Abstract
Soil health management and increase crop productivity are challenging issues for researchers and scientists. Many research publications have given multiple technological solutions for improving soil health and crop productivity but main problem is sustainability of those technologies under field condition and different agro-climatic zone. Due to the random industrialization, deforestation, mining and other environmental factor reduce soil fertility and human health. Many alternative options e.g., crop rotation, green manuring, integrated farming, biofertilizer (plant-growth-promoting microorganism, microbial consortium of rhizosphere soils), and vermicomposting are available for adapting and improving the soil heath and crop productivity by farmers. Recent trends of new research dimension for sustainable agriculture, endophytic microbes and its consortium is one of the better alternative for increasing crop productivity, soil health and fertility management. However, current trends are focuses on the endophytic microbes, which are present mostly in all plant species. Endophytic microbes are isolated from plant parts—root, shoot, leaf, flower and seeds which have very potential ability of plant growth promotion and bio-controlling agent for enhancing plant growth and development. Mostly plant endophytes showed multi-dimensional (synergistic, mutualistic, symbiotic etc.) interactions within the host plants. It promotes the plant growth, protects from pathogen, and induces resistance against biotic and abiotic environmental stresses, and improves the soil fertility. Till date, most of the scientific research has been done on assuming that interaction of plant endophytes with the host is similar like the plant-growth-promoting microorganism (PGPM). It would be very interesting to explore the functional properties of plant endophytes to modulate the essential gene expression during biotic and abiotic stresses. Endophytes have the ability to induce the soil fertility by improving soil essential nutrient, enzymatic activity and influence the other physiochemical property. In this study, we have discussed details about functional properties of plant endophytes and their mechanism for enhancing plant productivity and soil health and fertility management under climate-resilient agricultural practices. Our main objective is to promote and explore the beneficial plant endophytes for enhancing sustainable agricultural productivity.
Keywords: Plant endophytes, Soil fertility, Plant growth promotion, Phytopathogen, Sustainable agriculture, Crop yield, Stress tolerance, Soil health, Plant–microbe interaction
Introduction
Plants are mega species harboring wide diversity of microbes in their different parts such as seed, root, stems, leaf, pollens and flowers, which altogether is known as the plant microbiome (Zhang et al. 2017; Mukherjee et al. 2020). Plant-associated microbes play critical roles in crop yield and plant health through different direct and indirect mechanisms (Mukherjee et al. 2020; Trivedi et al. 2020b). Endophytes are a unique group of plant microbiome that reside asymptomatically inside plant parts and tissues having a symbiotic relationship (Wilson 1995). The group constitutes bacteria, fungi, and archaea that inhabit the plant tissue as a whole or a part of their lifecycle (Hassan 2017; Harrison and Griffin 2020). The majority of plant endophytes belong to genera of Bacillus, Pseudomonas, Streptomyces, Burkholderia, Klebsiella, Enterobacter, Penicillium, Aspergillus, Alternaria, and Fusarium (Hassan 2017; Khan et al. 2017a, b; Singh et al. 2017a; Mukherjee et al. 2020, 2021). The features of interest of all these endophytic microbial genera are provided in Table 1. However, from newer studies, it has been demonstrated that there is more diversity of plant endophytes which are subject to change according to the host and environmental factors (Kawasaki et al. 2016; Liu et al. 2020). With that being said, some microbial groups are present universally regardless of the environment and are part of the plant’s core microbiome (Hamonts et al. 2018). This core group of microbes has co-evolved with the host plant species and is inherited through generations (Song et al. 2020).
Table 1.
List of major endophytic microbial genera isolated from plants and their features of interest
| Endophyte | Host plants | Isolation point(s) | Features of interest | Reference(s) |
|---|---|---|---|---|
| Alternaria spp. | Salvia miltiorrhiza, Solanum nigrum, and Brassica napus | Root, shoot, and leaf |
Increased biomass, chlorophyll content, and secondary metabolite production Abiotic stress tolerance |
Khan et al. (2015b) Shi et al. (2017) Zhou et al. (2018) |
| Aspergillus spp. | Zea mays, Euphorbia indica, Soybean, and Sunflower | Root and leaf |
Production of secondary metabolites for plant growth Stress tolerance |
Mehmood et al. (2019) |
| Bacillus spp. | Zea mays, Saccharum officinarum, Aloe vera, Cucurbits, and Oryza sativa, Cicer arietinum | Seed, root, stem, and leaf |
Inhibition of phytopathogens Plant growth promotion |
Akinsanya et al. (2015) Gond et al. (2015a) Khalaf and Raizada (2018) Kumar et al. (2020) Mukherjee et al. (2020) Wang et al. (2020b) |
| Enterobacter spp. | Cicer arietinum, Zea mays, and Sorghum sudanense | Seed |
Improved productivity Phyto-stabilization of heavy metals Plant growth promotion |
Li et al. (2016) Ullah et al. (2020) Mowafy et al. (2021) Mukherjee et al. (2020) |
| Fusarium spp. | Brassica napus, Oxalis corniculate, and Glycine max | Root |
Abiotic stress tolerance Mineral solubilization Biomass production Secondary metabolite production |
Radhakrishnan et al. (2015) Shi et al. (2017) Bilal et al. (2018) |
| Klebsiella spp. | Zea mays, Saccharum officinarum, and Triticum aestivum | Root |
Enhance growth and yield N fixation Stress tolerance |
Lin et al. (2015) Zhang et al. (2017) Mowafy et al. (2021) |
| Penicillium spp. | Triticum aestivum and Capsicum annum | Root |
Resistance against abiotic stresses Production of IAA Nutrient mineralization |
Ikram et al. (2018) Oses-pedraza et al. (2020) |
| Pseudomonas spp. | Pisum sativum, Oryza sativa, Achyranthes aspera, Zea mays Brassica napus, and Cicer arietinum | Leaf, root, and seed |
Mineral solubilization N fixation Defense against phytopathogens Stress tolerance |
Otieno et al. (2015) Devi et al. (2017) Lally et al. (2017) Pham et al. (2017) Sandhya et al. (2017) Mukherjee et al. (2020) |
| Streptomyces spp. | Solanum lycopersicum, Glycine max, and Sorghum | Root and stem |
Plant growth promotion Biocontrol Production of active secondary metabolites |
Goudjal et al. (2016) Patel et al. (2018) Liu et al. (2019) |
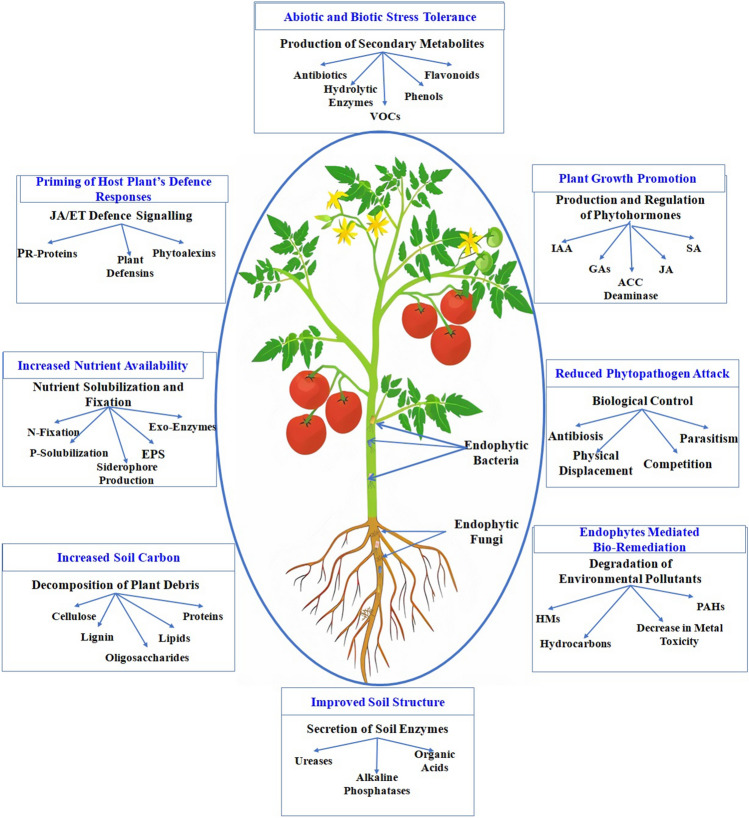
Plants in natural communities preserve their symbiotic associations with endophytes that help in growth promotion and protection against different stresses (Rodriguez et al. 2009; Johnston-Monje et al. 2016; Trivedi et al. 2020b). The actinorhizal and rhizobial endophytes enhance nutrient availability especially nitrogen through the process of biological nitrogen fixation (BNF) (Pawlowski and Demchenko 2012) in specialized root structures called nodules (Coba de la Peña et al. 2017). The mycorrhizal endophytic fungal families also help in nutrient acquisition to plant especially phosphorous. Many of the endophytes produce siderophores which increase iron availability to plants (Mukherjee et al. 2020). The enzymatic activities of endophytes mobilize the soil nutrients making them readily available to plants (Behie and Bidochaka 2013; White et al. 2019). In a fashion similar to human gut microbes, the endophytes improve plant health by protecting against phytopathogens. They have the ability to induce systemic resistance and upregulate defense gene expression in the host plant and suppress the growth and fitness of phytopathogens (Hardoim et al. 2015; Irizarry and White 2017; White et al. 2018). Endophytes start modulation of defense gene in host plant right from the seedling stage to maturation (Ongena and Jacques 2008; Gond et al. 2015a, b). Systemic resistance is induced against a broad spectrum of phytopathogen through jasmonic acid, salicylic acid, and ethylene pathways and the production of pathogenesis-related proteins (Bastias et al. 2017). The growth suppression of phytopathogen by endophytes is through the production of antimicrobial compounds such as pyrrolnitrin, pyoleutirin, 2, 4-diacetylphloroglucinol, phenazine-1-carboxylic acid, and hydrogen cyanide (Mousa et al. 2016; Bastias et al. 2017). The different underlying mechanisms of plant endophytes in the improvement of plant and soil health are represented in Fig. 1.
Fig. 1.
