CDK4/6) inhibitors in combination with endocrine therapy (ET) have become the mainstay of treatment for patients with hormone receptor‐positive/human epidermal growth factor receptor 2‐negative (HR+/HER2−) advanced breast cancer (ABC) [1, 2, 3]. Palbociclib, a first‐in‐class CDK4/6 inhibitor, demonstrated anticancer activity in preclinical tests [4, 5] and is approved to treat patients with HR+/HER2− ABC in combination with ET [6]. The PALOMA‐3 clinical trial demonstrated the efficacy and safety of palbociclib plus fulvestrant in patients with HR+/HER2− ABC, regardless of menopausal status, whose disease had progressed on prior ET [7, 8, 9]. Overall survival (OS) was numerically longer for patients in the palbociclib arm versus the placebo arm (median OS, 34.9 vs. 28.0 months, respectively; stratified hazard ratio [95% confidence interval (CI)], 0.81 [0.64–1.03]; two‐sided p = .09; absolute difference, 6.9 months) but did not meet the threshold for statistical significance [8].
Prespecified subgroup analyses of PALOMA‐3 indicated that patients with sensitivity to prior ET (approximately 79% of the PALOMA‐3 overall population) derived an OS benefit from palbociclib plus fulvestrant compared with placebo plus fulvestrant (median OS, 39.7 vs. 29.7 months; unstratified hazard ratio [95% CI], 0.72 [0.55–0.94]; absolute difference, 10 months) [8]. In contrast, median OS was similar between treatment arms for those patients who had received prior chemotherapy in the ABC setting (34% of the overall population; 25.6 vs. 26.2 months, respectively), who had visceral disease (60% of overall; 27.6 vs. 24.7 months, respectively), or who were pre‐ or perimenopausal (21% of overall; 38.0 vs. 38.0 months) [8]. These findings suggested that prior chemotherapy in the ABC setting, visceral disease, and pre‐ or perimenopausal status may potentially hinder the OS benefit provided by palbociclib combination therapy. In this post hoc analysis, we examined the efficacy of palbociclib plus fulvestrant versus placebo plus fulvestrant in subgroups of patients with and without prior chemotherapy for ABC, by visceral and nonvisceral disease, and by menopausal status in both the overall and ET‐sensitive patient populations from PALOMA‐3.
Subjects, Materials, and Methods
Study Design
The PALOMA‐3 (NCT01942135) study design has been previously published [7, 8, 9]. In brief, patients with HR+/HER2− ABC, regardless of menopausal status, whose disease had progressed on prior ET were randomized 2:1 to receive either palbociclib (oral, 125 mg/day, 3/1 schedule) plus fulvestrant (500 mg intramuscular injection, administered on days 1 and 15 of cycle 1, and then day 1 of each subsequent cycle) or matching placebo plus fulvestrant. Patients were permitted to have received no more than one prior chemotherapy regimen for ABC. Pre‐ and perimenopausal patients received concurrent ovarian suppression with goserelin. Patients were stratified according to menopausal status, presence or absence of visceral metastases, and sensitivity to prior ET, defined as documented clinical benefit response (complete response, partial response, or stable disease for ≥24 weeks) to at least one previous ET regimen in the context of ABC, or ≥ 24 months of adjuvant ET prior to recurrence.
This clinical trial was carried out in accordance with principles of Good Clinical Practice and the Declaration of Helsinki and was approved by the internal review board at each site. Written informed consent was obtained from all participants.
Outcomes and Assessments
The primary endpoint was investigator‐assessed progression‐free survival (PFS); additional endpoints included OS, tumor response, and safety, all of which have been published previously. In this post hoc analysis, PFS and OS were analyzed in the following subgroups in both the overall and ET‐sensitive populations: patients without and with prior chemotherapy for ABC; patients with visceral and nonvisceral disease; patients without prior chemotherapy for ABC and two or fewer regimens of prior systemic therapy (i.e., any systemic therapy in any setting) by visceral versus nonvisceral disease; and by menopausal status.
Statistical Analysis
In this post hoc analysis, median OS and PFS were estimated using the Kaplan‐Meier method based on the Brookmeyer and Crowley method. Hazard ratios and corresponding 95% CIs were calculated using a one‐sided unstratified log‐rank test and an unstratified Cox proportional hazards model. For the multivariable analysis, a Cox proportional hazards model was fitted controlling for the following baseline factors simultaneously along with treatment: age (≥65 vs. <65), race (White, Black, Asian, other), baseline Eastern Cooperative Oncology Group performance status (ECOG PS) (1 vs. 0), disease site (nonvisceral vs. visceral), sensitivity to prior hormonal therapy (yes vs. no), prior chemotherapy for ABC, menopausal status at study entry (pre‐ or perimenopausal vs. postmenopausal), geographical region (North America, Europe, Asia Pacific), liver metastases (yes vs. no), bone‐only disease (yes vs. no), prior line of therapy in the context of metastatic disease (0, 1, 2, 3, 4, ≥5), and disease sites (1, 2, ≥3). A stepwise selection was used to only retain factors with p < .05 in the model.
