INTRODUCTION
Physical functioning is a universal health value that strongly predicts mortality and healthcare utilization and is significantly associated with multimorbidity in aging adults.1 Unfortunately, current metrics2–6 fail to capture the severity and range of chronic conditions using person-centered outcomes. Simple disease count and comorbidity indices originally designed to predict inpatient mortality and healthcare utilization and cost have frequently been used to measure multimorbidity, despite their limitations.2–6 For example, the highly cited Charlson index2 was developed as a mortality predictor in 559 patients admitted to New York Hospital in 1984, applied in 685 primary breast cancer patients treated in the 1960s, and mapped to the International Classification of Diseases, Ninth Revision (ICD-9) codes using a cohort of patients hospitalized for lumbar spine surgery in 1985.3 Small sample sizes from specific populations in prior indices underscore the need to challenge current paradigms for multimorbidity metrics, their intended utility, and harm when overgeneralized.
Recent metrics for health-related quality of life (HRQOL) and other self-reported outcomes show promise for capturing disease severity using person-centered approaches7–14 but lack a comprehensive disease inventory and do not directly relate to the construct of multimorbidity. To overcome this, we developed and validated a multimorbidity-weighted index (MWI) that weights 81 self-reported candidate diseases and conditions by their impact on physical functioning in three cohorts of community-dwelling adults15 and externally validated it in the nationally representative Health and Retirement Study (HRS).16 MWI has since been validated against mortality, disability as measured by basic and instrumental activities of daily living (ADLs and IADLs), physical, cognitive, and social functioning, and other aspects of HRQOL15, 17–19 and mapped to ICD-9 CM codes.20
To increase the utility of the MWI for clinical, research, and administrative purposes, this study adopted the approach used to create the original MWI but adapted it to yield a new greatly expanded ICD-coded MWI (MICD). We started with all observed ICD-9 CM codes in the HRS, adopting an unbiased approach across the full gamut of coded conditions, and related them to physical functioning. We then compared the distribution and performance of the newly developed MICD with the Elixhauser comorbidity score21 and simple disease count.
METHODS
Study Participants and Eligibility
The HRS is a nationally representative longitudinal cohort of > 37,000 US adults aged ≥ 51 years followed since 1992. HRS participants are interviewed biennially, starting with a face-to-face interview at baseline and subsequent telephone or face-to-face interviews. Participants provide information on physician-diagnosed medical conditions, functional status, living situation, employment, income, and health behaviors.19 HRS data are linked at the individual level to Medicare claims from the Centers for Medicare and Medicaid Services (CMS) for consenting participants. We included Medicare-eligible HRS participants who contributed repeated measures of self-reported physical functioning from the HRS (2000–2012) linked to their Medicare claims (1991–2012) with follow-up through 2014 for future physical functioning and 2016 for mortality outcomes. This study was approved by the University of Michigan Institutional Review Board (HUM00128383).
Physical Functioning Assessment
The Short Form (SF)-3622 has been used to monitor disease burden and health status and predict outcomes such as mortality.23 The SF-36 physical functioning scale covers a broad range of component items, from activities of daily living such as bathing to vigorous activities such as lifting heavy objects.24 It is a standard measure for understanding the impact and scope of disease burden on physical functioning, particularly at lower levels.
In each biennial wave of the HRS, physical functioning was assessed using items closely resembling the SF-36 physical functioning scale.17 To calibrate the HRS physical functioning measure to the SF-36, the score was rescaled and standardized. The sum of these 10 items (eAppendix 1) forms a continuous measure ranging 0–100 for lowest to highest functioning.16
We excluded 49 (0.27%) participants without self-reported physical functioning and 4 (0.02%) participants who completed less than half of the 10-item physical functioning scale per SF-36 protocol.21
Chronic Condition Assessment
We included chronic conditions and common procedures with long-term effects. We defined “chronic” conditions to be largely irreversible, lasting ≥ 3 months in duration including relapsing/remitting patterns (e.g., multiple sclerosis, depression), or acute on chronic exacerbations, and requiring lifelong management for treatment or prevention (e.g., heartburn). We also included serious illness and significant acute events with long-term sequela (e.g., stroke). Finally, we included common procedures for conditions no longer resolved by pharmacologic treatment (e.g., knee replacement for osteoarthritis) that were assessed previously in the MWI based on self-report.16 Given our study sample of Medicare-eligible adults, we excluded conditions of childhood, gestation, and early adulthood.
