Abstract
Fragile X Syndrome (FXS) is a neurodevelopmental disorder caused by silencing of the Fragile X Mental Retardation (FMR1) gene. The resulting loss of Fragile X Mental Retardation Protein (FMRP) leads to excessive glutamate signaling via metabotropic glutamate subtype 5 receptors (mGluR5) which has been implicated in the pathogenesis of the disorder. In the present study we used the radioligand 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB) in simultaneous PET-MR imaging of males with FXS and age- and gender-matched controls to assess the availability of mGlu5 receptors in relevant brain areas. Patients with FXS showed lower [18F]FPEB binding potential (p < 0.01), reflecting reduced mGluR5 availability, than the healthy controls throughout the brain, with significant group differences in insula, anterior cingulate, parahippocampal, inferior temporal and olfactory cortices, regions associated with deficits in inhibition, memory, and visuospatial processes characteristic of the disorder. The results are among the first to provide in vivo evidence of decreased availability of mGluR5 in the brain in individuals with FXS than in healthy controls. The consistent results across the subjects, despite the tremendous challenges with neuroimaging this population, highlight the robustness of the protocol and support for its use in drug occupancy studies; extending our radiotracer development and application efforts from mice to humans.
Subject terms: Neuroscience, Biomarkers
Introduction
Fragile X Syndrome (FXS) is a leading cause of inherited intellectual disability1 and the most common, known single gene cause of autism spectrum disorder2,3. Successful treatment of FXS has the potential to positively impact the larger field of neurodevelopmental disabilities. A number of studies have implicated metabotropic glutamate subtype 5 receptors (mGluR5) in FXS4; the use of mGluR5 antagonists in Fmr1 Knockout (KO) mouse models has demonstrated a variety of benefits including reduced seizures, anxiety, and behavioral issues5,6. The Fmr1 KO mouse model has shown that the absence of FMRP leads to increased protein synthesis at the synapse and excessive mGluR5 glutamatergic signaling7, building on the work of Weiler and colleagues who first identified the connection between FMRP and mGluR5 pathways8. However, despite these promising preclinical findings, clinical trials of mGluR5-targeted drugs in FXS have met with limited success. It is unclear as to whether these trials were conducted with optimal outcome endpoints or in the most appropriate age group9–11.
The severity of FXS phenotype is known to be correlated with the magnitude of the FMRP deficit12. The FMR1 gene responsible for FXS is located on the X chromosome, thereby affecting males with FXS more severely than their female counterpart who have one unaffected X chromosome to fall back on13. We focused on measuring the availability and distribution of mGluR5 in males with FXS and age- and gender-matched healthy controls to better understand the role of mGluR5 expression in the pathophysiology of FXS in humans. Given the heterogeneity found in neurodevelopmental disorders like FXS, stringent methodological standards were used to obtain reliable delineation and quantification of mGluR5 occupancy differences between healthy and disorder groups, critical for biomarker research.
In the present study, we examined PET data from five adult males with FXS (> 200 CGG repeats, full mutation, 26–48 years of age; mean: 34.8 years ± 7.8) and seven age-matched males with normal development (22–43 years of age; mean 31.4 years ± 7.5) from whom PET and MRI data was successfully obtained (see “Methods” for details). Participants with FXS were recruited from the hospital database, local clinics and Fragile X organizations; typical controls (TC) were drawn from the local community. All subjects completed a high resolution MRI and 90-min dynamic PET examination on an integrated whole-body PET-MR (Siemens Biograph mMR) following an intravenous bolus injection of 5 mCi of [18F]FPEB, a safe and reliable radioligand for quantifying regional brain concentrations of mGluR514. Based on Fmr1 mouse studies of the mGluR theory and the established affinity and specificity of [18F]FPEB to mGluR5, we hypothesized that there would be a significant difference in regional brain concentrations of mGluR5 between the two groups of subjects, validating the use of [18F]FPEB in clinical trials as a tool to confirm target engagement of novel drug agents for mGluR5s in FXS and to monitor dose response.
In a recent study, Brašić and colleagues17 used the same [18F]FPEB tracer in males with FXS, slightly younger than those in the present study, though incorporating different acquisition protocols and data analysis methods for PET across collaborating sites; they found mGluR5 density was significantly reduced in multiple brain regions including the cingulate, cortex, thalamus and striatum, compared to age-matched males with typical development, thereby supporting the use of [18F]FPEB to measure drug occupancy in clinical trials for mGLuR5 in FXS.
