Abstract
Purpose
To provide a summarized state of the art of the relative efficacy and rapidity of action of pharmacological treatments to prevent imminent osteoporotic fractures.
Methods
We reviewed metanalyses (MA) and network metaanalyses (NMA) published during the last 10 years concerning the pharmacological treatment of osteoporosis. We compared the anti-fracture efficacy and the rapidity of action of various agents versus placebo and versus risedronate.
Results
All bisphosphonates decrease the incidence of vertebral fractures compared with placebo. Ibandronate is the only one without demonstrated efficacy against non-vertebral and hip fractures. Zoledronate, denosumab and anabolic therapy are associated with a higher fracture risk reduction than oral bisphosphonates. Compared with risedronate, which significantly reduces the rate of hip fractures, zoledronate, denosumab, teriparatide, abaloparatide and romosozumab are more efficient for vertebral fractures but not for non-vertebral or hip fractures reduction. No studies have compared bone anabolic treatments with zoledronate or denosumab. Oral bisphosphonates significantly reduce fracture risk only after more than one year of therapy. A faster reduction of fracture risk is observed with zoledronate and denosumab, or with anabolic agents. For denosumab and anabolic agents, a sequential treatment is required to keep gains after treatment withdrawal.
Conclusions
In patients at high risk of imminent fracture, starting therapy with potent antiresorptive agents or with an anabolic agent seems most appropriate to promptly reduce the fracture risk. Available NMA/MA suggest that, compared to zoledronate and denosumab, anabolic agents have a higher efficacy for vertebral fractures but head-to-head studies are lacking.
Keywords: Imminent fracture, Osteoporosis, Bisphosphonate, Denosumab, Anabolic
Highlights
-
•
The concept of imminent fracture has implications for the choice of therapy
-
•
We reviewed metanalyses and network metaanalyses published in the last 10 years
-
•
We compared the efficacy and rapidity of treatments to prevent imminent fractures
-
•
Potent antiresorptive and anabolic agents are most appropriate to promptly reduce fracture risk
-
•
Anabolic agents seem to be more efficient to reduce vertebral fracture risk
1. Introduction
Osteoporotic fractures are a major and increasing cause of mortality, morbidity, loss of independence and altered quality of life worldwide (Alarkawi et al., 2020). Because of population aging, osteoporosis is among the most important health crises for industrialized countries, with a high cost of incident fragility fractures, estimated at € 37 billion in European Union, and a predicted increase of 25% from now to 2025. The cost of treatment and long-term care of patients with fractures are considerably higher than those of pharmacological prevention, which remains largely underused (Hernlund et al., 2013).
Guidelines for the assessment and treatment of osteoporosis imperatively recommend work up and treatment for patients after a first fragility fracture, with secondary fracture prevention as an obvious first step in the development of a systematic approach (Hernlund et al., 2013). The risk for recurrent fractures is maximal during the first two years after a fragility fracture (“imminent fractures” period) and decreases gradually afterward (Kanis et al., 2020a). This concept of imminent fracture is therefore central to the categorization of very high risk and has implications for the choice of therapy: these patients at high risk of imminent fractures are most at need of immediate treatment with agents that reduce fracture risk most efficiently and as promptly as possible. Hence the need to identify such agents.
Several anti-osteoporotic agents have a high antifracture efficacy, proven in many randomized controlled trials (RCT): anti-resorptive drugs such as oral bisphosphonates, denosumab and zoledronate, or anabolic agents of the first-generation, teriparatide, or newer ones, namely abaloparatide and romosozumab. However, they differ by their potency and the lag time before observing a significant fracture risk reduction.
The aim of the present paper is to provide the reader with a summarized view of the relative potency and rapidity of action of pharmacological treatments available to prevent osteoporotic fractures. For this purpose, we did not perform another metaanalysis but rather synthetized available metanalyses (MA) and network metaanalyses (NMA) published in the last 10 years. We analysed the anti-fracture efficacy of active treatments versus placebo. To better appreciate differences in efficiency, the power of the more recent agents (parenteral anti-resorptives and anabolics) was also systematically compared with that of risedronate, chosen as representative of oral bisphosphonate activity.
Three explicit questions were defined:
-
1.
Which treatment would be the most powerful to prevent fracture?
-
2.
What are the fastest anti-osteoporotic agents to promptly reduce fracture risk?
-
3.
How to maintain the early benefits of treatment?
2. Methods
A search of Scopus was performed to find NMAs and MAs published in the last 10 years. The language was limited to English for pragmatic reasons. Furthermore, the reference lists of studies selected for inclusion in the present review were screened for additional relevant reports.
Studies were eligible for this review if they met the following criteria: (a) MA/NMA included RCTs for which only postmenopausal women with primary osteoporosis or osteopenia were included; (b) one or more active agents were compared to placebo or to each other; and (c) the outcomes of interest (vertebral, hip, and nonvertebral fragility fractures) were reported as a primary or secondary outcome.