We detailed a multistep process to examine ICD codes and respective chronic conditions (eAppendix 2; eFigure 1). We considered a comprehensive list of 14,568 diagnosis and 3883 procedure codes and their corresponding conditions and procedures.25 Among these, we identified 55 condition groups with negative coefficients and 14 condition groups with positive coefficients for physical functioning. MICD included 55 condition groups with negative coefficients. We intentionally did not include conditions with positive coefficients in MICD since they do not contribute to the role of MICD as a measure of disease burden.
We compared MICD with two widely used measures. The Elixhauser algorithm includes 30 conditions associated with inpatient mortality, cost, and length of hospitalization that are weighted 1 point each. We calculated a simple disease count from all 69 condition groups (negative and positive coefficients) derived above and assigned each 1 point.
Mortality Assessment
We assessed mortality in the CMS Master Beneficiary Summary File between January 1, 2007, and March 31, 2016. Most (98%) death information was obtained from the Social Security Administration. Additional sources included Medicare claims data from the Medicare Common Working File and the Railroad Retirement Board, and death status updates submitted by family members.26 All deaths were validated.27
Statistical Analysis
We fit linear mixed-effects models to obtain regression coefficients for chronic conditions on physical functioning to enable between- and within-person variation, with unstructured covariance. Each chronic condition diagnosis was examined with the modified SF-36 physical functioning scale as a continuous measure. Models were adjusted for all other conditions.
We required that individual conditions were sufficiently prevalent in our sample population so that regression coefficients could be stably estimated. Where necessary, we grouped ICD codes from rare conditions (N < 100) with similar neighboring codes and conditions. We also grouped ICD codes where coefficients suggested adverse effects on physical functioning but standard errors exceeded half the coefficient (i.e., were not statistically significant). Combining rare and/or unstably estimated codes increased the likelihood of obtaining more accurate coefficients for combined group of similar but rarer conditions. Due to the large number of potential of ICD-9 codes, we grouped ICD codes based on the existing hierarchical system25 when biologically sensible. If similar neighboring conditions could not be identified, we performed sequential groupings of similar conditions by organ system. Groupings were identified by two reviewers (MYW, JEL) and checked for agreement by a third reviewer (DR).
We internally validated the regression estimates through bootstrapping. For sufficiently prevalent conditions, we estimated the bias and confidence intervals for our estimates. With unrestricted sampling, we created 100 random independent samples with replacement that were each the same size as our original cohort. For each sample, we again computed the estimate of the associations between diseases with observed physical functioning and compared them with the original regression coefficients through relative and absolute differences.
For conditions with positive regression coefficients (i.e., that appeared to improve physical functioning), which suggested confounding by concurrent conditions, we further grouped their ICD codes with similar conditions. If the regression coefficient could not be grouped further, we excluded it from MICD (eTable 5). Finally, if a stable estimate could not be obtained from the original coefficient or bootstrapping, we provide an option to use a suggested imputed value based on the median of regression coefficients for conditions in the same organ system that reached significance (eTable 4).
Given the large number of predictors, we examined for potential multicollinearity among conditions. We assessed for multicollinearity by examining the variance inflation factor and additionally examined Spearman correlation coefficients between all conditions.
To create MICD, chronic conditions were weighted by their cross-sectional association with the HRS physical functioning scale and then, the absolute value was summed to form each individual’s MICD. MICD represents both the cumulative burden of ICD-coded chronic conditions and decrease in physical functioning.
We next compared the performance of the multimorbidity measures. First, we examined their distribution and number of unique values. Second, we assessed their associations with age, a known predictor of multimorbidity. Third, we examined 10-year mortality prediction. We applied Cox proportional hazard models19 and report hazard ratios and 95% confidence intervals for each measure with mortality. Multimorbidity measures were examined categorically in quartiles due to right-skewed distributions particularly in Elixhauser. To assess model fit, we compared the Akaike Information Criterion (AIC), where the lowest AIC indicated best performance. To assess how accurately the models discriminated between survival outcomes, we computed the concordance (C) statistic, or area under the receiver operating characteristic (ROC) curve, for survival.21 Finally, we determined the associations between each multimorbidity measure with future 8-year physical functioning using general linear models. To compare the strengths of the association, we assessed the magnitude of regression coefficients and P and T-values. We also computed the coefficient of determination (R2) to quantify how well each measure explained and predicted future physical functioning.
