Abstract
Background
Neratinib has efficacy in central nervous system (CNS) metastases from HER2‐positive metastatic breast cancer (MBC). We report outcomes among patients with CNS metastases at baseline from the phase III NALA trial of neratinib plus capecitabine (N + C) versus lapatinib plus capecitabine (L + C).
Materials and Methods
NALA was a randomized, active‐controlled trial in patients who received two or more previous HER2‐directed regimens for HER2‐positive MBC. Patients with asymptomatic/stable brain metastases (treated or untreated) were eligible. Patients were assigned to N + C (neratinib 240 mg per day, capecitabine 750 mg/m2 twice daily) or L + C (lapatinib 1,250 mg per day, capecitabine 1,000 mg/m2 twice daily) orally. Independently adjudicated progression‐free survival (PFS), overall survival (OS), and CNS endpoints were considered.
Results
Of 621 patients enrolled, 101 (16.3%) had known CNS metastases at baseline (N + C, n = 51; L + C, n = 50); 81 had received prior CNS‐directed radiotherapy and/or surgery. In the CNS subgroup, mean PFS through 24 months was 7.8 months with N + C versus 5.5 months with L + C (hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.41–1.05), and mean OS through 48 months was 16.4 versus 15.4 months (HR, 0.90; 95% CI, 0.59–1.38). At 12 months, cumulative incidence of interventions for CNS disease was 25.5% for N + C versus 36.0% for L + C, and cumulative incidence of progressive CNS disease was 26.2% versus 41.6%, respectively. In patients with target CNS lesions at baseline (n = 32), confirmed intracranial objective response rates were 26.3% and 15.4%, respectively. No new safety signals were observed.
Conclusion
These analyses suggest improved PFS and CNS outcomes with N + C versus L + C in patients with CNS metastases from HER2‐positive MBC.
Implications for Practice
In a subgroup of patients with central nervous system (CNS) metastases from HER2‐positive breast cancer after two or more previous HER2‐directed regimens, the combination of neratinib plus capecitabine was associated with improved progression‐free survival and CNS outcomes compared with lapatinib plus capecitabine. These findings build on previous phase II and III studies describing efficacy of neratinib in the prevention and treatment of CNS metastases, and support a role for neratinib as a systemic treatment option in the management of patients with HER2‐positive brain metastases following antibody‐based HER2‐directed therapies.
Keywords: Capecitabine; Central nervous system neoplasms; Lapatinib; Neratinib; Receptor, ErbB‐2
Short abstract
This article reports outcomes among HER2‐positive breast cancer patients with central nervous system metastases at baseline from the phase III NALA trial of neratinib plus capecitabine versus lapatinib plus capecitabine.
Introduction
HER2‐directed agents have altered the natural history of HER2‐positive breast cancer, achieving marked improvements in the control of systemic disease but with an accompanying increase in the occurrence or progression of central nervous system (CNS) metastases [1]. In early‐stage disease, the brain is a common first site of metastasis after HER2‐directed adjuvant therapy (~35%–55% of distant recurrences) [2, 3, 4], whereas in the metastatic setting, 30%–55% of patients develop CNS metastases [5], highlighting the need for multiple lines of safe and effective CNS‐directed treatments.
Management of CNS metastases primarily involves local therapy including stereotactic radiosurgery, whole‐brain radiotherapy, and surgery. Historically, systemic treatment options were limited because patients with CNS metastasis were often excluded from clinical trials and because there was a reluctance to use HER2‐directed monoclonal antibodies based on a presumed inability to cross the blood‐brain barrier [6]. However, accumulating clinical evidence suggests that HER2‐directed therapies can ameliorate brain metastases [7, 8, 9], and, for the first time, a systemic treatment, the reversible HER2‐specific tyrosine kinase inhibitor (TKI) tucatinib, has been approved for use in brain metastases from HER2‐positive breast cancer in combination with trastuzumab and capecitabine [10].
Neratinib (Nerlynx; Puma Biotechnology Inc, Los Angeles, CA), an irreversible small‐molecule pan‐HER TKI, has been approved by the US Food and Drug Administration for use in combination with capecitabine in patients with advanced/metastatic HER2‐positive breast cancer who have received two or more prior anti‐HER2–based regimens in the metastatic setting, as well as for the extended adjuvant treatment of patients with early‐stage HER2‐positive breast cancer following trastuzumab‐based therapy [11]. Of note, neratinib has demonstrated efficacy in both the prevention [12, 13] and treatment [14, 15, 16] of CNS metastases from HER2‐positive breast cancer. In the recent phase III NALA trial, neratinib plus capecitabine (N + C) significantly improved progression‐free survival (PFS) compared with lapatinib plus capecitabine (L + C) in patients with HER2‐positive metastatic breast cancer (MBC) who had received at least two previous HER2‐directed regimens for metastatic disease (hazard ratio [HR], 0.76; 95% confidence interval [CI], 0.63–0.93; p = .0059) [16]. There was also a numerical difference favoring N + C for overall survival (OS), but statistical significance was not reached (HR, 0.88; 95% CI, 0.72–1.07; p = .2086). Fewer interventions for CNS disease were required with N + C versus L + C (p = .043) [16].
Within the NALA study population, a cohort of 101 patients had CNS metastases at baseline. We report efficacy, safety, and health‐related quality‐of‐life (HRQoL) outcomes in this patient subgroup, with a particular focus on CNS‐specific endpoints. Lapatinib, a reversible dual TKI, plus capecitabine was selected as the active comparator regimen for NALA as it is a recognized treatment option for patients with previously treated HER2‐positive MBC [17] and has well‐documented efficacy in the CNS [18, 19, 20].
Materials and Methods
Study Design and Patients
NALA was an international, randomized, multicenter, open‐label, active‐controlled, parallel‐design study conducted at 203 sites in 28 countries in Europe, North and South America, Asia, and Australia (clinicaltrials.gov: NCT01808573), the details of which have been described previously [16]. Randomization was stratified by hormone receptor status (hormone receptor‐positive or hormone receptor‐negative), number of previous HER2‐directed therapies for metastatic disease (2 or ≥ 3), geographic region (North America or Europe [including Israel] or rest of world), and location of disease (visceral or nonvisceral).
Within the NALA study population (n = 621), there were 101 patients (16.3%) with CNS metastases at baseline, defined as patients with treated or untreated disease in the “brain” as assessed by the investigator at enrollment. Baseline brain imaging (magnetic resonance imaging [MRI], etc). was not mandatory for any patient, regardless of history of CNS involvement, but performed if clinically indicated per investigator assessment. According to the study inclusion criteria, asymptomatic patients with metastatic brain disease, including leptomeningeal disease (LMD), who were on stable doses of corticosteroids (without any dose limit) for the treatment of brain metastases for at least 14 days prior to randomization were eligible. Previous surgery and radiation therapy was permitted if completed within 28 days and 14 days, respectively, before starting study treatment. Patients with symptomatic or unstable brain metastases were not allowed. The protocol was approved by national/institutional ethics committees at participating sites, and the study was conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent prior to enrollment.