Three different types of pharmacological treatments were studied: 1) oral and parenteral bisphosphonates (alendronate, ibandronate, risedronate, and zoledronate), 2) denosumab, and 3) anabolic therapy (teriparatide, abaloparatide, and romosozumab). We present the efficacy versus placebo, versus risedronate and the head-to-head comparisons. Risedronate was chosen as representative of oral anti-resorptive treatments that has been shown in a placebo-controlled trial to reduce the rate of hip fractures (Barrionuevo et al., 2019; Murad et al., 2012).
Studies on the following topics were excluded: acute fracture care, high-energy fractures, fracture healing, secondary osteoporosis (including osteoporosis induced by glucocorticoid therapy or by cancer therapy), male osteoporosis, premenopausal osteoporosis, and studies in languages other than English.
We summarize the results of NMAs/MAs published during the last 10 years with available information on timing of action and efficacy of available osteoporosis treatments in relation to fracture risk reduction. No specific statistical analysis was performed.
3. Results
3.1. Which treatment would be the most powerful to prevent fracture?
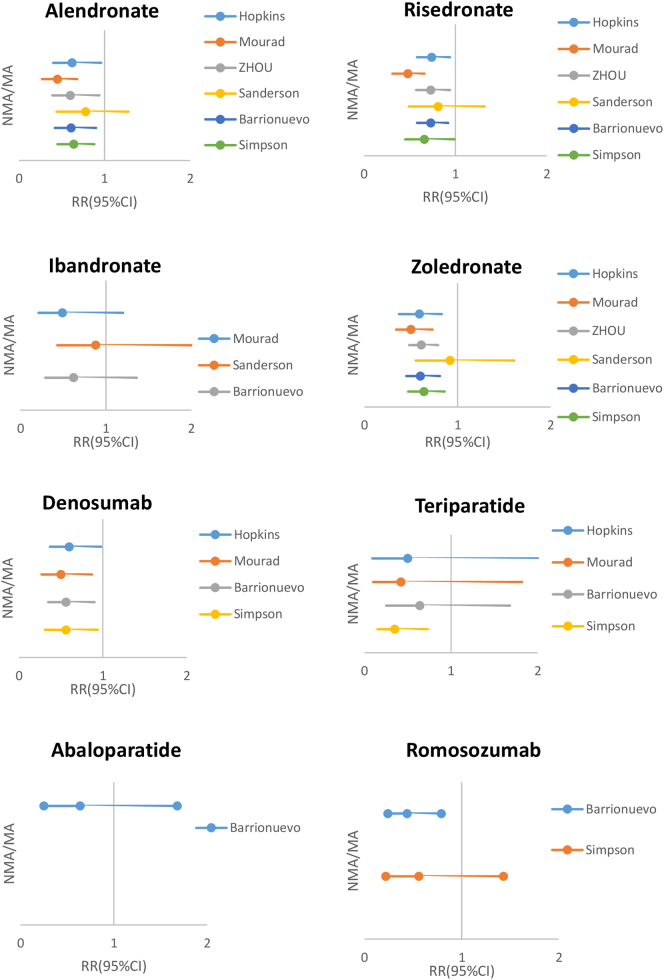
For vertebral fractures, all bisphosphonates showed efficacy in preventing fractures compared with placebo (see Fig. 1 and Supplementary Table 1). Zoledronate was associated with a higher reduction (RR = 0.28–0.42) than the three oral bisphosphonates: alendronate (RR = 0.45–0.65), risedronate (RR = 0.46–0.60) and ibandronate (RR = 0.46–0.67). The efficacy of denosumab was similar to that of zoledronate (RR = 0.30–0.32). With respect to anabolics, the reduction of fracture risk following treatment with teriparatide and abaloparatide was substantially greater than that after anti-resorptives, even zoledronate or denosumab, with a RR at 0.23–0.31 and 0.13–0.15 vs placebo, respectively. In a head-to-head comparative trial (Kendler et al., 2017), teriparatide was more efficient than risedronate for prevention of vertebral fractures (RR = 0.44, p < 0.0001) (see Table 1). Moreover, the VERO study showed that the antifracture efficacy was superior in a subgroup of patients with imminent fracture risk (those with a prior clinical vertebral fracture (VFx) in the year before entering the study), with a reduction of new VFx, new and worsened VFx, and clinical fractures by 65%, 68%, and 62%, respectively, in patients treated with teriparatide as compared with risedronate (Geusens et al., 2018).
Fig. 1.
Data reported in NMA/MA (efficacy vs placebo) for vertebral fractures.
Table 1.