As sensitivity analysis, we included MICD with simple disease count and Elixhauser simultaneously in the models and included a three-way test for significance to determine which index had the greatest associations with future physical functioning and mortality, even in the presence of expected collinearity. We also compared an unweighted MICD (count of negative coefficient conditions) with “complete” simple disease count encompassing negative and positive coefficient conditions (eTable 7). All analyses were performed using Statistical Analysis System software (release 9.4; SAS Institute, Cary, NC, 2013) and Stata MP15-64 (Stata Corp, College Station, TX).
RESULTS
Participant Characteristics
Between 2000 and 2012, 20,167 participants contributed 98,019 surveys. Of these, 18,223 participants were age-eligible for the HRS linkage to CMS. The final sample included 18,212 participants who contributed 73,830 observations over a mean of 6.3 ± 4.3 years. Participants contributed a mean of 4.1 observations (SD 2.1, range 1–7). At baseline, participants had a mean age of 70.7 ± 8.1 years and HRS physical functioning scale of 59.3 ± 30.3 physical functioning units (Table 1). Over the 10-year follow-up for mortality, 42.1% of the participants died.
Table 1.
Participant Characteristics, in the Health and Retirement Study and Medicare Claims Linked Data at Baseline
| N = 18,212 | ||
|---|---|---|
| Variable (range) | N (%) | Mean ± SD |
| Age, years | 70.7 ± 8.1 | |
| Female | 10,289 (56.5%) | |
| Multimorbidity-weighted index ICD-coded conditions (MICD) (0–77.8) | 6.7 ± 8.5 | |
| Elixhauser comorbidity score (0–22) | 2.9 ± 2.4 | |
| Simple disease count (0–38) | 4.7 ± 5.1 | |
| HRS physical functioning scale (0–100) | 59.3 ± 30.3 | |
Chronic Condition Groupings by ICD-9 Codes
We considered 14,568 diagnosis and 3,883 procedure codes from the CMS list of ICD-9 CM codes for consideration in the analysis with physical functioning. After grouping and refinement, the final categorization corresponded to 69 chronic condition groups (eTable 3) representing 2021 ICD codes (3067 when imputed conditions from eTable 4 are included). We did not detect collinearity in the final model. The maximum variation inflation factor was 1.66 (mean 1.21), well below a conservative cutoff of 2.5.28
Prevalent Chronic Conditions and Physical Functioning
Among the 69 groups of conditions, the median impact on physical functioning by ICD code groups varied several fold (median − 1.71, range − 17.65, 2.89). Among conditions that had a negative association with physical functioning (N = 55) and comprised MICD, the median was − 2.02 (range − 17.65, − 0.12). The most prevalent conditions spanned several organ systems and included hypertension, osteoarthritis, other musculoskeletal conditions, coronary artery disease, back pain, diabetes, anemia, fluid, electrolyte and acid-base balance disorders, and chronic obstructive pulmonary disease (COPD) (eTable 1; eFigure 2).
Among the most prevalent conditions, osteoarthritis, COPD, and back pain had the worst impact on physical functioning. Even among the most prevalent diseases, the impact of physical functioning varied several fold, from a 1.33-unit increase in physical functioning (cataract) to a 4.65-unit decline for congestive heart failure.
Conditions with the Worst Physical Functioning
Conditions that had the greatest impact on physical functioning were predominantly progressive neurologic (ALS, Parkinson disease, paralysis) and musculoskeletal (hemiplegia/paraplegia, limb amputation, hip fracture) conditions, select cancers (lung), and end-stage organ conditions (congestive heart failure, COPD, dementia) (eTable 2; eFigure 2). ALS had a tenfold greater impact on physical functioning than the median impact of all conditions.
Internal Validation
Through bootstrapping, we obtained estimates for physical functioning for 69 groups of conditions covering a range of severity and prevalence. Most estimates for physical functioning in MICD consistently approached the original parameter estimates (eFigure 3), and the tight confidence intervals from 100 bootstrapped resamplings suggest reliable and precise results (eTable 6). Based on these resamplings, we retained original coefficient estimates for computing MICD.