Results
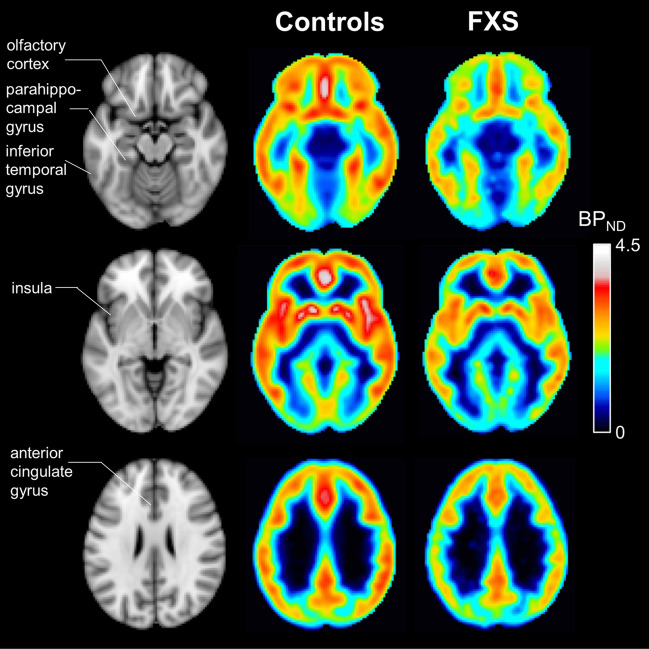
Regional and voxel-wise estimates of [18F]FPEB non-displaceable binding potential (BPND), a measure of mGluR5 availability, were obtained for each subject by multi-linear reference tissue analysis of dynamic PET data15,16. We found regional brain uptake was consistent with known mGlu5 receptors based on previous studies1,17. A global reduction of [18F]FPEB BPND was observed in males with FXS compared to control subjects (Fig. 1).
Figure 1.
Group-average images of [18F]FPEB BPND in control subjects and subjects with FXS, and corresponding MR template images. Regions exhibiting significant differences in BPND between control subjects and subjects with FXS (olfactory cortex, parahippocampal gyrus, inferior temporal gyrus, insula, and anterior cingulate gyrus) are indicated in the MR images.
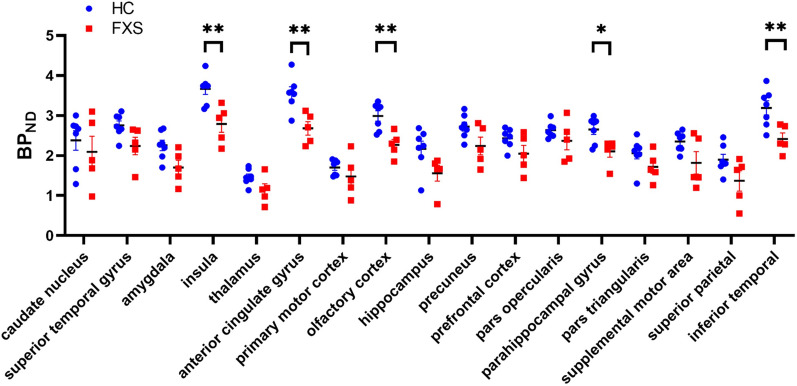
Region of interest analyses confirmed BPND differences between FXS and control groups (Fig. 2). [18F]FPEB BPND were significantly lower in subjects with FXS compared to controls in the anterior cingulate gyrus (2.67 ± 0.38 vs. 3.56 ± 0.41; p = 0.004), insula (2.79 ± 0.47 vs. 3.67 ± 0.36; p = 0.009), inferior temporal gyrus (2.41 ± 0.34 vs. 3.19 ± 0.47; p = 0.008), parahippocampal gyrus (2.10 ± 0.32 vs. 2.65 ± 0.32; p = 0.018) and olfactory cortex (2.27 ± 0.30 vs. 2.98 ± 0.34; p = 0.004) (two-tailed unpaired t-tests, corrected for multiple comparisons using FDR18 (q = 0.05). BPND was also significantly lower for subjects with FXS subjects in the amygdala (1.70 ± 0.41 vs. 2.25 ± 0.34; p = 0.04), although it did not survive correction for multiple brain region testing. The findings correspond to those in the neuroimaging literature on FXS19,20.
Figure 2.