Treatment
Patients received neratinib 240 mg once daily plus capecitabine 750 mg/m2 twice daily or lapatinib 1,250 mg once daily plus capecitabine 1,000 mg/m2 twice daily orally. Neratinib and lapatinib were given continuously, whereas capecitabine was administered on days 1–14 of a 21‐day cycle. Treatment was discontinued on disease progression (even for isolated brain metastases), initiation of alternative anticancer therapy, or other specified criteria. Dose modifications and/or discontinuation specified by protocol were performed in cases of treatment‐emergent adverse events (TEAEs). Antidiarrheal prophylaxis with loperamide as described previously [16] was required per protocol for all patients randomized to N + C for the duration of cycle 1. Hormonal therapy during the active study treatment phase was not permitted.
Assessments
Tumor assessments were performed using MRI or computerized tomography at baseline and then every 6 weeks from first dose of study treatment. CNS tumor assessments were not a prerequisite for enrollment and were followed according to the same schedule of assessments as extracranial tumors; for all patients, ad hoc CNS imaging was performed if clinically indicated per investigator assessment. Patients who discontinued study therapy were contacted via telephone every 12 weeks for survival status and to collect data concerning interventions for CNS disease. TEAEs were monitored until 28 days after the last dose of study drug and graded according to National Cancer Institute Common Terminology Criteria Adverse Events, v4.0. HRQoL was assessed using the European Organisation for Research and Treatment of Cancer (EORTC) Quality‐of‐Life Questionnaire (QLQ‐C30; version 3) and breast cancer‐specific module (QLQ‐BR23) at screening, every 6 weeks during treatment, and at treatment discontinuation.
Endpoints
Independently adjudicated PFS and OS were evaluated in the CNS population. CNS‐specific endpoints were time to intervention for metastatic CNS disease, defined as time from randomization to start of therapy for CNS disease, with interventions for CNS disease including anticancer medication, cancer‐related radiation therapy, cancer‐related surgery/procedure, or concomitant medication/therapy as collected on the CNS case report form; time to progressive CNS lesions, defined as time from randomization until newly diagnosed or progressive CNS lesions (scans read centrally); CNS‐PFS, defined as time from randomization to disease progression in the brain or death from any cause, whichever occurred first (scans read centrally); and intracranial objective response rate (ORR), defined as confirmed complete or partial responses in patients with target CNS lesions according to local investigator assessment using RECIST v1.1 [21]. See supplemental online Table 1 for definitions of all endpoints.
Statistical Analysis
All analyses in this CNS subgroup are descriptive, and p values presented are without multiplicity adjustments. Time‐to‐event endpoints were analyzed using the Kaplan‐Meier method, and treatment groups were compared using a log‐rank test and Cox proportional hazards model to estimate HR and 95% CI. The restricted mean survival time method was used as a prespecified sensitivity analysis for PFS (24‐month time point) and OS (48‐month time point) in the patient population with CNS metastases at baseline and was performed in the intention‐to‐treat population [16]. Cumulative incidence of interventions for CNS was analyzed by competing risks analysis and tested via Gray's method [22], where competing events were deaths without intervention for CNS disease. CNS progression was analyzed by competing risks analysis, where competing events were progression at other sites and deaths prior to CNS progression. Confirmed intracranial ORR with exact 95% CI was provided. The analysis cutoff date was September 28, 2018. SAS statistical software, version 9.1 or later, was used.
Results
Patients
Between May 2013 and July 2017, 621 patients were randomized to study treatment. Overall, 101 patients (16.3%) had CNS metastases at baseline (N + C, n = 51; L + C, n = 50); 99 patients from this subgroup received study treatment and were evaluated for safety (N + C, n = 50; L + C, n = 49; see Fig. 1). Compared with patients without CNS metastases (n = 520), patients with CNS metastases were more likely to have an ECOG performance status of 1 (57.4% vs. 43.3%) and hormone receptor‐negative disease (49.5% vs. 39.2%). Accompanying visceral (mainly lung and liver) and nonvisceral lesions (mainly bone and lymph nodes) were common (Table 1).
Figure 1.
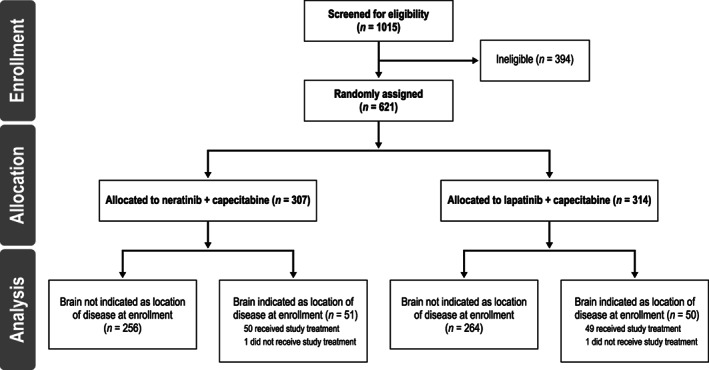
CONSORT flowchart.
Table 1.