Multimorbidity Measure Comparison
At baseline in 2000, MICD had the most unique values to quantify multimorbidity, with 7138 values for all conditions with negative coefficients. In contrast, there were only 34 observed unique values for disease count and 22 for Elixhauser.
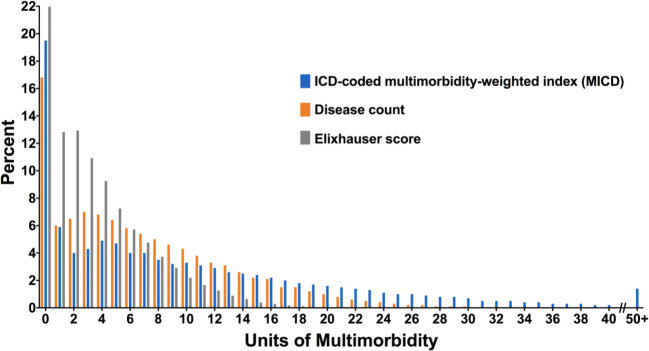
MICD had the broadest distribution, with a maximum value of 77.8, compared with 38 for disease count and 22 for Elixhauser. There was left-censoring of scores for all measures (Fig. 1), with scores of 0 observed for 22% of participants for Elixhauser, 19.5% for MICD, and 16.8% for disease count. MICD was strongly correlated with disease count (Spearman’s rho = 0.95, p < 0.001) and Elixhauser (Spearman’s rho = 0.89, p < 0.001).
Figure 1.
Distribution of multimorbidity-weighted index ICD-coded conditions (MICD), simple disease count, and Elixhauser comorbidity score.
All measures were correlated with age, with Pearson’s r values of 0.34 for MICD, 0.36 for disease count, and 0.29 for Elixhauser.
For mortality risk, a one SD increase in MICD was associated with HR = 1.69 (95% CI: 1.65–1.73), which is 55% worse than the impact of 1 year of aging on 10-year mortality risk in the average participant. Mortality risk increased with increasing quartiles for all measures (Table 2). For all three measures, each standardized point increase was associated with a nearly 70% increase in mortality. The AIC was lowest for MICD, suggesting it provided the numerically better model fit. The C-statistics were similar for MICD and Elixhauser (C-statistic = 0.65) and disease count (C-statistic = 0.64). As sensitivity analysis when all three measures were simultaneously included in the model, the association between MICD with mortality (HR = 1.46, 95% CI: 1.32–1.61, p < 0.001) was of marginally greater magnitude than that of Elixhauser with mortality (HR = 1.34, 95% CI: 1.27–1.42, p < 0.001), while disease count was no longer associated with mortality (HR = 0.90, 95% CI: 0.81–1.00, p = 0.06) (p for 3-way test < 0.001).
Table 2.
Hazard Ratios for Mortality After 10-Year Follow-up, in the Health and Retirement Study and Medicare Claims Linked Data, 2007–2016
| N = 10,616 | ||||
|---|---|---|---|---|
| Deaths | N = 4473* | |||
| Model | HR (95% CI) | p value | AIC | C-statistic |
| Multimorbidity-weighted index ICD-coded conditions, continuous, standardized | 1.69 (1.65–1.73) | < 0.001 | 79318.65 | 0.652 |
| Multimorbidity-weighted index ICD-coded conditions, quartiles | 79522.45 | 0.639 | ||
| Q4 | 3.62 (3.32–3.94) | < 0.001 | ||
| Q3 | 1.89 (1.73–2.08) | < 0.001 | ||
| Q2 | 1.12 (1.02–1.24) | 0.02 | ||
| Q1 (reference) | 1.0 | |||
| Elixhauser score, continuous, standardized | 1.69 (1.65–1.74) | < 0.001 | 79335.88 | 0.651 |
| Elixhauser score, quartiles | 79446.19 | 0.644 | ||
| Q4 | 3.77 (3.48–4.07) | < 0.001 | ||
| Q3 | 2.05 (1.87–2.25) | < 0.001 | ||
| Q2 | 1.34 (1.22–1.47) | < 0.001 | ||
| Q1 (reference) | 1.0 | |||
| Simple disease count, continuous, standardized | 1.64 (1.60–1.68) | < 0.001 | 79553.05 | 0.639 |
| Simple disease count, quartiles | 79650.66 | 0.630 | ||
| Q4 | 3.44 (3.17–3.74) | < 0.001 | ||
| Q3 | 1.89 (1.74–2.07) | < 0.001 | ||
| Q2 | 1.14 (1.04–1.26) | 0.005 | ||
| Q1 (reference) | 1.0 | |||
AIC, Akaike Information Criterion; C, concordance; CI, confidence interval; HR, hazard ratio; Q, quartile; ICD, International Classification of Diseases
*Participants contributed a total of 29,982,087 person-days (82,143 person-years), during which there were 4473 deaths
