Abstract
Lessons Learned
MET overexpression is uncommon, and positive MET immunohistochemistry (1+/2+) was an independent positive prognostic factor for response rate and progression‐free survival.
Whether MET overexpression can be considered a potential predictive biomarker and be used as an inclusion criterion is worth investigating in a future study.
Background
Metatinib tromethamine tablet (metatinib) is a small molecule receptor kinase inhibitor targeting both c‐MET and vascular endothelial growth factor receptor 2. This phase I trial aimed to determine the dose‐limiting toxicity (DLT) and maximum tolerated dose (MTD), pharmacokinetics, safety, and efficacy of metatinib in patients with advanced solid tumors.
Methods
Eligible patients received a single dose of metatinib in a 3 + 3 dose‐escalation design with dose levels of 25–800 mg/day, after a single dose on day 1, then 2 days off, and then a multidose schedule of once‐daily doses for 25 consecutive days (days 4–28). Primary endpoints were MTD and safety; secondary and exploratory endpoints included pharmacokinetics (PK), efficacy, and biomarkers.
Results
Eighteen patients (including nine patients with hepatocellular carcinoma [HCC]) received at least one dose of study drug (one patient quit the study without continuous multiple‐dose administration after receiving a single dose of metatinib). Hand‐foot skin reaction, diarrhea, and liver dysfunction were the DLTs, and 200 mg/day was the MTD. The most common treatment‐related adverse events (TRAEs) were skin toxicity (50%), diarrhea (33.3%), and liver dysfunction (27.8%). Three patients (only one of six in the 200 mg/day cohort; the other two in the 300 mg/day cohort) experienced severe TRAEs: one patient with severe liver dysfunction and two patients with severe liver dysfunction and skin toxicity, respectively. Pharmacokinetics assessment indicated that metatinib was rapidly absorbed and metabolized to the formation of reactive metabolite, SCR‐1510, after single‐dose administration. The mean time taken to achieve maximum concentration and terminal elimination half‐life of SCR‐1510 was approximately 2.0–3.0 hours and ranged from 8 to 14 hours. Two patients had partial responses. The objective response rate and disease control rate (DCR) were 11.1% and 61.1%, respectively. The median progression‐free survival (PFS) was 2.75 months.
Conclusion
Metatinib administration of 200 mg/day was well tolerated, safe, and effective. The MTD was 200 mg/day, which should be recommended in further investigations.
Keywords: Metatinib tromethamine tablet, Advanced cancer, Phase I
Discussion
The toxicity profile of metatinib has been shown to be similar to that of other analogous agents, such as apatinib, sunitinib, anlotinib, and regorafenib. Hand‐foot skin reaction, diarrhea, and liver dysfunction were the most frequent serious TRAEs and were also the DLTs observed in the present study (Table 1). Although almost all patients had mild to moderate TRAEs, none in the lower dose group (<200 mg/day) developed grade 3–5 TRAEs. Although grade 3 skin toxicity in the 200 mg/day group was observed, it could be quickly controlled with drug discontinuation and dose reduction, suggesting continuous accumulation of toxicity that might be manageable by changing the administration protocol to a 2‐weeks‐on/1‐week‐break schedule. Nine patients with advanced liver cancer presented higher tumor load and limited liver reserve capacity; this may account for why liver toxicity was also a common adverse event (AE). The lower incidence of TRAEs (hypertension, proteinuria, fatigue) supported the advantages over similar tyrosine kinase inhibitor drugs. Palmar‐plantar erythema syndrome was remarkable and manageable. The incidence of common TRAEs in metatinib was as follows: diarrhea (33.3%), grade 3 diarrhea (5.6%), palmar‐plantar erythema syndrome (50%), and grade 3 palmar‐plantar erythema syndrome (5.6%). Similarly, in the cabozantinib group in a randomized, phase III trial [19, 26], the incidence of diarrhea and palmar‐plantar erythema syndrome was 74% and 42%, respectively, and the incidence of the common grade 3 or 4 AEs were diarrhea (13%) and palmar‐plantar erythema syndrome (8%). Discontinuation of study treatment because of AEs not related to disease occurred in 22.2% of patients and in 100% of patients administered 300 mg/day, suggesting that overall tolerability of the agent was acceptable and that 200 mg/day should be the optimal dose investigated in further study. The longest PFS was 8.5 months in a patient with rectal cancer and 5.5 months in a patient with HCC, who both received ≥100 mg/day, which suggested a substantial and promising antitumor activity of metatinib. Considering that the terminal elimination half‐life of SCR‐1510 ranges from 8 to 14 hours, daily administration is reasonable. The continuous accumulation of SCR‐1510 at day 29 was more than twice than that at day 1, and significantly, toxicity was observed in the 300 mg/day cohort. To adapt to the PK feature, whether the administration protocol should be changed to the daily treatment in a 2‐weeks‐on/1‐week‐off cycle to support the feasibility needs further study.
Table 1.