Demographics and baseline characteristics of patients with and without CNS metastases at baseline
| Characteristic | CNS metastases | No CNS metastases, total (n = 520) | ||
|---|---|---|---|---|
| N + C (n = 51) | L + C (n = 50) | Total (n = 101) | ||
| Age, yr | ||||
| Median (range) | 53 (25–71) | 54 (26–75) | 54 (25–75) | 55 (25–84) |
| Sex | ||||
| Female | 51 (100) | 50 (100) | 101 (100) | 517 (99.4) |
| Geographic region | ||||
| Europe | 18 (35.3) | 14 (28.0) | 32 (31.7) | 212 (40.8) |
| North America | 9 (17.6) | 13 (26.0) | 22 (21.8) | 102 (19.6) |
| Rest of world | 24 (47.1) | 23 (46.0) | 47 (46.5) | 206 (39.6) |
| ECOG performance status | ||||
| 0 | 21 (41.2) | 22 (44.0) | 43 (42.6) | 295 (56.7) |
| 1 | 30 (58.8) | 28 (56.0) | 58 (57.4) | 225 (43.3) |
| Time from diagnosis to randomization, yr | ||||
| Median (range) | 2.9 (0.9–16.2) a | 3.2 (0.8–20.2) | 2.9 (0.8–20.2) | 3.6 (0.5–25.1) |
| Disease location | ||||
| Visceral | 51 (100) | 50 (100) | 101 (100) | 430 (82.7) |
| Brain b | 51 (100) | 50 (100) | 101 (100) | 0 (0) |
| Lung | 29 (56.9) | 34 (68.0) | 63 (62.4) | 267 (51.3) |
| Liver | 29 (56.9) | 27 (54.0) | 56 (55.4) | 229 (44.0) |
| Nonvisceral | 39 (76.5) | 46 (92.0) | 85 (84.2) | 441 (84.8) |
| Lymph node | 27 (52.9) | 29 (58.0) | 56 (55.4) | 289 (55.6) |
| Bone | 28 (54.9) | 34 (68.0) | 62 (61.4) | 256 (49.2) |
| Hormone receptor status c | ||||
| Negative | 28 (54.9) | 22 (44.0) | 50 (49.5) | 204 (39.2) |
| Positive | 23 (45.1) | 28 (56.0) | 51 (50.5) | 316 (60.8) |
| Prior HER2 regimens | ||||
| 2 | 42 (82.4) | 33 (66.0) | 75 (74.3) | 355 (68.3) |
| ≥3 | 9 (17.6) | 17 (34.0) | 26 (25.7) | 165 (31.7) |
| Prior anticancer medication | ||||
| Neoadjuvant | 5 (9.8) | 8 (16.0) | 13 (12.9) | 112 (21.5) |
| Adjuvant | 16 (31.4) | 23 (46.0) | 39 (38.6) | 256 (49.2) |
| Metastatic/locally advanced | 51 (100) | 50 (100) | 101 (100) | 519 (99.8) |
| Prior CNS‐directed therapies d | ||||
| No prior surgery or radiation | 7 (13.7) | 13 (26.0) | 20 (19.8) | |
| Prior radiation | 43 (84.3) | 35 (70.0) | 78 (77.2) | |
| Whole‐brain radiation e | 34 (66.7) | 30 (60.0) | 64 (63.4) | |
| Stereotactic radiation only | 9 (17.6) | 5 (10.0) | 14 (13.9) | |
| Prior surgery | 2 (3.9) | 3 (6.0) | 5 (5.0) | |
| Prior surgery plus radiation | 1 (2.0) | 1 (2.0) | 2 (2.0) | |
| Concomitant CNS medications at baseline | ||||
| Antiepileptic agents | 7 (13.7) | 3 (6.0) | 10 (9.9) | |
| Corticosteroids | 12 (23.5) | 9 (18.0) | 21 (20.8) | |
| Dexamethasone dose equivalent, mg/day f | 12 | 6 | 18 | |
| Median (range) | 2.5 (0.8–8.0) | 4.0 (0.8–4.0) | 3.5 (0.8–8.0) | |
Data presented as n (%) unless otherwise stated.
n = 50.
Two patients in the N + C group and one patient in the L + C group indicated location as other, with additional explanations indicating brain.
Hormone receptor‐positive: estrogen receptor‐positive, progesterone receptor‐positive, or both. Hormone receptor‐negative: estrogen receptor‐ and progesterone receptor‐negative.
CNS‐directed therapies were only reviewed for patients with CNS metastases (n = 101) at baseline.
Patients may have documented both targeted and whole‐brain radiotherapy.
Three patients had incorrect doses or units and were not included. Corticosteroid doses were converted to dexamethasone dose equivalents according to Parente [23].
Abbreviations: CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; L + C, lapatinib plus capecitabine; N + C, neratinib plus capecitabine.
Within the CNS subgroup, demographics and disease characteristics were generally well balanced between treatment groups (Table 1), except hormone receptor‐negative disease was more common in the N + C group (54.9% vs. L + C, 44.0%), and patients in the N + C group were less likely to have received three or more HER2‐directed therapies in the metastatic setting (17.6% vs. 34.0%). Twenty patients (19.8%) had received no CNS‐directed radiotherapy or surgery at the time of enrollment, 78 patients (77.2%) had received radiotherapy (whole brain or stereotactic), and 5 patients (5.0%) had undergone surgery (Table 1). Furthermore, 21 patients (20.8%) reported taking corticosteroids, and 10 patients (9.9%) reported taking antiepileptics at baseline. Of the 101 patients with CNS disease at baseline, 70 had baseline CNS scans that underwent retrospective central radiology review, and 3 of these were found to have LMD (N + C, n = 2; L + C, n = 1; supplemental online Table 2). Additional patients with LMD may have been enrolled, but information was not collected.
Efficacy
In the CNS subgroup, independently adjudicated PFS and OS were numerically improved with N + C compared with L + C (Table 2). Mean PFS restricted at 24 months was 7.8 months (95% CI, 5.6–10.1) with N + C and 5.5 months (95% CI, 3.8–7.2) with L + C, and median PFS was 5.6 months (95% CI, 3.7–7.1) and 4.3 months (95% CI, 2.8–5.6), respectively (HR, 0.66; 95% CI, 0.41–1.05; Fig. 2). Mean OS restricted at 48 months was 16.4 months (95% CI, 13.4–19.5) with N + C and 15.4 months (95% CI, 12.1–18.8) with L + C, and median OS was 13.9 months (95% CI, 8.9–17.5) and 12.4 months (95% CI, 9.7–16.9), respectively (HR, 0.90; 95% CI, 0.59–1.38; Fig. 2).
Table 2.
Efficacy in patients with CNS metastases at baseline
| Endpoint | N + C (n = 51) | L + C (n = 50) |
|---|---|---|
| Progression‐free survival a | ||
| Restricted mean (95% CI), b mo | 7.8 (5.6–10.1) | 5.5 (3.8–7.2) |
| Median (95% CI), mo | 5.6 (3.7–7.1) | 4.3 (2.8–5.6) |
| Hazard ratio (95% CI) | 0.66 (0.41–1.05) | |
| p value (Log‐rank) | .074 | |
| Overall survival | ||
| Restricted mean (95% CI), b mo | 16.4 (13.4–19.5) | 15.4 (12.1–18.8) |
| Median (95% CI), mo | 13.9 (8.9–17.5) | 12.4 (9.7–16.9) |
| Hazard ratio (95% CI) | 0.90 (0.59–1.38) | |
| p value (Log‐rank) | .635 | |
| Time to intervention for CNS disease | ||
| 12‐month cumulative incidence (95% CI), % | 25.5 (14.4–38.1) | 36.0 (22.9–49.3) |
| p value (Gray's test) | .430 | |
| Progressive CNS disease c | ||
| 12‐month cumulative incidence (95% CI), % | 26.2 (13.8–40.3) | 41.6 (25.4–57.1) |
| p value (Gray's test) | .364 | |
| CNS progression‐free survival c | ||
| Median (95% CI), mo | 12.4 (5.6–17.9) | 8.3 (4.3–NE) |
| Hazard ratio (95% CI) | 0.62 (0.32–1.18) | |
| p value (Log‐rank) | .143 | |
| Objective response, a n (%) | 10/35 (28.6) | 11/39 (28.2) |
| Objective response rate (95% CI), % | 28.6 (14.6–46.3) | 28.2 (15.0–44.9) |
| p value (CMH test) | .972 | |
| Duration of response a , d | ||
| Median (95% CI), mo | 8.3 (2.7–8.3) | 5.3 (4.1–6.8) |
| Hazard ratio (95% CI) | 0.47 (0.10–1.60) | |
| p value (Log‐rank) | .252 | |
| Clinical benefit,a no. (%) | 14/35 (40.0) | 12/39 (30.8) |
| Clinical benefit rate (95% CI), % | 40.0 (23.9–57.9) | 30.8 (17.0–47.6) |
| p value (CMH test) | .410 | |
Independently adjudicated.
