Abstract
Compounds in microalgae-derived feed ingredients in poultry diets may improve intestinal physiology and immunity to protect against damage induced by physiological and pathogen challenges, but mechanisms are examined sparingly. The study objective was to evaluate changes to intestinal morphology, permeability, and systemic immunity in broilers fed a proprietary microalgae ingredient during 2 separate challenge studies. In study 1, two replicate 28 d battery cage trials used 200 Ross 308 broilers each (n = 400) fed a control diet ± 0.175% algae ingredient. Half of the birds were subjected to a 12 h feed restriction challenge and fluorescein isothiocyanate dextran (FITC-D) intestinal permeability assay on d 28. Study 2 used 800 broilers randomly assigned to the same dietary treatments and housed in floor pens for 42 d. At d 14, intestine and spleen samples were collected from 10 birds/ diet. Half of the remainder was orally inoculated with 10X Coccivac-B52 vaccine in a 2 × 2 factorial treatment design (diet and Eimeria inoculation). The FITC-D assay was conducted at 1, 3, 7, and 14 d post-inoculation (pi) while intestinal and spleen samples were collected at 3, 7, 14, and 28 dpi for histomorphology and flow cytometric immune cell assessment. Study 1 validated intestinal leakage via FITC-D absorbance induced by feed restriction but showed no algae-associated protective effects. In study 2, algae preserved intestinal integrity during coccidiosis (P = 0.04) and simultaneously protected jejunal villus height as early as 7dpi (P < 0.0001), whereas intestinal damage resolution in control birds did not occur until 14 dpi. Algae inclusion increased splenic T cells in unchallenged broilers at d 14 by 29.6% vs. control (P < 0.0001), specifically γδ T cell populations, without impacting performance (P < 0.03). During Eimeria challenge, splenic T cells in algae-fed birds did not show evidence of recruitment to peripheral tissues, while control birds showed a 16.7% reduction compared to their uninoculated counterparts from 3 to 7 dpi (P < 0.0001). This evidence suggests the algae ingredient altered the immune response in a manner that reduced recruitment from secondary lymphoid organs in addition to protecting intestinal physiology.
Key words: broiler, algae, intestinal integrity, immunity, coccidiosis
INTRODUCTION
Microalgae feed ingredients in poultry diets have typically been investigated as an alternative protein source, but may provide bioactive compounds at lower inclusion levels (Christaki et al., 2011; Austic et al., 2013; Tavernari et al., 2018). Microalgae is a rich source of n-3 polyunsaturated fatty acids (PUFAs), carotenoids, B vitamins, and non-starch polysaccharides such as beta-glucans (Christaki et al., 2011; Światkiewicz et al., 2015). Individually, these compounds provide documented health benefits associated with the anti-inflammatory and antioxidant properties of n-3 PUFAs and carotenoids, respectively (Zhang et al., 2014; Calder 2017; Eggersdorfer and Wyss, 2018). Though published literature consistently indicates microalgae as a source of these compounds, their composition and consequent effects on health outcomes such as host immunity and intestinal integrity vary between different genera and strains (Yaakob et al., 2014; Światkiewicz et al., 2015; Madeira et al., 2017). Furthermore, when dietary algae is used as a protein replacement, the mechanism of potential improvements to alter immune outcomes is typically not investigated, in favor of placing emphasis on growth, feed intake, and feed efficiency outcomes.
Research into microalgae's effects on poultry immunity and intestinal health focuses primarily on products derived from Spirulina and Chlorella. Dietary Spirulina inclusion as low as 0.001% in both layer and broiler diets increased macrophage phagocytic potential and natural killer cell activity, while 0.01% inclusions were associated with increased white blood cell counts in unchallenged 42-day-old broilers (Qureshi et al., 1996; Mariey et al., 2014). In broilers fed 10.0% Spirulina platensis, there was no effect on blood lymphocyte profiles; however, the cytokine-upregulating effects of a low crude protein diet were ameliorated (Mullenix et al., 2021). Heat-stressed broilers fed 1% Spirulina diets had greater anti-sheep red blood cell titers compared to birds fed control diets (Mirzaie et al., 2018). Chlorella inclusion in all forms at 1.0% of the diet increased plasma immunoglobulin (Ig)A in unchallenged broilers, but only fresh product increased white blood cell counts (Kang et al., 2013). Similarly, serum IgG, IgM, and IgA was positively associated with increasing Chlorella by-product inclusion from 2.5 to 7.5% (Kang et al., 2017). While specific outcomes related to intestinal integrity are not well-documented, 2.5% Chlorella in broiler diets increased ileal villus height (VH) and crypt depth (CrD) in unchallenged broilers and 10.0% Spirulina reduced bacterial translocation from the gastrointestinal tract to the liver (Kang et al., 2017; Mullenix et al., 2021).