Adverse events of patients in the extended period (n = 10)
| Adverse events | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Total, n (%) |
|---|---|---|---|---|
| Any TRAE | 1 (10) | 5 (50) | 3 (30) | 9 (90) |
| Skin toxicities | 3 (30) | 2 (20) | 1 (10) | 6 (60) |
| Palmar‐plantar erythema syndrome (peeling) | 3 (30) | 2 (20) | 1 (10) | 6 (60) |
| Skin pain | 1 (10) | 1 (10) | 1 (10) | 3 (30) |
| Rash maculo‐papular | 0 (0) | 1 (10) | 0 (0) | 10 (10) |
| Circumoral numbness | 2 (20) | 0 (0) | 0 (0) | 2 (20) |
| Edema limbs | 1 (10) | 0 (0) | 0 (0) | 1 (10) |
| Diarrhea | 1 (10) | 2 (20) | 1 (10) | 4 (40) |
| Abdominal pain | 2 (10) | 0 (0) | 0 (0) | 2 (10) |
| Anorexia | 0 (0) | 1 (10) | 0 (0) | 1 (10) |
| Dysphagia | 1 (10) | 0 (0) | 0 (0) | 1 (10) |
| Constipation | 1 (10) | 0 (0) | 0 (0) | 1 (10) |
| Blood bilirubin elevation | 0 (0) | 1 (10) | 1 (10) | 2 (20) |
| AST elevation | 1 (10) | 1 (10) | 1 (10) | 3 (30) |
| ALT elevation | 2 (20) | 1 (10) | 1 (10) | 4 (40) |
| Hypertension | 0 (0) | 1 (10) | 0 (0) | 1 (10) |
| Thrombocytopenia | 0 (0) | 2 (20) | 0 (0) | 2 (20) |
| Anemia | 0 (0) | 1 (10) | 0 (0) | 1 (10) |
| Fatigue | 0 (0) | 1 (10) | 0 (0) | 1 (10) |
| Fever | 1 (10) | 0 (0) | 0 (0) | 1 (10) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TRAE, treatment‐related adverse event.
Trial Information
| Disease |
Advanced cancer/solid tumor only Colorectal cancer Esophageal cancer Gastric cancer Hepatocellular carcinoma |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study | Phase I, 3 + 3 |
| Primary Endpoints |
Maximum tolerated dose Toxicity Safety Recommended phase II dose |
| Secondary Endpoints |
Pharmacokinetics Efficacy Correlative endpoint |
| Additional Details of Endpoints or Study Design | |
| Patient Eligibility: This study was a single‐center, single‐arm, open‐label, prospective phase I trial, registered in May 2013 (registration no. NCT02004548), carried out in the Department of Abdominal Oncology and Institute of Clinical Pharmacology, GCP Center, West China Hospital, Sichuan University, China, which was approved by the ethics committee and registered in a clinical trial registry. | |
| Eligibility criteria: Patients with pathologically and/or cytologically proven advanced or metastatic digestive tract cancer with no standard therapy were enrolled in the study. Eligibility criteria included age 18–74 years, Eastern Cooperative Oncology Group (ECOG) performance status 0–2, expected survival duration of more than 3 months, weight ≥ 45 kg for men and weight ≥ 40 kg for women, and body mass index (BMI) between 18 and 28 kg/m2. Routine blood test, bone marrow, liver and kidney function, and heart function must be determined to be within normal range and with no major organ dysfunction. Patients must be capable of understanding and be willing to comply with the protocol and follow‐up and had to give signed informed consent document for the study. | |
| Exclusion criteria: Patients were not eligible if they presented the following: brain metastases; an uncontrolled intercurrent illness including, but not limited to, ongoing or active infection, symptomatic congestive heart failure, unstable angina pectoris, or cardiac arrhythmia, active hepatitis B or hepatitis C, active tuberculosis, or HIV positivity; involvement of major blood vessels or nerves by tumor; uncontrollable hypertension or left ventricular ejection fraction <50%; disease history of bleeding or thromboembolic events occurring within the past 6 months and need for preventive anticoagulant therapy; or third lacunar effusion with difficulty to control. | |
| Treatment Plan: All patients received escalating doses of metatinib tromethamine tablet for only a single dose of metatinib on day 1 and were evaluated for toxicity for 3 days (days 1–3). After 3 days following the first dose (single dose), subjects continued to receive metatinib once daily (multidose schedule) at the intended dose level for 25 consecutive days (days 4–28) and were closely observed in the following 4 days (days 29–32). The single dose was administered orally 2 hours before breakfast, and in the multidose schedule, the dose was daily administered orally 1 hour before breakfast, which could be modified according to the following PK data. A standard 3+3 design was applied in this study, with terms of cohort expansion to six evaluable patients if a DLT was observed in the first cycle (days 1–32) in the initial three patients. If two DLTs were observed during the first cycle (days 1–32) in a cohort, dose escalation was stopped, and dosage continued at a lower level until the MTD was identified. The planned dose levels were 25, 50, 100, 200, 300, 450, 600, and 800 mg/day, respectively. | |
| Extended period: After the patients completed the single dose and then the multidose schedule daily for 25 consecutive days, if clinical benefits were achieved at the efficacy evaluation on the 32nd day (±3 days), then the patients continued the corresponding daily dose treatment in the extended period. The treatment period was to be extended until the disease progressed, intolerable AEs occurred, or the patient withdrew informed consent. | |
| Efficacy assessment: Efficacy assessment was evaluated according to the National Cancer Institute RECIST version 1.1. The evaluated lesions were assessed on day 32 (±3 days) compared with the baseline imaging examination done within 4 weeks before the first dose. For all patients enrolled into the extended period of treatment, the imaging evaluation was performed every 6 weeks, and the standard imaging examination (computed tomography or magnetic resonance imaging) and physical examination (for superficial lesions) was used for every evaluation. | |