For future physical functioning, a one SD increase in MICD was associated with a − 15.47 (95% CI: − 16.39, − 14.55) unit change in future 8-year physical functioning, which is 20-fold worse than the impact of 1 year of aging on 8-year physical functioning in the average participant. To compare the association between multimorbidity measures and future physical functioning, the coefficient of determination values was marginally higher for MICD (R2 = 0.15) than simple disease count (R2 = 0.14) and Elixhauser (R2 = 0.12) (Table 3). Using a sample-based measure of effect, the T-value for MICD (T = − 33.09) was higher than that of simple disease count (T = − 30.23). Finally, when all three measures were simultaneously included in the model, the association between MICD with future physical functioning (β = − 11.24, 95% CI: − 14.67, − 7.81, T = − 6.42, p < 0.001) persisted while simple disease count (β = − 3.19, 95% CI: − 6.77, 0.39, T = − 1.75, p = 0.08) and Elixhauser (β = − 1.22, 95% CI: − 3.08, 0.63, T = − 1.29, p = 0.20) were no longer associated with future physical functioning (p for 3-way test < 0.001); in addition, models with MICD had the highest T-value compared with simple disease count and Elixhauser.
Table 3.
Future 8-Year HRS Physical Functioning Scale in the Health and Retirement Study and Medicare Claims Linked Data, 2006–2014
| Model | β coefficient (95% CI) | p value | T-value | R2 |
|---|---|---|---|---|
| Multimorbidity-weighted index ICD-coded conditions, continuous, standardized | − 15.47 (− 16.39, − 14.55) | < 0.001 | − 33.09 | 0.15 |
| Multimorbidity-weighted index ICD-coded conditions, quartiles | ||||
| Q4 | − 33.55 (− 35.81, − 31.30) | < 0.001 | − 29.12 | 0.14 |
| Q3 | − 19.19 (− 21.17, − 17.21) | < 0.001 | − 19.00 | |
| Q2 | − 5.28 (− 7.16, − 3.39 | < 0.001 | − 5.50 | |
| Q1 (reference) | ||||
| Elixhauser score, continuous, standardized | − 13.55 (− 14.47, − 12.62) | < 0.001 | − 28.62 | 0.12 |
| Elixhauser score, quartiles | ||||
| Q4 | − 30.23 (− 32.44, − 28.02) | < 0.001 | − 26.81 | 0.12 |
| Q3 | − 19.91 (− 22.11, − 17.71) | < 0.001 | − 17.75 | |
| Q2 | − 9.36 (− 11.16, − 7.55) | < 0.001 | − 10.14 | |
| Q1 (reference) | ||||
| Simple disease count, continuous, standardized | − 13.47 (− 14.34, − 12.59) | < 0.001 | − 30.23 | 0.13 |
| Simple disease count, quartiles | ||||
| Q4 | − 32.32 (− 34.69, − 29.94) | < 0.001 | − 26.67 | 0.12 |
| Q3 | − 19.00 (− 21.00, − 16.99) | < 0.001 | − 18.55 | |
| Q2 | − 7.42 (− 9.26, − 5.58) | < 0.001 | − 7.91 | |
| Q1 (reference) | ||||
ICD, International Classification of Diseases; Q, quartile; R2, coefficient of determination
For both future physical functioning and mortality outcomes, unweighted MICD (simple disease count with only negative coefficients) outperformed simple disease count (eTable 7).
DISCUSSION
This study used unique data linkages between self-reported physical functioning in the nationally representative HRS and ICD-coded claims from Medicare to develop a new and greatly expanded MICD derived from claims data. We identified ICD codes for chronic diseases and conditions with clinically significant associations with physical functioning and provide internally validated weights for 69 groups of conditions representing 2021 stably estimated conditions. The resulting MICD is a useful measure of multimorbidity that captures the impact of coexisting chronic conditions on physical functioning in older adults and has implications for patient care, research, and policy.