Regional [18F]FPEB BPND in control subjects (blue circle) and subjects with FXS (red square). Error bars indicate standard error of the means. *p < 0.05, **p < 0.01 (corrected for multiple comparisons).
Discussion
Among the core symptoms of FXS is a deficit in visuospatial learning and memory mediated by hippocampus and parahippocampal and inferior temporal regions21,22. Our findings of significantly lower [18F]FPEB binding potential (BPND) in these areas provide support for the role of mGluR5 in the neurobiology of FXS. Anxiety is frequently reported in this population, which may explain the significant group differences we observed in the insula and olfactory cortex19,23. Evidence in support of these findings also comes from preclinical studies of the Fmr1 KO mouse using the Morris water maze task and the mirrored chamber test24–26. Whereas only one of our participants with FXS was on prescription medication for anxiety, a high rate of anxiety in FXS, especially social anxiety, is reported in the literature regardless of gender12,27,28. The amygdala group differences we observed did not hold up after multiple comparison, but would likely have survived correction in a larger sample. Thus, the finding clearly suggests a need for larger studies, perhaps a narrower age range, and outcome measures to fully capture the profile of the disorder in males and females. Executive functions have also been consistently documented as impaired in FXS29, implicating the prefrontal cortex. While this area did not show a signal in the present study, it deserves to be examined, especially the gyrus rectus which has been shown to have lower levels of FMRP in individuals with FXS than in controls13. Taken together, these approaches along with the genetic etiology of FXS will help advance our understanding of gene-brain-behavior relationships in FXS30 and related neurodevelopmental conditions like autism spectrum disorder31.
The role of mGluR5 has been extensively studied in mouse models of FXS. However, findings related to mGluR5 expression in animals and autopsies of humans with FXS have been inconsistent. And clinical trials targeting mGluR5 in FXS have met with repeated failure, pointing to a need for better designed studies and reliable biomarkers in individuals with FXS9,32.
FXS is currently treated symptomatically using behavioral, educational and psychopharmacological strategies33 often with unsatisfying results34,35. A targeted treatment is lacking.The results of the present study are important as they pave the way for successful human trials by providing a potential biomarker of the impaired mGluR5 mechanism for use in developing targeted drug therapies in FXS. Specifically, using the radioligand [18F]FPEB, which has been shown to reliably bind to mGlu5 receptors, we found the binding potential to mGlu5 receptors to be lower throughout the brain in participants with FXS compared with typical controls. The reduced BP appears to reflect reduced density of unoccupied mGlu5 receptors, potentially a downstream consequence of excessive mGluR5 signaling caused by the lack of FMRP protein in individuals with FXS. However, given the lack of consistency of mGlu5 receptor expression findings across human and mouse models, more studies are needed to understand the functional relationship between these variables. Importantly, this difference in BPND between the subjects with FXS and control group was significant in areas consistent with known distribution of mGluR5, and which have been functionally implicated in FXS symptomatology. Our results of overall reduced mGlu5 receptor expression in relevant regions of the brain in individuals with FXS replicate recent findings17 and advance ongoing efforts for a much-needed measure of mGluR5 target engagement for drugs and treatment of symptoms in clinical trials of FXS and related disorders36,37.
The participants with FXS in the study had the full Fmr1 mutation with CGG repeats greater than 200. However, some individuals with FXS may present with mosaicism (size or methylation mosaics), which can affect FMRP levels and hence the severity of the condition38. There has been significant progress in understanding the physiological role of mGluR5, but treatment with selective mGluR5 antagonists (e.g., MPEP, CTEP, fenobam) have met with limited success in FXS6,39,40 despite evidence for use of this approach41. Factors like age are known to influence treatment efficacy as was seen when MPEP was administered to 2-week- vs. 6-week-old Fmr1 KO mice; the immature morphological phenotype of pyramidal neurons in the somatosensory cortex were rescued in the younger and less so in the older KO mice.