| Safety assessment: All adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, and the attribution between AE and the study drug was determined. Safety was evaluated daily after the first dose administration in the following 29 days, then every 3 weeks during the first four follow‐up visits during the extended treatment period; then, every 6 weeks after that, the AE and laboratory tests were evaluated. During treatment, vital signs (body temperature, pulse, respiration, blood pressure) were monitored every day, and physical examination was carried out daily from day 1 to day 29. The safety endpoints were DLT, MTD, AEs, laboratory examination (hematology, clinical biochemistry, urinary routine, and coagulation), ECG, adrenocorticotropic hormone level, and thyroid function. The safety follow‐up continued until 30 days after the treatment. All patients were followed up by telephone every 3 months until the patient died. | |
| Pharmacokinetic assessment: In single‐dose studies, the serial blood samples were collected before and at 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 48, and 72 hours after the first dose. In multiple‐dose studies, blood samples were collected 0.5 hour before dosing on days 8, 15, and 29 and 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 hours after the last dosing on day 29. All blood samples were centrifuged to prepare plasma fractions and stored at −70°C for subsequently analysis. The main PK parameters in single‐dose studies were as follows: the maximum concentration (Cmax) and the time taken to achieve Cmax (Tmax), the area under concentration‐time curve up to the last measured time point (AUC0–t), the area under concentration‐time curve up to positive infinity (AUC0–∞), and terminal elimination half‐life (t1/2). The PK parameters in multiple‐dose studies were Tmax, Cmax, AUC0–t, AUC0–∞, and fluctuation coefficient. The plasma concentration of the reactive metabolites of metatinib tromethamine tablet (SCR‐1510 and SCR‐1512) after single‐dose and multiple‐dose administration at each dose level, were detected by Phoenix WinNonlin 6.1 (Certara USA, Inc., Princeton, NJ). | |
| Statistical analysis: The measurement data use means ± SD or medians (min, max) for statistical description and paired t test to compare the difference within the group with baseline data. Frequency (constituent ratio) is used for statistical description to the enumeration data and the changes in each dose group before and after the treatments. The two‐sided test is used for all statistical tests, and a value of p < .05 was considered to be statistically significant. A noncompartmental analysis model was used to calculate the plasma pharmacokinetic parameters AUC0–t, AUC0–∞, and t1/2. Cmax and Tmax were adopted as the measured value. The correlation between dosage and drug plasma exposure was evaluated by the power model method, and the ratio of the main parameters between fed and fasted as well as the 90% confidence interval (CIs) were calculated. Phoenix WinNonlin version 6.3 was used to perform pharmacokinetic analyses. A value of p < .05 was considered to be statistically significant. | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information: Metatinib Tromethamine Tablet
| Generic/Working Name | Metatinib tromethamine tablet |
| Company Name | Jiangsu Simcere Pharmaceutical Co., Ltd |
| Drug Type | Small molecule |
| Drug Class | MET ‐ c‐MET |
| Dose | 25–300 mg (tablets) per day milligrams (mg) per flat dose |
| Route | Oral (p.o.) |
| Schedule of Administration | All patients received escalating doses of metatinib tromethamine tablet with a single dose on day 1, then 2 days off, and then a multidose schedule, meaning once daily for 25 consecutive days (days 4–28). The single dose was administered orally 2 hours before the breakfast; in the multidose schedule, the daily dose was administered orally 1 hour before the breakfast and could be modified according to the following PK data. A standard 3 + 3 design was applied in this study, with terms of cohort expansion to six evaluable patients if a DLT was observed in the first cycle (days 1–32) in the initial three patients. If two DLTs were observed during the first cycle (days 1–32) in a cohort, dose escalation was stopped, and dosage continued at a lower level until the MTD was identified. The planned doses were 25, 50, 100, 200, 300, 450, 600, and 800 mg/day, respectively. |
Patient Characteristics
| Number of patients, male | 15 |
| Number of patients, female | 3 |
| Stage | All patients had stage IV advanced or metastatic digestive tract cancer with no standard therapy options; pathological and/or cytological confirmation was required. |
| Age | Median (range): 49 (22–72) years |
| Number of prior systemic therapies | Median (range): 2 (1–3) |
| Performance status: ECOG |
0 — 8 1 — 8 2 — 2 3 — 0 Unknown — 0 |
| Other | The median BMI (kg/m2) was 20.79, ranging from 17.1 to 27.9. The numbers of patients with Child‐Pugh scores of A and B were 17 and 1, respectively. The numbers of patients with two lesions (primary and metastatic lesions) and with at least three lesions were 6 and 12, respectively. |
| Cancer types or histologic subtypes |
Esophageal cancer, 4 Liver cancer, 9 Gastric cancer, 2 Rectal cancer, 2 Jejunal cancer, 1 |
Primary Assessment Method
| Number of patients screened | 20 |
| Number of patients enrolled | 18 |
| Number of patients evaluable for toxicity | 18 |
| Number of patients evaluated for efficacy | 18 |
| Evaluation method | RECIST version 1.1 |
| Response assessment CR | n = 0 (0%) |
| Response assessment PR | n = 11.1 (2%) |
| Response assessment SD | n = 50 (9%) |
| Response assessment PD | n = 38.9 (7%) |
| Response assessment OTHER | n = 0 (0%) |
| Median duration assessments PFS | 84 days, CI: 59.887–120.891 |
| Median duration assessments TTP | 84 days, CI: 59.887–120.891 |
| Median duration of treatment | 75 days |
Outcome Notes
According to the RECIST version 1.1 criteria, 18 patients (including one patient who discontinued the study after receiving a single dose of metatinib) were assessed radiologically at day 32. In the first cycle, one patient (5.6%) in the 300 mg/day group had partial response (PR), 10 patients (55.6%) maintained stable disease (SD), 8 patients presented progressive disease (PD), and all 3 patients in the 25 mg/day group had PD.