Results
Baseline Demographic and Disease Characteristics
The overall population in PALOMA‐3 comprised 521 randomized patients (palbociclib arm, n = 347; placebo arm, n = 174). This analysis used data from the cutoff dates of October 23, 2015 (PFS), and April 13, 2018 (OS). Patient demographics and baseline disease characteristics were generally similar among treatment groups (Table 1). Patients without prior chemotherapy for ABC had fewer prior regimens of systemic therapy in any setting, fewer prior lines of therapy in the advanced setting, fewer involved disease sites, and more nonvisceral disease compared with patients with prior chemotherapy for ABC.
Table 1.
Select patient demographics and baseline disease characteristics (overall population)
| Demographics and characteristics | Without prior CT for ABC | With prior CT for ABC | ||
|---|---|---|---|---|
| PAL + FUL(n = 234) | PBO + FUL(n = 110) | PAL + FUL(n = 113) | PBO + FUL(n = 64) | |
| Age, median (range), yr | 58.0 (30–88) | 55.0 (29–80) | 53.0 (31–79) | 60.0 (39–79) |
| Race, a % | ||||
| White | 71.8 | 79.1 | 74.3 | 71.9 |
| Black or other | 5.1 | 4.5 | 7.1 | 6.3 |
| Asian | 22.6 | 15.5 | 18.6 | 21.9 |
| Disease site, % | ||||
| Visceral | 55.1 | 56.4 | 68.1 | 67.2 |
| Nonvisceral | 44.9 | 43.6 | 31.9 | 32.8 |
| Number of regimens b of prior systemic therapy, % | ||||
| ≤2 | 68.8 | 68.2 | 35.4 | 53.1 |
| 3+ | 31.2 | 31.8 | 64.6 | 46.9 |
| Number of prior lines of therapy in ABC setting, % | ||||
| 0 | 31.6 | 36.4 | 0 | 0 |
| 1 | 47.0 | 51.8 | 27.4 | 42.2 |
| 2 | 17.1 | 9.1 | 47.8 | 42.2 |
| 3+ | 4.3 | 2.7 | 24.8 | 15.6 |
| Number of involved disease sites, % | ||||
| ≤2 | 64.1 | 67.3 | 49.6 | 57.8 |
| 3+ | 35.9 | 32.7 | 48.7 | 40.6 |
| Sensitivity to prior ET, % | ||||
| Yes | 80.3 | 74.5 | 76.1 | 84.4 |
| No | 19.7 | 25.5 | 23.9 | 15.6 |
| Menopausal status, % | ||||
| Pre‐ or perimenopausal | 20.9 | 21.8 | 20.4 | 18.8 |
| Postmenopausal | 79.1 | 78.2 | 79.6 | 81.3 |
Race was unspecified for two patients.
Any prior systemic regimen in any setting.
Abbreviations: ABC, advanced breast cancer; CT, chemotherapy; ET, endocrine therapy; FUL, fulvestrant; PAL, palbociclib; PBO, placebo.
Prognostic Factors for OS
The four significant prognostic factors for OS in the overall population identified from the multivariable analysis were sensitivity to prior ET, nonvisceral disease, no prior chemotherapy for ABC, and an ECOG PS of 0 (Table 2). An OS benefit was observed with palbociclib plus fulvestrant versus placebo plus fulvestrant after adjusting for these four prognostic factors. No significant interactions were observed between treatment and each prognostic factor, including between prognostic factors. When examining the treatment effect in patients with an ECOG PS of 0 or 1, the hazard ratio was similar between the two groups, favoring palbociclib plus fulvestrant versus placebo plus fulvestrant (ECOG PS 0: median OS, 40 months vs. 29.7 months; hazard ratio, 0.75 [95% CI 0.55–1.01]; ECOG PS 1: median OS, 30.1 months vs. 22.8 months; hazard ratio, 0.79 [95% CI 0.54–1.15]). Since the treatment effect was similar in patients with an ECOG PS of 0 and those with an ECOG PS of 1, further analyses were deemed not warranted. Based on the findings from the multivariable analysis, the effect of prior chemotherapy in the advanced setting on OS was investigated.
Table 2.