Diagrammatic representation of plant endophytes’ mechanisms involved in improvement of plant and soil health
Soil is a mystic resource on this planet harboring both biological and chemical entities. Agricultural soil in particular linked to human health, production economics, water and soil quality, and food safety and security either directly and/or indirectly (Karlen et al. 2019). Healthy soils are the backbone of agricultural productivity as it provides support to healthy plant growth and development. The quantity and quality of about 95% of our food depend on soil functional properties (Kemper and Lal 2017; Brevik et al. 2018, 2020) which indirectly dictates human and animal nourishment as nutrient deficiency of food grains cause many human diseases. It has been known through various studies that the application of organic fertilizers improves soil quality and health by stimulating microbial population and diversity in the soil (Jannoura et al. 2014; Verma et al. 2014; Mukherjee et al. 2019). However, both organic and inorganic fertilizers are applied in common agricultural practices for better crop production (Naab et al. 2017; Mukherjee et al. 2021), in which the proportion of the latter is quite higher. Inorganic fertilizers can be replaced with the use of endophytes as a sustainable approach in modern agricultural practices (Kumar et al. 2020). Endophytes possess the ability to solubilize micro and macronutrients in soil without hampering the natural properties and microbial community of the soil. They also play an important role in soil mineral cycling and the removal of pollutants from soil (He et al. 2020; Liu et al. 2020), due to which they are deemed to be a better alternative. Hence, our main aim of this review is to present the functional properties of endophytes keeping in view the current demand of their application as bioinoculants for improvement of soil health, plant productivity, and protection against phytopathogens under a sustainable approach.
Screening and molecular characterization of endophytic microbes from plant material
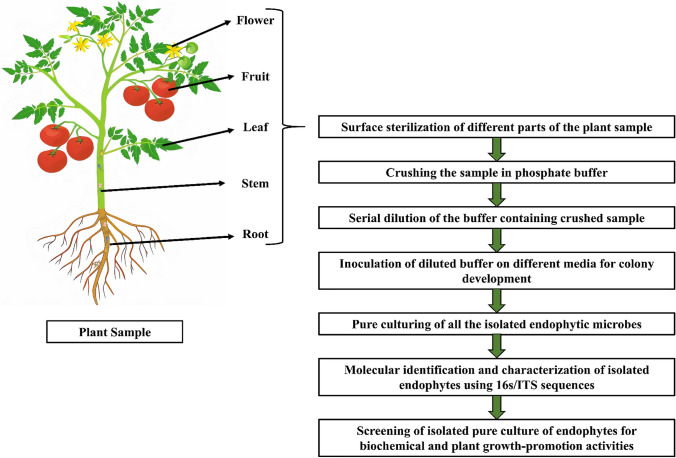
Before going for screening and molecular characterization, one should have to attain a pure culture of endophytic microbe. Endophytes can be isolated by different plating methods in respective culture media from different plant parts (root, shoot, leaf, flower, fruits, and seeds) after sterilization of that particular part with 1% sodium hypochlorite solution for 1 min and 70% ethanol followed by washing two to three times with sterilized distilled water (Mukherjee et al. 2020). Pure culture of the respective isolated endophyte can be then done by single spore culture for fungi and single colony culture for bacteria. DNA should be extracted from this pure culture of isolated bacteria and fungi as the next step for molecular characterization using polymerase chain reaction (PCR) amplification of conserved regions namely: 16S rRNA or 18S rRNA for bacterial endophytes and ITS for fungal endophytes followed by sequencing. The sequence obtained must be aligned using the Basic Local Alignment Search Tool (BLAST) in National Center for Biotechnology Information (NCBI) for obtaining a similarity index to match the microbial organism from the database. For screening of biochemical and plant growth promotion properties, pure culture of microbes should be grown in respective liquid media. A diagrammatic representation of isolation, identification, and characterization of endophytes from different plant parts is given in Fig. 2. These screening results should be used for the development of potential single inoculants and consortiums. The endophytic microbial consortium is, however, more effective plant-growth-promoting and biocontrol agent for enabling better plant growth under abiotic and biotic stresses (Singh et al. 2018).
Fig. 2.
Flowchart of isolation, identification, and characterization of plant endophytes from different parts
Endophytes for agricultural soil health
Almost all the plants have their world of endophytic microbes which are non-aggressive and ubiquitous (Schulz and Boyle 2006). Once colonized, endophytes stimulate the growth and physiology of host plants and properties of soil through various direct and indirect mechanisms (Singh et al. 2018; Mukherjee et al. 2020; Chouhan et al. 2021). Nitrogen fixation, phosphate solubilization, siderophore production, exopolysaccharide (EPS) secretion, exoenzymes’ production, and biocontrol activities are some of the functional properties of endophytes (Jasim et al. 2014; Mukherjee et al. 2019, 2020) that aid restoration of soil health and fertility. The exoenzymes produced by endophytes specifically have the capacity of solubilizing essential plant nutrients from their insoluble for to soluble ones (Puri et al. 2020). These exoenzymes also constitute organic acids which lead to lowering of soil pH (Verma et al. 2017). The changes in pH additionally inhibit the activities of phytopathogenic microbes and also alter the growth of some invasive plants thereby increasing nutrient availability (Shahrtash and Brown 2021). Many endophytes synthesize soil invertase, urease, and soil alkaline phosphatases which directly modulate soil organic carbon (SOC), soil nitrogen, and microbial biomass (Hou et al. 2020). Endophytes also degrade plant debris present in soil having macromolecules like lignin, pectin, oligosaccharides, cellulose, hemicellulose, lipids, and proteins with the help of exoenzymes (Wang and Dai 2011; Uzma et al. 2016) into their simpler forms. This adds to the nutrient status of soil, enhancing soil quality, nutrient cycling, and soil micro-environment. Puente et al. (2009) reported in a study on endophytic bacteria associated with Pachycereus pringlei produces organic acids which help in weathering and transformation of minerals under in vitro conditions.
Plant–microbe interaction is a complex system that involves a vast array of microbes, the plant, and the soil. The interaction not only affects the physiology of plant but also regulate soil flora and fauna, soil microbial respiration rate, soil health, and nutrient cycling (Chaparro et al. 2012). Plants communicate with the soil microbial community through chemical signals constituting proteins, fatty acids, flavonoids, sugars, aliphatic acids, and amino acids which create a unique environment for the survival of soil microbes. These secreted chemical signals establish interaction with endophytes and neighboring plants leading to the formation of soil aggregates which improves soil porosity by designing the soil structure (Miller and Jastrow 2000). A wide range of endophytic bacteria and fungi viz. Bacillus, Arthrobacter, Enterobacter, Clostridium, Pseudomonas, Microbacterium, Mucor, Microsphaeropsis, Phoma, Alternaria, Steganosporium, and Aspergillus have been reported resistant to metals (Guo et al. 2010; Li et al. 2012). These endophytes can thus be helpful in removing heavy metal toxicity from the soil. Moreover, different studies have suggested that endophytes play a crucial role in the phytoremediation of organic contaminants such as hydrocarbons as well. Most of the soil contaminants are toxic for plants and cannot be degraded by them alone. This problem can be alleviated through plant–endophytes interaction (Li et al. 2012). Endophytes reduce phytotoxicity due to soil contaminants by increasing their immobilization, chelation, and degradation. For this, they secrete organic acids of low molecular weight, siderophores, and enzymes. Siderophores can bind efficiently with iron (Fe), zinc (Zn), cadmium (Cd), gallium (Ga), aluminum (Al), and lead (Pb) to form a stable complex which increases their soluble concentration (Rajkumar et al. 2010).
Many of the endophytic bacteria and fungi are antagonistic and are drawing special attention as an alternative for the management of soil-borne diseases with minimal environmental impact and soil pollution. These antagonistic endophytes control the population of soil-borne phytopathogens through different mechanisms namely: parasitism, competition, production of lytic enzymes, and antibiosis (De Silva et al. 2019). EPS produced by endophytes plays important role in plant–endophyte interactions and also exhibit many biological functions. EPS of endophytic origin have antioxidative, antiallergic, and prebiotic properties (Liu et al. 2017) along with metal complexation ability (Liu et al. 2021). Thus, EPS can be helpful in regulating the population of soil phytopathogens and reducing the bioaccessibility of heavy metals. From the above-presented statements, it can be very well concluded that augmentation of soil with specific endophyte or endophytic consortium can be significantly supportive in restoring soil health. A list of identified endophytes that have studied in the management of soil health, their host plant(s), and features of interest is provided in Table 2.
Table 2.
Endophytes in improvement of soil health
| Endophytes | Host plant | Features of interest in soil health management | Reference(s) |
|---|---|---|---|
| Neotyphodium coenophialum | Festuca arundinacea | Enhancement of soil carbon and nitrogen | Franzluebbers et al. (1999) |
| Neotyphodium occultans | Lolium multiflorum |
Modulation of soil catabolic activity Enhancement of soil microbial (fungal) population and their activities |
Casas et al. (2011) |
| Bacillus spp. | Solanum nigrum | Hyperaccumulation of metal (Cu, Cd, and Cr) in soil | Guo et al. (2010) |
| Burkholderia cepacia | Zea mays | Phytoremediation of organic contaminants (toluene and phenols) | Wang et al. (2010) |
| Phomopsis liquidambari | Bischofia polycarpam |
Promotion of litter mass degradation Alleviation of soil nitrogen concentration |
Chen et al. (2013) |
| Phialocephala fortinii | Asparagus officinalis |
Degradation of organic compounds Improvement of nutrient cycling in the soil |
Narisawa (2017) |
| Chamaecyparis obtusa and Rubus spp. | Surono and Narisawa (2017) | ||
| Enterobacter spp., Microbacterium arborescens, and Pantoea stewartii | Leptochloa fusca and Brachiaria mutica | Enhancement in the uptake, translocation, accumulation, and phyto-stabilization of heavy metal (Cr) in contaminated soil | Ahsan et al. (2018) |
| Phomopsis liquidambari | Oryza sativa | Increment in decomposition of straw and total soil nitrogen | Sun et al. (2019) |
| Epichloë gansuensis | Achnatherum inebrians | Improvement of soil fertility and soil nutrients availability by inducing soil enzyme activity such as invertase, alkaline phosphatase and urease | Hou et al. (2020) |
| Serratia spp., and Arthrobacter spp. | Brassica juncea |
Improvement in organic matter content of soil Phytoremediation of vanadium contaminated soil Improvement of plant growth and soil health |
Wang et al. (2020a) |
| Phomopsis liquidambaris | Acharis hypogaea |
Alleviation of soil health through improved root exudation Improvement of soil carbon metabolism Increment of rhizospheric bacterial community |
Xie et al. (2020) |
|
Acrocalymma vagum and Paraboeremia putaminum |
Glycyrrhiza uralensis | Ensure plant growth under drought stress conditions by structuring soil microbiome and maintaining soil water content, soil organic matter, and nutrient availability | He et al. (2021) |
Endophytes for sustainable plant protection and its stress management
Many appreciative efforts have been made to study the role of endophytes in a plant’s defense system against different stresses. Application of different endophytes can assist in the adjustment of plant’s tolerance to various abiotic and biotic stresses (Wani et al. 2015). Biofertilization, biocontrol, and phytostimulation are the three mechanisms through which endophytes help plants in combating unfavorable conditions. It is a well established fact that plants regulate their defense system through phytohormonal signaling and its crosstalk. The phytohormones induce innate immunity in a plant for protection against different phytopathogens. As per Waqas et al. (2015), endophytic fungus Penicillium citrinum provided protection to Sclerotium rolfsii (phytopathogen) by increasing the level of jasmonic acid (JA) and salicylic acid (SA)-mediated hormone signaling. In addition, they also reported that another endophytic species of the same fungus, P. formosus, increased plant growth by lowering the level of phytohormones associated with stress signaling namely abscisic acid (ABA) and JA during heat stress. They also regulated other phytohormones levels and produced different secondary metabolites for alleviating the same stress. Similarly in another study, endophytic Aspergillus niger increased the level of gibberellins and auxin to promote plant growth under stress (Lubna et al. 2018). During stress conditions, the level of ethylene increases in plants causing inhibition in root length, root hair, and lateral root development. During such instances, endophytes produce an enzyme known as 1-aminocyclopropane-1-carboxylate (ACC) deaminase which functions in lowering ethylene levels and promoting plant growth (Santoyo et al. 2016). Sun et al. (2009), compared ACC deaminase production capacity in mutated and wild type endophytic Burkholderia phytofirmans and their impact in canola. They observed that the mutated strain was unable to promote the growth of canola seedlings while the wild type strain showed remarkable growth promotion. The result ascertains that endophytes affect the growth and development of plants through ACC deaminase enzyme production.