In the first cycle, the DCR was 61.1% (11/18); among the patients who was achieved DCR, 10 patients continued on the treatment in the following extended period. During the extended period, three patients had PD, and seven patients had SD at the first efficacy evaluation; another patient in the 200 mg/day group achieved PR after 3 months entering into the extended period, although he experienced severe skin toxicity and reduced the dose to 100 mg/day. In total, in the current phase I study, two patients had PR, and nine patients exhibited SD, including four patients with tumor burden shrinkage; the objective response rate and DCR were 11.1% and 61.1%, respectively.
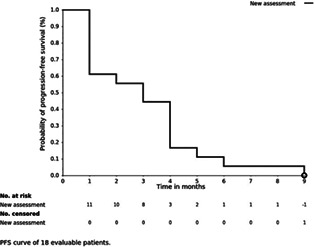
The median PFS in 18 patients was 2.75 months, ranging from 1.0 to 8.5 months, and the median PFS in 10 patients included in the extended period was 4.0 months, ranging from 2 to 8.5 months.
MET analysis: Samples from a total of 18 patients, in 162 paraffin‐embedded tumor slides, were evaluated for MET expression status by MET immunohistochemistry (IHC) and dual‐color fluorescence in situ hybridization (FISH), and for MET mutation by sequence analysis (Table 2). There were 2 cases with IHC 2+, 3 cases with IHC 1+, and 13 cases with IHC 0. MET gene amplification was not detected, and MET exon 14 skipping was exhibited in one patient with IHC 2+, showing a weak concordance between IHC and FISH. One patient in the 200 mg/day group whose tumor showed both MET IHC 2+ and exon 14 skipping achieved PR and 8.5 months’ PFS. A 5.5‐month PFS was also observed in another patient with MET IHC 2+, suggesting that MET status might be associated with the efficacy in the study.
Kaplan‐Meier Time Units (days)
| Time of scheduled assessment and/or time of event | No. progressed (or deaths) | No. censored | Percent at start of evaluation period | Kaplan‐Meier % | No. at next evaluation/No. at risk |
|---|---|---|---|---|---|
| 0 | 0 | 0 | 100.00 | 100.00 | 18 |
| 32 | 7 | 0 | 100.00 | 61.11 | 11 |
| 75 | 1 | 0 | 61.11 | 55.55 | 10 |
| 78 | 1 | 0 | 55.55 | 49.99 | 9 |
| 90 | 1 | 0 | 49.99 | 44.44 | 8 |
| 120 | 3 | 0 | 44.44 | 27.77 | 5 |
| 121 | 1 | 0 | 27.77 | 22.22 | 4 |
| 122 | 1 | 0 | 22.22 | 16.66 | 3 |
| 135 | 1 | 0 | 16.66 | 11.11 | 2 |
| 166 | 1 | 0 | 11.11 | 5.55 | 1 |
| 256 | 1 | 0 | 5.55 | 0.00 | 0 |

Waterfall plot of evaluable patients (n = 18) showing the largest decrease in the sum of the target lesions compared with baseline.
Adverse Events
| All Dose Levels, Cycle 1 | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
| Diarrhea | 67 | 17 | 11 | 6 | 0 | 0 | 33 |
| Palmar‐plantar erythrodysesthesia syndrome | 50 | 17 | 28 | 6 | 0 | 0 | 50 |
| Pain of skin | 83 | 0 | 11 | 6 | 0 | 0 | 17 |
| Rash maculo‐papular | 89 | 6 | 6 | 0 | 0 | 0 | 11 |
| Lower gastrointestinal hemorrhage | 94 | 0 | 6 | 0 | 0 | 0 | 6 |
| Abdominal pain | 94 | 0 | 6 | 0 | 0 | 0 | 6 |
| Back pain | 94 | 0 | 6 | 0 | 0 | 0 | 6 |
| Proteinuria | 94 | 0 | 6 | 0 | 0 | 0 | 6 |
| Hearing impaired | 94 | 6 | 0 | 0 | 0 | 0 | 6 |
| Insomnia | 94 | 0 | 6 | 0 | 0 | 0 | 6 |
| Neutrophil count decreased | 94 | 0 | 6 | 0 | 0 | 0 | 6 |
| Hematuria | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Aspartate aminotransferase increased | 78 | 17 | 0 | 6 | 0 | 0 | 22 |
| Alanine aminotransferase increased | 78 | 11 | 6 | 6 | 0 | 0 | 22 |
| Blood bilirubin increased | 83 | 6 | 0 | 11 | 0 | 0 | 17 |
| Electrocardiogram QT corrected interval prolonged | 78 | 11 | 11 | 0 | 0 | 0 | 22 |
| Anorexia | 78 | 11 | 11 | 0 | 0 | 0 | 22 |
| Hypoglossal nerve disorder | 83 | 17 | 0 | 0 | 0 | 0 | 17 |
| Fatigue | 83 | 17 | 0 | 0 | 0 | 0 | 17 |
| Hypertension | 83 | 17 | 0 | 0 | 0 | 0 | 17 |