Vertebral fracture data reported in head-to-head studies.
| Trial name: first author and year (Ref.) | Treatments, n analysed | Follow-up (months) | Vertebral fracture outcomes n (%) (reported between-group difference) |
|---|---|---|---|
| Panico et al., 2011 postmenopausal women with severe osteoporosis | ALN, 39 TPTD, 42 |
18 | ALN 6/39 (15.7) TPTD 1/42 (2.4) RR 0.15, 95% CI 0.02–1.23 NS |
| Hadji et al., 2012 postmenopausal women with osteoporosis | RIS, 350 TPTD, 360 |
6 | RIS, 18/350 (5.1) TPTD, 15/360 (4.2), RR 0.83, 95% CI 0.41–1.58 NS |
| Miller et al., 2016a postmenopausal women with osteoporosis | ABL 824 TPTD 818 |
18 | ABL 4/824 TPTD 6/818 RR 0.66, 95% CI 0.18–2.40, NS |
| ARCH: Saag et al., 2017 postmenopausal women with osteoporosis | ALN, 2047 ROMO, 2046 | 12 | ALN, 128/2047 (6.3) ROMO, 82/2046 (4.0) RR 0.63, 95% CI 0.47 to 0.85 p = 0.003 |
| VERO: Kendler et al., 2017 postmenopausal women with osteoporosis | RIS, 533 TPTD, 516 |
24 | RIS, 64/533 (12.0) TPTD, 28/516 (5.0) RR 0.44, 95% CI 0.29–0.68; p < 0.0001 |
ALN – Alendronate, DEN – Denosumab, ROMO – Romosozumab, RIS – Risedronate, TPTD – Teriparatide, ZOL – Zoledronate, ABL-Abaloparatide, NS- not significant.
No difference in efficacy was apparent between risedronate and other oral bisphosphonates. Zoledronate, however, was more efficient, at the same level as denosumab. The three anabolic agents were slightly more potent than parenteral antiresorptives, particularly abaloparatide (for which there are only few data). The NMA of Ding et al. (2020) is the only one to assess the comparative anti-fracture effectiveness of various drugs according to the proportion of prevalent vertebral fractures (PVF): in the subgroup where more than 50% of the patients had a PVF, the greatest risk reduction was obtained for romosozumab (RR = 0.28); in the subgroup where PVF was <50%, abaloparatide was the most potent (RR = 0.16).
For non-vertebral fractures, bisphosphonates associated with a significant reduction in fractures were alendronate (RR = 0.53–0.83), risedronate (RR = 0.55–0.81) and zoledronate (RR = 0.30–0.76) (see Fig. 2 and Supplementary Table 1). Ibandronate did not reduce non-vertebral fractures in the majority of NMA/MA. The same efficacy was obtained for teriparatide, denosumab and abaloparatide (RR = 0.31–0.81; 0.26–0.63 and 0.13–0.54, respectively). The few head-to-head comparisons showed no significant differences between the evaluated drugs (see Table 2). However, in the study of Saag (Saag et al., 2017), the risk of nonvertebral fractures was lower by 19% in the romosozumab-to-alendronate group than in the alendronate-to-alendronate group (P = 0.04). Cosman et al. (2016) did not obtain with romosozumab a significant reduction of the fracture risk within 12 months (p = 0.10) or 24 months (p = 0.06). In a post hoc analysis of the role of regional background fracture risk (Cosman et al., 2018), risk reductions were observed in “rest-of-world” (p = 0.012), with no treatment effect observed in Latin America.
Fig. 2.
Data reported in NMA/MA (efficacy vs placebo) for non-vertebral fractures.
Table 2.
Non - vertebral fracture data reported in head-to-head studies.
| Trial name: first author and year (Ref.) | Treatments, n analysed | Follow-up (months) | Non-vertebral fracture outcomes n (%) (reported between-group difference) |
|---|---|---|---|
| STAND: Kendler et al., 2010 postmenopausal women with osteoporosis | ALN, 249 DEN, 253 |
12 | ALN, 4/251 (1.6) DEN, 8/253 (3.2) RR 0.5, 95% CI 0.15–1.65 NS |
| Hadji et al., 2012 postmenopausal women with osteoporosis | RIS, 350 TPTD, 360 |
6 | RIS, 29/350 (8.30) TPTD, 28/360 (7.80) RR 0.94, 95% CI 0.57–1.54 NS |
| DAPS: Freemantle et al., 2012 postmenopausal women with osteoporosis | ALN, 124 DEN, 126 |
12 | ALN, 1/118 (0.85) DEN, 1/125 (0.80) RR 1.06 95% CI 0.06–16.7 NS |
| Miller et al., 2016b postmenopausal women with osteoporosis | ZOL, 320 DEN, 320 |
12 | ZOL, 11/320 DEN, 7/320 RR 0.63 95% CI 0.24–1.67 NS |
| ARCH: Saag et al., 2017 postmenopausal women with osteoporosis | ALN, 2047 ROMO, 2046 |
12 | ALN, 95/2047 (4.60) ROMO, 70/2046 (3.40) RR 0.74, 95% CI 0.54–0.99 p = 0.05 |
| VERO: Kendler et al., 2018 postmenopausal women with osteoporosis | RIS 680 TPTD 680 |
24 | RIS, 38/680 (6.00) TPTD, 25/680 (4.00) RR 0.66, 95% CI 0.39–1.10 NS |
ALN – Alendronate, DEN – Denosumab, ROMO – Romosozumab, RIS – Risedronate, TPTD – Teriparatide, ZOL – Zoledronate, NS- not significant.