Following a long course of coexistence, endophytes have developed the ability to mimic host plant metabolism and produce effective bioactive compounds similar to their host in vitro as a result of close contact and horizontal gene transfer (Wang et al. 2010). Endophytes produce a vast array of secondary metabolites constituting antibiotics, hydrolytic enzymes, toxins, and volatile organic compounds (VOCs) that play a significant role in alleviating a plant’s defense system for mitigation of stresses (Afzal et al. 2019). Hence, endophytes are also considered as an emerging source of novel bioactive compounds (Singh et al. 2017a). Endophytic Streptomyces spp. provides resistance in chickpea by enhancing the level of defense-related compounds such as phenols and flavonoids (Singh and Gaur 2017). Kang et al. (2018) observed an increased level of nematicidal compounds such as 4-vinyl phenol, L-methionine, palmitic acid, and piperine in plants colonized by Bacillus simplex, inhibited soybean cyst nematode. Co-inoculation of endophytic fungi Beauveria bassiana and mycorrhizae increases terpenoids levels in tomato plant leaves, reducing the foliar feeding by herbivores (Shrivastava et al. 2015). Endophytes also activate the defense pathway by modulating systemic acquired resistance in the plant. Endophyte actinobacteria isolated from the wheat plant induced the genes of SAR such as PR-1 and PR-5 genes and PDF-1.2 and Hel genes to regulate JA and ethylene pathway and confers resistance against several fungal phytopathogens in A. thaliana (Conn et al. 2008). A similar study was also reported by Gond et al. (2015a), that endophytic bacteria, Bacillus amyloliquefaciens activate JA-dependent defense pathway by increasing the expression of PR-1 and PR-10 genes against the attack of fungal pathogens and enhanced the growth and development of maize plant. Endophytes also protect plants from oxidation through excessive pesticidal application by producing antioxidants (Jan et al. 2020).
Quorum sensing (QS) is responsible for communication between host and pathogenic microbes as well as other bacterial symbionts via signaling molecules like N-acyl-homoserine lactone (AHL). Quorum sensing is a density-dependent gene expression in bacteria. As the density increases, the signaling also increases and all cells act somewhat like multicellular organisms (Rosenblueth and Martínez-Romero 2006). The regulation of gene expression in phytopathogenic bacteria needs to produce antibiotics, virulence factors, and exoenzymes to degrade cell walls and to infect plants (Von Bodman et al. 2003). Plant under stress conditions produce signal molecules or mimic the bacterial QS to manipulate the QS-regulated behavior of phytopathogenic bacteria (Bauer and Mathesius 2004). Endophytic bacteria isolated from Cannabis sativa were investigated to disrupt cell-to-cell quorum sensing signals in Chromobacterium violaceum and were proved to act as biocontrol agents for bacterial phytopathogens (Kusari et al. 2014). In the same way, endophytic isolates of phylum Actinobacteria isolated from Phaseolus vulgaris provide resistance from phytopathogenic Gram-positive bacteria disruption of QS (Lopes et al. 2015). A detailed list of endophytes that have been studied in plant protection and stress management and their respective features of interest is provided in Table 3.
Table 3.
Endophytes in plant health and defense
| Endophytes | Host plant | Features of interest | Reference(s) |
|---|---|---|---|
| Neotyphodium coenophialum | Festuca arundinacea | Alteration of root activity and mineral transport from root to shoot under phosphate limited condition | Malinowski et al. (2000) |
| Penicillium verruculosum RS7PF | Potentilla fulgens L. | Promotion of seed germination in green-gram and chickpea by IAA modulation | Bhagobaty et al. (2010) |
| Acinetobacter johnsonii | Beta vulgaris |
Production of IAA for plant growth and development Production of phosphatase enzyme for enhanced nutrient absorption by host plant |
Shi et al. (2011) |
| Bacillus weihenstephanensis, Serratia marcescens, and Cornyebacterium minutissimum | Solanum lycopersicum and Capsicum annum |
Antagonism against wilt-causing bacteria Ralstonia solanacearum Production of secondary metabolites for increased plant growth |
Amaresan et al. (2012) |
| Sporosarcina aquimarina | Avicennia marina |
Promotion of plants growth by IAA production Enhancement of nutrient availability by production of siderophore and phosphatase |
Janarthine and Eganathan (2012) |
| Piriformospora indica | Hordeum vulgare L. | Increased grain yield and shoot biomass under low temperature condition | Murphy et al. (2014) |
| Trichoderma brevicompactum | Allium sativum |
Production of bioactive compound (trichodermin) Antifungal activity against fungal phytopathogens |
Shentu et al. (2014) |
| Bacillus spp. | Zea mays |
Inhibition of phytopathogens through antifungal lipopeptide Regulation of genes associated to production of pathogenesis-related (PR) proteins |
Gond et al. (2015b) |
| Pantoea spp. and Paenibacillus spp. | Triticum aestivum |
Biocontrol activity against F. graminearum by biofilm formation Modulation of plant growth through production of IAA, siderophore, and phosphatase |
Herrera et al. (2016) |
| Pseudomonas aeruginosa, Bacillus spp., Enterobacter spp., Shinella spp. | Saccharum officinarum | Promotion of plant growth by IAA production, phosphorous solubilization, siderophore production, and biocontrol activity |
Taulé et al. (2016) Pirhadi et al. (2018) |
| Bacillus subtilis | Cicer arietinum L. |
Improvement in plant growth under salinity condition Protection against root rot causing fungal phytopathogen (F. solani) |
Egamberdieva et al. (2017) |
| Stenotrophomonas maltophilia, Prototheora geniculata, Bacillus amyloliquefaciens, Stenotrophomonas maltophilia, and Bacillus licheniformis | Solanum lycopersicum | Production of IAA and phosphatase for increased plant growth and development | Abdallah et al. (2018) |
| Hypocrea lixii F3ST1 | Allium cepa | Boosting of plant immunity and reducing damage caused by Iris yellow spot virus and its vector Thrips tabaci | Muvea et al. (2018) |
| Curtobacterium spp., Methylobacterium spp., Microbacterium spp., and Bacillus amyloliquefaciens | Urochloa ramosa L. |
Inhibition of fungal phytopathogens by lipopeptide Promotion of seedling growth Regulation of expression of defense-related genes |
Verma (2018) |
| Acinetobacter calcoaceticus, Enterobacter cloacae, and Bacillus cereus | Glycine max |
Promotion of plant growth through IAA and siderophore production Fixation of atmospheric nitrogen Phosphate solubilization |
Zhao et al. (2018) |
| Acinetobacter baumannii | Capsicum annum | Induction of secondary metabolites production having antioxidant property | Monowar et al. (2019) |
| Pseudomonas aeruginosa | Cucumis sativus |
Suppression of damping off phytopathogen (Pythium aphanidermatum) Plant growth promotion activity |
Priyanka et al. (2019) |
| Bacillus subtilis, Bacillus pumilus, and Klebsiella pneumoniae | Oryzae sativa | Antagonism against fungal phytopathogens | Kumar et al. (2020) |
| Enterobacter cloacae, Enterobacter spp., Bacillus subtilis, Pseudomonas aeruginosa, Bacillus subtilis, Enterobacter spp., Enterobacter hormaechei, Staphylococcus equorum, Pantoea spp., and Mixta intestinalis | Cicer arietinum L. |
Production of plant growth-promoting components (IAA, mineral solubilization, NH3 production, siderophore production, protease activity) Antagonism against phytopathogens |
Mukherjee et al. (2020) |
Endophytes for sustainable management of environmental pollution
Detoxification of heavy metals (HMs)
Rapid industrialization and urbanization without proper planning is adversely affecting the environment through contamination or pollution. One such pollution is the increasing deposition of HMs and pesticides in soil which is has a direct impact on crop production and human health. HMs and their isotopes are categorized under elemental pollutants while residual pesticides are categorized under organic pollutants. As per WHO (1996), the maximum permissible limit of HMs is (0.8, 50, 36, 100, 85, and 35 mg kg−1) in soils and (0.02, 0.6, 1, 1.3, 2, and 10 mg kg−1) in plant with respect of Cd, Zn, Cu, Cr, Pb, and Ni. However, the amount of these HMs is ever-increasing in the soil and the plants leading to several fatal human diseases. Endophytes perform the remediation process more effectively than rhizospheric microbes because of their close contact with host plants, since plants growing in HM contaminated soil naturally employ endophytes with HM-degrading genes. Siciliano et al. (2001) support this fact as they reported that endophytes perform degradation of nitroaromatic compounds more effectively than the rhizospheric microbial community. This was due to the presence of nitro-aromatics degradation genes being prevalent in endophytes than other soil microbes. Microbes and/or genetically engineered microbes have the capacity of reducing soil contamination (Pilon Smits et al. 1999). Research studies have reported that endophytes such as A. calcoaceticus, B. cereus, P. putida, Trichoderma spp., Cladosporium spp., P. polymyxa, P. fluorescens, Paecilomyces spp., B. subtilis, Rhizobium spp., E. pisciphila, R. rubrum, P. agglomerans, Aspergillus spp., Mucor spp., Microsphaeropsis spp., Alternaria spp., Phoma spp., Peyronellaea spp., Steganosporium spp., and Azotobacter spp. have the ability to produce different extracellular oxidase enzymes such as manganese peroxidase, laccase, and lithium peroxidase that helps to degrade various phenolics substance and it directly linked to the remediation process (Ongena and Jacques 2008; Nandy et al. 2020). The removal of HMs is mostly done through absorption, transformation, phytoextraction, hyperaccumulation, and translocation. The organic pollutants are mostly removed by the process of mineralization, degradation, and detoxification (Meagher 2000). A list of endophytes studied for HMs detoxification and their respective host(s) is provided in Table 4.