| White blood cell decreased | 89 | 0 | 11 | 0 | 0 | 0 | 11 |
| Platelet count decreased | 89 | 0 | 11 | 0 | 0 | 0 | 11 |
| Palpitations | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Hemorrhoids | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Mucositis oral | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Vomiting | 89 | 6 | 6 | 0 | 0 | 0 | 11 |
| Nausea | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Fever | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Hypokalemia | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Pain in extremity | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Palmar‐plantar erythrodysesthesia syndrome | 40 | 30 | 20 | 10 | 0 | 0 | 60 |
| Pain of skin | 70 | 10 | 10 | 10 | 0 | 0 | 30 |
| Rash maculo‐papular | 90 | 0 | 10 | 0 | 0 | 0 | 10 |
| Circumoral numbness | 80 | 20 | 0 | 0 | 0 | 0 | 20 |
| Edema limbs | 90 | 10 | 0 | 0 | 0 | 0 | 10 |
| Diarrhea | 60 | 10 | 20 | 10 | 0 | 0 | 40 |
| Abdominal pain | 80 | 20 | 0 | 0 | 0 | 0 | 20 |
| Anorexia | 90 | 0 | 10 | 0 | 0 | 0 | 10 |
| Dysphagia | 90 | 10 | 0 | 0 | 0 | 0 | 10 |
| Constipation | 90 | 10 | 0 | 0 | 0 | 0 | 10 |
| Blood bilirubin increased | 80 | 0 | 10 | 10 | 0 | 0 | 20 |
| Aspartate aminotransferase increased | 70 | 10 | 10 | 10 | 0 | 0 | 30 |
| Alanine aminotransferase increased | 60 | 20 | 10 | 10 | 0 | 0 | 40 |
| Hypertension | 90 | 0 | 10 | 0 | 0 | 0 | 10 |
| Platelet count decreased | 80 | 0 | 20 | 0 | 0 | 0 | 20 |
| Anemia | 90 | 0 | 10 | 0 | 0 | 0 | 10 |
| Fatigue | 90 | 0 | 10 | 0 | 0 | 0 | 10 |
| Fever | 90 | 10 | 0 | 0 | 0 | 0 | 10 |
Adverse Events Legend
Treatment‐related adverse events of patients in the first cycle (n = 18). Abbreviation: NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Hand‐foot skin reaction | 3 | Definite |
| Diarrhea | 3 | Definite |
| Liver dysfunction | 3 | Definite |
Serious Adverse Events Legend
Hand‐foot skin reaction, diarrhea, and liver dysfunction were the most frequent serious TRAEs and were also the DLTs observed in the present study.
Pharmacokinetics/Pharmacodynamics
| See Table 3. |
Table 3.
Pharmacokinetic parameters of SCR‐1510 and SCR‐1512, concentration of the reactive metabolites of metatinib tromethamine tablet after a single dose and multiple‐dose schedule in all patients
| Parameter | Unit | Measurement of single‐dose dependent pharmacokinetics at day 1 | Measurement of multiple‐dose dependent pharmacokinetics at day 29 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 mg/day (n = 3) | 50 mg/day (n = 4) | 100 mg/day (n = 3) | 200 mg/day (n = 6) | 300 mg/day (n = 2) | 25 mg/day (n = 3) | 50 mg/day (n = 3) | 100 mg/day (n = 3) | 200 mg/day (n = 5) | 300 mg/day (n = 2) | ||
| SCR‐1510 | |||||||||||
| Cmax | ng/mL | 180.35 ± 90.27 | 341.04 ± 175.61 | 792.44 ± 105.12 | 1,312.23 ± 682.62 | 2,810.83 ± 2,040.37 | 268.40 ± 66.99 | 471.62 ± 159.40 | 981.28 ± 100.04 | 2,066.41 ± 1,048.30 | 3,629.26 ± 157.97 |
| Tmax | hour | 2.67 ± 0.58 | 4.50 ± 5.07 | 2.67 ± 1.15 | 4.00 ± 2.00 | 2.50 ± 0.71 | 1.67 ± 0.28 | 1.33 ± 0.58 | 4.33 ± 1.53 | 3.60 ± 0.55 | 2.50 ± 0.71 |
| AUC0‐t |
hour*ng/ mL |
2,116.79 ± 1,554.88 | 4,861.34 ± 2,656.57 | 14,350.00 ± 6,581.95 | 21,037.57 ± 9,671.28 | 38,622.97 ± 24,309.57 | 118.53 ± 44.33 | 7,393.18 ± 3,127.88 | 23,924.26 ± 4,301.22 | 50,134.35 ± 24,593.97 | 122,532.94 ± 22,855.14 |
| AUC0‐∞ |
hour*ng/ mL |
2,161.32 ± 1,575.95 | 4,967.39 ± 2,747.22 | 14,552.67 ± 6,640.64 | 21,183.29 ± 9,717.07 | 39,340.88 ± 24,188.51 | 3,708.11 ± 1,657.55 | 7,485.62 ± 3,219.96 | 24,523.83 ± 4,837.07 | 50,832.92 ± 24,930.50 | 159,154.87 ± 55,721.86 |