For hip fractures, similar efficacies were observed for alendronate (RR = 0.45–0.64), risedronate (RR = 0.48–0.74), zoledronate (RR = 0.50–0.64) and denosumab (RR = 0.50–0.60). Teriparatide was efficient with RR = 0.35 (0.15–0.73) only in the NMA of Simpson et al., 2020. For romosozumab, only Barrionuevo et al. (2019) indicated a significant effect on the risk of hip fracture (RR = 0.44) (Fig. 3). Romosozumab followed by alendronate reduced the risk of hip fracture to a greater extent that alendronate alone (P = 0.02).
Fig. 3.
Data reported in NMA/MA (efficacy vs placebo) for hip fractures.
None of the parenteral drugs were apparently more potent than risedronate for non-vertebral and hip fractures prevention (see Supplementary Table 2 and Fig. 4).
Fig. 4.
Data reported in NMA/MA (efficacy vs Risedronate) for vertebral fractures.
3.2. What are the fastest anti-osteoporotic agents to reduce fracture risk?
For vertebral fractures, a significant reduction of fracture risk was only demonstrated after more than one year of treatment with oral bisphosphonates (Black et al., 2000; Chesnut et al., 2004; Harris et al., 1999; Liberman et al., 1995) (Table 3): after the first year for risedronate (p < 0.001) (Harris et al., 1999) and alendronate (Cosman et al., 2018), and during the second year for ibandronate (p < 0.001) (Chesnut et al., 2004). With zoledronate (Dennis et al., 2007) and denosumab (Steven et al., 2007), a significant risk reduction was already observed after 6 months (p < 0.001). The protective effect of teriparatide became evident after 9 to 12 months (Neer et al., 2001). With romosozumab, a significant reduction of the risk of vertebral fracture was obtained within 12 months (P < 0.001) (Cosman et al., 2016). Abaloparatide had a similar efficacy (p < 0.001), but no data are available for the rapidity of action (Cosman et al., 2017; Miller et al., 2016a). However, only a small number of fracture events occurred across treatment groups, with the event rate in the placebo group being smaller than anticipated. Moreover, the result could be influenced by the fact that 63% of participants had a prior fracture.
Table 3.
Minimal duration of treatment before obtaining a significant risk reduction for a) vertebral fractures; b) hip fractures and c) non-vertebral fractures according to the included studies. (*p < 0.05, **p < 0.01, ***p < 0.001, NS – not significant, NA - not analysed).
| a) For vertebral fractures | ||
|---|---|---|
| Before 12 months | After 12 months | |
|
Oral bisphosphonates (Black et al., 2000; Chesnut et al., 2004; Harris et al., 1999; Liberman et al., 1995): Alendronate Risedronate Ibandronate |
NS |
*** ** ** |
| Zoledronate (Dennis et al., 2007) | *** | *** |
| Denosumab (Steven et al., 2007; Boonen et al., 2011) | *** | *** |
| Teriparatide (Body et al., 2020; Lindsay et al., 2009) | ** | ** |
| Abaloparatide (Cosman et al., 2017; Miller et al., 2016a) | NA | *** |
| Romosozumab (Cosman et al., 2016; Saag et al., 2017) | ** | ** |
| b) For non-vertebral fractures | ||
|---|---|---|
| Before 12 months | After 12 months | |
|
Oral bisphosphonates (Black et al., 2000; Harris et al., 1999; Liberman et al., 1995): Alendronate Risedronate |
NS |
* * |
| Zoledronate (Dennis et al., 2007) | *** | *** |
| Denosumab (Steven et al., 2007) | * | * |
| Teriparatide (Body et al., 2020) | ** | ** |
| Abaloparatide (Cosman et al., 2017; Miller et al., 2016a) | * | * |
| Romosozumab (Cosman et al., 2016; Saag et al., 2017) | NS | * |
| c) For hip fractures |
||
|---|---|---|
| Before 12 months | After 12 months | |
|
Oral bisphosphonates (Black et al., 2000; Liberman et al., 1995): Alendronate |
NS |
* |
| Zoledronate (Dennis et al., 2007) | *** | *** |
| Denosumab (Steven et al., 2007; Boonen et al., 2011) | * | * |
| Teriparatide (Eriksen et al., 2014; Lindsay et al., 2009) | * | * |
| Romosozumab (Saag et al., 2017) | NS | * |
In the few available head-to-head comparisons, both teriparatide and romosozumab seem to be more efficient than the oral bisphosphonate already during the first year (Saag et al., 2017; Body et al., 2020). Body et al. (2020) compared teriparatide to risedronate. The largest difference in incidence rates of clinical fractures occurred during the 6- to 12-month period (p = 0.03). With regards to romosozumab, in the study of Saag et al., 2017, a significantly lower risk was observed in the romosozumab-to-alendronate group than in the alendronate-to-alendronate group (P < 0.001), but the difference was significant only after the first year of treatment (p = 0.003). Comparing the efficacy of abaloparatide and teriparatide, Miller et al. (2016a) found a significant reduction in the risk of new vertebral fractures in the abaloparatide vs placebo group (RR 0.14, P < 0.001) but without data for the rapidity of effect.