Significant prognostic factors for overall survival (overall population)
| Prognostic factors | Hazard ratio a (95% CI) |
|---|---|
| PAL+FUL vs. PBO + FUL | 0.808 (0.639–1.023) |
| Sensitivity to prior hormonal therapy (yes vs. no) | 0.591 (0.455–0.767) |
| Disease site (nonvisceral vs. visceral) | 0.556 (0.436–0.709) |
| Prior chemotherapy for ABC (yes vs. no) | 1.329 (1.052–1.678) |
| Baseline ECOG PS (≥1 vs. 0) | 1.439 (1.147–1.805) |
A hazard ratio <1 indicates a reduced hazard in the first category of the variable; a hazard ratio >1 indicates a reduced hazard in the last category of the variable.
Abbreviations: ABC, advanced breast cancer; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; FUL, fulvestrant; PAL, palbociclib; PBO, placebo.
Efficacy in Patients Without or With Prior Chemotherapy for ABC
To better understand the impact of chemotherapy on survival benefit, PFS and OS were analyzed in subgroups of patients who received and had not received prior chemotherapy for ABC. Median PFS has been previously reported in the overall population: 11.2 versus 4.6 months in the palbociclib and placebo arms, respectively (hazard ratio, 0.50; 95% CI, 0.40–0.62; stratified p < .0001) [8]. In all subgroups analyzed in this exploratory analysis, median PFS was prolonged with palbociclib plus fulvestrant compared with placebo plus fulvestrant. In the subgroup of patients who had not received prior chemotherapy in the advanced setting (n = 344), median PFS was 12.9 and 5.5 months in the palbociclib and placebo arms, respectively (hazard ratio, 0.49; 95% CI, 0.37–0.65; Fig. 1A). In the subgroup of patients who received prior chemotherapy in the advanced setting (n = 177), median PFS was 9.5 months with palbociclib plus fulvestrant and 3.5 months with placebo plus fulvestrant (hazard ratio, 0.53; 95% CI, 0.37–0.77; Fig. 1B).
Figure 1.
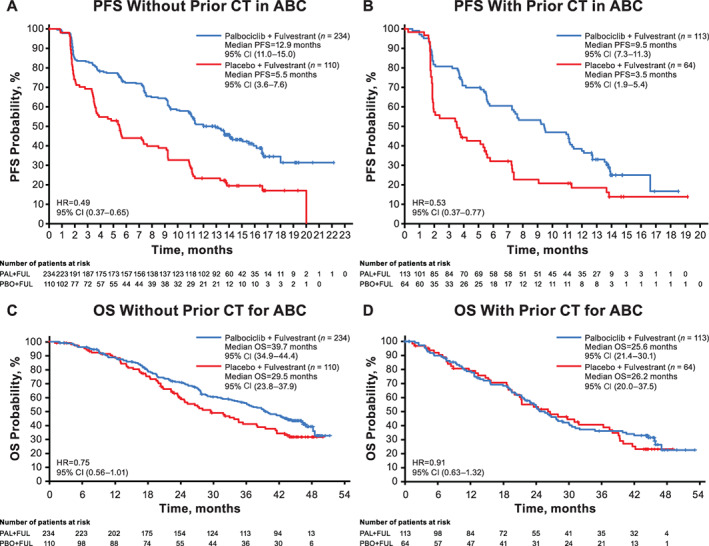
PFS and OS in patients without and with prior chemotherapy for ABC (overall population). (A): PFS in patients without prior chemotherapy. (B): PFS in patients with prior chemotherapy. (C): OS in patients without prior chemotherapy. (D): OS in patients with prior chemotherapy. Abbreviations: ABC, advanced breast cancer; CI, confidence interval; CT, chemotherapy; FUL, fulvestrant; HR, hazard ratio. OS, overall survival; PAL, palbociclib; PBO, placebo; PFS, progression‐free survival.
Endocrine sensitivity was also identified in the multivariable analysis as a prognostic factor for OS in PALOMA‐3. For ET‐sensitive patients, median PFS was previously reported to be 12.0 and 4.2 months in the palbociclib and placebo arms, respectively (hazard ratio, 0.46; 95% CI, 0.36–0.59) [8]. In the subgroup of ET‐sensitive patients who had not received prior chemotherapy in the advanced setting (n = 270), median PFS in the palbociclib and placebo arms was 13.6 and 5.5 months, respectively (hazard ratio, 0.46; 95% CI, 0.33–0.64; supplemental online Fig. 1A). In the subgroup of ET‐sensitive patients who received prior chemotherapy in the advanced setting (n = 140), median PFS was 9.5 months with palbociclib plus fulvestrant and 3.5 months with placebo plus fulvestrant (hazard ratio, 0.50; 95% CI, 0.33–0.76; supplemental online Fig. 1B).