| t1/2 | hour | 9.91 ± 1.27 | 8.35 ± 3.69 | 12.41 ± 4.48 | 8.48 ± 2.14 | 13.31 ± 2.70 | 11.26 ± 2.16 | 10.66 ± 2.51 | 11.30 ± 4.15 | 11.55 ± 1.40 | 31.05 ± 12.09 |
| Fluctuation coefficient | % | — | — | — | — | — | 201.45 ± 72.01 | 177.76 ± 70.61 | 86.52 ± 15.29 | 87.82 ± 22.00 | 67.91 ± 16.59 |
| SCR‐1512 | |||||||||||
| Cmax | ng/mL | 73.77 ± 43.33 | 127.18 ± 63.18 | 232.79 ± 252.96 | 335.06 ± 189.87 | 382.90 ± 323.83 | 139.25 ± 46.80 | 146.75 ± 101.19 | 311.88 ± 205.76 | 470.85 ± 327.17 | 1,415.69 ± 723.72 |
| Tmax | hour | 3.67 ± 2.08 | 13.50 ± 12.15 | 14.67 ± 8.33 | 12.17 ± 9.35 | 9.00 ± 4.24 | 3.33 ± 4.16 | 8.67 ± 5.77 | 7.33 ± 1.15 | 1.80 ± 1.10 | 1.50 ± 0.71 |
| AUC0‐t |
hour*ng/ mL |
1,607.10 ± 774.35 | 3,930.15 ± 3,826.66 | 6,772.35 ± 6,579.02 | 10,012.86 ± 6,421.09 | 10,977.71 ± 8,712.42 | 3,979.45 ± 1,799.53 | 3,994.10 ± 3,199.80 | 10,546.48 ± 7,981.98 | 14,830.55 ± 8,935.82 | 59,035.61 ± 53,496.82 |
| AUC0‐∞ |
hour*ng/ mL |
1,701.08 ± 764.09 | 6,010.75 ± 7,615.43 | 6,916.06 ± 6,705.61 | 10,582.82 ± 7,202.00 | 11,112.95 ± 8,672.48 | 4,224.96 ± 2,016.43 | 4,303.23 ± 3,572.96 | 12,059.26 ± 10,262.54 | 15,565.86 ± 9,588.14 | 219,535.16 ± 273,159.07 |
| t1/2 | hour | 14.84 ± 4.03 | 20.03 ± 20.51 | 12.04 ± 3.62 | 11.14 ± 5.36 | 10.83 ± 3.29 | 15.21 ± 3.22 | 15.14 ± 4.48 | 17.18 ± 7.95 | 15.00 ± 2.02 | 92.81 ± 91.03 |
| Fluctuation coefficient | % | — | — | — | — | — | 89.20 ± 20.81 | 24.75 ± 15.47 | 65.93 ± 37.33 | 55.60 ± 17.58 | 99.10 ± 39.89 |
Abbreviations: —, not available ; AUC0–t, area under the concentration‐time curve up to the last measured time point; AUC0‐∞, area under the concentration‐time curve up to positive infinity; Cmax, maximum concentration; t1/2, terminal elimination half‐life; Tmax, time to achieve maximum concentration.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
The receptor–tyrosine kinase c‐MET, which is encoded by the MET proto‐oncogene, was identified as the receptor for hepatocyte growth factor (HGF) [1, 2]. The HGF‐MET pathway was found to be vital for the growth, survival, and invasive potential of cancers by activating MAPK/ERK cascades, the PI3K/Akt axis, and the STAT3 pathway [3]; therefore, both HGF and MET are targets in anticancer drug discovery [4].
Changes in serum or tissue HGF levels and MET expression/phosphorylation in tumors were reported in several kinds of tumors, such as gastric cancer, lung cancer, breast cancer, renal cell carcinoma, and hepatocellular carcinoma [5, 6, 7, 8]. The prevalence of HGF/MET pathway activation in human malignancies has driven the development of drug programs.
Tumor angiogenesis is crucial to tumor growth, progression, and metastasis [9, 10], and antiangiogenic therapy has been a successfully used strategy for cancer treatment [11]. Whereas the therapeutic resistance toward antiangiogenic therapy is a limitation of vascular endothelial growth factor receptor (VEGFR) in clinical treatment, HGF mediates angiogenesis through positive vascular endothelial growth factor (VEGF) [12], and increased MET expression has been implicated in the development of resistance to VEGFR inhibitors in preclinical models of several cancers [13, 14, 15]. Hence, the HGF/c‐MET pathway is one of the most investigated signaling pathways in anti‐VEGF therapy/VEGFR‐resistant tumors [16, 17]. Recent studies have suggested that blockade of both MET and VEGFR pathways may achieve better treatment outcome in cancer treatment [18]. Cabozantinib, an inhibitor of tyrosine kinases, including VEGF receptors 1, 2, and 3, MET, and AXL, exhibited its efficacy in several types of solid tumor and became the standard of care for patients with hepatocellular carcinoma (HCC) and renal cell carcinoma [19, 20, 21].