Insofar as the precise modulation of synaptic transmission either through the use of selective mGluR5 inhibitors to reduce synaptic excitability or through activation of presynaptic GABAB receptors is critical for drug efficacy, [18F]FPEB binding potential, an index of mGluR5 availability, may prove to be an invaluable biomarker for optimizing the outcomes of drug trials and improving the lives of individuals with FXS42. None of the participants with FXS in the study had an autism diagnosis. However, given the high co-morbidity of FXS and ASD43, the use of [18F]FPEB to examine differences in mGluR5 binding potential between these disorder groups could significantly contribute to our understanding of the differences in underlying pathophysiology of the disorders; towards development of mechanism-based novel therapeutics in neurodevelopmental disorders. In one such study, Brašić and colleagues37, using [18F]FPEB, replicated their earlier findings with FXS; but found that compared to subjects with typical development (TD), individuals with autism spectrum disorder (ASD) had higher mGluR5 expression in cortical areas and no difference in subcortical regions. The participants with ASD were younger than the controls. The PET data were acquired and analyzed at collaborating sites using different scanners, scan protocols and analysis methods, as in the original study. Despite potential methodological confounds, these preliminary findings hint at exciting new opportunities for the development of drugs targeting the observed differences in the underlying pathophysiology of the two disorders.
The failure of a clinical trial may not necessarily reflect a failure to understand the underlying mechanism but rather may reveal treatment parameters to be optimized beyond target identification. Future studies could draw on the protocol and findings from the current study to systematically interrogate larger samples with FXS to address questions of disorder severity, age and cognitive abilities as they relate to mGluR5 availability and its regional distribution in the brain. Zhao and colleagues44 showed that by taking Nutlin-3, an experimental cancer drug which serves as an inhibitor, mice with FXS lacking the FMRP protein regained their ability to remember what they had seen or smelled in their first study session; this apparent reversal of a memory impairment appeared to specifically relate to reactivation of FMRP affecting neural stem cells and new neurons that they form in the hippocampus. In a recent study, Berry-Kravis and colleagues45 found that a phosphodiesterase-4D (PRE4D) allosteric modulator helped improve cognitive functions and behavioral outcomes in patients with FXS. Taken together with the results from the present study, the field of FXS appears poised for major breakthroughs in treatment using inhibitors targeting increased mGluR5 signaling or cyclic AMP (cAMP) production; offering promising new directions for future pharmacological research, as well as approaches to the challenges associated with scanning this population.
In conclusion, the current study supports a role for reduced mGluR5 expression in the underlying pathophysiology of FXS in humans; it holds promise as a potential biomarker for use in clinical trials of FXS that could prove critical in improving pharmacological interventions. Importantly, it builds on our research over the years, from the early development of [18F]FPEB as a PET tracer46, to its application in the mouse model47, and in humans48 and most recently, to humans with FXS evident in the present study.
Methods
Study subjects
The study was approved by the Institutional Review Board at Massachusetts General Hospital; and performed in keeping with the Human Subjects Research Committee guidelines, and in accordance with the Declaration of Helsinki. We enrolled eight males with FXS and eight age- and gender-matched Controls. Of the eight participants recruited in each group, three with FXS were unable to complete the PET-MR protocol and had to be excluded, as also the data from one Control participant due to technical issues. Subjects with FXS were recruited through the hospital’s patient database, local clinics, and Fragile X organizations. Those with confirmed FMR1 full mutation (> 200 CGG repeats, based on medical records) at screening were eligible to participate. They ranged in age from 26 to 48 years (mean: 34.8 years ± 7.8), had no comorbid diagnosis and were not on any antipsychotic medications. Two of the participants with FXS were on medication (viz., Losartan, Famotidine) for blood pressure and indigestion, and a third on Ritalin (belonging to the stimulant class of drugs) for ADHD symptoms, and Clonazepam (belonging to benzodiazepines class of drugs), as needed, when anxious. While the caregivers reported a tendency for participants to be anxious in new settings and with strangers, only one of the participants with FXS was on prescription medication for anxiety on an as-needed basis. Control participants were recruited from the local community. They had no history of neurological or psychological problems and were not on any prescription medication; they had completed at least two years of college, and were between 22 and 43 years (mean: 31.4 years ± 7.5). The two groups did not differ in age (p = 0.416). All participants provided informed consent and/or assent prior to the experiment and were compensated for their involvement in the study. Additionally, participants with FXS underwent acclimatization training to familiarize them with the actual scanner and environment prior to their PET-MR session to help alleviate their anxiety.
Data acquisition
Study subjects were scanned for up to 90-min on an integrated whole-body PET-MR (Siemens Biograph mMR) at the MGH Martinos Center for Biomedical Imaging, following an intravenous bolus injection of 5 mCi of 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB) (injected dose: mean 5.15 mCi, SD 0.28; average specific activity at time of injection: 2046 mCi /µmol). Using the published method48, the radioligand was locally synthesized at the Gordon Center for Medical Imaging in Massachusetts General Hospital under the guidelines of the Radioactive Drug Research Committee and the approval from the FDA.