In contrast to the consistent PFS benefit observed with palbociclib combination therapy, patients who had not received and who had received prior chemotherapy for ABC in both the overall and ET‐sensitive populations derived varying degrees of OS benefit (Fig. 1C, D; supplemental online Fig. 1C, D). In patients who had not received prior chemotherapy for ABC, median OS was prolonged in the palbociclib arm compared with the placebo arm (overall population [n = 344]: 39.7 vs. 29.5 months; hazard ratio, 0.75; 95% CI, 0.56–1.01; ET‐sensitive population [n = 270]: 42.3 vs. 32.1 months; hazard ratio, 0.68; 95% CI, 0.48–0.96). In contrast, in patients who received prior chemotherapy for ABC, median OS in the palbociclib versus placebo arms was 25.6 versus 26.2 months (hazard ratio, 0.91; 95% CI, 0.63–1.32) in the overall population (n = 177) and 27.6 versus 28.0 months (hazard ratio, 0.84; 95% CI, 0.54–1.28) in the ET‐sensitive population (n = 140).
Efficacy by Visceral or Nonvisceral Disease Status
In the overall population, median OS in patients with visceral disease (n = 311) was similar between the palbociclib and placebo arms (27.6 and 24.7 months, respectively; hazard ratio, 0.85; 95% CI, 0.64–1.13; Table 3). In patients with visceral disease who had not received prior chemotherapy for ABC (n = 191), median OS was 29.5 and 25.2 months in the palbociclib and placebo arms, respectively (hazard ratio, 0.78; 95% CI, 0.54–1.15). Patients with visceral disease who had no prior chemotherapy and two or fewer prior systemic therapy regimens (i.e., patients who had at least one prior line of ET in either the adjuvant or ABC setting; n = 129) had a median OS of 28.8 and 24.7 months in the palbociclib and placebo arms, respectively (hazard ratio, 0.74; 95% CI, 0.47–1.15). For patients with nonvisceral disease (n = 210), median OS was 46.9 months with palbociclib plus fulvestrant and 35.4 months with placebo plus fulvestrant (hazard ratio, 0.69; 95% CI, 0.46–1.04). Patients with nonvisceral disease and no prior chemotherapy in the advanced setting (n = 153) had a median OS of 46.9 and 37.9 months in the palbociclib and placebo arms, respectively (hazard ratio, 0.69; 95% CI, 0.42–1.12). In patients with nonvisceral disease, no prior chemotherapy, and two or fewer prior systemic therapy regimens (n = 107), median OS was 46.9 and 35.4 months with palbociclib and placebo, respectively (hazard ratio, 0.66; 95 CI, 0.37–1.16). PFS was prolonged with palbociclib plus fulvestrant versus placebo plus fulvestrant in all subgroups of patients with visceral or nonvisceral disease who had not received and who had received prior chemotherapy (Table 3).
Table 3.
Overall survival and progression‐free survival in patients with visceral and nonvisceral disease
| Survival | Visceral [8] | Visceral without prior CT for ABC | Visceral without prior CT for ABC and ≤ 2 regimens a , b | Visceral with prior CT for ABC | Nonvisceral [8] | Nonvisceral without prior CT for ABC | Nonvisceral without prior CT for ABC and ≤ 2 regimens a , b | Nonvisceral with prior CT for ABC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAL + FUL(n = 206) | PBO + FUL(n = 105) | PAL + FUL(n = 129) | PBO + FUL(n = 62) | PAL + FUL(n = 85) | PBO + FUL(n = 44) | PAL + FUL(n = 77) | PBO + FUL(n = 43) | PAL + FUL(n = 141) | PBO + FUL(n = 69) | PAL + FUL(n = 105) | PBO + FUL(n = 48) | PAL + FUL(n = 76) | PBO + FUL(n = 31) | PAL + FUL(n = 36) | PBO + FUL(n = 21) | |
| mOS, mo | 27.6 | 24.7 | 29.5 | 25.2 | 28.8 | 24.7 | 24.1 | 21.4 | 46.9 | 35.4 | 46.9 | 37.9 | 46.9 | 35.4 | 45.8 | 28.0 |
| HR (95% CI) | 0.85 (0.64–1.13) | 0.78 (0.54–1.15) | 0.74 (0.47–1.15) | 1.00 (0.65–1.54) | 0.69 (0.46–1.04) | 0.69 (0.42–1.12) | 0.66 (0.37–1.16) | 0.74 (0.35–1.57) | ||||||||
| mPFS, mo | 9.2 | 3.5 | 10.2 | 3.5 | 11.1 | 3.5 | 7.6 | 2.9 | 16.6 | 5.6 | NR | 9.2 | NR | 9.2 | 11.3 | 3.8 |
| HR (95% CI) | 0.50 (0.38–0.65) | 0.46 (0.32–0.65) | 0.34 (0.22–0.52) | 0.57 (0.37–0.88) | 0.48 (0.33–0.71) | 0.50 (0.31–0.80) | 0.47 (0.27–0.84) | 0.44 (0.22–0.87) | ||||||||
Any prior systemic regimen in any setting.