Table 4.
Endophytes in bioremediation of heavy metals (HMs)
| Host plant | Microbes | HMs bioremediated | Reference(s) |
|---|---|---|---|
| Salix variegate Franch | Chromosporium spp., Fusarium spp., and Gonatobotrysi spp. | Cd | An et al. (2015) |
| Solanum lycopersicum L. | P. janthinellum LK5 | Al | Khan et al. (2015a) |
| Populus spp. | Serenpidita vermifera P04 | Cd, Zn, Pb, and Cu | Lacercat-Didier et al. (2016) |
| Dysphania ambrosioides L. | Plectosphaerella spp., Cladosporium spp., and Verticillium spp. | Pb and Zn | Li et al. (2016) |
| Penicillium spp. FT2G59 and P. columnaris FT2G7 | |||
| Imperata cylindrica L. and Bothriochloa ischaemum L. | Leotiomycetes and Pezizomycetes | Pb and Cd | Tong et al. (2017) |
| Solanum nigrum L. | Colletotrichum spp., Alternaria spp., and Fusarium spp. | Cd | Khan et al. 2017a, b |
| F. tricinctum, and A. alternata | Cd | ||
| Brassica napus L. | Fusarium spp., Penicillium spp., and Alternaria spp. | Cd and Pb | Shi et al. (2017) |
| Zea mays | Westerdykella spp. | Hg | Pietro-Souza et al. (2020) |
Detoxification of pesticides
Injudicious use of fertilizers and pesticides has caused many physical and physiological discomforts in plants as well as in animals. Discolouration, necrosis, and deformation are the major physical impact of excessive pesticides on plants (Geetha 2019) which have significant effects on physiological and biochemical processes (Chaudhary et al. 2020; Giménez-Moolhuyzen et al. 2020). The pesticides accumulate in soil mostly through the process of leaching which leads to deterioration of soil fertility and soil microbial community. Endophytic microbes play an important role to minimize and degrade inside the plant body. A study on bark, xylem tissue, and leaves of tea plants showed that there are no significant changes in community structure and number of endophytic colonies in the phyllosphere after pesticide treatment (Win et al. 2021). Seed treatment with pesticides resulted in alteration of rhizosphere fungal and bacterial community in maize plant but leaf fungal endophytic colonies remain unaffected (Nettles et al. 2016). Another study on the community-level effect of different concentrations of pesticide N-(3,5-dichlorophenyl) succinimide on Nicotiana tabacum phyllosphere showed that there was no significant impact on alpha and beta diversity of beneficial endophytic bacterial community, viz., Alphaproteobacteria, Gammaproteobacteria, Sphingomonas, and Pseudomonas (Chen et al. 2021). All these reports suggest that leaf endophytes are more resistant to pesticides and are well-suited candidates for degradation agrochemicals.
There are a number of studies on endophytes revealing that these microbes establish a symbiotic relationship with their host and secrete enzymes to metabolize and detoxify various pesticides. For example, an endophytic Pseudomonas spp. possesses gene encoding organophosphate hydrolase enzyme which is responsible for degradation of 97% of organophosphate pesticide such as chlorpyrifos (Barman et al. 2014a, b). A group of endophytes having a symbiotic relationship with P. fugax (one of the major winter weeds in the oilseed rape field in China) helped to promote resistance from quizalofop-p-ethyl, an acetyl CoA carboxylase-inhibiting herbicide (Liu et al. 2020). In another study, it was shown that endophytic bacteria Pantoea ananatis Sd-1 degrade carbaryl by secreting hydrolytic enzyme carbohydrate esterase (Yao et al. 2020). This enzyme esterase is a major enzyme in hydrolysis of other pesticidal compounds as well namely: organochlorines, pyrethroids, and carbamates (Sharma et al. 2018). Since endophytes exhibit significant growth and multiplication rate within plant tissue, they can be used as a potential tool for the bioremediation of environmental contaminants such as xenobiotics and pesticides (Gupta et al. 2020; Win et al. 2021). Moreover, plant–endophyte interaction not only enhances phytoextraction or phytoremediation of environmental pollutants but they perform an excellent job for plant growth promotion even under biotic and abiotic stress conditions (Waller et al. 2005; Becerra-Castro et al. 2013). A detailed list of endophytes studied in the bioremediation of pesticides and their respective host(s) and properties is provided in Table 5.
Table 5.
Endophytes in bioremediation of pesticides
| Endophytes | Host plant | Pesticide bioremediated | Properties | Reference(s) |
|---|---|---|---|---|
| Sphingomonas spp. | Cytisus striatus | Hexachlorocyclohexane |
Phytoremediation of HCH Enhancement of plant growth |
Becerra-Castro et al. (2013) |
| Pseudomonas spp. | Balloon flower | Chlorpyrifos | Synthesis of organophosphate hydrolase enzyme | Barman et al. (2014a, b) |
| Polypogon fugax | Quizalofop-p-ethyl | Degradation of acetyl-CoA carboxylase-inhibiting herbicide | Liu et al. (2020) | |
| Rhizobium leguminosarum | Pisum sativum | Kitazin | Production of plant growth-promoting bioactive compounds to enhance plant growth under pesticide stress condition | Shahid et al. (2018) |
| Alphaproteobacteria, Gammaproteobacteria, Sphingomonas, and Pseudomonas spp. | Nicotiana tabacum | N-(3,5-Dichlorophenyl) succinimide | Negative responders of broad-spectrum pesticide treatments | Chen et al. (2020) |
| Pantoea ananatis | Oryza sativa | Carbaryl | Secretion of carbohydrate esterase to hydrolyze carbaryl | Yao et al. (2020) |
Endophytes for human health
Endophytes are a very precious source of secondary metabolites of which many are antioxidant, antimicrobial, and anticancerous. They are just like a treasure house of bioactive molecules that needs to be explored. Many of these bioactive molecules can be used for the management of human diseases either directly or after transformation (Devi and Prabakaran 2014; Gouda et al. 2016). The trait of producing bioactive molecules have been incorporated in them through the transfer of genetic information from higher plants during evolution as explained earlier. The classical example of this fact is taxol-producing endophyte Metarhizium anisopliae isolated from the bark of Taxus spp., which is a very important life-saving anticancer agent (Zhang et al. 2009). Going by the classical example, endophytes associated with medicinal plants can be a very eminent source of bioactive molecules and can be utilized for producing natural drugs (Singh and Dubey 2015). Several bioactive compounds constituting vinblastine, paclitaxel, camptothecin, hypericin, etc. are already produced at a commercial scale from the endophytes isolated from their respective plants and are of pharmaceutical importance (Nicoletti and Fiorentino 2015). Endophytes are also gaining the limelight for human health because many studies suggest that novel bioactive molecules produced by them are important for combating antibiotic resistance by human pathogenic microbes (Fadiji and Babalola 2020).
Endophytes are also a good source of antioxidant compounds that are now deemed to be a potential alternative for the prevention and treatment of human diseases linked with reactive oxygen species (ROS). Thus, diseases such as diabetes, hypertension, cancer, Alzheimer, ischemia, and Parkinson can be treated with the help of antioxidants derived from endophytes (Mishra et al. 2014). Many prevalent human deficiency diseases can be overcome by taking that particular nutrient through diet. Plants form a major part of the diet and their biofortification with nutrients can help in providing the deficient nutrient to the human population naturally as a replacement of chemical supplements. Endophytes can be an integral part of this concept as well, since, many of the reports have proved that endophytic microbes associated to crop also helps in biofortification (Singh et al. 2017b; Trivedi et al. 2020a). The underlying mechanisms in crop biofortification by endophytes are improvement of nutrient absorption, direct synthesis and release of micronutrients, and induction of micronutrient synthesis in plants (Ku et al. 2019). The list of potential use of endophytes for human health also continues to grow with the advancements in science.
Conclusions
Excessive use of synthetic fertilizers and pesticides and changing environment has led to unfertile agricultural lands causing a major problem in feeding the growing population. The inevitable concern arising due to this is enhancing the crop productivity under shrinking land and minimization of chemical inputs. Hence, we have provided some critical insights about an emerging alternative of utilizing the plant endophytic microbiome for combating the concern. Endophytes are significantly influential and are providing us with the opportunity to overcome the global problem of agricultural productivity. Augmentation of indigenous and effective beneficial endophytes has the potential to bring consequential positive impacts on the current agriculture scenario by improving soil and yield quality. Endophytes have more potential than other rhizosphere microbes as they can be inoculated in the same plant species from which they are isolated and can easily colonize inside the plant body to provide sustainable crop productivity and food security under different environmental stresses. A consortium of endophytic microbes can be more effective as climate-resilient biofertilizers and biocontrol agents. In addition, the consortium can be a powerful approach for boosting plant growth and productivity along with the maintenance of the soil microbial community. The approach is environment friendly, ecologically sound, and socially acceptable. However, the studies on the effects of endophytic microbial consortium are very limited and should be explored further in combination with plant-growth-promoting microbes (PGPMs) for boosting the productivity in agricultural crops and improvement of soil health under different environmental conditions.
Acknowledgements
The authors want to thank the Head of the Department, Institute of Environment and Sustainable Development, Banaras Hindu University. The authors especially are grateful to Science and Engineering Research Board (Ref. File No. EEQ/2017/000775) and the Government of India, for financial assistance to research endophytic microbes of chickpea. The authors are also thankful to IoE (6031) BHU, Varanasi for providing funds for research related to endophytes. MMR is thankful to University Grants Commission for providing financial assistance through NF-OBC (NFO-2018-19-OBC-BIH-68765).
Author contributions
AM contributed to the preparation of the manuscript, its formatting and collection of data for writing and preparing figures. MMR, GKC and JKV contributed to creating figures and editing the manuscript. SB, GKC and SY contributed to the collection of data for preparing the tables. JPV contributed to the design, idea, final editing, and revision of the whole manuscript.
Declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
References
- Abdallah RA, Jabnoun-Khiareddine H, Nefzi A, Daami-Remadi M. Evaluation of the growth-promoting potential of endophytic bacteria recovered from healthy tomato plants. J Hortic for. 2018;5:2. doi: 10.4172/2376-0354.1000234. [DOI] [Google Scholar]
- Afzal I, Shinwari ZK, Sikandar S, Shahzad S. Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol Res. 2019;221:36–49. doi: 10.1016/j.micres.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Ahsan MT, Saeed A, Mustafa T, Afzal M. Augmentation with potential endophytes enhances phytostabilization of Cr in contaminated soil. Environ Sci Pollut Res. 2018;25(7):7021–7032. doi: 10.1007/s11356-017-0987-x. [DOI] [PubMed] [Google Scholar]
- Akinsanya MA, Goh JK, Lim SP, Ting AS. Diversity, antimicrobial and antioxidant activities of culturable bacterial endophyte communities in Aloe vera. FEMS Microbiol Lett. 2015;362(23):fnv184. doi: 10.1093/femsle/fnv184. [DOI] [PubMed] [Google Scholar]
- Amaresan N, Jayakumar V, Kumar K, Thajuddin N. Endophytic bacteria from tomato and chilli, their diversity and antagonistic potential against Ralstonia solanacearum. Arch Phytopathol. 2012;45:344–355. doi: 10.1080/03235408.2011.587273. [DOI] [Google Scholar]
- An H, Liu Y, Zhao X, Huang Q, Yuan S, Yang X, Dong J. Characterization of cadmium-resistant endophytic fungi from Salix variegata Franch. in three Gorges Reservoir Region. China Microbiol Res. 2015;176:29–37. doi: 10.1016/j.micres.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Barman DN, Haque MA, Islam SMA, Yun HD, Kim MK. Cloning and expression of ophB gene encoding organophosphorus hydrolase from endophytic Pseudomonas spp. BF1-3 degrades organophosphorus pesticide chlorpyrifos. Ecotoxicol Environ Saf. 2014;108:135–141. doi: 10.1016/j.ecoenv.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Barman DN, Haque MA, Islam SMA, Yun HD, Kim MK. Cloning and expression of ophB gene encoding organophosphorus hydrolase from endophytic Pseudomonas sp. BF1-3 degrades organophosphorus pesticide chlorpyrifos. Ecotoxicol Environ Saf. 2014;108:135–141. doi: 10.1016/j.ecoenv.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Bastias DA, Martínez-Ghersa MA, Ballaré CL, Gundel PE. Epichloë fungal endophytes and plant defenses: not just alkaloids. Trends Plant Sci. 2017;22:939–948. doi: 10.1016/j.tplants.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Bauer WD, Mathesius U. Plant responses to bacterial quorum sensing signals. Curr Opin Plant Biol. 2004;7(4):429–433. doi: 10.1016/j.pbi.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Becerra-Castro C, Prieto-Fernández Á, Kidd PS, Weyens N, Rodríguez-Garrido B, Touceda-González M, Acea MJ, Vangronsveld J. Improving performance of Cytisus striatus on substrates contaminated with hexachlorocyclohexane (HCH) isomers using bacterial inoculants: developing a phytoremediation strategy. Plant Soil. 2013;362(1):247–260. doi: 10.1007/s11104-012-1276-6. [DOI] [Google Scholar]
- Behie SW, Bidochaka MJ. Insects as a nitrogen source for plants. Insects. 2013;4:413–424. doi: 10.3390/insects4030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagobaty RK, Joshi SR, Kumar R. Penicillium verruculosum RS7PF: a root fungal endophyte associated with an ethno-medicinal plant of the indigenous tribes of Eastern India. Afr J Microbiol Res. 2010;4(9):766–770. doi: 10.5897/AJMR.9000548. [DOI] [Google Scholar]
- Bilal L, Asaf S, Hamayun M, Gul H, Iqbal A, Ullah I, Lee IJ, Hussain A. Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis. 2018;76(2):117–127. doi: 10.1007/s13199-018-0545-4. [DOI] [Google Scholar]
- Brevik EC, Pereg L, Steffan JJ, Burgess LC. Soil ecosystem services and human health. Curr Opin Environ Sci Health. 2018;5:87–92. doi: 10.1016/j.coesh.2018.07.003. [DOI] [Google Scholar]
- Brevik EC, Slaughter L, Singh BR, Steffan JJ, Collier D, Barnhart P, Pereira P. Soil and human health: current status and future needs. Air, Soil Water Res. 2020;13:1–23. doi: 10.1177/1178622120934441. [DOI] [Google Scholar]
- Casas C, Omacini M, Montecchia MS, Correa OS. Soil microbial community responses to the fungal endophyte Neotyphodium in Italian ryegrass. Plant Soil. 2011;340(1):347–355. doi: 10.1007/s11104-010-0607-8. [DOI] [Google Scholar]
- Chaparro JM, Sheflin AM, Manter DK, Vivanco JM. Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils. 2012;48(5):489–499. doi: 10.1007/s00374-012-0691-4. [DOI] [Google Scholar]
- Chaudhary N, Choudhary KK, Agrawal SB, Agrawal M. Pesticides usage, uptake and mode of action in plants with special emphasis on photosynthetic characteristics. Pesticides in crop production. Physiol Biochem Action. 2020 doi: 10.1002/9781119432241.ch9. [DOI] [Google Scholar]
- Chen Y, Ren CG, Yang B, Peng Y, Dai CC. Priming effects of the endophytic fungus Phomopsis liquidambari on soil mineral N transformations. Microb Eco. 2013;65(1):161–170. doi: 10.1007/s00248-012-0093-z. [DOI] [PubMed] [Google Scholar]
- Chen QL, Ding J, Zhu D, Hu HW, Delgado-Baquerizo M, Ma YB, He JZ, Zhu YG. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils. Soil Biol Biochem. 2020;141:107686. doi: 10.1016/j.soilbio.2019.107686. [DOI] [Google Scholar]
- Chen X, Wicaksono WA, Berg G, Cernava T. Bacterial communities in the plant phyllosphere harbour distinct responders to a broad-spectrum pesticide. Sci Total Environ. 2021;751:141799. doi: 10.1016/j.scitotenv.2020.141799. [DOI] [PubMed] [Google Scholar]
- Chouhan GK, Veram JP, Jaiswal DK, Mukherjee A, Singh S, Pereira APA, Liu H, Abd-Allah EF, Singh BK. Phytomicrobiome for promoting sustainable agriculture and food security: opportunities, challenges, and solutions. Microbiol Res. 2021;248:126763. doi: 10.1016/j.micres.2021.126763. [DOI] [PubMed] [Google Scholar]
- Coba de la Peña T, Fedorova E, Pueyo JJ, Lucas MM. The Symbiosome: legume and rhizobia co-evolution toward a nitrogen-fixing organelle? Front Plant Sci. 2017;8:2229. doi: 10.3389/fpls.2017.02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VM, Walker AR, Franco CMM. Endophytic actinobacteria induce defense pathways in Arabidopsis thaliana. Mol Plant Microbe Interact. 2008;21(2):208–218. doi: 10.1094/MPMI-21-2-0208. [DOI] [PubMed] [Google Scholar]
- De Silva NI, Brooks S, Lumyong S, Hyde KD. Use of endophytes as biocontrol agents. Fungal Biol Rev. 2019;33(2):133–148. doi: 10.1016/j.fbr.2018.10.001. [DOI] [Google Scholar]
- Devi KA, Pandey G, Rawat AK, Sharma GD, Pandey P. The endophytic symbiont-Pseudomonas aeruginosa stimulates the antioxidant activity and growth of Achyranthes aspera L. Front Microbiol. 2017;8:1897. doi: 10.3389/fmicb.2017.01897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi NN, Prabakaran JJ. Bioactive metabolites from an endophytic fungus Penicillium sp. isolated from Centella asiatica. Curr Res Environ Appl Mycol. 2014;4(1):34–43. doi: 10.5943/cream/4/1/3. [DOI] [Google Scholar]
- Egamberdieva D, Wirth SJ, Shurigin VV, Hashem A, Abd-Allah EF. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicerarietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front Microbiol. 2017;8:1887. doi: 10.3389/fmicb.2017.01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiji AE, Babalola OO. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front Bioeng Biotechnol. 2020;8:467. doi: 10.3389/fbioe.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzluebbers AJ, Nazih N, Stuedemann JA, Fuhrmann JJ, Schomberg HH, Hartel PG. Soil carbon and nitrogen pools under low-and high-endophyte-infected tall fescue. Soil Sci Soc Am J. 1999;63(6):1687–1694. doi: 10.2136/sssaj1999.6361687x. [DOI] [Google Scholar]
- Geetha A. Phytotoxicity due to fungicides and herbicides and its impact in crop physiological factors. New Delhi, India: AkiNik Publications; 2019. [Google Scholar]
- Giménez-Moolhuyzen M, Blom JVD, Lorenzo-Mínguez P, Cabello T, Crisol Martínez E. Photosynthesis inhibiting effects of pesticides on sweet pepper leaves. Insects. 2020;11(2):69. doi: 10.3390/insects11020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gond SK, Bergen MS, Torres MS, White JF, Kharwar RN. Effect of bacterial endophyte on expression of defense genes in Indian popcorn against Fusarium moniliforme. Symbiosis. 2015;66(3):133–140. doi: 10.1007/s13199-015-0348-9. [DOI] [Google Scholar]
- Gond SK, Bergen MS, Torres MS, White JF. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol Res. 2015;172:79–87. doi: 10.1016/j.micres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Gouda S, Das G, Sen SK, Shin HS, Patra JK. Endophytes: a treasure house of bioactive compounds of medicinal importance. Front Microbiol. 2016;7:1538. doi: 10.3389/fmicb.2016.01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudjal Y, Zamoum M, Sabaou N, Mathieu F, Zitouni A. Potential of endophytic Streptomyces spp. for biocontrol of Fusarium root rot disease and growth promotion of tomato seedlings. Biocontrol Sci Technol. 2016;26(12):1691–1705. doi: 10.1080/09583157.2016.1234584. [DOI] [Google Scholar]
- Guo H, Luo S, Chen L, Xiao X, Xi Q, Wei W, Zeng G, Liu C, Wan Y, Chen J, He Y. Bioremediation of heavy metals by growing hyperaccumulaor endophytic bacterium Bacillus sp. L14. Bioresour Technol. 2010;101(22):8599–8605. doi: 10.1016/j.biortech.2010.06.085. [DOI] [PubMed] [Google Scholar]
- Gupta A, Singh SK, Singh VK, Singh MK, Modi A, Zhimo VY, Singh AV, Kumar A. Endophytic microbe approaches in bioremediation of organic pollutants. In: Kumar A, Singh VP, editors. Microbial endophytes. Woodhead Publishing; 2020. pp. 157–174. [Google Scholar]