Each dynamic study was divided into three scanning sessions of up to 35-min, with breaks between sessions. A structural MRI scan was acquired for each participant at the beginning of each session using a 3-D T1 weighted multi-echo MPRAGE sequence49 with the following parameters: TR = 2530 ms, TEs = 1.69, 3.55, 5.41, 7.27 ms, inversion time = 1100 ms, matrix size = 256 × 256 × 176 and voxel size = 1 × 1 × 1 mm3. An attenuation map for PET was generated based on the structural MRI data using a hybrid segmentation and atlas-based technique50.
Data processing and analysis
Dynamic list-mode PET data for each scanning session were binned into temporal frames of up to 30 s and were reconstructed and corrected for motion using the following multi-step procedure. First, an initial dynamic reconstruction was performed without correction of attenuation, followed by application of spatial Gaussian smoothing (6-mm full width at half maximum—FWHM) to each reconstructed frame and rigid-body alignment of the activity volume for each frame to a selected reference frame. The attenuation map was then registered to the resulting time-averaged volume and transformed using the previously calculated registration parameters, yielding an attenuation map for each frame. Second, another dynamic reconstruction was performed, incorporating the frame-dependent attenuation map obtained in the first step as well as standard corrections for dead-times, random and scattered coincidences. Note that the attenuation map used during reconstruction also accounted for “static” attenuating medium, such as the scanner’s table and MR head coil. Third, activity volumes in each scanning session were smoothed with a 4-mm FWHM Gaussian filter and rigidly registered to a selected reference frame, followed by another registration to the resulting time-averaged volume. Lastly, all the frames in each session were aligned to a common reference frame, taken as the first session’s time-average image volume. All PET reconstructions were performed using OP-OSEM 3D with 3 iterations and 21 subsets on a 344 × 344 × 127 array with voxel size 2.08 × 2.08 × 2.03 mm3. Image registrations were performed using FSL’s flirt method with normalized mutual information as the data consistency criterion and 6 degrees of freedom51. To further mitigate motion effects, realignment transformations estimated during the third step were inspected to discard frames associated with substantial frame-to-frame shifts (> 2.5 mm) from the analysis. Linear interpolation was used to compute time points corresponding to discarded frames as well as missing time points between scanning sessions.
Afterwards, the structural MRI scan for each subject was rigidly aligned to PET space (FSL, flirt), followed by non-rigid registration (FSL, fnirt) of the Montreal Neurological Institute (MNI) template to MRI space for definition of regions of interest (ROIs). In keeping with the range of behavioral symptoms associated with FXS, we explored 18 brain regions that have been implicated across neuroimaging studies of FXS52,53. [18F]FPEB concentration histories were extracted in the following bilateral regions: caudate nucleus, superior temporal gyrus, amygdala, insula, thalamus, anterior cingulate gyrus, primary motor cortex, olfactory cortex, hippocampus, precuneus, prefrontal cortex, pars opercularis, parahippocampal gyrus, pars triangularis, supplementary motor area, superior parietal gyrus, inferior temporal gyrus and cerebellar white matter. Regional time-activity curves (TACs) were fitted using MRTM254 to estimate [18F]FPEB binding potential (BPND) with the cerebellum white matter as reference16. The t* value was fixed at 10 and the value was estimated by first-pass MRTM analysis of the average TACs9. In addition, for visualization purposes, images of [18F]FPEB BPND were generated for each subject by application of MRTM2 analysis at the voxel level. BPND images were then transformed to MNI space, followed by spatial Gaussian smoothing (5-mm FWHM) and averaging over subjects in each group.
Note that no region completely devoid of mGlu5 receptors exists in the human brain55. As such, kinetic approaches based on reference region modeling could be biased by individual variations in specific radiotracer uptake. A close examination revealed no substantial differences in reference region TACs which appeared similar for the two groups (Fig. 3).
Figure 3.