In the progression‐free survival analysis, but not the overall survival analysis, anticancer treatment included surgery containing a lesion removal or subsequent anticancer systemic therapies. For progression‐free survival analysis, n = 86 in the PAL+FUL arm for patients with visceral disease, no prior CT for ABC, and two or fewer regimens; and n = 79 and n = 32 for the PAL+FUL and PBO + FUL arms, respectively, for patients with nonvisceral disease, no prior CT for ABC, and two or fewer regimens.
Abbreviations: ABC, advanced breast cancer; CI, confidence interval; CT, chemotherapy; FUL, fulvestrant; HR, hazard ratio; mOS, median overall survival; mPFS, median progression‐free survival; PAL, palbociclib; PBO, placebo.
Efficacy in Patients by Menopausal Status
Approximately 21% of patients in PALOMA‐3 were pre‐ or perimenopausal; median OS and PFS were analyzed by menopausal status in both the overall and ET‐sensitive populations (Table 4). In the overall population, median OS in pre‐ and perimenopausal patients (n = 108) was 38.0 months in both treatment arms (hazard ratio, 1.07; 95% CI, 0.61–1.86) [8], and median PFS was 11.3 months with palbociclib plus fulvestrant and 5.6 months with placebo plus fulvestrant (hazard ratio, 0.46; 95% CI, 0.28–0.75). Both median OS and median PFS were prolonged for postmenopausal patients in the overall population (n = 413) who received palbociclib combination therapy (OS: hazard ratio, 0.73; 95% CI, 0.57–0.95; PFS: hazard ratio, 0.52; 95% CI, 0.40–0.66) [8]. Pre‐ and perimenopausal patients with prior sensitivity to ET (n = 76) also derived clinical benefit from palbociclib plus fulvestrant (OS: 48.3 vs. 34.6 months; hazard ratio, 0.73; 95% CI, 0.37–1.46; PFS: 13.6 vs. 5.6 months; hazard ratio, 0.38; 95% CI, 0.21–0.68). Benefit from palbociclib plus fulvestrant was also demonstrated in pre‐ and perimenopausal patients who had not received prior chemotherapy (OS: 48.3 vs. 34.6 months; hazard ratio, 0.69; 95% CI, 0.34–1.40; PFS: 15.0 vs. 5.6 months; hazard ratio, 0.39; 95% CI, 0.21–0.72).
Table 4.
Overall survival and progression‐free survival in patients by menopausal status
| Survival | Overall pre‐ or perimenopausal [8] (21% of PALOMA‐3) | ET‐sensitive pre‐ or perimenopausal (15% of PALOMA‐3) | Without prior CT pre‐ or perimenopausal (14% of PALOMA‐3) | Overall postmenopausal [8] (79% of PALOMA‐3) | ET‐sensitive postmenopausal (64% of PALOMA‐3) | Without prior CT postmenopausal (52% of PALOMA‐3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAL + FUL(n = 72) | PBO + FUL(n = 36) | PAL + FUL(n = 51) | PBO + FUL(n = 25) | PAL + FUL(n = 49) | PBO + FUL(n = 24) | PAL + FUL(n = 275) | PBO + FUL(n = 138) | PAL + FUL(n = 223) | PBO + FUL(n = 111) | PAL + FUL(n = 185) | PBO + FUL(n = 86) | |
| mOS, mo | 38.0 | 38.0 | 48.3 | 34.6 | 48.3 | 34.6 | 34.8 | 27.1 | 38.8 | 29.7 | 37.3 | 29.5 |
| HR (95% CI) | 1.07 (0.61–1.86) | 0.73 (0.37–1.46) | 0.69 (0.34–1.40) | 0.73 (0.57–0.95) | 0.72 (0.54–0.97) | 0.76 (0.55–1.06) | ||||||
| mPFS, mo | 11.3 | 5.6 | 13.6 | 5.6 | 15.0 | 5.6 | 11.2 | 3.9 | 11.4 | 3.8 | 11.3 | 5.4 |
| HR (95% CI) | 0.46 (0.28–0.75) | 0.38 (0.21–0.68) | 0.39 (0.21–0.72) | 0.52 (0.40–0.66) | 0.49 (0.37–0.65) | 0.53 (0.39–0.73) | ||||||
Abbreviations: CI, confidence interval; CT, chemotherapy; ET, endocrine therapy; FUL, fulvestrant; HR, hazard ratio; mOS, median overall survival; mPFS, median progression‐free survival; PAL, palbociclib; PBO, placebo.