- Hamayun M, Hussain A, Iqbal A, Khan SA, Lee IJ. Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. BioMed Res Int. 2018;2018:7696831. doi: 10.1155/2018/7696831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamayun M, Hussain A, Afzal Khan S, Iqbal A, Lee IJ. Aspergillus flavus promoted the growth of soybean and sunflower seedlings at elevated temperature. BioMed Res Int. 2019;2019:1295457. doi: 10.1155/2019/1295457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamonts K, Trivedi P, Garg A, Janitz C, Grinyer J, Holford P, Botha FC, Anderson IC, Singh BK. Field study reveals core plant microbiota and relative importance of their drivers. Environ Microbiol. 2018;20:124–140. doi: 10.1111/1462-2920.14031. [DOI] [PubMed] [Google Scholar]
- Hardoim PR, Van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring M, Sessitsch A. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev. 2015;79:293–320. doi: 10.1128/MMBR.00050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JG, Griffin EA. The diversity and distribution of endophytes across biomes, plant phylogeny, and host tissuesâ how far have we come and where do we go from here? Environ Microbiol. 2020;22(6):2107–2123. doi: 10.1111/1462-2920.14968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan SED. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J Adv Res. 2017;8(6):687–695. doi: 10.1016/j.jare.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Megharaj M, Wu CY, Subashchandrabose SR, Dai CC. Endophyte-assisted phytoremediation: mechanisms and current application strategies for soil mixed pollutants. Crit Rev Biotechnol. 2020;40(1):31–45. doi: 10.1080/07388551.2019.1675582. [DOI] [PubMed] [Google Scholar]
- He C, Zeng Q, Chen Y, Chen C, Wang W, Hou J, Li X. Colonization by dark septate endophytes improves the growth and rhizosphere soil microbiome of licorice plants under different water treatments. Appl Soil Ecol. 2021;166:103993. doi: 10.1016/j.apsoil.2021.103993. [DOI] [Google Scholar]
- Herrera SD, Grossi C, Zawoznik M, Groppa MD. Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol Res. 2016;186:37–43. doi: 10.1016/j.micres.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Hou WP, Wang JF, Nan ZB, Christensen MJ, Xia C, Chen T, Zhang ZX, Niu XL. Epichloë gansuensis endophyte-infection alters soil enzymes activity and soil nutrients at different growth stages of Achnatherum inebrians. Plant Soil. 2020;455(1):227–240. doi: 10.1007/s11104-020-04682-2. [DOI] [Google Scholar]
- Ikram M, Ali N, Jan G, Jan FG, Rahman IU, Iqbal A, Hamayun M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS ONE. 2018;13(11):0208150. doi: 10.1371/journal.pone.0208150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry I, White JF. Application of bacteria from non-cultivated plants to promote growth, alter root architecture and alleviate salt stress of cotton. J Appl Microbiol. 2017;122:1110–1120. doi: 10.1111/jam.13414. [DOI] [PubMed] [Google Scholar]
- Jan S, Singh R, Bhardwaj R, et al. Plant growth regulators: a sustainable approach to combat pesticide toxicity. 3 Biotech. 2020;10:466. doi: 10.1007/s13205-020-02454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janarthine S, Eganathan P. Plant growth promoting of endophytic Sporosarcina aquimarina SjAM16103 isolated from the pneumatophores of Avicennia marina L. Int J Microbiol. 2012 doi: 10.1155/2012/532060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannoura R, Joergensen RG, Bruns C. Organic fertilizer effects on growth, crop yield, and soil microbial biomass indices in sole and intercropped peas and oats under organic farming conditions. Eur J Agron. 2014;52:259–270. doi: 10.1016/j.eja.2013.09.001. [DOI] [Google Scholar]
- Jasim B, Anisha C, Rohini S, Kurian JM, Jyothis M, Radhakrishnan EK. Phenazine carboxylic acid production and rhizome protective effect of endophytic Pseudomonas aeruginosa isolated from Zingiber officinale. J Microbiol Biotechnol. 2014;30(5):1649–1654. doi: 10.1007/s11274-013-1582-z. [DOI] [PubMed] [Google Scholar]
- Johnston-Monje D, Lundberg DS, Lazarovits G, Reis VM, Raizada MN. Bacterial populations in juvenile maize rhizospheres originate from both seed and soil. Plant Soil. 2016;405:337–355. doi: 10.1007/s11104-016-2826-0. [DOI] [Google Scholar]
- Kang W, Zhu X, Wang Y, Chen L, Duan Y. Transcriptomic and metabolomic analyses reveal that bacteria promote plant defense during infection of soybean cyst nematode in soybean. BMC Plant Biol. 2018;18(1):1–14. doi: 10.1186/s12870-018-1302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen DL, Veum KS, Sudduth KA, Obrycki JF, Nunes MR. Soil health assessment: past accomplishments, current activities, and future opportunities. Soil Tillage Res. 2019;195:104365. doi: 10.1016/j.still.2019.104365. [DOI] [Google Scholar]
- Kawasaki A, Donn S, Ryan PR, Mathesius U, Devilla R, Jones A, Watt M. Microbiome and exudates of the root and rhizosphere of Brachypodium distachyon, a model for wheat. PloSOne. 2016;11(10):e0164533. doi: 10.1371/journal.pone.0164533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper KJ, Lal R. Pay dirt! human health depends on soil health. Complement Ther Med. 2017;32:A1–A2. doi: 10.1016/j.ctim.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Khalaf EM, Raizada MN. Bacterial seed endophytes of domesticated cucurbits antagonize fungal and oomycete pathogens including powdery mildew. Front Microbiol. 2018;9:42. doi: 10.3389/fmicb.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AL, Waqas M, Hussain J, Al-Harrasi A, Hamayun M, Lee IJ. Phytohormones enabled endophytic fungal symbiosis improve aluminum phytoextraction in tolerant Solanum lycopersicum: An examples of Penicillium janthinellum LK5 and comparison with exogenous GA3. J Hazard Mater. 2015;295:70–78. doi: 10.1016/j.jhazmat.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Khan AR, Ullah I, Waqas M, Shahzad R, Hong SJ, Park GS, Jung BK, Lee IJ, Shin JH. Plant growth-promoting potential of endophytic fungi isolated from Solanum nigrum leaves. World J Microbiol Biotechnol. 2015;31(9):1461–1466. doi: 10.1007/s11274-015-1888-0. [DOI] [PubMed] [Google Scholar]
- Khan AR, Ullah I, Waqas M, Park GS, Khan AL, Hong SJ, Ullah R, Jung BK, Park CE, Ur-Rehman S, Lee IJ. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotox Environ Safe. 2017;136:180–188. doi: 10.1016/j.ecoenv.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Khan AR, Waqas M, Ullah I, Khan AL, Khan MA, Lee IJ, Shin JH. Culturable endophytic fungal diversity in the cadmium hyperaccumulator Solanum nigrum L. and their role in enhancing phytoremediation. Environ Exp Bot. 2017;135:126–135. doi: 10.1016/j.envexpbot.2016.03.005. [DOI] [Google Scholar]
- Ku YS, Rehman HM, Lam HM. Possible roles of rhizospheric and endophytic microbes to provide a safe and affordable means of crop biofortification. Agronomy. 2019;9(11):764. doi: 10.3390/agronomy9110764. [DOI] [Google Scholar]
- Kumar V, Jain L, Jain SK, Chaturvedi S, Kaushal P. Bacterial endophytes of rice (Oryza sativa L.) and their potential for plant growth promotion and antagonistic activities. S Afr J Bot. 2020;134:50–63. doi: 10.1016/j.sajb.2020.02.017. [DOI] [Google Scholar]
- Kusari P, Kusari S, Lamshöft M, Sezgin S, Spiteller M, Kayser O. Quorum quenching is an antivirulence strategy employed by endophytic bacteria. Appl Microbiol Biotechnol. 2014;98(16):7173–7183. doi: 10.1007/s00253-014-5807-3. [DOI] [PubMed] [Google Scholar]
- Lacercat-Didier L, Berthelot C, Foulon J, Errard A, Martino E, Chalot M, Blaudez D. New mutualistic fungal endophytes isolated from poplar roots display high metal tolerance. Mycorrhiza. 2016;26(7):657–671. doi: 10.1007/s00572-016-0699-y. [DOI] [PubMed] [Google Scholar]
- Lally RD, Galbally P, Moreira AS, Spink J, Ryan D, Germaine KJ, Dowling DN. Application of endophytic Pseudomonas fluorescens and a bacterial consortium to Brassica napus can increase plant height and biomass under greenhouse and field conditions. Front Plant Sci. 2017;8:2193. doi: 10.3389/fpls.2017.02193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Wei DQ, Shen M, Zhou ZP. Endophytes and their role in phytoremediation. Fungal Divers. 2012;54(1):11–18. doi: 10.1007/s13225-012-0165-x. [DOI] [Google Scholar]
- Li Y, Wang Q, Wang L, He LY, Sheng XF. Increased growth and root Cu accumulation of Sorghum sudanense by endophytic Enterobacter sp. K3–2: implications for Sorghum sudanense biomass production and phytostabilization. Ecotox Environ Safe. 2016;124:163–168. doi: 10.1016/j.ecoenv.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Lin L, Wei C, Chen M, Wang H, Li Y, Li Y, Yang L, An Q. Complete genome sequence of endophytic nitrogen-fixing Klebsiella variicola strain DX120E. Stand Genom Sci. 2015;10(1):1–7. doi: 10.1186/s40793-015-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang X, Pu H, Liu S, Kan J, Jin C. Recent advances in endophytic exopolysaccharides: production, structural characterization, physiological role and biological activity. Carbohydr Polym. 2017;157:1113–1124. doi: 10.1016/j.carbpol.2016.10.084. [DOI] [PubMed] [Google Scholar]
- Liu H, Brettell LE, Qiu Z, Singh BK. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020 doi: 10.1016/j.tplants.2020.03.014. [DOI] [PubMed] [Google Scholar]
- Liu H, Li J, Carvalhais LC, Percy CD, Verma JP, Schenk PM, Singh BK. Evidence for the plant recruitment of beneficial microbes to suppress soil borne pathogens. New Phytol. 2021;229:2873–2885. doi: 10.1111/nph.17057. [DOI] [PubMed] [Google Scholar]
- Liu D, Yan R, Fu Y, Wang X, Zhang J, Xiang W. Antifungal, plant growth-promoting, and genomic properties of an endophytic actinobacterium Streptomyces sp. NEAU-S7GS2. Front Microbiol. 2019;10:2077. doi: 10.3389/fmicb.2019.02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes RBM, de Oliveira Costa LE, Vanetti MCD, de Araújo EF, de Queiroz MV. Endophytic bacteria isolated from common bean (Phaseolus vulgaris) exhibiting high variability showed antimicrobial activity and quorum sensing inhibition. Curr Microbiol. 2015;71(4):509–516. doi: 10.1007/s00284-015-0879-6. [DOI] [PubMed] [Google Scholar]