In this sample of Medicare-HRS participants, the rank order of the top prevalent conditions from claims was similar to those from self-report, including the top condition groups: hypertension, osteoarthritis, other musculoskeletal conditions, and coronary artery disease (which included hyperlipidemia).16 The prevalence was also similar to those reported for 21 select conditions in Medicare fee-for-service beneficiaries aged ≥ 65 years in 2012: hypertension (65% vs 60%), hyperlipidemia (56% vs 49%), arthritis (37% vs 31%).29 Differences may be due to the number and type of claims based on the Chronic Conditions Warehouse criteria30 versus our criteria of ≥ 1 inpatient diagnosis or two outpatient diagnoses within a 2-year period, but the similarity provides useful validation to our approach.
We also compared MICD with simple disease count and the Elixhauser score that was developed in administrative data using ICD-9 codes. Compared with these measures, MICD had the widest distribution and several fold more unique values to quantify multimorbidity. Furthermore, it performed at least as well as other metrics that were far less granular but based similarly on readily extracted administrative codes. MICD outperformed disease count and Elixhauser for future physical functioning prediction and was similar to Elixhauser for mortality prediction. Even an unweighted MICD (disease count of negative coefficients) outperformed a “complete” simple disease count (negative and positive coefficients) for future physical functioning and mortality risk, demonstrating that indiscriminate disease counts are suboptimal.
Unlike prior studies, we considered all reliably measured ICD-coded conditions from the full CMS list of ICD-9 CM codes for fiscal year 2015 (October 1, 2014, to September 30, 2015) as potential morbidities. Most administrative databases record only a limited number of diagnoses and invariably omit several important conditions.2–6 We created one of the most comprehensive mappings to date, with 69 groups of conditions mapped from 14,568 diagnosis and 3883 procedure codes. We agnostically considered any potential condition that could impact physical functioning rather than selecting candidate conditions a priori. Nonetheless, the overall benefit of including several more conditions was limited, suggesting that the most impactful conditions have been captured in existing indices—a reassuring finding.
There are potential limitations of this study. First, we could not provide regression coefficients for all ICD codes. The index includes sufficiently prevalent chronic conditions and acute but significant conditions with a long-lasting impact on physical functioning afflicting our sample population of Medicare beneficiaries aged 65 years and older. We did not capture conditions afflicting younger adults or children. Nonetheless, we evaluated 69 groups of conditions spanning a range of organ systems and disease prevalences in a population-based sample that is representative of the population of older adults for whom multimorbidity is most prevalent and problematic.
Second, our index relies on ICD codes alone, and the overall value of any measure of multimorbidity built from administrative data will depend on the accuracy of ICD coding. While additional information from chart review may improve the positive predictive value of an ICD code mapping to a chronic condition, the type and quality of data available vary for conditions.31 Not all conditions may rely upon ancillary laboratory, procedural, or subjective clinical data for diagnosis. Variation in the accuracy of coding is an inherent limitation in claims data but these data remain critical in health services research. That MICD relies upon ICD codes enhances its scalability and ease of use.
Third, methods to crosswalk ICD-9 codes with respective conditions vary. When available, we compiled ICD codes from established indices, but given the marked variation in these codes, we ultimately used the 2015 CMS ICD version 32 diagnosis and procedure codes for the most comprehensive listing of ICD codes. Our list of ICD codes and mapped conditions may be further refined in future studies. Fourth, ICD codes are provided for ICD-9 codes only. Medicare data were not yet available for ICD-10 conditions. Nonetheless, the MICD will be useful for existing data through 2015. Future studies to crosswalk MICD to ICD-10 are already underway. Finally, MICD is weighted to physical functioning and adjusted for mental health conditions but does not weight conditions based on their impact on mental health. We previously demonstrated that a multimorbidity-weighted index using self-reported conditions weighted to the SF-36 physical functioning scale also captures mental health, as it was significantly associated with all mental health scales and even the mental component summary.32 Further studies are needed to assess the impact of MICD on mental health.