Subsequent Therapies
A total of 249 patients (71.8%) in the palbociclib group and 140 (80.5%) patients in the placebo group received subsequent systemic anticancer therapy after discontinuation from study treatment (supplemental online Table 1). The type of subsequent systemic anticancer therapy received generally did not differ between patients who had received and who had not received prior chemotherapy for ABC, although patients with prior chemotherapy received more subsequent chemotherapy and less antihormonal therapy, especially in earlier lines poststudy treatment, than those without prior chemotherapy for ABC. Furthermore, regardless of prior chemotherapy subgroup, patients in the palbociclib plus fulvestrant group received fewer poststudy treatments than those in the placebo plus fulvestrant group (supplemental online Tables 2 and 3); however, among patients in the palbociclib arm, there was no apparent selection against a specific subsequent chemotherapy or targeted agent.
Discussion
This exploratory analysis identified four significant prognostic factors for overall survival in patients with HR+/HER2− ABC: the absence of prior chemotherapy in the advanced setting, endocrine sensitivity, nonvisceral disease, and an ECOG PS of 0. Patients without prior chemotherapy in the ABC setting in both the overall and ET‐sensitive populations experienced longer median PFS and OS with palbociclib plus fulvestrant versus placebo plus fulvestrant. The ET‐sensitive subgroup of patients who did not have prior chemotherapy for ABC had a slightly longer median PFS and OS compared with the patients who did not have prior chemotherapy in the overall population. Furthermore, for patients with nonvisceral or visceral disease and no prior chemotherapy for ABC, the OS and PFS benefit with palbociclib‐fulvestrant was further enhanced in the subgroup of patients who had fewer prior regimens of therapy. Overall, a PFS benefit was observed with palbociclib plus fulvestrant compared with placebo plus fulvestrant regardless of subgroup (including patients with or without prior chemotherapy in ABC), although the median PFS was shorter in the subgroup of patients with prior chemotherapy in ABC compared with those without prior chemotherapy in ABC. Patients with prior chemotherapy for ABC had a similar median OS regardless of treatment intervention or ET sensitivity, and median OS was lower in these subgroups compared with that in patients without prior chemotherapy for ABC. Together, these findings suggest that patients may receive greater clinical benefit from palbociclib plus fulvestrant if they receive the combination before chemotherapy in the advanced setting.
This hypothesis is also supported by clinical trial results for other CDK4/6 inhibitors and is based on all currently available evidence. Three clinical trials, PALOMA‐3, MONARCH 2, and MONALEESA‐3, have reported OS data with a CDK4/6 inhibitor in combination with fulvestrant [10, 11, 12, 13]. PALOMA‐3 enrolled a heterogeneous patient population compared with the other two clinical trials. In PALOMA‐3, the patient population was more heavily pretreated, as patients had progressed after previous endocrine therapy, patients who had received up to one line of prior chemotherapy in the advanced setting were included (34% of the overall population), and 35% of the overall study population received at least two prior systemic therapy regimens in the ABC setting; both the MONARCH 2 and MONALEESA‐3 studies excluded patients who received prior chemotherapy or at least two prior lines of therapy for advanced disease [10, 11, 12, 13]. The patient populations of the three trials also differed regarding the menopausal status of the participants. Both PALOMA‐3 and MONARCH 2 allowed pre‐ and perimenopausal women to enroll (approximately 21% of patients in PALOMA‐3 were pre‐ or perimenopausal), and concurrent treatment with goserelin was mandatory, whereas MONALEESA‐3 participation was restricted to only postmenopausal patients [10, 11, 12, 13]. In MONARCH 2, abemaciclib in combination with fulvestrant prolonged median OS compared with placebo plus fulvestrant in patients with HR+/HER2− ABC whose disease had progressed on prior ET regardless of menopausal status (hazard ratio, 0.76; 95% CI, 0.61–0.95) [11]. In MONALEESA‐3, median OS was prolonged with ribociclib plus fulvestrant versus placebo plus fulvestrant as first‐ or second‐line treatment in postmenopausal women with HR+/HER2− ABC (hazard ratio, 0.72; 95% CI, 0.57–0.92) [10]. In PALOMA‐3, palbociclib in combination with fulvestrant prolonged median OS compared with placebo in patients who had not received prior chemotherapy for ABC (hazard ratio, 0.75; 95% CI, 0.56–1.01) and in patients with sensitivity to prior ET who had not received prior chemotherapy for ABC (hazard ratio, 0.68; 95% CI, 0.48–0.96). The hazard ratios for OS observed in all three trials favored combination treatment with the CDK4/6 inhibitor and fulvestrant and were generally similar. Although cross‐trial comparisons must be interpreted with caution, particularly when the patient populations are different, the data from these three trials provide further evidence that early use of a CDK4/6 inhibitor may be beneficial in patients with HR+/HER2− ABC.