At 24 months (progression‐free survival) and 48 months (overall survival).
Scans read centrally.
Assessed in 10 and 11 patients in the N + C and L + C groups, respectively.
Abbreviations: CI, confidence interval; CMH, Cochran Mantel‐Haenszel; CNS, central nervous system; L + C, lapatinib plus capecitabine; N + C, neratinib plus capecitabine; NE, not estimable.
Figure 2.
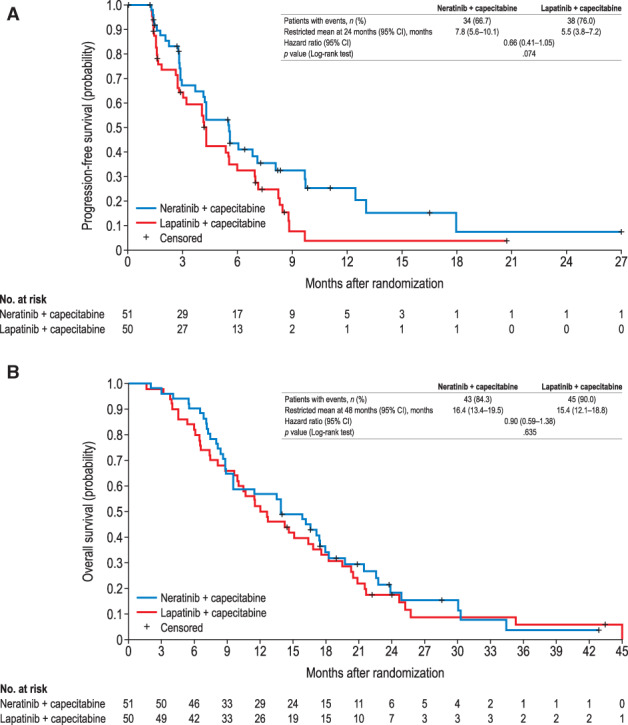
Progression‐free survival by independent adjudication (A) and overall survival (B) in patients with central nervous system metastases at baseline. Abbreviation: CI, confidence interval.
Cumulative incidence of interventions for CNS disease at 6 months was 15.7% (95% CI, 7.3–27.0%) for N + C versus 24.0% (95% CI, 13.2–36.6%) for L + C and at 12 months was 25.5% (95% CI, 14.4–38.1%) versus 36.0% (95% CI, 22.9–49.3%), respectively (Fig. 3A). Overall, 43 of 101 patients required interventions for CNS disease (including radiotherapy, surgery, anti‐cancer medications, and concomitant medications): 20 (39.2%) with N + C and 23 (46.0%) with L + C; radiotherapy was the most commonly used post‐treatment cancer‐related intervention (N + C, n = 10; L + C, n = 14; supplemental online Table 3). Cumulative incidence of progressive CNS disease at 12 months was 26.2% (95% CI, 13.8–40.3%) for N + C versus 41.6% (95% CI, 25.4–57.1%) for L + C (Fig. 3B). Median CNS‐PFS was 12.4 months (95% CI, 5.6–17.9) for N + C versus 8.3 months (95% CI, 4.3 to not estimable) for L + C (HR, 0.62; 95% CI, 0.32–1.18; Fig. 3C). A summary of efficacy outcomes in patients with baseline CNS metastases is presented in Table 2.
Figure 3.
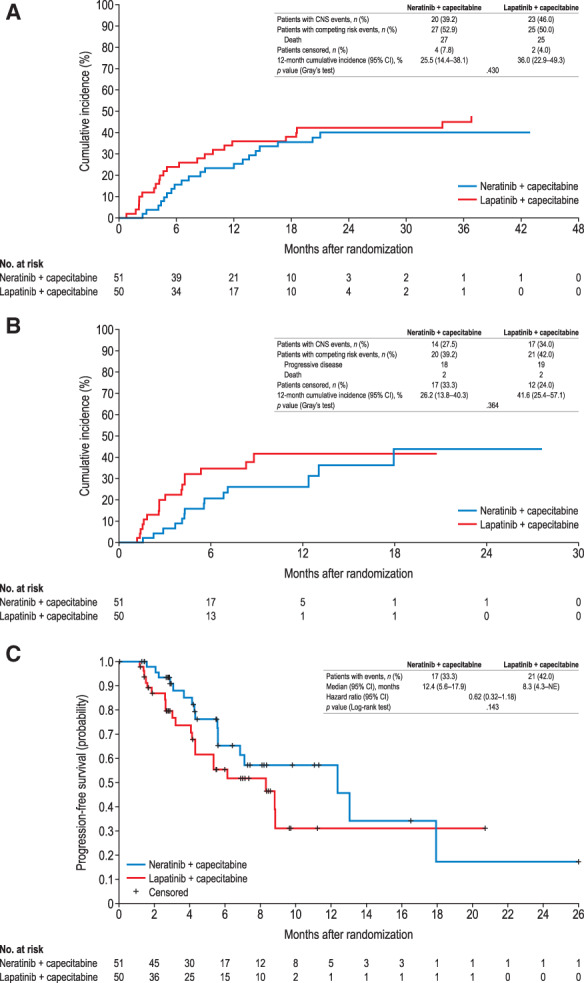
Time to intervention for CNS disease (A), progressive CNS disease (B), and CNS progression‐free survival (C) in patients with CNS metastases at baseline. For (B) and (C), scans read centrally.
Abbreviations: CI, confidence interval; CNS, central nervous system; NE, not estimable.
Among the 32 patients with at least one target CNS lesion identified at screening by the investigator, the confirmed intracranial ORR per local assessment was 26.3% with N + C and 15.4% with L + C. Intracranial response rates and waterfall plots of best changes in tumor size from baseline by treatment group are shown in Figure 4.
Figure 4.
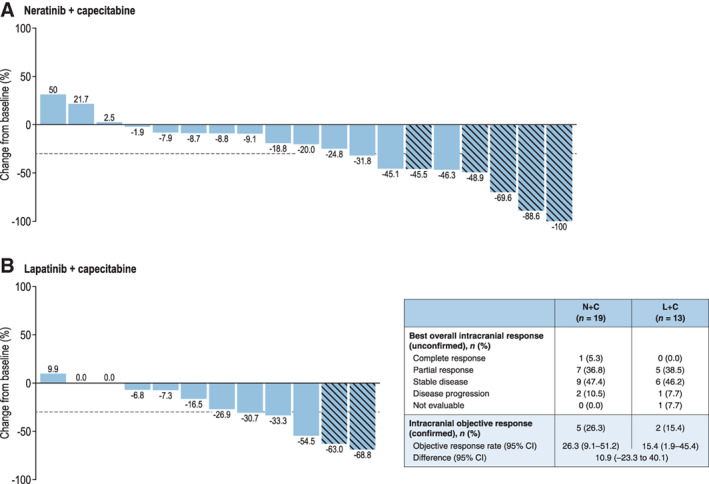
Best changes in intracranial tumor size from baseline for patients with target CNS lesions at screening per local assessment. Hatched bar indicates patient has confirmed response. Note: One patient in the lapatinib plus capecitabine group did not have a follow‐up assessment and is not included in Fig. 4B.