- Lubna S, Hamayun M, Gul H, Lee IJ, Hussain A. Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J Plant Interact. 2018;13(1):100–111. doi: 10.1080/17429145.2018.1436199. [DOI] [Google Scholar]
- Malinowski DP, Alloush GA, Belesky DP. Leaf endophyte Neotyphodium coenophialum modifies mineral uptake in tall fescue. Plant Soil. 2000;227(1):115–126. doi: 10.1023/A:1026518828237. [DOI] [Google Scholar]
- Meagher RB. Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol. 2000;3(2):153–162. doi: 10.1016/S1369-5266(99)00054-0. [DOI] [PubMed] [Google Scholar]
- Mehmood A, Hussain A, Irshad M, Hamayun M, Iqbal A, Khan N. In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis. 2019;77(3):225–235. doi: 10.1007/s13199-018-0583-y. [DOI] [Google Scholar]
- Miller RM, Jastrow JD. Mycorrhizal fungi influence soil structure. In: Kapulnik Y, Douds DD, editors. Arbuscular Mycorrhizas: physiology and function. Springer, Dordrecht; 2000. [Google Scholar]
- Mishra Y, Singh A, Batra A, Sharma M. Understanding the biodiversity and biological applications of endophytic fungi: A review. J Microb Biochem Technol. 2014;8:4. doi: 10.4172/1948-5948.S8-004. [DOI] [Google Scholar]
- Monowar T, Rahman M, Bhore SJ, Raju G, Sathasivam KV. Secondary metabolites profiling of Acinetobacter baumannii associated with chili (Capsicumannuum L.) leaves and concentration dependent antioxidant and prooxidant properties. BioMed Res Int. 2019;2019:6951927. doi: 10.1155/2019/6951927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa WK, Shearer CR, Limay-Rios V, Ettinger CL, Eisen JA, Raizada MN. Root-hair endophyte stacking in finger millet creates a physicochemical barrier to trap the fungal pathogen Fusarium graminearum. Nature Microbiol. 2016;1:16167. doi: 10.1038/nmicrobiol.2016.167. [DOI] [PubMed] [Google Scholar]
- Mowafy AM, Fawzy MM, Gebreil A, Elsayed A. Endophytic Bacillus, Enterobacter, and Klebsiella enhance the growth and yield of maize. Acta Agric Scand B Soil Plant Sci. 2021;71(4):1–10. doi: 10.1080/09064710.2021.1880621. [DOI] [Google Scholar]
- Mukherjee A, Gaurav AK, Singh S, Chouhan GK, Kumar A, Das S. Role of potassium (K) solubilising microbes (KSM) in growth and induction of resistance against biotic and abiotic stress in plant: a book review. Climate Change Environ Sustain. 2019;7(2):212–214. [Google Scholar]
- Mukherjee A, Singh B, Verma JP. Harnessing chickpea (Cicerarietinum L.) seed endophytes for enhancing plant growth attributes and bio-controlling against Fusarium sp. Microbiol Res. 2020 doi: 10.1016/j.micres.2020.126469. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Chouhan GK, Gaurav AK, Jaiswal DK, Verma JP. Development of indigenous microbial consortium for biocontrol management. In: Verma JP, Macdonald C, Gupta V, Podile A, editors. New and future developments in microbial biotechnology and bioengineering phytomicrobiome for sustainable agriculture. Amsterdam: Elsevier; 2021. pp. 91–110. [Google Scholar]
- Murphy BR, Doohan FM, Hodkinson TR. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis. 2014;62(1):29–39. doi: 10.1007/s13199-014-0268-0. [DOI] [Google Scholar]
- Muvea AM, Subramanian S, Maniania NK, Poehling HM, Ekesi S, Meyhöfer R. Endophytic colonization of onions induces resistance against Viruliferous thrips and virus replication. Front Plant Sci. 2018;9:1785. doi: 10.3389/fpls.2018.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naab JB, Mahama GY, Yahaya I, Prasad PVV. Conservation agriculture improves soil quality, crop yield, and incomes of smallholder farmers in North Western Ghana. Front Plant Sci. 2017;8:996. doi: 10.3389/fpls.2017.00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandy S, Das T, Tudu CK, Pandey DK, Dey A, Ray P. Fungal endophytes: futuristic tool in recent research area of phytoremediation. S Afr J Bot. 2020;134:285–295. doi: 10.1016/j.sajb.2020.02.015. [DOI] [Google Scholar]
- Narisawa K. The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol. 2017;28:1–10. doi: 10.1016/j.funeco.2017.04.001. [DOI] [Google Scholar]
- Nettles R, Watkins J, Ricks K, Boyer M, Licht M, Atwood LW, Peoples M, Smith RG, Mortensen DA, Koide RT. Influence of pesticide seed treatments on rhizosphere fungal and bacterial communities and leaf fungal endophyte communities in maize and soybean. Appl Soil Ecol. 2016;102:61–69. doi: 10.1016/j.apsoil.2016.02.008. [DOI] [Google Scholar]
- Nicoletti R, Fiorentino A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture. 2015;5:918–970. doi: 10.3390/agriculture5040918. [DOI] [Google Scholar]
- Ongena M, Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16(3):115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Oses-Pedraza R, Torres-Díaz C, Lavín P, Retamales-Molina P, Atala C, Gallardo-Cerda J, Acuña-Rodríguez IS, Molina-Montenegro MA. Root endophytic Penicillium promotes growth of Antarctic vascular plants by enhancing nitrogen mineralization. Extremophiles. 2020;24(5):721–732. doi: 10.1007/s00792-020-01189-7. [DOI] [PubMed] [Google Scholar]
- Otieno N, Lally RD, Kiwanuka S, Lloyd A, Ryan D, Germaine KJ, Dowling DN. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JK, Madaan S, Archana G. Antibiotic producing endophytic Streptomyces spp. colonize above-ground plant parts and promote shoot growth in multiple healthy and pathogen-challenged cereal crops. Microbiol Res. 2018;215:36–45. doi: 10.1016/j.micres.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Pawlowski K, Demchenko KN. The diversity of actinorhizal symbiosis. Protoplasma. 2012;249:967–979. doi: 10.1007/s00709-012-0388-4. [DOI] [PubMed] [Google Scholar]
- Pham VT, Rediers H, Ghequire MG, Nguyen HH, De Mot R, Vanderleyden J, Spaepen S. The plant growth-promoting effect of the nitrogen-fixing endophyte Pseudomonas stutzeriA15. Arch Microbiol. 2017;199(3):513–517. doi: 10.1007/s00203-016-1332-3. [DOI] [PubMed] [Google Scholar]
- Pietro-Souza W, de Campos PF, Mello IS, Stachack FF, Terezo AJ, da Cunha CN, White JF, Li H, Soares MA. Mercury resistance and bioremediation mediated by endophytic fungi. Chemosphere. 2020;240:124874. doi: 10.1016/j.chemosphere.2019.124874. [DOI] [PubMed] [Google Scholar]
- Pilon Smits EAH, De Souza MP, Hong G, Amini A, Bravo RC, Payabyab ST, Terry N. Selenium volatilization and accumulation by twenty aquatic plant species. J Environ Qual. 1999;28(3):1011–1018. doi: 10.2134/jeq1999.00472425002800030035x. [DOI] [Google Scholar]
- Pirhadi M, Enayatizamir N, Motamedi H, Sorkheh K. Impact of soil salinity on diversity and community of sugarcane endophytic plant growth promoting bacteria (Saccharumofficinarum L. Var. CP48) Appl Ecol Environ. 2018;16:725–739. doi: 10.15666/aeer/1601_725739. [DOI] [Google Scholar]
- Priyanka S, Krishnamoorthy AS, Latha P, Kalaiselvi T. Exploration of bacterial endophytes in cucumber (Cucumissativus L.) Madras Agri J. 2019;106(4–6):406–414. [Google Scholar]
- Puente ME, Li CY, Bashan Y. Endophytic bacteria in cacti seeds can improve the development of cactus seedlings. Environ Exp Bot. 2009;66(3):402–408. doi: 10.1016/j.envexpbot.2009.04.007. [DOI] [Google Scholar]
- Puri A, Padda KP, Chanway CP. In vitro and in vivo analyses of plant-growth-promoting potential of bacteria naturally associated with spruce trees growing on nutrient-poor soils. Appl Soil Ecol. 2020;149:103538. doi: 10.1016/j.apsoil.2020.103538. [DOI] [Google Scholar]
- Radhakrishnan R, Khan AL, Kang SM, Lee IJ. A comparative study of phosphate solubilization and the host plant growth promotion ability of Fusariumverticillioides RK01 and Humicola sp. KNU01 under salt stress. Ann Microbiol. 2015;65(1):585–593. doi: 10.1007/s13213-014-0894-z. [DOI] [Google Scholar]
- Rajkumar M, Ae N, Prasad MNV, Freitas H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010;28(3):142–149. doi: 10.1016/j.tibtech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, Woodward C, Kim YO, Redman RS. Habitat-adapted symbiosis as a defense against abiotic and biotic stresses. In: White JF, Torres MS, editors. Defensive mutualism in microbial symbiosis. Boca Raton, FL: CRC Press; 2009. pp. 335–346. [Google Scholar]
- Rosenblueth M, Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact. 2006;19(8):827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- Sandhya V, Shrivastava M, Ali SZ, Prasad VS. Endophytes from maize with plant growth promotion and biocontrol activity under drought stress. Russ Agric Sci. 2017;43(1):22–34. doi: 10.3103/S1068367417010165. [DOI] [Google Scholar]
- Santoyo G, Moreno-Hagelsieb G, del Carmen O-M, Glick BR. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Schulz B, Boyle C. What are endophytes? In: Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial root endophytes. Berlin: Springer; 2006. pp. 1–13. [Google Scholar]
- Shahid M, Ahmed B, Khan MS. Evaluation of microbiological management strategy of herbicide toxicity to green gram plants. Biocatal Agric Biotechnol. 2018;14:96–108. doi: 10.1016/j.bcab.2018.02.009. [DOI] [Google Scholar]
- Shahrtash M, Brown SP. A path forward: promoting microbial-based methods in the control of invasive plant species. Plants. 2021;10(5):943. doi: 10.3390/plants10050943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Kumar V, Handa N, Bali S, Kaur R, Khanna K, Thukral AK, Bhardwaj R. Potential of endophytic bacteria in heavy metal and pesticide detoxifcation. In: Egamberdieva D, Ahmad P, editors. Plant microbiome: stress response. Microorganisms for Sustainability. Springer Belin; 2018. pp. 307–336. [Google Scholar]
- Shentu X, Zhan X, Ma Z, Yu X, Zhang C. Antifungal activity of metabolites of the endophytic fungus Trichoderma brevicompactum from garlic. Braz J Microbiol. 2014;45(1):248–254. doi: 10.1590/S1517-83822014005000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Xie H, Cao L, Zhang R, Xu Z, Wang Z, Deng Z. Effects of Cd-and Pb-resistant endophytic fungi on growth and phytoextraction of Brassica napus in metal-contaminated soils. Environ Sci Pollut Res. 2017;24(1):417–426. doi: 10.1007/s11356-016-7693-y. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lou K, Li C. Growth promotion effects of the endophyte Acinetobacterjohnsonii strain 3–1 on sugar beet. Symbiosis. 2011;54(3):159–166. doi: 10.1007/s13199-011-0139-x. [DOI] [Google Scholar]