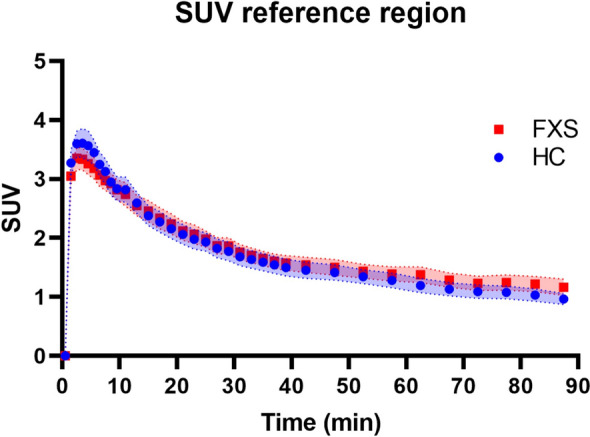
Mean time activity curves in the cerebellum white matter (reference region) for the control subjects (blue circle) and subjects with FXS (red square). Envelope represents standard error of the mean. SUV, standardized uptake value.
Statistical analyses
Unpaired t-tests (2-tailed) were applied to test the null hypothesis of no difference in [18F]FPEB BPND between healthy controls and subjects with FXS for all surveyed regions. The p-values were corrected for multiple comparisons by applying the Benjamini, Krieger and Yekutieli false discovery rate method18 with q = 0.05.
Acknowledgements
We would like to thank Grae Arabasz, Regan Butterfield and Shirley Hsu for technical assistance with data collection, and Seppo Ahlfors for advice on subject setup, acclimatization, and training. Our gratitude goes to the participants with FXS and their families for their willingness to take part and help with making this study possible. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Medical Research Program under Award No. W81XWH-17-1-0228 to ALB.
Author contributions
A.L.B. and M.M. conceived and designed this study. P.H., C.M. and M.M. conducted the experiments. D.K. assisted with recruiting and screening subjects; R.N. and D.Y. were responsible for locally synthesizing FPEB; D.Y. supervised the production, scheduling and delivery of the radioligand; Y.P. analyzed the data with guidance from M.N., M.M. and A.L.B. M.M. and Y.B. prepared the manuscript assisted by A.L.B. All authors contributed to critically reviewing this work and approved the final version.
Data availability
All datasets generated and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Maria Mody and Yoann Petibon.
References
- 1.Verkerk AJ, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-H. [DOI] [PubMed] [Google Scholar]
- 2.Budimirovic DB, Subramaniam M. Neurobiology of autism and intellectual disability: Fragile X syndrome. In: Johnston MV, editor. Neurobiology of Disease. 2. Oxford University Press; 2016. pp. 375–384. [Google Scholar]
- 3.Hagerman RJ, Rivera SM, Hagerman PJ. The fragile x family of disorders; a model for autism and targeted treatments. Curr. Ped. Rev. 2008;4:40–52. doi: 10.2174/157339608783565770. [DOI] [Google Scholar]
- 4.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile x mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Burket JA, et al. Complex effects of mGluR5 antagonism on sociability and stereotypic behaviors in mice: Possible implications for the pharmacology of autism spectrum disorder. Brain Res. Bull. 2011;86:152–158. doi: 10.1016/j.brainresbull.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Thomas AM, et al. Group 1 metabotropic glutamate receptor antagonists alter select behaviors in a mouse model of Fragile X syndrome. Psychopharmacology. 2012;219:47–58. doi: 10.1007/s00213-011-2375-4. [DOI] [PubMed] [Google Scholar]
- 7.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of Fragile X mental retardation. PNAS USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiler IJ, et al. Fragile X Mental Retardation Protein is translated near synapses in response to neurotransmitter activation. PNAS USA. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budimirovic DB, Berry-Kravis E, Erickson CA, Hall SS, Hessl D, Reiss AL, King MK, Abbeduto L, Kaufmann WE. Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. J. Neurodev. Disord. 2017;9:14. doi: 10.1186/s11689-017-9193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duy PQ, Budimirovic DB. Fragile X Syndrome: Lessons learned from the most translated neurodevelopmental disorder in clinical trials. Transl. Neurosci. 2017;8:7–8. doi: 10.1515/tnsci-2017-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson CA, et al. Fragile X targeted pharmacotherapy: Lessons learned and future directions. J. Neurodev. Disord. 2017;9:7. doi: 10.1186/s11689-017-9186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budimirovic DB, et al. A genotype-phenotype study of high-resolution FMR1 nucleic acid and protein analyses in Fragile X patients with neurobehavioral assessments. Brain Sci. 2020;10:694. doi: 10.3390/brainsci10100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variations and FMRP levels in Fragile X. Mental Retard. Dev. Disabil. Res. Rev. 2004;10:31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- 14.Wang J-Q, Tueckmantel W, Zhu A, Pellegrino D, Brownell A-L. Synthesis and preliminary biological evaluation of 3-[18F] Fluoro-5-(2-pyridinylethynyl) benzonitrile ([18FFPEB) as a PET radiotracer for imaging metabotropic glutamate receptor subtype 5. Synapse. 2007;61(12):951–961. doi: 10.1002/syn.20445. [DOI] [PubMed] [Google Scholar]