Abbreviations: CI, confidence interval; CNS, central nervous system; L + C, lapatinib + capecitabine; N + C, neratinib plus capecitabine.
Among the three patients who had LMD at enrollment (supplemental online Table 2), two patients treated with N + C had disease progression after 5.6 and 9.8 months, and OS times of 17.4 and 19.8 months, respectively. The other patient received L + C and had disease progression after 4.3 months and OS of 6.5 months.
Safety
The median duration of study treatment was 5.7 months (range, 0.4–28.6) for neratinib and 3.5 months (range, 0.5–20.8) for lapatinib (supplemental online Table 4). Similar proportions of patients in both groups required dose modifications. The general safety profile observed was consistent with the safety profile in the overall NALA safety population [16], with diarrhea, nausea, vomiting, and palmar‐plantar erythrodysesthesia syndrome reported as the most common TEAEs of any grade (Table 3). Grade 3 diarrhea occurred in 11 patients (22.0%) with N + C and 5 patients (10.2%) with L + C, with no grade 4 diarrhea reported. No patients discontinued N + C because of diarrhea, compared with 2 patients (4.1%) receiving L + C. Common CNS adverse events (grade 1–4) included headache (N + C, 18.0% vs. L + C, 28.6%), dizziness (18.0% vs. 16.3%), hemiparesis (4.0% vs. 4.1%), seizure (4.0% vs. 4.1%), and gait disturbance (0% vs. 8.2%; supplemental online Table 5). CNS‐related TEAEs were slightly more common in the CNS subgroup than the overall safety population [16]; for example, headache was reported in 23.2% of patients versus 13.5% in the overall safety population and dizziness in 17.2% versus 12.1%, respectively.
Table 3.
Common treatment‐emergent adverse events in patients with CNS metastases at baseline (occurring in ≥10 patients)
| Adverse event | N + C (n = 50) | L + C (n = 49) | ||
|---|---|---|---|---|
| Grade 1–4 | Grade 3/4 | Grade 1–4 | Grade 3/4 | |
| Any event | 50 (100) | 30 (60.0) | 48 (98.0) | 30 (61.2) |
| Diarrhea | 41 (82.0) | 11 (22.0) | 33 (67.3) | 5 (10.2) |
| Nausea | 28 (56.0) | 2 (4.0) | 19 (38.8) | 3 (6.1) |
| Vomiting | 25 (50.0) | 1 (2.0) | 16 (32.7) | 1 (2.0) |
| Palmar‐plantar erythrodysesthesia syndrome | 23 (46.0) | 6 (12.0) | 22 (44.9) | 6 (12.2) |
| Constipation | 19 (38.0) | 0 (0.0) | 8 (16.3) | 0 (0.0) |
| Fatigue | 19 (38.0) | 2 (4.0) | 18 (36.7) | 2 (4.1) |
| Decreased appetite | 18 (36.0) | 1 (2.0) | 11 (22.4) | 1 (2.0) |
| Weight decreased | 17 (34.0) | 1 (2.0) | 9 (18.4) | 1 (2.0) |
| Stomatitis | 11 (22.0) | 0 (0.0) | 14 (28.6) | 1 (2.0) |
| Aspartate aminotransferase increased | 9 (18.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Dizziness | 9 (18.0) | 1 (2.0) | 8 (16.3) | 0 (0.0) |
| Headache | 9 (18.0) | 0 (0.0) | 14 (28.6) | 0 (0.0) |
| Hypokalemia | 9 (18.0) | 4 (8.0) | 8 (16.3) | 3 (6.1) |
| Paronychia | 9 (18.0) | 1 (2.0) | 11 (22.4) | 0 (0.0) |
| Abdominal pain | 7 (14.0) | 0 (0.0) | 8 (16.3) | 1 (2.0) |
| Anemia | 7 (14.0) | 0 (0.0) | 9 (18.4) | 1 (2.0) |
| Pyrexia | 4 (8.0) | 0 (0.0) | 7 (14.3) | 1 (2.0) |
| Rash | 4 (8.0) | 0 (0.0) | 17 (34.7) | 1 (2.0) |
| Back pain | 4 (8.0) | 0 (0.0) | 7 (14.3) | 0 (0.0) |
| Asthenia | 3 (6.0) | 1 (2.0) | 9 (18.4) | 3 (6.1) |
Data presented as n (%).
Abbreviations: CNS, central nervous system; L + C, lapatinib plus capecitabine; N + C, neratinib plus capecitabine.
HRQoL
In the CNS subgroup, mean EORTC QLQ‐C30 summary and global health status scores and QLQ‐BR23 systemic therapy side effect scores over time were similar between treatment groups (supplemental online Fig. 1) and consistent with the overall HRQoL population [16, 24].
Discussion
Patients with asymptomatic or stable brain metastases (treated or untreated) from HER2‐positive MBC were eligible to enroll in the phase III NALA trial and made up 16% of the total population. All patients in this subgroup had received two or more lines of systemic HER2‐directed therapy, and 80% had undergone CNS‐directed radiotherapy and/or surgery prior to study entry. Descriptive analyses of this patient subgroup suggest that N + C is associated with improved PFS compared with L + C as in the NALA intention‐to‐treat population [16], despite having a poorer prognosis (mean OS 7–8 months shorter vs. intention‐to‐treat population [16]). In addition, N + C was associated with improvements in all CNS endpoints (time to intervention for CNS disease, time to progressive CNS disease, CNS‐PFS, and intracranial ORR) when compared with L + C. These CNS benefits were apparent despite the use of a lower dose of capecitabine with N + C than L + C (1,500 vs. 2,000 mg/m2 per day). A unique feature of NALA was that patients with LMD were eligible to enroll. Three patients with LMD were identified after independent radiology review, two of whom were treated with N + C with good outcomes, supporting similar observations from another recent study of N + C [14]. Neurological adverse events were slightly more common in patients with CNS metastases, as reported elsewhere [7], but no new safety signals were observed.
These findings build on prior prospective studies demonstrating neratinib activity in both the prevention and treatment of CNS metastases from HER2‐positive breast cancer [12, 13, 14, 15] and provide further support for the recommendation to use neratinib‐based therapy for brain metastases [25]. In early‐stage HER2‐positive breast cancer, fewer patients developed CNS metastases as site of first distant recurrence with neratinib monotherapy compared with placebo in the extended adjuvant setting [12, 13]. In the setting of locally advanced or metastatic HER2‐positive breast cancer, neratinib plus chemotherapy (i.e., paclitaxel or capecitabine) reduced the incidence of CNS‐related events [15, 16] and delayed time to CNS metastases [15]. Promising CNS ORR were also reported with N + C in patients with measurable, progressive CNS metastases (lapatinib‐naive patients, 49%; lapatinib‐pretreated patients, 33%) [14].