Metatinib tromethamine tablet, an inhibitor targeting both c‐MET and VEGFR‐2, has exhibited antitumor activity (in MET‐dependent and non–MET‐dependent tumor models), good pharmacokinetics (PK), and reversible toxicities.
The toxicity profile of metatinib was similar to that of other analogous agents, such as apatinib, sunitinib, anlotinib, and regorafenib [22, 23, 24, 25]. Hand‐foot skin reaction, diarrhea, and liver dysfunction were the most frequent serious treatment‐related adverse events (TRAEs) and were also the dose‐limiting toxicities observed in the present study. Whereas almost all patients had mild to moderate TRAEs, none in the lower dose group (<200 mg/day) developed grade 3–5 TRAEs. Although severe skin toxicity in the 200 mg/day group was observed, it could be quickly controlled with drug discontinuation and dose decrease, suggesting a continuous accumulation of toxicity that might be manageable with a change in administration protocol to the 2‐weeks‐on/1‐week‐break schedule. Nine patients with advanced liver cancer presented higher tumor load and limited liver reserve capacity, which may account for why liver toxicity is also a common adverse event. The lower incidence of TRAEs (hypertension, proteinuria, fatigue) supported the advantages over similar tyrosine kinase inhibitor drugs. Palmar‐plantar erythema syndrome was remarkable and manageable. Similarly, in the cabozantinib group in a randomized, phase III trial [19, 26], the incidence of diarrhea and palmar‐plantar erythema syndrome was 74% and 42%, respectively; even the common grade 3 or 4 adverse events were diarrhea (13%) and palmar‐plantar erythema syndrome (8%). Discontinuation of study treatment because of adverse events not related to disease occurred in 22.2% of patients and in 100% of patients administered 300 mg/day, suggesting that overall tolerability of the agent was acceptable and that 200 mg/day should be the optimal dose investigated in further study. The longest progression‐free survival (PFS) was 8.5 months in a patient with rectal cancer and 5.5 months in a patient with HCC, who both received ≥100 mg/day, which suggested a substantial and promising antitumor activity of metatinib. Considering the t1/2 of SCR‐1510 ranged from 8 to 14 hours, daily administration is reasonable. The continuous accumulation of SCR‐1510 at day 29 was more than double that at day 1, and significantly, toxicity was observed in the 300 mg/day cohort (Figs. 1 and 2). To adapt to the PK feature, whether the administration protocol should be changed to the daily treatment in a 2‐weeks‐on/1‐week‐off cycle to support the feasibility needs further study.
Figure 1.

The mean (+1 SD) plasma concentration‐time curve of SCR‐1510 in different cohorts after a single dose on the first cycle, day 1 (A) and after multidose administration on day 29 (B).
Figure 2.

The chemical structure of metatinib.
MET overexpression is uncommon, and positive MET immunohistochemistry (1+/2+) was an independent positive prognostic factor for response rate and PFS; whether MET overexpression can be considered a potential predictive biomarker and even be used as an inclusion criterion is worth investigating in future studies.
Disclosures
The authors indicated no financial relationships.
Figures and Tables
Table 2.
Correlation among MET overexpression, gene amplification, and tumor response in patients
| Patient number | Dose cohort (mg/day) | Primary tumor location | Tumor response | PFS (months) | MET IHC score | MET amplification | MET mutation |
|---|---|---|---|---|---|---|---|
| 1 | 25 | Esophageal cancer | PD | 1 | — | — | None |
| 2 | 25 | Esophageal cancer | PD | 1 | — | — | None |
| 3 | 25 | Esophageal cancer | PD | 1 | + | — | None |
| 4 | 50 | Liver cancer | PD | 1 | — | — | None |
| 5 | 50 | Liver cancer | PD | 1 | + | — | None |
| 6 | 50 | Gastric cancer | SD | 3 | — | — | None |
| 7 | 50 | Liver cancer | SD | 2.5 | — | — | None |
| 8 | 100 | Liver cancer | SD | 4.5 | + | — | None |
| 9 | 100 | Gastric cancer | PD | 1 | — | — | None |
| 10 | 100 | Liver cancer | SD | 4 | — | — | None |
| 11 | 200 | Liver cancer | SD | 5.5 | ++ | — | None |
| 12 | 200 | Liver cancer | PD | 1 | — | — | None |
| 13 | 200 | Liver cancer | SD | 4 | — | — | None |
| 14 | 200 | Liver cancer | SD | 4 | — | — | None |
| 15 | 200 | Rectal cancer | PR | 8.5 | ++ | — | Exon 14 skipping |
| 16 | 200 | Rectal cancer | SD | 4 | — | — | None |
| 17 | 300 | Jejunal cancer | SD | 3 | — | — | None |
| 18 | 300 | Esophageal cancer | PR | 3 | — | — | None |
Abbreviations: —, negative; IHC, immunohistochemistry; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Acknowledgments
We thank the patients who participated in this study, their families, and the investigators from the study center. This study was sponsored by Jiangsu Simcere Pharmaceutical Co., Ltd. This research was supported by Major Specific Project of Sichuan Province of China (2020YFS0034)
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT02004548
- Sponsor: Jiangsu Simcere Pharmaceutical Co., Ltd
- Principal Investigator: Feng Bi and Li Zheng
- IRB Approved: Yes
Contributor Information
Feng Bi, Email: bifeng@scu.edu.cn.
Li Zheng, Email: lzheng2005618@163.com.