For non-vertebral fractures, the effect of oral bisphosphonates became significant after the first year only (Black et al., 2000; Chesnut et al., 2004; Harris et al., 1999; Liberman et al., 1995) (Table 3). For zoledronate (Dennis et al., 2007) and denosumab (Steven et al., 2007), a significant reduction of the risk of fractures was already observed after 6 months (p < 0.001). Regarding the effect of romosozumab, in the study of Saag et al. (2017), a significantly lower risk (19%) was observed in the romosozumab-to-alendronate group than in the alendronate-to-alendronate group for new non-vertebral fractures during the second year of treatment (p = 0.037). With abaloparatide, Miller et al., 2016a, Miller et al., 2016b obtained an early significant reduction (RR = 0.57, p = 0.049). Kaplan-Meier curves for time to first nonvertebral fracture showed early separation (before 12 months) between the abaloparatide group and both the placebo and teriparatide groups. The curve of the abaloparatide- group continued to diverge from the placebo group and maintained consistent separation from the teriparatide group over the full course of the 18-month trial (Cosman et al., 2017; Miller et al., 2016a, Miller et al., 2016b).
For hip fractures, the same delay as for vertebral and non-vertebral fractures was observed after bisphosphonates and denosumab. For teriparatide, the effect became significant after 6 months (p < 0.05) (Eriksen et al., 2014). No significant effect was observed for romosozumab at 1 and 2 years by Cosman et al. (p = 0.18 and 0.06 respectively) (Cosman et al., 2016). However in the study of Saag et al., 2017, hip fractures occurred in 2.0% of patients in the romosozumab-to-alendronate group as compared with 3.2% in the alendronate-to-alendronate group at the time of the primary analysis, representing a 38% lower risk with romosozumab (hazard ratio, 0.62; 95% CI, 0.42 to 0.92; P = 0.02) during the second year.
3.3. How to maintain the early benefits of treatment?
Because they are stored in bone, the anti-fracture effect of bisphosphonates (oral or parenteral) persists for several months or years after they are stopped. It is not the case with denosumab or anabolics. When denosumab is withdrawn, there is a rebound of bone turn-over and bone loss, and several cases of multiple vertebral fractures have been described (3.4% of patients in the post hoc analysis of the Freedom trial) (Anastasilakis et al., 2017; Cummings et al., 2018; Popp et al., 2016). Cummings et al. reported that the odds of developing multiple vertebral fractures after stopping denosumab were 3.9 times higher in those with prior vertebral fractures, sustained before or during treatment, than those without, and 1.6 times higher with each additional year of off-treatment follow-up. Thus, denosumab should be given lifelong (Hansen et al., 2020) or, if stopped, replaced with another potent antiresorptive. Results of studies are still limited and controversial: one infusion of zoledronate did not prevent bone loss after discontinuing treatment according to studies of Solling and Horne (Horne et al., 2018; Sølling et al., 2020); conversely, Anastasilakis (Anastasilakis et al., 2017) showed that a single intravenous infusion of zoledronate given 6 months after the last denosumab injection prevented bone loss for at least 2 years independently of the rate of bone turnover.
The optimal timing to start a bisphosphonate treatment after denosumab is unknown, nor the dosage, nor the duration of treatment. A recent review (Tsourdi et al., 2021) concluded that the duration of denosumab treatment is an important determinant of the extent of the rebound phenomenon. A short duration of denosumab treatment (i.e. up to 2.5 years) in patients with otherwise low fracture risk could justify treatment with an oral bisphosphonate for 1–2 years. Patients having been treated with denosumab for a longer period (i.e., more than 2.5 years) or who are at persistently high risk for fracture should receive zoledronate.
Because the use of anabolic drugs for postmenopausal osteoporosis is limited to 12 to 24 months and the beneficial anti-fracture effect of anabolic therapy decreases rapidly when the treatment is stopped, a sequential treatment of antiresorptive therapy is required (Eastell et al., 2019; McClung et al., 2018). In postmenopausal women who have completed a course of teriparatide or abaloparatide, guidelines recommend treatment with antiresorptive therapy to maintain bone density gains (V; Shoback et al., 2020). In the case of abaloparatide, efficacy was maintained with a subsequent 24-month treatment with alendronate; eighteen months of abaloparatide followed by 24 months of alendronate reduced the risk of vertebral, nonvertebral, clinical, and major osteoporotic fractures (Bone et al., 2017). For romosozumab, 12 months of alendronate after 12 months of romosozumab showed superior efficacy on fracture outcomes compared with alendronate alone (Saag et al., 2017). Treatment effects of romosozumab are reversible upon discontinuation and further augmented by denosumab (McClung et al., 2018): women receiving romosozumab who transitioned to denosumab continued to accrue BMD, with additional mean gains of 2.6% at the lumbar spine, 1.9% at the total hip, and 1.4% at the femoral neck, whereas BMD returned toward pretreatment levels with placebo.