The recent approval of tucatinib based on the HER2CLIMB study [26] has been a key milestone in confirming the role of systemic therapy in HER2‐positive patients with brain metastases. Our analyses from NALA provide further support for the efficacy of TKIs, although differences in the design and inclusion criteria of these two studies should be noted. HER2CLIMB compared tucatinib‐trastuzumab‐capecitabine with placebo‐trastuzumab‐capecitabine in patients with HER2‐positive metastatic breast cancer previously treated with HER2‐directed therapy and was positive both for PFS and OS in the intention‐to‐treat and brain metastases populations [26]. The presence of brain metastases was a stratification factor, and brain MRI at baseline was mandatory. Patients with untreated, treated and stable, and treated and progressing brain metastases were eligible, whereas patients with LMD were excluded [27]. Additional inclusion criteria were specified regarding the size of untreated lesions (<2.0 cm or requiring further approval) and restrictions for corticosteroid doses (dexamethasone ≤2 mg per day or equivalent) [27]. In NALA, patients with treated or untreated asymptomatically stable brain metastases, including LMD, were eligible but without restrictions on tumor size or corticosteroid doses (doses up to 8 mg per day were documented in some patients during the study). No CNS imaging was required at enrollment, so patients with stable or progressing brain metastases may have been included. It is likely NALA included a more heterogeneous population, including some patients with more advanced CNS disease.
Within the context of current treatment guidelines, first‐line treatment for patients with HER2‐positive metastatic breast cancer remains unchanged regardless of CNS involvement. Local CNS‐directed therapy is typically recommended upon first intracranial progression followed by systemic therapy [25], the choice of which will likely depend on the status of extracranial and intracranial disease (i.e., treated stable, treated progressive, or untreated asymptomatic). Although there are currently no clinical data on which to base decisions about sequencing tucatinib‐based and neratinib‐based regimens beyond the second‐line setting in patients with CNS progression, it is likely that the choice will depend on multiple patient‐specific factors including prior treatments, feasibility of oral versus intravenous administration, preference for triplet versus doublet therapy, as well as tolerance for capecitabine.
Effective drug delivery into the brain remains a significant challenge in the treatment of CNS metastases. In theory, small‐molecule TKIs, such as neratinib, may cross the blood‐brain barrier more effectively than macromolecular HER2‐directed monoclonal antibodies (trastuzumab, pertuzumab) and antibody‐drug conjugates (trastuzumab emtansine, trastuzumab deruxtecan). This is supported by the TBCRC‐022 study, which showed the presence of neratinib in CNS surgical specimens, although distribution within the samples was heterogeneous [28]. Variable drug levels within brain metastases have also been observed with other TKIs [29], suggesting that molecular size and other factors (e.g., disruption of blood‐brain barrier in the presence of brain metastases, mechanisms regulating transport across the blood‐brain barrier [30]) are likely to influence CNS penetration. It has been suggested that irreversible TKIs, such as neratinib, may require less brain penetration than reversible inhibitors in order to achieve the same pharmacodynamic effect [31], but this has yet to be formally investigated. Neratinib also inhibits the transporter ATP‐binding cassette B1 (ABCB1; P‐glycoprotein), which is found in the blood‐brain barrier [32] and may act to reverse ABCB1‐mediated chemotherapeutic resistance. Further tissue‐based and imaging studies are required to improve understanding of CNS bioavailability, and to what extent it determines therapeutic efficacy [5, 28].
In the NALA CNS population, approximately half of the patients had hormone receptor‐positive (HR+) tumors, but concomitant endocrine therapy was not permitted as it is not considered standard of care [17]. The ExteNET and SUMMIT trials showed that neratinib has appreciable efficacy in patients with HR+/HER2‐positive or HER2‐mutated breast cancer who are also receiving endocrine therapy [12, 33], an observation attributed to inhibition of bidirectional cross‐talk between HER2 and estrogen receptor (ER) signaling. Because estrogen can cross the blood‐brain barrier [34], it is possible that neratinib efficacy in CNS metastases may be further improved by adding endocrine therapy in HR+ patients, although a possible confounding factor is that the intrinsic molecular subtype of primary breast tumors differs from matched brain metastases in up to 20% of cases (predominantly through loss of hormone receptor expression or gain of HER2 overexpression) [35, 36].
We acknowledge limitations of the present analyses. Although patients with CNS metastases and LMD were eligible for inclusion in NALA, the study was not specifically designed to evaluate these patients in detail. As such, the present analyses rely on available data and on the assessments of local investigators and radiologists for some endpoints. The study protocol also did not require universal CNS imaging at screening, and CNS disease stability was based on clinical assessment and absence of CNS symptoms. As a result, disease progression due to a new lesion in the brain could have been due to an undetected preexisting lesion at baseline. It is also possible that a few patients with baseline CNS metastases or LMD may have been missed. Prior radiotherapy was permitted if completed within 14 days before study treatment, but the exact washout times for each patient were not available. Patients with unstable or symptomatic brain metastases were not eligible for NALA and our findings should not be extended to these patients.
Conclusion
These subgroup analyses support the benefits of N + C in patients with CNS metastases from HER2‐positive MBC compared with L + C as demonstrated in the intention‐to‐treat population. Our findings support a role for neratinib as a systemic treatment option in the management of patients with HER2‐positive brain metastases following antibody‐based HER2‐directed therapies.
Author Contributions
Conception or design of the work: Sara A. Hurvitz, Judith Bebchuk, Fairooz Kabbinavar, Richard Bryce, Kiana Keyvanjah, Adam M. Brufsky
Provided study material or patients: Sara A. Hurvitz, Cristina Saura, Mafalda Oliveira, Maureen E. Trudeau, Beverly Moy, Suzette Delaloge, William Gradishar, Sung‐Bae Kim, Barbara Haley, Larisa Ryvo, Ming‐Shen Dai, Vladimir Milovanov, Jesús Alarcón, Sujith Kalmadi, Eduardo Cronemberger, Cristiano Souza, Luciana Landeiro, Ron Bose, Judith Bebchuk, Fairooz Kabbinavar, Richard Bryce, Kiana Keyvanjah, and Adam M. Brufsky
Collecting and/or assembling data: Judith Bebchuk, Kiana Keyvanjah.