Existing literature on improved growth performance associated with algae-derived ingredients reports varying outcomes. In many cases, algae inclusion did not alter broiler FCR but improved body weight gain (BWG), suggesting that changes to underlying physiology do not have a negative effect on bird performance (Qureshi et al., 1996; Kang et al., 2013; Kang et al., 2017; Mirzaie et al., 2018). Others similarly report improved weight gain in broilers at 21 d, but at considerably higher inclusion levels than other studies (16%) or no effect on performance at all (Waldenstedt et al., 2003; Evans et al., 2015).
Many of the documented responses to algae inclusion were observed without implementation of specific challenges to provide functional insight into mechanistic changes associated with different strains and inclusion rates. Such challenges could implement pathogenic or physiological stressors such as feed restriction (FR), which is known to stimulate significant stress in broilers, inducing translocation of large molecules like fluorescein isothiocyanate-labeled dextran (FITC-D) across the broiler intestine (Kuttappan et al., 2015; Baxter et al., 2017; Maguey‐Gonzalez et al., 2018). Additional challenges include specific pathogens such as Eimeria. Infection with these parasites results in coccidiosis, a disease of interest to commercial poultry production that can cause nutrient malabsorption, reduced intestinal integrity, villus atrophy, and depressed feed intake, all of which contribute to reduced performance and consequent economic loss (Baba et al., 1982; Williams, 2005; Collier et al., 2008). Current mitigation strategies do not provide satisfactory protection and shifts toward antibiotic-free production limit treatment of secondary bacterial infections (Blake and Tomley, 2014). A viable strategy for addressing coccidiosis is the use of bioactive feed ingredients like those derived from microalgae that may beneficially alter immunity and intestinal integrity to reduce negative physiological outcomes and protect bird performance. Broilers fed algae-based products at 0.015% dietary inclusion during Eimeria challenge had increased intestinal immune cell density and reduced lesion scores compared to infected controls, but differed on performance outcomes between studies, with some showing no changes and others reporting improved BW and FCR compared to infected controls at inclusions as low as 0.005% (Pieniazek et al., 2016; Levine et al., 2018). Varied experimental outcomes could additionally reflect the strength or type of inoculation (live oocyst vs. vaccine) and potential genetic strain response differences, which are not well-documented based on inoculation and genetic strain combinations within the same trial.
Combined outcomes associated with algae inclusion in the diets of unchallenged and Eimeria-inoculated broilers support the use of algae-based ingredients to protect broiler health during a challenge. Reported alterations to broiler immunity and intestinal health emphasize broad changes related to antibody titer, cell counts and presence, intestinal histomorphology, and lesion scores, but do not provide functional insights or a detailed explanation of immune cells present in unchallenged vs. challenged animals. Similarly, promising results observed in birds fed Spirulina and Chorella reinforce the use of algae ingredients to improve animal health; however, the varied outcomes from these products also support continued investigation into novel algae strains that may be enriched in underlying bioactive compounds. The research objectives of the present work were divided into 2 studies utilizing a propriety algae-based ingredient derived from a strain that has not previously been cultivated or investigated mechanistically. This strain has shown promising effects in pilot studies, but the mechanisms by which this strain support broiler health during a challenge are uncertain (unpublished data). Study 1 evaluated performance and intestinal integrity responses to a proprietary algae-based ingredient in broilers subjected to FR challenge. Study 2 examined the effects of the same algae product on performance, systemic immunity, and intestinal health of Eimeria-challenged broilers.
MATERIALS AND METHODS
Birds and Experimental Diets
All animal protocols were approved by the Iowa State University Institutional Animal Care and Use Committee. In both studies, straight-run Ross 308 broilers from Welp Hatchery (Bancroft, IA) were transported to the Iowa State Poultry Research and Teaching Farm, wing-banded upon arrival, and randomly assigned to dietary treatments. Dietary treatments in both studies consisted of a corn-soybean meal control diet formulated based on NRC requirements (National Research Council, 1994; Table 1) ± 0.175% algae. In both studies, lighting schedule for the first 7 d of the trial was 23 h light before transition to 20 h light for the remainder. In study 1, two sets of 200 chicks were obtained and 2 replicate experiments were conducted sequentially (n = 400; average d 0 BW = 0.04kg). Broilers were housed in chick brooder units (Petersime Model 2SD20RE; Gettysburg, OH) in a total of 40 cages across 2 brooder units (5 birds/cage; stocking density 1.34 ft2/ bird), with ad libitum access to mash feed and water from an attached trough for 28 d. Birds were weighed individually at the end of each 2-wk performance period and feed intake was recorded throughout. The mortality rate in study 1 was 5.3%.