4. Discussion
There is a substantial body of evidence that the risk of a subsequent osteoporotic fracture is the highest immediately after the index fracture and wanes progressively with time (Kanis et al., 2020b). Therefore, the first 2 years after the index event is a period of “imminent risk” which requires that a pharmacological treatment should be given as soon as possible. Also, the chosen treatment should be most efficient to reduce the risk, and act promptly. The available pharmacologic treatments can be classified as anti-resorptive drugs: oral and parenteral bisphosphonates or inhibitors of RANK-ligand, and anabolic agents: activators of the PTH receptor and sclerostin inhibitors. These treatments differ in their mechanism of action and do not have the same power to reduce fracture risk. Also, the lag time before observing a significant risk reduction is variable. A number of RCT's, MA and NMA have been published about their relative efficiency and timing of action. The aim of the present narrative review was to summarize their results in order to help choosing the best therapeutic approach in the prevention of imminent fractures.
The NMAs and MAs showed that all pharmacological treatments significantly reduce the fracture risk. All bisphosphonates decrease the incidence of vertebral fractures compared with placebo. In contrast to other oral bisphosphonates, ibandronate has no significant efficacy against non-vertebral and hip fractures in the majority of NMA/MA. Zoledronate and denosumab are associated with a higher fracture risk reduction than the oral bisphosphonates. Anabolic therapy (romosozumab, abaloparatide or teriparatide) are more efficient for fracture risk reduction than an oral bisphosphonate. Compared with risedronate, which has a proven efficacy in the reduction of fracture risk, chosen as representative of oral anti-resorptive treatments, zoledronate, denosumab, teriparatide, abaloparatide and romosozumab are more efficient for vertebral fractures reduction but not for non-vertebral and hip fractures. Therefore, given their greater antifracture efficacy on vertebral fractures, zoledronate, denosumab or an anabolic treatment would be a better option than oral bisphosphonates for patients at high and imminent risk of such fractures.
Regarding the rapidity of action, a significant reduction of fracture risk was demonstrated only after more than one year of treatment with oral bisphosphonates. A faster reduction of fracture risk is observed with more potent antiresorptive agents, intravenous zoledronate and denosumab, or with anabolic agents.
The rapidity of action of these parenteral antiresorptives is probably due to their much faster inhibition of bone remodeling (within a week), compared to the 3–6 months it takes to achieve remodeling inhibition with oral agents. These drugs have a protective effect already during the first year, especially for non-vertebral fractures and should be recommended for patients at very high risk of imminent fracture, even if they are more costly. Davis et al., 2020 showed indeed in a systematic review and economic evaluation that the incremental cost-effectiveness ratios for newer treatments are generally greater than the commonly applied threshold of £20,000–30,000 (23,000-34,000€) per quality-adjusted life-year. However, the incremental cost-effectiveness ratio for denosumab may fall below £30,000 (34,000€) per quality-adjusted life-year at very high levels of risk or for high-risk patients with specific characteristics. Nevertheless, a major problem arises from the fact that the beneficial anti-fracture effect of anabolic therapy and denosumab is reversible and quickly disappears when therapy is stopped (Eastell et al., 2019; McClung et al., 2018). Thus, when these treatments are discontinued, a bisphosphonate should be given to avoid a rebound fracture risk after denosumab (Hansen et al., 2020) and an anti-resorptive to keep the gains after an anabolic agent (Shoback et al., 2020). The optimal regimen to prevent rebound after denosumab, particularly if given for long periods, has yet to be investigated.
Unfortunately, despite the availability of effective treatments, the prescription and adherence to an osteoporosis therapy after a sentinel fracture is low (around 20% of eligible patients) and declining (Iconaru et al., 2020). The estimated probability of osteoporosis medication used in the year after hip fracture decreased significantly from 40% to 21% over a 10-year study period (Kanis et al., 2017; Solomon et al., 2014). This highlights the urgent need of additional education for the medical profession and patients regarding the risk-benefit balance of treatment (Iconaru et al., 2020).
A limitation of our review is that we could not find enough specific studies where the efficacy of treatment was investigated in patients with a recent fracture. However, studies of efficacy based only on patients with a recent index fracture would be quite impractical. We hypothesize that published data in patients with osteoporosis can be applied for those with an imminent fracture risk. Another limitation is the small number of studies and subjects with new agents, romosozumab and abaloparatide, which could explain the homogeneity of results concerning these treatments in the analysed MA/NMA. On the other hand, there are only a limited number of head-to-head studies which could allow a better comparison of the drug's efficiency and rapidity of action. Additionally, no studies have compared bone anabolic treatments with zoledronate or denosumab, so it was not possible to analyse the benefit risk ratios of the anabolics compared with these drugs. Moreover, these analyses are done in different populations and there may be differences in many characteristics of the trials accounting for differences in fracture incidence other than the therapy.