Data analysis or interpretation: Sara A. Hurvitz, Cristina Saura, Mafalda Oliveira, Maureen E. Trudeau, Beverly Moy, Suzette Delaloge, William Gradishar, Sung‐Bae Kim, Barbara Haley, Larisa Ryvo, Ming‐Shen Dai, Vladimir Milovanov, Jesús Alarcón, Sujith Kalmadi, Eduardo Cronemberger, Cristiano Souza, Luciana Landeiro, Ron Bose, Judith Bebchuk, Fairooz Kabbinavar, Richard Bryce, Kiana Keyvanjah, and Adam M. Brufsky
Drafting the work or revising it critically for important intellectual content: Sara A. Hurvitz, Cristina Saura, Mafalda Oliveira, Maureen E. Trudeau, Beverly Moy, Suzette Delaloge, William Gradishar, Sung‐Bae Kim, Barbara Haley, Larisa Ryvo, Ming‐Shen Dai, Vladimir Milovanov, Jesús Alarcón, Sujith Kalmadi, Eduardo Cronemberger, Cristiano Souza, Luciana Landeiro, Ron Bose, Judith Bebchuk, Fairooz Kabbinavar, Richard Bryce, Kiana Keyvanjah, and Adam M. Brufsky
Final approval of the version to be published: Sara A. Hurvitz, Cristina Saura, Mafalda Oliveira, Maureen E. Trudeau, Beverly Moy, Suzette Delaloge, William Gradishar, Sung‐Bae Kim, Barbara Haley, Larisa Ryvo, Ming‐Shen Dai, Vladimir Milovanov, Jesús Alarcón, Sujith Kalmadi, Eduardo Cronemberger, Cristiano Souza, Luciana Landeiro, Ron Bose, Judith Bebchuk, Fairooz Kabbinavar, Richard Bryce, Kiana Keyvanjah, and Adam M. Brufsky
Disclosures
Sara A. Hurvitz: Genentech/Roche, Novartis, GlaxoSmithKline, Sanofi, Pfizer, Amgen, OBI Pharma, Puma Biotechnology, Dignitana, Bayer, Biomarin, Eli Lilly & Co, Merrimack, Cascadian Therapeutics, Seattle Genetics, Daiichi Sankyo, Macrogenics, Ambryx, Immunomedics, Pieris, Radius, Arvinas (RF–institution), Roche, Pfizer (Other relationship); Cristina Saura: Puma Biotechnology, Roche, Pfizer, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Genomic Health, Novartis, Pierre Fabre, Synthon, PIQUR Therapeutics, Bristol‐Myers Squibb, Philips Healthwork, Merck Sharpe & Dohme, Sanofi (C/A), Genentech, AstraZeneca, Roche, Macrogenics, Novartis, Pfizer, Puma Biotechnology, Synthon, PIQUR Therapeutics (RF–institution), Pfizer, Novartis, Roche, AstraZeneca, Genomic Health, Puma Biotechnology (Other–travel, accommodations, expenses); Mafalda Oliveira: Roche, Novartis, Seattle Genetics (H), Roche/Genentech, GlaxoSmithKline, Puma Biotechnology, AstraZeneca, Seattle Genetics (C/A), Philips Healthcare, Roche/Genentech, Novartis, AstraZeneca, Immunomedics, Seattle Genetics, Boehringer Ingelheim, GlaxoSmithKline, Cascadian Therapeutics, Sanofi, Celldex Therapeutics, Bayer, PIQUR Therapeutics, Puma Biotechnology, Zenith Epigenetics (RF–institution), Roche, Novartis, Grünenthal Group, Pierre Fabre, GP Pharm, Eisai (Other–travel, accommodations, expenses); Maureen E. Trudeau: RNA Diagnostics; Roche Canada, Novartis, Pfizer, Eisai, AstraZeneca, Astellas Pharma, Genomic Health (RF–institution); Beverly Moy: Motus (C/A–institution), Puma Biotechnology (RF–institution); Suzette Delaloge: AstraZeneca (C/A); AstraZeneca, Pfizer, Roche/Genentech, Puma, Eli Lilly & Co, Novartis, Sanofi (RF–institution), Pfizer, AstraZeneca, Roche (Other–travel, accommodations, expenses); William Gradishar: Genentech/Roche, AstraZeneca, Pfizer, Puma (C/A); Sung‐Bae Kim: DAEHWA Pharmaceutical, ISU Abxis (H), AstraZeneca, DAEHWA Pharmaceutical, ISU Abxis (C/A), Eli Lilly & Co (C/A–institution); Novartis, Dongkook Pharma, Genzyme, Kyowa Kirin (RF–institution); Barbara Haley: Pfizer, Eli Lilly & Co, Daiichi Sankyo, Roche, Puma Biotechnology, AstraZeneca, Sanofi (RF–institution); Jesús Alarcón: GlaxoSmithKline, Clovis, Roche, AstraZeneca (H), GlaxoSmithKline, Clovis (C/A), GlaxoSmithKline, Clovis, Roche (Other–Speaker's Bureau), Clovis (ET), GlaxoSmithKline (Other–travel, accommodations, expenses); Sujith Kalmadi: Immunomedics, AstraZeneca (OI), Bayer, Abbvie/Genentech, AstraZeneca/MedImmune, EMD Serono, Eli Lilly & Co (C/A), Genentech/Roche, Pharmacyclics/Janssen, AstraZeneca/MedImmune, Takeda, Bayer, Novartis/Pfizer (Other–Speaker's Bureau), Genentech/Roche, Tesaro, Pfizer, EMD Serono, Novocure, Abbvie/Genentech (RF); Eduardo Cronemberger: AstraZeneca/MedImmune, Roche/Genentech, Merck Sharpe & Dohme Oncology, Janssen‐Cilag, Merck Serono (RF–institution); AstraZeneca, Pfizer (Other–travel, accommodations, expenses), Ron Bose: Puma Biotechnology (RF–institution), Genentech (C/A); Judith Bebchuk: Puma Biotechnology (E, OI); Fairooz Kabbinavar: Puma Biotechnology (E, OI); Richard Bryce: Puma Biotechnology (E, OI, Other–travel, accommodations, expenses); Kiana Keyvanjah: Puma Biotechnology (E, OI, Other–travel, accommodations, expenses); Adam M. Brufsky: Pfizer, Genentech/Roche, Agendia, Celgene, Novartis, Bayer, Eli Lilly & Co, Biotheranostics, NanoString Technologies, Genomic Health, Puma Biotechnology, Bioarray Therapeutics, Merck, Myriad Pharmaceuticals, Eisai, Immunomedics, Seattle Genetics, Daiichi Sankyo/Eli Lilly & Co (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Figure S1
Appendix S2. Tables.
Acknowledgments
The authors would like to acknowledge Aimee Frazier for her contribution to the NALA study, Bin Yao for reviewing the manuscript, and the statistical programming team at Puma Biotechnology for providing the analyses described. We also thank Bethann Hromatka at Puma Biotechnology for review and revision of the manuscript and Lee Miller and Harriet Lamb (Miller Medical Communications Ltd) for writing/editorial support.
Funded by Puma Biotechnology, Inc. Medical writing support was also funded by Puma Biotechnology Inc. and provided by Miller Medical Communications.