- 15.Ichise M, et al. Linearized reference tissue parametric imaging methods: Application to [11C] DASB positron emission tomography studies of the serotonin transporter in human brain. J. Cereb. Blood Flow Metab. 2003;23(9):1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan JM, et al. Kinetic analysis of the metabotropic glutamate subtype 5 tracer [18F]FPEB in bolus and bolus-plus-constant-infusion studies in humans. J. Cereb. Blood Flow Metab. 2013;33(4):532–541. doi: 10.1038/jcbfm.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brašić JR, et al. Reduced expression of cerebral metabotropic glutamate receptor subtype 5 in men with Fragile X Syndrome. Brain Sci. 2020;10(12):899. doi: 10.3390/brainsci10120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93(3):491–507. doi: 10.1093/biomet/93.3.491. [DOI] [Google Scholar]
- 19.Hall SS, Jiang H, Reiss AL, Greicius MD. Identifying large scale brain networks in Fragile X Syndrome. JAMA Psychiat. 2013;70(11):1215–1223. doi: 10.1001/jamapsychiatry.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razak KA, Dominick KC, Erickson CA. Developmental studies in Fragile X Syndrome”. J. Neurodev. Disord. 2020;12:13. doi: 10.1186/s11689-020-09310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon H, et al. Functional neuroanatomy of visuospatial working memory in Fragile X Syndrome: Relation to behavior and molecular measures. Am. J. Psychiatry. 2001;158(7):1040–1051. doi: 10.1176/appi.ajp.158.7.1040. [DOI] [PubMed] [Google Scholar]
- 22.MacLeod LS, et al. A comparative study of the performance of individuals with fragile X syndrome and Fmr1 knockout mice on Hebb-Williams mazes. Genes Brain Behav. 2009;9(1):53–64. doi: 10.1111/j.1601-183X.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 23.Bodaleo F, et al. Structural and functional abnormalities in the olfactory system of Fragile X Syndrome models. Front. Mol. Neurosci. 2019;12:135. doi: 10.3389/fnmol.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbab T, Pennartz CMA, Battaglia FP. Impaired hippocampal representation of place in the Fmr1-knockout mouse model of fragile X syndrome. Sci. Rep. 2018;8:8889. doi: 10.1038/s41598-018-26853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazdoba TM, Leach PT, Silverman JL, Crawley JN. Modeling fragile X syndrome in the Fmr1 knockout mouse. Intractable Rare Dis. Res. 2014;3:118–133. doi: 10.5582/irdr.2014.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer CM, et al. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 27.Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in Fragile X Syndrome: Prevalence and characterization. J. Neurodev. Disord. 2011;3:57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufmann WE, Capone G, Clarke M, Budimirovic DB. Autism in genetic intellectual disability: Insights into idiopathic autism. In: Zimmerman AW, editor. Autism: Current Theories and Evidence. The Humana Press; 2008. pp. 81–108. [Google Scholar]
- 29.Schmitt LM, Shaffer RC, Hessl D, Erickson C. Executive function in Fragile X Syndrome: A systematic review. Brain Sci. 2019;9:15. doi: 10.3390/brainsci9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruno JL, Hosseini SMH, Saggar M, Quintin E-M, Raman MM, Reiss AL. Altered brain network segregation in FXS revealed by structural connectomics. Cereb. Cortex. 2016;27:2249–2259. doi: 10.1093/cercor/bhw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat. Neurosci. 2006;9:1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- 32.Berry-Kravis, et al. Drug development for neurodevelopmental disorders: Lessons learned from fragile x syndrome. Nat. Rev. Drug Discov. 2018;17:280–299. doi: 10.1038/nrd.2017.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Protic D, Salcedo-Arellano MJ, Dy JB, Potter LA, Hagerman RJ. New Targeted treatments for Fragile X Syndrome. Curr. Pediatr. Rev. 2019;15:251–258. doi: 10.2174/1573396315666190625110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry-Kravis E, Sumis A, Hervey C, Mathur S. Clinic-based retrospective analysis of psychopharmacology for behavior in fragile x syndrome. Int. J. Pediatr. 2012;2012(5):843016. doi: 10.1155/2012/843016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee AW, Ventola P, Budimirovic D, Berry-Kravis E, Visootsak J. Clinical development of targeted Fragile X treatments: An industry perspective. Brain Sci. 2018;8:214. doi: 10.3390/brainsci8120214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brašić JR, Mathur AK, Budimirovic DB. The urgent need for molecular imaging to confirm target engagement for clinical trials of fragile x syndrome and other subtypes of autism spectrum disorder. Arch. Neurosci. 2019;6:e91832. doi: 10.5812/ans.91831. [DOI] [Google Scholar]