In conclusion, in patients at high risk of imminent fracture, starting therapy with potent antiresorptive agents, intravenous zoledronate or denosumab, or anabolic agent seems most appropriate to promptly reduce the fracture risk because of their higher potency and faster effect on fracture risk reduction. In the absence of head-to-head studies to compare anabolic treatments with zoledronate and denosumab, the synthesis of NMA/MA suggests a higher efficacy of anabolics for vertebral fractures prevention, a moderate advantage for non-vertebral fractures and not enough data for hip fractures. For denosumab and anabolics, a sequential treatment is required to keep gains after treatment withdrawal, but the optimal regimen of these treatments remains to be defined with certainty. As these treatments are much more costly, a rigorous choice of patients is needed, underlying the need to develop a model for predicting imminent fractures.
Transparency document
Transparency document
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
BP, BJJ, IL designed the study.
IL wrote the first draft of the manuscript.
IL, BJJ, BP revised subsequent versions of the manuscript. All authors read and approved the final version of the paper. IL accepts responsibility for the integrity of the data analyses.
The FRISBEE study is supported by CHU Brugmann and IRIS-Recherche.
Iconaru Laura, Baleanu Felicia, Charles Alexia, Mugisha Aude, Benoit Florence, Surquin Murielle, Karmali Rafik, Body Jean-Jacques, Bergmann Pierre declare that they have no conflict of interest.
Footnotes
The Transparency document associated with this article can be found, in online version.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2021.101105.
Appendix A. Supplementary data
Supplementary tables
References
- Alarkawi D. Impact of osteoporotic fracture type and subsequent fracture on mortality: the Tromsø study. Osteoporos. Int. 2020;31:119–130. doi: 10.1007/s00198-019-05174-5. [DOI] [PubMed] [Google Scholar]
- Anastasilakis Clinical features of 24 patients with rebound-associated vertebral fractures after Denosumab discontinuation: systematic review and additional cases. J. Bone Miner. Res. 2017;32(6):1291–1296. doi: 10.1002/jbmr.3110. [DOI] [PubMed] [Google Scholar]
- Barrionuevo P. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J. Clin. Endocrinol. Metab. 2019;104:1623–1630. doi: 10.1210/jc.2019-00192. [DOI] [PubMed] [Google Scholar]
- Black Fracture intervention trial. Fracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial. FIT Research Group. J. Clin. Endocrinol. Metab. 2000;85(11):4118–4124. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- Body Efficacy of teriparatide compared with risedronate on FRAX®-defined major osteoporotic fractures: results of the VERO clinical trial. Osteoporos. Int. 2020;31:1935–1942. doi: 10.1007/s00198-020-05463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone Sustained fracture risk reduction with sequential abaloparatide/alendronate: results of ACTIVE extend. J. Bone Miner. Res. 2017;32(1):S25–S26. [Google Scholar]
- Boonen Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J. Clin. Endocrinol. Metab. 2011;96(6):1727–1736. doi: 10.1210/jc.2010-2784. [DOI] [PubMed] [Google Scholar]
- Chesnut Oral Ibandronate osteoporosis vertebral fracture trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J. Bone Miner. Res. 2004;19(8):1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- Cosman Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016;375(2016):1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- Cosman Eighteen months of treatment with subcutaneous Abaloparatide followed by 6 months of treatment with alendronate in postmenopausal women with osteoporosis: results of the ACTIVExtend trial. Mayo Clin. Proc. 2017;92(2):200–210. doi: 10.1016/j.mayocp.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Cosman F. 2018 Romosozumab FRAME study: a post hoc analysis of the role of regional background fracture risk on nonvertebral fracture outcome. J. Bone Miner. Res. 2018;33(8):1407–1416. doi: 10.1002/jbmr.3439. [DOI] [PubMed] [Google Scholar]
- Cummings S.R. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J. Bone Miner. Res. 2018;33(2):190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]
- Davis Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation. Health Technol. Assess. 2020;24(29):1–314. doi: 10.3310/hta24290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis Once-yearly Zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- Ding Osteoporosis drugs for prevention of clinical fracture in white postmenopausal women: a network meta-analysis of survival data. Osteoporos. Int. 2020;31(5):961–971. doi: 10.1007/s00198-019-05183-4. [DOI] [PubMed] [Google Scholar]
- Eastell Pharmacological Management of Osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J. Clin. Endocrinol. Metab. 2019;104:1595–1622. doi: 10.1210/jc.2019-00221. [DOI] [PubMed] [Google Scholar]
- Eriksen . Vol. 67. 2014. Literature Review: The Effects of Teriparatide Therapy at the Hip in Patients With Osteoporosis; pp. 246–256. (Epub 2014 Jul 15. PMID: 25053463) [DOI] [PubMed] [Google Scholar]