Study limitations include its exploratory, post hoc nature and the small numbers of patients in some of the subgroups. As such, these data must be interpreted with caution. Despite these limitations, in this exploratory analysis, improved OS benefit was observed for palbociclib plus fulvestrant versus placebo plus fulvestrant in patients (regardless of menopausal status or visceral involvement) without prior chemotherapy for ABC, with prior endocrine sensitivity, and who received fewer lines of prior systemic therapy. However, it is important to note that patients who received palbociclib plus fulvestrant versus placebo plus fulvestrant achieved a clinical benefit, regardless of subgroup. Thus, patients with prior chemotherapy, endocrine resistance, or multiple lines of systemic therapy should not be excluded from receiving a CDK4/6 inhibitor as treatment for HR+/HER2− ABC.
Conclusion
Taken together, these findings in combination with the current body of literature regarding OS benefit with CDK4/6 inhibitors suggest that patients receive greater clinical benefit from palbociclib plus fulvestrant if the combination is received prior to chemotherapy for treatment of HR+/HER2− ABC.
Author Contributions
Conception/design: Hope S. Rugo, Massimo Cristofanilli, Sybille Loibl, Nadia Harbeck, Angela DeMichele, Hiroji Iwata, Yoon Hee Park, Adam Brufsky, Kathy Puyana Theall, Xin Huang, Lynn McRoy, Eustratios Bananis, Nicholas C. Turner
Provision of study material or patients: Hope S. Rugo, Massimo Cristofanilli, Sybille Loibl, Nadia Harbeck, Angela DeMichele, Hiroji Iwata, Yoon Hee Park, Adam Brufsky, Kathy Puyana Theall, Xin Huang, Lynn McRoy, Eustratios Bananis, Nicholas C. Turner
Collection and/or assembly of data: Xin Huang
Data analysis and interpretation: Hope S. Rugo, Massimo Cristofanilli, Sybille Loibl, Nadia Harbeck, Angela DeMichele, Hiroji Iwata, Yoon Hee Park, Adam Brufsky, Kathy Puyana Theall, Xin Huang, Lynn McRoy, Eustratios Bananis, Nicholas C. Turner
Manuscript writing: Hope S. Rugo, Massimo Cristofanilli, Sybille Loibl, Nadia Harbeck, Angela DeMichele, Hiroji Iwata, Yoon Hee Park, Adam Brufsky, Kathy Puyana Theall, Xin Huang, Lynn McRoy, Eustratios Bananis, Nicholas C. Turner
Final approval of manuscript: Hope S. Rugo, Massimo Cristofanilli, Sybille Loibl, Nadia Harbeck, Angela DeMichele, Hiroji Iwata, Yoon Hee Park, Adam Brufsky, Kathy Puyana Theall, Xin Huang, Lynn McRoy, Eustratios Bananis, Nicholas C. Turner
Disclosures
Hope S. Rugo: Pfizer Inc, Merck, Novartis, Eli Lilly & Co., Genentech, Odonate, Daiichi, Seattle Genetics, Eisai, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, AstraZeneca, Immunomedics (RF), PUMA, Samsung, Mylan (H); Massimo Cristofanilli: Pfizer Inc, Novartis, Menarini, Eli Lilly & Co., G1 Therapeutics (RF), Novartis, Menarini, Eli Lilly & Co., CytoDyn, Sermonix, G1 Therapeutics, Pfizer Inc (C/A); Sybille Loibl: AbbVie, Amgen, AstraZeneca, Celgene, Novartis, Pfizer Inc, Roche, Teva, Vifor (RF); Nadia Harbeck: AstraZeneca, Eli Lilly & Co., Novartis, Pfizer Inc, Roche (C/A); Angela DeMichele: Pfizer Inc, Novartis, Menarini Biosystems, Calithera, Incyte, Genentech (RF), Pfizer Inc (H), Pfizer Inc, Novartis, Context Therapeutics (C/A); Hiroji Iwata: Chugai, Daiichi‐Sankyo (C/A), AstraZeneca, Chugai, Eisai, Novartis (H), Pfizer Inc, AstraZeneca, Chugai, Daiichi‐Sankyo, Novartis, Eli Lilly & Co. (other—personal fees); Yoon Hee Park: AstraZeneca, Eisai, Merck, Pfizer Inc, Novartis, Roche (RF), AstraZeneca, Pfizer Inc, Eisai, Novartis (C/A); Adam Brufsky: Pfizer Inc (C/A); Kathy Puyana Theall: Pfizer Inc (E, OI); Xin Huang: Pfizer Inc (E, OI); Lynn McRoy: Pfizer Inc (E, OI); Eustratios Bananis: Pfizer Inc (E, OI); Nicholas C. Turner: Pfizer Inc, Eli Lilly & Co., Novartis (RF), Pfizer Inc (C/A, H).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 PFS and OS in Patients Without and With Prior CT for ABC (ET‐Sensitive Population).