References
- 1. Bottaro DP, Rubin JS, Faletto DL et al. Identification of the hepatocyte growth factor receptor as the c‐Met proto‐oncogene product. Science 1991;251:802–804. [DOI] [PubMed] [Google Scholar]
- 2. Bradley CA, Salto‐Tellez M, Laurent‐Puig P et al. Targeting c‐MET in gastrointestinal tumours: rationale, opportunities and challenges. Nat Rev Clin Oncol 2017;14:562–576. [DOI] [PubMed] [Google Scholar]
- 3. Trusolino L, Bertotti A, Comoglio PM. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010;11:834–848. [DOI] [PubMed] [Google Scholar]
- 4. Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin Ther Targets 2012;16:553–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noguchi E, Saito N, Kobayashi M et al. Clinical significance of hepatocyte growth factor/c‐Met expression in the assessment of gastric cancer progression. Mol Med Rep 2015;11:3423–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Awad MM, Oxnard GR, Jackman DM et al. MET exon 14 mutations in non‐small‐cell lung cancer are associated with advanced age and stage‐dependent MET genomic amplification and c‐Met overexpression. J Clin Oncol 2016;34:721–730. [DOI] [PubMed] [Google Scholar]
- 7. Raghav KP, Wang W, Liu S et al. cMET and phospho‐cMET protein levels in breast cancers and survival outcomes. Clin Cancer Res 2012;18:2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tran HT, Liu Y, Zurita AJ et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal‐cell cancer: A retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 2012;13:827–837. [DOI] [PubMed] [Google Scholar]
- 9. Kerbel RS. Tumor angiogenesis. N Engl J Med 2008;358:2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weis SM, Cheresh DA. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat Med 2011;17:1359–1370. [DOI] [PubMed] [Google Scholar]
- 11. Kuczynski EA, Vermeulen PB, Pezzella F et al. Vessel co‐option in cancer. Nat Rev Clin Oncol 2019;16:469–493. [DOI] [PubMed] [Google Scholar]
- 12. Zhang YW, Su Y, Volpert OV et al. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci USA 2003;100:12718–12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shojaei F, Lee JH, Simmons BH et al. HGF/c‐Met acts as an alternative angiogenic pathway in sunitinib‐resistant tumors. Cancer Res 2010;70:10090–10100. [DOI] [PubMed] [Google Scholar]
- 14. Ebos JM, Kerbel RS. Antiangiogenic therapy: Impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 2011;8:210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ciamporcero E, Miles KM, Adelaiye R et al. Combination strategy targeting VEGF and HGF/c‐Met in human renal cell carcinoma models. Mol Cancer Ther 2015;14:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to anti‐angiogenic therapy in cancer‐alterations to anti‐VEGF pathway. Int J Mol Sci 2018;19:1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cascone T, Xu L, Lin HY et al. The HGF/c‐MET pathway is a driver and biomarker of VEGFR‐inhibitor resistance and vascular remodeling in non‐small cell lung cancer. Clin Cancer Res 2017;23:5489–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiang Q, Chen W, Ren M et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res 2014;20:2959–2970. [DOI] [PubMed] [Google Scholar]
- 19. Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daud A, Kluger HM, Kurzrock R et al. Phase II randomised discontinuation trial of the MET/VEGF receptor inhibitor cabozantinib in metastatic melanoma. Br J Cancer 2017;116:432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abou‐Alfa GK, Meyer T, Cheng AL et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gore ME, Szczylik C, Porta C et al. Safety and efficacy of sunitinib for metastatic renal‐cell carcinoma: An expanded‐access trial. Lancet Oncol 2009;10:757–763. [DOI] [PubMed] [Google Scholar]
- 23. Sun Y, Niu W, Du F et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi‐target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Røed Skårderud M, Polk A, Kjeldgaard Vistisen K et al. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: A systematic review. Cancer Treat Rev 2018;62:61–73. [DOI] [PubMed] [Google Scholar]
- 25. Li J, Qin S, Xu J et al. Apatinib for chemotherapy‐refractory advanced metastatic gastric cancer: Results from a randomized, placebo‐controlled, parallel‐arm, phase II trial. J Clin Oncol 2013;31:3219–3225. [DOI] [PubMed] [Google Scholar]
- 26. Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open‐label, phase 3 trial. Lancet Oncol 2016;17:917–927. [DOI] [PubMed] [Google Scholar]
- 27. Matsumoto K, Umitsu M, De Silva DM et al. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci 2017;108:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Engelman JA, Zejnullahu K, Mitsudomi T et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039–1043. [DOI] [PubMed] [Google Scholar]
- 29. Nandagopal L, Sonpavde GP, Agarwal N. Investigational MET inhibitors to treat renal cell carcinoma. Expert Opin Investig Drugs 2019;28:851–860. [DOI] [PubMed] [Google Scholar]
- 30. Strickler JH, Weekes CD, Nemunaitis J et al. First‐in‐human phase I, dose‐escalation and ‐expansion study of telisotuzumab vedotin, an antibody‐drug conjugate targeting c‐Met, in patients with advanced solid tumors. J Clin Oncol 2018;36:3298–3306. [DOI] [PubMed] [Google Scholar]
- 31. Ma PC. (Not giving up) the marathon race of MET targeting therapy: Are we there yet? Clin Cancer Res 2019;25:2375–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]