- Shrivastava G, Ownley BH, Augé RM, et al. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis. 2015;65:65–74. doi: 10.1007/s13199-015-0319-1. [DOI] [Google Scholar]
- Siciliano SD, Fortin N, Mihoc A, Wisse G, Labelle S, Beaumier D, Greer CW. Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl Environ Microbiol. 2001;67(6):2469–2475. doi: 10.1128/AEM.67.6.2469-2475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Gaur R. Endophytic Streptomyces spp. underscore induction of defense regulatory genes and confers resistance against Sclerotium rolfsii in chickpea. Biol Control. 2017;104:44–56. doi: 10.1016/j.biocontrol.2016.10.011. [DOI] [Google Scholar]
- Singh A, Sharma S, Singh B. Effect of germination time and temperature on the functionality and protein solubility of sorghum flour. J Cereal Sci. 2017;76:131–139. doi: 10.1016/j.jcs.2017.06.003. [DOI] [Google Scholar]
- Singh D, Rajawat MVS, Kaushik R, Prasanna R, Saxena AK. Beneficial role of endophytes in biofortification of Zn in wheat genotypes varying in nutrient use efficiency grown in soils sufficient and deficient in Zn. Plant Soil. 2017;416(1):107–116. doi: 10.1007/s11104-017-3189-x. [DOI] [Google Scholar]
- Singh BK, Trivedi P, Singh S, Macdonald CA, Verma JP. Emerging Microbiome technologies for sustainable increase in farm productivity and environmental security. Microbiol Aust. 2018;39(1):17–23. doi: 10.1071/MA18006. [DOI] [Google Scholar]
- Singh R, Dubey AK. Endophytic actinomycetes as emerging source for therapeutic compounds. Indo Global J Pharm Sci. 2015;5:106–116. doi: 10.35652/IGJPS.2015.11. [DOI] [Google Scholar]
- Song C, Zhu F, Carrión VJ, Cordovez V. Beyond plant microbiome composition: exploiting microbial functions and plant traits via integrated approaches. Front Bioeng Biotechnol. 2020;8:896. doi: 10.3389/fbioe.2020.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Cheng Z, Glick BR. The presence of a 1-aminocyclopropane-1-carboxylate (ACC) deaminase deletion mutation alters the physiology of the endophytic plant growth-promoting bacterium Burkholderia phytofirmans PsJN. FEMS Microbiol Lett. 2009;296(1):131–136. doi: 10.1111/j.1574-6968.2009.01625.x. [DOI] [PubMed] [Google Scholar]
- Sun K, Cao W, Hu LY, Fu WQ, Gong JH, Kang N, Dai CC. Symbiotic fungal endophyte Phomopsis liquidambari-rice system promotes nitrogen transformation by influencing below-ground straw decomposition in paddy soil. J Appli Microbiol. 2019;126(1):191–203. doi: 10.1111/jam.14111. [DOI] [PubMed] [Google Scholar]
- Surono, Narisawa K (2017) The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol 28:1–10. 10.1016/j.funeco.2017.04.001
- Taulé C, Castillo A, Villar S, Olivares F, Battistoni F. Endophytic colonization of sugarcane (Saccharumofficinarum) by the novel diazotrophs Shinella sp. UYSO24 and Enterobacter sp. UYSO10. Plant Soil. 2016;403(1):403–418. doi: 10.1007/s11104-016-2813-5. [DOI] [Google Scholar]
- Tong J, Miaowen C, Juhui J, Jinxian L, Baofeng C. Endophytic fungi and soil microbial community characteristics over different years of phytoremediation in a copper tailings dam of Shanxi, China. Sci Total Environ. 2017;574:881–888. doi: 10.1016/j.scitotenv.2016.09.161. [DOI] [PubMed] [Google Scholar]
- Trivedi G, Patel P, Saraf M. Synergistic effect of endophytic selenobacteria on biofortification and growth of Glycine max under drought stress. S Afr J Bot. 2020;134:27–35. doi: 10.1016/j.sajb.2019.10.001. [DOI] [Google Scholar]
- Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK. Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol. 2020;18(11):607–621. doi: 10.1038/s41579-020-0412-1. [DOI] [PubMed] [Google Scholar]
- Ullah A, Farooq M, Hussain M. Improving the productivity, profitability and grain quality of kabuli chickpea with co-application of zinc and endophyte bacteria Enterobacter sp MN17. Arch Agron Soil Sci. 2020;66(7):897–912. doi: 10.1080/03650340.2019.1644501. [DOI] [Google Scholar]
- Uzma F, Konappa NM, Chowdappa S. Diversity and extracellular enzyme activities of fungal endophytes isolated from medicinal plants of Western Ghats, Karnataka. Egypt J Basic Appl Sci. 2016;3(4):335–342. doi: 10.1016/j.ejbas.2016.08.007. [DOI] [Google Scholar]
- Verma JP. Functional importance of the plant microbiome: implications for agriculture, forestry and bioenergy: a book review. J Clean Prod. 2018;178:877–879. doi: 10.1016/j.jclepro.2018.01.043. [DOI] [Google Scholar]
- Verma JP, Jaiswal DK, Sagar R. Pesticide relevance and their microbial degradation: a-state-of-art. Rev Environ Sci Biotechnol. 2014;13:429–466. doi: 10.1007/s11157-014-9341-7. [DOI] [Google Scholar]
- Verma JP, Jaiswal DK, Singh S, Kumar A, Prakash S, Curá JA. Consequence of phosphate solubilising microbes in sustainable agriculture as efficient microbial consortium: a review. Climate Change Environ Sustain. 2017;5:1–19. doi: 10.5958/2320-642x.2017.00001.1. [DOI] [Google Scholar]
- Von Bodman SB, Bauer WD, Coplin DL. Quorum sensing in plant-pathogenic bacteria. Annu Review Phytopathol. 2003;41(1):455–482. doi: 10.1146/annurev.phyto.41.052002.095652. [DOI] [PubMed] [Google Scholar]
- Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci. 2005;102(38):13386–13391. doi: 10.1073/pnas.0504423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dai CC. Endophytes: a potential resource for biosynthesis, biotransformation, and biodegradation. Ann Microbiol. 2011;61(2):207–215. doi: 10.1007/s13213-010-0120-6. [DOI] [Google Scholar]
- Wang Y, Li H, Zhao W, He X, Chen J, Geng X, Xiao M. Induction of toluene degradation and growth promotion in corn and wheat by horizontal gene transfer within endophytic bacteria. Soil Biol Biochem. 2010;42(7):1051–1057. doi: 10.1016/j.soilbio.2010.03.002. [DOI] [Google Scholar]
- Wang L, Lin H, Dong Y, Li B, He Y. Effects of endophytes inoculation on rhizosphere and endosphere microecology of Indian mustard (Brassica juncea) grown in vanadium-contaminated soil and its enhancement on phytoremediation. Chemosphere. 2020;240:124891. doi: 10.1016/j.chemosphere.2019.124891. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yu ZX, Solanki MK, Yang LT, Xing YX, Dong DF, Li YR. Diversity of sugarcane root-associated endophytic Bacillus and their activities in enhancing plant growth. J Appl Microbiol. 2020;128(3):814–827. doi: 10.1111/jam.14512. [DOI] [PubMed] [Google Scholar]
- Wani ZA, Ashraf N, Mohiuddin T, Riyaz-Ul-Hassan S. Plant-endophyte symbiosis, an ecological perspective. Appl Microbiol Biotech. 2015;99(7):2955–2965. doi: 10.1007/s00253-015-6487-3. [DOI] [PubMed] [Google Scholar]
- Waqas M, Khan AL, Hamayun M, Shahzad R, Kang SM, Kim JG, Lee IJ. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: an example of Penicillium citrinum and Aspergillus terreus. J Plant Interact. 2015;10(1):280–287. doi: 10.1080/17429145.2015.1079743. [DOI] [Google Scholar]
- White JG, Kingsley KL, Verma SK, Kowalski K. Rhizophagy cycle: an oxidative process in plants for nutrient extraction from symbiotic microbes. Microorganisms. 2018;6:95. doi: 10.3390/microorganisms6030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JF, Kingsley KL, Zhang Q, Verma R, Obi N, Dvinskikh S, Elmore MT, Verma SK, Gond SK, Kowalski KP. Review: Endophytic microbes and their potential applications in crop management. Pest Manag Sci. 2019;75:2558–2565. doi: 10.1002/ps.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (1996) Permissible limits of heavy metals in soil and plants. (Geneva: World Health Organization), Switzerland
- Wilson D. Endophyte: the evolution of a term, and clarification of its use and definition. Oikos. 1995;73:274–276. doi: 10.2307/3545919. [DOI] [Google Scholar]
- Win PM, Matsumura E, Fukuda K. Effects of pesticides on the diversity of endophytic fungi in tea plants. Microb Ecol. 2021 doi: 10.1007/s00248-020-01675-7. [DOI] [PubMed] [Google Scholar]
- Xie XG, Zhao YY, Yang Y, Lu F, Dai CC. Endophytic fungus alleviates soil sickness in peanut crops by improving the carbon metabolism and rhizosphere bacterial diversity. Microb Ecol. 2020 doi: 10.1007/s00248-020-01555-0. [DOI] [PubMed] [Google Scholar]
- Yao Q, Huang M, Bu Z, Zeng J, Wang X, Liu Z, Ma J, Zhang K, Liu X, Zhu Y. Identification and characterization of a novel bacterial carbohydrate esterase from the bacterium Pantoea ananatis Sd-1 with potential for degradation of lignocellulose and pesticides. Biotechnol Lett. 2020;42(8):1479–1488. doi: 10.1007/s10529-020-02855-8. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhou PP, Yu LJ. An endophytic taxol-producing fungus from Taxus media, Cladosporium cladosporioides MD2. Curr Microbiol. 2009;59:227–232. doi: 10.1007/s00284-008-9270-1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhong J, Liu H, Xin K, Chen C, Li Q, Wei Y, Wang Y, Chen F, Shen X. Complete genome sequence of the drought resistance-promoting endophyte Klebsiella sp. LTGPAF-6F. J Biotechnol. 2017;246:36–39. doi: 10.1016/j.jbiotec.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Zhao L, Xu Y, Lai X. Antagonistic endophytic bacteria associated with nodules of soybean (Glycinemax L.) and plant growth-promoting properties. Brazilian J Microbiol. 2018;49(2):269–278. doi: 10.1016/j.bjm.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LS, Tang K, Guo SX. The plant growth-promoting fungus (PGPF) Alternaria sp. A13 markedly enhances Salviamiltiorrhiza root growth and active ingredient accumulation under greenhouse and field conditions. Int J Mol Sci. 2018;19(1):270. doi: 10.3390/ijms19010270. [DOI] [PMC free article] [PubMed] [Google Scholar]