- Freemantle Final results of the DAPS (Denosumab adherence preference satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos. Int. 2012;23:317–326. doi: 10.1007/s00198-011-1780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geusens Effects of Teriparatide compared with Risedronate on the risk of fractures in subgroups of postmenopausal women with severe osteoporosis: the VERO trial. J. Bone Miner. Res. 2018;33(5):783–794. doi: 10.1002/jbmr.3384. [DOI] [PubMed] [Google Scholar]
- Hadji The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos. Int. 2012;23:2141–2150. doi: 10.1007/s00198-011-1856-y. [DOI] [PubMed] [Google Scholar]
- Hansen Subsequent fracture rates in a nationwide population-based cohort study with a 10-year perspective. Osteoporos Int.2015; 26:513–519. Sanchez-Rodriguez D, Bergmann P, body JJ, cavalier E, Gielen E, Goemaere S, et al. the Belgian bone Club 2020 guidelines for the management of osteoporosis in postmenopausal women. Maturitas. 2020;139:69–89. doi: 10.1016/j.maturitas.2020.05.006. [DOI] [PubMed] [Google Scholar]
- Harris Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral efficacy with Risedronate therapy (VERT) study group. JAMA. 1999;282(14):1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- Hernlund Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch. Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne Bone loss after romosozumab/denosumab: effects of bisphosphonates. Calcif. Tissue Int. 2018;103(1):55–61. doi: 10.1007/s00223-018-0404-6. [DOI] [PubMed] [Google Scholar]
- Iconaru Osteoporosis treatment gap in a prospective cohort of volunteer women. Osteoporos. Int. 2020;31(7):1377–1382. doi: 10.1007/s00198-020-05339-7. [DOI] [PubMed] [Google Scholar]
- Kanis Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos. Int. 2017;28:2023–2034. doi: 10.1007/s00198-017-4009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis A decade of FRAX: how has it changed the management of osteoporosis? Aging Clin. Exp. Res. 2020;32:187–196. doi: 10.1007/s40520-019-01432-y. [DOI] [PubMed] [Google Scholar]
- Kanis Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos. Int. 2020;31:1–12. doi: 10.1007/s00198-019-05176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J. Bone Miner. Res. 2010;25:72–81. doi: 10.1359/jbmr.090716. [DOI] [PubMed] [Google Scholar]
- Kendler Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2017;S0140-6736(17) doi: 10.1016/S0140-6736(17)32137-2. (32137–32132) [DOI] [PubMed] [Google Scholar]
- Liberman Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate phase III osteoporosis treatment study group. N. Engl. J. Med. 1995;333(22):1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- Lindsay Relationship between duration of teriparatide therapy and clinical outcomes in postmenopausal women with osteoporosis. Osteoporos. Int. 2009;20(6):943–948. doi: 10.1007/s00198-008-0766-0. [DOI] [PubMed] [Google Scholar]
- McClung Effects of 24 months of treatment with Romosozumab followed by 12 months of Denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J. Bone Miner. Res. 2018;33:1397–1406. doi: 10.1002/jbmr.3452. [DOI] [PubMed] [Google Scholar]
- Miller Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722–733. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- Miller Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J. Clin. Endocrinol. Metab. 2016;101:3163–3170. doi: 10.1210/jc.2016-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J. Clin. Endocrinol. Metab. 2012;97(6):1871–1880. doi: 10.1210/jc.2011-3060. [DOI] [PubMed] [Google Scholar]
- Neer Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Panico Teriparatide vs. alendronate as a treatment for osteoporosis: changes in biochemical markers of bone turnover, BMD and quality of life. Med. Sci. Monit. 2011;17:CR442–448. doi: 10.12659/MSM.881905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp Rebound-associated vertebral fractures after discontinuation of denosumab - from clinic and biomechanics. Osteoporos. Int. 2016;27(5):1917–1921. doi: 10.1007/s00198-015-3458-6. [DOI] [PubMed] [Google Scholar]
- Saag Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 2017;377:1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- Shoback Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline update. J. Clin. Endocrinol. Metab. 2020;104(3) doi: 10.1210/clinem/dgaa048.595–1622. [DOI] [PubMed] [Google Scholar]
- Simpson Clinical effectiveness of denosumab, raloxifene, romosozumab, and teriparatide for the prevention of osteoporotic fragility fractures: a systematic review and network meta-analysis. Bone. 2020;130 doi: 10.1016/j.bone.2019.115081. [DOI] [PubMed] [Google Scholar]
- Sølling Treatment with Zoledronate subsequent to Denosumab in osteoporosis: a randomized trial. J. Bone Miner. Res. 2020;35(10):1858–1870. doi: 10.1002/jbmr.4098. [DOI] [PubMed] [Google Scholar]
- Solomon Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J. Bone Miner. Res. 2014;29:1929–1937. doi: 10.1002/jbmr.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2007;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- Tsourdi Characterization of fracture risk and management of discontinuation of denosumab therapy for osteoporosis: a systematic review and updated position statement by European calcified tissue society (ECTS) JCEM. 2021;106:264–281. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document
Supplementary tables