MICD has several clinical and research implications. Underspecified multimorbidity measures are detrimental for patients, providers, and health systems, and contribute to the underrating of multimorbidity on health and function. For example, Medicare evaluates health system performance in their care of seniors without regard to current functional impairment. However, the success and viability of payment programs, including the CMS 2020 Primary Care First model,33 will depend on accurate adjustment of the health and risk of patients. MICD provides a more comprehensive and patient-centered measure for quantifying multimorbidity in research. Ultimately, MICD was designed with the intent of operationalizing at the point of clinical care. With its twofold clinically meaningful interpretation of both cumulative disease burden and physical functioning impairment, MICD may be used to prospectively identify patients who are high risk or “rising risk” in an automated, easily implemented fashion. Its performance in specific use cases should be assessed using data from the electronic health record so it may be maximally utilized.
In summary, chronic conditions from Medicare claims had a wide range of associations with physical functioning. MICD provided the broadest distribution and most unique values to quantify multimorbidity and outperformed Elixhauser and simple disease count for future physical functioning prediction and was similarly associated with mortality as Elixhauser. MICD is a useful measure of multimorbidity that captures the impact of coexisting chronic conditions on physical functioning in older adults crucial for patient care, research, and policy. This newly developed MICD is feasible and readily implemented to quantify multimorbidity in a variety of clinical settings.
Supporting Information
(DOCX 434 kb)
Authors’ Contributions
Study concept or design (MYW, KJM), data acquisition, analysis or interpretation of data (MYW, DR, KJM, JEL), drafting of manuscript (MYW), reviewing the manuscript for feedback, and approval of the final version for publication (all authors).
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (grant number K23AG056638), the University of Michigan Claude D. Pepper Older Americans Independence Center (grant numbers P30AG024824 and UL1TR002240), the Michigan Center on the Demography of Aging (grant number P30AG012846-24), and the Society of General Internal Medicine (Founder’s Award). The Health and Retirement Study is supported by the National Institute on Aging at the National Institutes of Health (grant number U01AG009740) and conducted at the Institute for Social Research, University of Michigan.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kenneth J. Mukamal and Kenneth M. Langa contributed equally to this work.
References
- 1.Wei MY, Kabeto MU, Galecki AT, Langa KM. Physical functioning decline and mortality in older adults with multimorbidity: joint modeling of longitudinal and survival data. J Gerontol A Biol Sci Med Sci. 2019;74(2):226–232. doi: 10.1093/gerona/gly038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 3.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 4.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–9. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 6.Quan H, Khan N, Hemmelgarn BR, et al. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54(6):1423–8. doi: 10.1161/HYPERTENSIONAHA.109.139279. [DOI] [PubMed] [Google Scholar]
- 7.Brettschneider C, Leicht H, Bickel H, et al. relative impact of multimorbid chronic conditions on health-related quality of life-results from the Multicare Cohort study. PLoS One. 2013;8(6):e66742. doi: 10.1371/journal.pone.0066742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tooth L, Hockey R, Byles J, Dobson A. Weighted multimorbidity indexes predicted mortality, health service use, and health-related quality of life in older women. J Clin Epidemiol. 2008;61(2):151–9. doi: 10.1016/j.jclinepi.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Agborsangaya CB, Lau D, Lahtinen M, Cooke T, Johnson JA. Health-related quality of life and healthcare utilization in multimorbidity: results of a cross-sectional survey. Qual Life Res. 2013;22(4):791–9. doi: 10.1007/s11136-012-0214-7. [DOI] [PubMed] [Google Scholar]
- 10.John R, Kerby DS, Hennessy CH. Patterns and impact of comorbidity and multimorbidity among community-resident American Indian elders. Gerontologist. 2003;43(5):649–60. doi: 10.1093/geront/43.5.649. [DOI] [PubMed] [Google Scholar]
- 11.McDaid O, Hanly MJ, Richardson K, Kee F, Kenny RA, Savva GM. The effect of multiple chronic conditions on self-rated health, disability and quality of life among the older populations of northern Ireland and the Republic of Ireland: a comparison of two nationally representative cross-sectional surveys. BMJ Open. 2013;3(6). 10.1136/bmjopen-2013-002571. [DOI] [PMC free article] [PubMed]