(A, B) PFS. (C, D) OS. ABC = advanced breast cancer; CI = confidence interval; CT = chemotherapy; ET = endocrine therapy; FUL = fulvestrant; HR = hazard ratio; OS = overall survival; PAL = palbociclib; PBO = placebo; PFS = progression‐free survival.
Table S1 Frequency of Number of Regimens for Follow‐up Systemic Anticancer Therapy
Table S2. Systemic Anticancer Therapies Received as First, Second, and Third or Greater Subsequent Treatment by >10% of Patients Without Prior CT for ABC in Either Treatment Arm Who Discontinued Study Treatment
Table S3. Systemic Anticancer Therapies Received as First, Second, and Third or Greater Subsequent Treatment by >10% of Patients With Prior CT for ABC in Either Treatment Arm Who Discontinued Study Treatment
Acknowledgments
This study (NCT01942135) was sponsored by Pfizer Inc. Editorial/medical writing support was provided by Jennifer Fetting, Ph.D., of ICON plc (North Wales, PA) and was funded by Pfizer Inc. Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (a) for indications that have been approved in the U.S. and/or European Union or (b) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer. Version 2.2020. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2020. https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf. [Google Scholar]
- 2. Rugo HS, Rumble RB, Macrae E et al. Endocrine therapy for hormone receptor‐positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 2016;34:3069–3103. [DOI] [PubMed] [Google Scholar]
- 3. Cardoso F, Senkus E, Costa A et al. 4th ESO‐ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 2018;29:1634–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Finn RS, Dering J, Conklin D et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor‐positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fry DW, Harvey PJ, Keller PR et al. Specific inhibition of cyclin‐dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004;3:1427–1438. [PubMed] [Google Scholar]
- 6. IBRANCE capsules (palbociclib). Full prescribing information. New York: Pfizer Inc, 2019. [Google Scholar]
- 7. Turner NC, Ro J, Andre F et al. Palbociclib in hormone‐receptor–positive advanced breast cancer. N Engl J Med 2015;373:209–219. [DOI] [PubMed] [Google Scholar]
- 8. Turner NC, Slamon DJ, Ro J et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018;379:1926–1936. [DOI] [PubMed] [Google Scholar]
- 9. Cristofanilli M, Turner NC, Bondarenko I et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone‐receptor‐positive, HER2‐negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA‐3): Final analysis of the multicentre, double‐blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–439. [DOI] [PubMed] [Google Scholar]
- 10. Slamon DJ, Neven P, Chia S et al. Overall survival (OS) results of the phase III MONALEESA‐3 trial of postmenopausal patients (pts) with hormone receptor‐positive (HR+), human epidermal growth factor 2‐negative (HER2‐) advanced breast cancer (ABC) treated with fulvestrant (FUL) +/‐ ribociclib (RIB). Ann Oncol 2019;30:v851–v934. [Google Scholar]
- 11. Sledge GW Jr, Toi M, Neven P et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor‐positive, ERBB2‐negative breast cancer that progressed on endocrine therapy‐MONARCH 2: A randomized clinical trial. JAMA Oncol 2020;6:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sledge GW Jr, Toi M, Neven P et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–2884. [DOI] [PubMed] [Google Scholar]
- 13. Slamon DJ, Neven P, Chia S et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor‐positive, human epidermal growth factor receptor 2‐negative advanced breast cancer: MONALEESA‐3. J Clin Oncol 2018;36:2465–2472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 PFS and OS in Patients Without and With Prior CT for ABC (ET‐Sensitive Population).
(A, B) PFS. (C, D) OS. ABC = advanced breast cancer; CI = confidence interval; CT = chemotherapy; ET = endocrine therapy; FUL = fulvestrant; HR = hazard ratio; OS = overall survival; PAL = palbociclib; PBO = placebo; PFS = progression‐free survival.
Table S1 Frequency of Number of Regimens for Follow‐up Systemic Anticancer Therapy
Table S2. Systemic Anticancer Therapies Received as First, Second, and Third or Greater Subsequent Treatment by >10% of Patients Without Prior CT for ABC in Either Treatment Arm Who Discontinued Study Treatment
Table S3. Systemic Anticancer Therapies Received as First, Second, and Third or Greater Subsequent Treatment by >10% of Patients With Prior CT for ABC in Either Treatment Arm Who Discontinued Study Treatment