Presented in part at the San Antonio Breast Cancer Symposium (SABCS), San Antonio, Texas, December 10–14, 2019; and SABCS, Virtual, December 8–11 2020.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Lim E, Lin NU. Updates on the management of breast cancer brain metastases. Oncology (Williston Park) 2014;28:572–578. [PubMed] [Google Scholar]
- 2. von Minckwitz G, Procter M, de Azambuja E et al. Adjuvant pertuzumab and trastuzumab in early HER2‐positive breast cancer. N Engl J Med 2017;377:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Minckwitz G, Huang CS, Mano MS et al. Trastuzumab emtansine for residual invasive HER2‐positive breast cancer. N Engl J Med 2019;380:617–628. [DOI] [PubMed] [Google Scholar]
- 4. Piccart M, Procter M, Fumagalli D et al. GS1‐04. Interim overall survival analysis of APHINITY (BIG 4‐11): A randomized multicenter, double‐blind, placebo‐controlled trial comparing chemotherapy plus trastuzumab plus pertuzumab versus chemotherapy plus trastuzumab plus placebo as adjuvant therapy in patients with operable HER2‐positive early breast cancer. Cancer Res 2020;80(4 suppl):GS1‐04a. [Google Scholar]
- 5. Lin NU, Amiri‐Kordestani L, Palmieri D et al. CNS metastases in breast cancer: Old challenge, new frontiers. Clin Cancer Res 2013;19:6404–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hurvitz SA, O'Shaughnessy J, Mason G et al. Central nervous system metastasis in patients with HER2‐positive metastatic breast cancer: Patient characteristics, treatment, and survival from SystHERs. Clin Cancer Res 2019;25:2433–2441. [DOI] [PubMed] [Google Scholar]
- 7. Montemurro F, Delaloge S, Barrios CH et al. Trastuzumab emtansine (T‐DM1) in patients with HER2‐positive metastatic breast cancer and brain metastases: Exploratory final analysis of cohort 1 from KAMILLA, a single‐arm phase IIIb clinical trial. Ann Oncol 2020;31:1350–1358. [DOI] [PubMed] [Google Scholar]
- 8. Lin NU, Kumthekar P, Sahebjam S et al. Pertuzumab (P) plus high‐dose trastuzumab (H) for the treatment of central nervous system (CNS) progression after radiotherapy (RT) in patients (pts) with HER2‐positive metastatic breast cancer (MBC): Primary efficacy analysis results from the phase II PATRICIA study. Cancer Res 2020;80(4 suppl): P1‐18‐03a. [Google Scholar]
- 9. Krop IE, Lin NU, Blackwell K et al. Trastuzumab emtansine (T‐DM1) versus lapatinib plus capecitabine in patients with HER2‐positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in EMILIA. Ann Oncol 2015;26:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. TUKYSA (tucatinib) tablets, for oral use . Prescribing information. 2020. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213411s000lbl.pdf. Accessed February 18, 2021.
- 11. NERLYNX (neratinib) tablets, for oral use . Prescribing information. 2020. Available at: https://nerlynxhcp.com/pdf/full-prescribing-information.pdf. Accessed February 18, 2021.
- 12. Chan A, Delaloge S, Holmes FA et al. Neratinib after trastuzumab‐based adjuvant therapy in patients with HER2‐positive breast cancer (ExteNET): A multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2016;17:367–377. [DOI] [PubMed] [Google Scholar]
- 13. Martin M, Holmes FA, Ejlertsen B et al. Neratinib after trastuzumab‐based adjuvant therapy in HER2‐positive breast cancer (ExteNET): 5‐year analysis of a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2017;18:1688–1700. [DOI] [PubMed] [Google Scholar]
- 14. Freedman RA, Gelman RS, Anders CK et al. TBCRC 022: A phase II trial of neratinib and capecitabine for patients with Human Epidermal Growth Factor Receptor 2‐positive breast cancer and brain metastases. J Clin Oncol 2019;37:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Awada A, Colomer R, Inoue K et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2‐positive breast cancer: The NEfERT‐T randomized clinical trial. JAMA Oncol 2016;2:1557–1564. [DOI] [PubMed] [Google Scholar]
- 16. Saura C, Oliveira M, Feng YH et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2‐positive metastatic breast cancer previously treated with ≥2 HER2‐directed regimens: Phase III NALA trial. J Clin Oncol 2020;38:3138–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology. Breast cancer, version 4, 2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed May 25, 2021.
- 18. Bachelot T, Romieu G, Campone M et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2‐positive metastatic breast cancer (LANDSCAPE): A single‐group phase 2 study. Lancet Oncol 2013;14:647–641. [DOI] [PubMed] [Google Scholar]
- 19. Geyer CE, Forster J, Lindquist D et al. Lapatinib plus capecitabine for HER2‐positive advanced breast cancer. N Engl J Med 2006;355:2733–2743. [DOI] [PubMed] [Google Scholar]
- 20. Cameron D, Casey M, Press M et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008;112:533–543. [DOI] [PubMed] [Google Scholar]
- 21. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (Version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 22. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1140–1154. [Google Scholar]
- 23. Parente L. Deflazacort: Therapeutic index, relative potency and equivalent doses versus other corticosteroids. BMC Pharmacol Toxicol 2017;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moy B, Oliveira M, Saura C et al. Neratinib + capecitabine sustains health‐related quality of life (HRQoL) while improving progression‐free survival (PFS) in patients with HER2+ metastatic breast cancer and ≥2 prior HER2‐directed regimens. In: Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium; December 8–11, 2020, San Antonio, TX. 2021;81(4 Suppl):PS9‐02a. [Google Scholar]
- 25. National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology. Central nervous system cancer, version 5, 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed May 25, 2021.
- 26. Murthy RK, Loi S, Okines A et al. Tucatinib, trastuzumab, and capecitabine for HER2‐positive metastatic breast cancer. N Engl J Med 2020;382:597–609. [DOI] [PubMed] [Google Scholar]
- 27. Lin NU, Borges V, Anders C et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2‐positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol 2020;38:2610–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Freedman RA, Gelman RS, Agar NYR et al. Pre‐ and postoperative neratinib for HER2‐positive breast cancer brain metastases: Translational Breast Cancer Research Consortium 022. Clin Breast Cancer 2020;20:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morikawa A, Peereboom DM, Thorsheim HR et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: A prospective study. Neuro Oncol 2015;17:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pulgar VM. Transcytosis to cross the blood brain barrier, new advancements and challenges. Front Neurosci 2019;12:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim M, Laramy JK, Mohammad AS et al. Brain distribution of a panel of Epidermal Growth Factor Receptor inhibitors using cassette dosing in wild‐type and Abcb1/Abcg2‐deficient mice. Drug Metab Dispos 2019;47:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao X, Xie J, Chen X et al. Neratinib reverses ATP‐binding cassette B1‐mediated chemotherapeutic drug resistance in vitro, in vivo, and ex vivo. Mol Pharmacol 2012;82:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smyth LM, Piha‐Paul SA, Won HH et al. Efficacy and determinants of response to HER kinase inhibition in HER2‐mutant metastatic breast cancer. Cancer Discov 2020;10:198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rettberg JR, Yao J, Brinton RD. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol 2014;35:8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaidar‐Person O, Meattini I, Jain P et al. Discrepancies between biomarkers of primary breast cancer and subsequent brain metastases: An international multicenter study. Breast Cancer Res Treat 2018;167:479–483. [DOI] [PubMed] [Google Scholar]
- 36. Priedigkeit N, Hartmaier RJ, Chen Y et al. Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol 2017;3:666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Figure S1
Appendix S2. Tables.