- 37.Brašić JR, et al. Cerebral expression of metabotropic glutamate receptor subtype 5 in idiopathic autism spectrum disorder and Fragile X Syndrome: A pilot study. Int. J. Mol. Sci. 2021;22(6):2863. doi: 10.3390/ijms22062863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pretto D, et al. Clinical and molecular implications of mosaicism in FMR1 full mutations. Front Genet. 2014;5:318. doi: 10.3389/fgene.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michalon A, et al. Chronic mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74(1):49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry-Kravis E, et al. A pilot open label single dose trial of fenobam in adults with fragile X syndrome. J. Med. Genet. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dölen G, et al. Correction of fragile x syndrome in mice. Neuron. 2014;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohith TG, et al. Is metabotropic glutamate receptor 5 upregulated in prefrontal cortex in fragile x syndrome? Mol. Autism. 2013;4:15. doi: 10.1186/2040-2392-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagerman R, Au J, Hagerman P. FMR1 premutation and full mutation molecular mechanisms related to autism. J. Neurodev. Disord. 2011;3:211–224. doi: 10.1007/s11689-011-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, et al. MDM2 inhibition rescues neurogenic and cognitive deficits in fragile X mice. Sci. Transl. Med. 2016;8:336. doi: 10.1126/scitranslmed.aad9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berry-Kravis E, et al. Inhibition of phosphodiesterase-4D in adults with fragile X syndrome: A randomized, placebo-controlled, phase 2 clinical trial. Nat. Med. 2021;27:862–870. doi: 10.1038/s41591-021-01321-w. [DOI] [PubMed] [Google Scholar]
- 46.Neelamegam R, Yokell D, Rice P, El Fakhri G, Brownell A-L. Automated radiosynthesis of [18F]FPEB with clinically viable yields for human use. J. Nucl. Med. 2018;59(Suppl 1):404. [Google Scholar]
- 47.Brownell A-L, Zhu A, Kil K-E, Poutiainen P, Choi J-K. mGluR5 and not mGluR4 is regionally elevated in fragile X syndrome: Longitudinal PET studies in FXS mouse model. Mol. Imaging Biol. 2016;18:1554–1859. doi: 10.1007/s11307-016-0968-3. [DOI] [PubMed] [Google Scholar]
- 48.Wong DF, et al. 18F-FPEB, a PET radiopharmaceutical for quantifying metabotropic glutamate 5 receptors: A first-in-human study of radiochemical safety, biokinetics, and radiation dosimetry. J. Nucl. Med. 2013;54(3):388–396. doi: 10.2967/jnumed.112.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Kouwe AJW, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40(2):559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izquierdo-Garcia D, et al. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: Application to simultaneous PET/MR brain imaging. J. Nucl. Med. 2014;55(11):1825–1830. doi: 10.2967/jnumed.113.136341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenkinson M, Smith S. A global optimization method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 52.Gothelf D, et al. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Ann. Neurol. 2008;63(1):40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lightbody AA, Reiss AL. Gene, brain, and behavior relationships in Fragile X Syndrome: Evidence from neuroimaging studies. Dev. Disabil. Res. Rev. 2009;15(4):343–352. doi: 10.1002/ddrr.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J. Cereb. Blood Flow Metab. 2002;22(10):1271–1281. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- 55.Patel S, et al. Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F]F-PEB. Nucl. Med. Biol. 2007;34(8):1009–1017. doi: 10.1016/j.nucmedbio.2007.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated and/or analyzed during the current study are available from the corresponding authors on reasonable request.