- 12.Radner H, Yoshida K, Mjaavatten MD, et al. Development of a multimorbidity index: impact on quality of life using a rheumatoid arthritis cohort. Semin Arthritis Rheum. 2015;45(2):167–173. doi: 10.1016/j.semarthrit.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Loza E, Jover JA, Rodriguez L, Carmona L. Multimorbidity: prevalence, effect on quality of life and daily functioning, and variation of this effect when one condition is a rheumatic disease. Semin Arthritis Rheum. 2009;38(4):312–9. doi: 10.1016/j.semarthrit.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Meyer JP, Qiu J, Chen NE, Larkin GL, Altice FL. Frequent emergency department use among released prisoners with human immunodeficiency virus: characterization including a novel multimorbidity index. Acad Emerg Med. 2013;20(1):79–88. doi: 10.1111/acem.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei MY, Mukamal KJ. Multimorbidity, mortality, and long-term physical functioning in 3 prospective cohorts of community-dwelling adults. Am J Epidemiol. 2018;187(1):103–112. doi: 10.1093/aje/kwx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei MY, Kawachi I, Okereke OI, Mukamal KJ. Diverse cumulative impact of chronic diseases on physical health-related quality of life: implications for a measure of multimorbidity. Am J Epidemiol. 2016;184(5):357–365. doi: 10.1093/aje/kwv456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei MY, Kabeto MU, Langa KM, Mukamal KJ. Multimorbidity and physical and cognitive function: performance of a new multimorbidity-weighted index. J Gerontol A Biol Sci Med Sci. 2018;73(2):225–232. doi: 10.1093/gerona/glx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luster JE, Ratz D, Wei MY. Purpose in life and life satisfaction moderate the association between multimorbidity and social participation among US middle aged and older adults. 2019.
- 19.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS) Int J Epidemiol. 2014;43(2):576–85. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei MY, Ratz D, Mukamal KJ. Multimorbidity in medicare beneficiaries: performance of an ICD-coded multimorbidity-weighted index. J Am Geriatr Soc. 2020;68(5):999–1006. doi: 10.1111/jgs.16310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Bayliss MS, Rogers WH, Kosinski M, Tarlov AR. Differences in 4-year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems. Results from the Medical Outcomes Study. JAMA. 1996;276(13):1039–1047. doi: 10.1001/jama.1996.03540130037027. [DOI] [PubMed] [Google Scholar]
- 22.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rumsfeld JS, MaWhinney S, McCarthy M, et al. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. participants of the department of veterans affairs cooperative study group on processes, structures, and outcomes of care in cardiac surgery. JAMA. 1999;281(14):1298–03. doi: 10.1001/jama.281.14.1298. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Kosinski M, Gandek B. SF-36 health survey manual and interpretation guide. Lincoln, RI: Quality Metric Inc; 1993. [Google Scholar]
- 25.Centers for Medicare & Medicaid Services. ICD-9-CM Diagnosis and Procedure Codes: Abbreviated and Full Code Titles. Updated 2014 May. www.cms.gov/medicare/coding/ICD9providerdiagnosticcodes/codes.html. Accessed 2016 August.
- 26.Research Data Assistance Center (ResDAC). Death Information in the Research Identifiable Medicare Data. https://www.resdac.org/articles/death-information-research-identifiable-medicare-data. Accessed Jan 10, 2019.
- 27.Virnig B. Sources and Use of Medicare Enrollment Information. http://resdac.umn.edu/sites/resdac.umn.edu/files/Sources%20and%20Use%20of%20Medicare%20Enrollment%20Information%20(Slides).pdf. Accessed Jan 10, 2019.
- 28.Allison P. When can you safely ignore multicollinearity? https://statisticalhorizons.com/multicollinearity. Accessed Aug 30, 2019.
- 29.Centers for Medicare and Medicaid Services. Chronic Conditions. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/CC_Main.html. Accessed Aug 30, 2019.
- 30.Centers for Medicare and Medicaid Services. Chronic Conditions Data Warehouse: Condition Categories. www.ccwdata.org/web/guest/condition-categories. Accessed Aug 30, 2019.
- 31.Wei MY, Luster JE, Chan CL, Min L. Comprehensive review of ICD-9 code accuracies to measure multimorbidity in administrative data. BMC Health Serv Res. 2020;20(1):489. doi: 10.1186/s12913-020-05207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei MY, Mukamal KJ. Multimorbidity and mental health-related quality of life and risk of completed suicide. J Am Geriatr Soc. 2019;67(3):511–519. doi: 10.1111/jgs.15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Medicare and Medicaid Services. Primary Care First Model Options. https://innovation.cms.gov/initiatives/primary-care-first-model-options/. Accessed June 27, 2019.