Table 1.
Composition of basal and algae-containing1 starter, grower, and finisher diets fed to Ross 308 broilers over 28 d (study 1; starter and grower rations only) and 42 d (study 2).
| Experimental diet |
||||||
|---|---|---|---|---|---|---|
| Ingredient, % | Basal starter | Basal grower | Basal finisher | |||
| Corn | 55.32 | 58.69 | 62.78 | |||
| Soybean meal, 48% CP | 37.15 | 33.40 | 28.59 | |||
| Soybean oil | 2.02 | 2.98 | 3.97 | |||
| Salt | 0.40 | 0.40 | 0.40 | |||
| DL-Met | 0.33 | 0.30 | 0.27 | |||
| L-Lys × HCL | 0.25 | 0.23 | 0.21 | |||
| L-Thr | 0.15 | 0.15 | 0.15 | |||
| Limestone | 1.30 | 1.01 | 1.00 | |||
| Dicalcium phosphate | 2.05 | 1.81 | 1.60 | |||
| Choline chloride-60 | 0.40 | 0.40 | 0.40 | |||
| Vitamin-mineral premix2 | 0.63 | 0.63 | 0.63 | |||
| Calculated values, % | ||||||
|---|---|---|---|---|---|---|
| Crude fat | 4.59 | 5.59 | 6.64 | |||
| CP | 23.05 | 21.5 | 19.5 | |||
| Digestible Lys | 1.30 | 1.19 | 1.06 | |||
| Digestible Met | 0.61 | 0.57 | 0.52 | |||
| ME, kcal/Kg | 3,000.00 | 3,100.00 | 3,200.00 | |||
| Analyzed values (%) | Control | Algae | Control | Algae | Control | Algae |
|---|---|---|---|---|---|---|
| Moisture | 10.89 | 12.21 | 11.28 | 11.39 | 10.66 | 10.89 |
| DM | 89.11 | 87.79 | 88.72 | 88.61 | 89.34 | 89.11 |
| Crude fat | 4.79 | 4.51 | 5.62 | 5.57 | 6.76 | 6.26 |
| CP | 21.20 | 20.46 | 19.26 | 19.99 | 16.44 | 17.71 |
| GE, cal/g | 3,809.89 | 3,751.24 | 3,865.37 | 3,836.05 | 3,922.34 | 3,897.67 |
Algae-based feed ingredient incorporated into the basal diet at 0.175%.
Vitamin and mineral premix provided per kg of diet: selenium 250 μg; Vitamin A (retinyl acetate) 8,250 IU; cholecalciferol (vitamin D3) 2,750 IU; α-tocopherol acetate (vitamin E) 17.9 IU; menadione 1.1 mg; vitamin B12 12 μg; biotin 41 μg; choline 447 mg; folic acid 1.4 mg; niacin 41.3 mg; pantothenic acid 11 mg; pyridoxine 1.1 mg; riboflavin 5.5 mg; thiamine 1.4 mg; iron 282 mg; magnesium 125 mg; manganese 275 mg; zinc 275 mg; copper 27.5 mg; iodine 844 μg.
In study 2, three hundred broilers were housed in barn with 2 rooms physically separated by an anteroom (average d 0 BW = 0.05 kg). Birds were equally distributed between the 2 rooms in 40 4’ × 4’ pens (20 pens/ room; 20 birds/ pen; stocking density 0.8 ft2/bird) and given ad libitum access to mash feed and nipple waterers. Birds in each pen were weighed every 7 d and feed intake was measured throughout the study. On d 14, 10 birds/diet were euthanized for baseline tissue sample collection and half of the remaining birds were orally gavaged with 200 μL of 10X Coccivac-B52 (Merck, Kenilworth, NJ) in MilliQ water. The other half were sham-inoculated with MilliQ water only. Inoculated and sham-inoculated birds were physically separated between the 2 sides of the barn. Dietary treatment and inoculation status contributed to a 2 × 2 factorial treatment design accounting for 4 treatment groups total. Post-inoculation (pi), birds were euthanized for tissue sampling on 1, 3, 7, 14, and 28 dpi (5 birds/ treatment). The trial was concluded on d 42 (28 dpi) with a final count of 8 to 10 birds/pen. Non sampling mortality for the entire 42 d study was 2.1%.
Intestinal Permeability
The FITC-D intestinal permeability assay was conducted in study 1 at d 28 with half of the cages subjected to a 12 h FR on d 27. On d 28, 3,000 to 5,000 molecular weight FITC-D was administered by oral gavage to all birds in 36 cages at an 8.32 mg/kg dose based on individual bird weights taken on d 27. Two cages/ trial without FITC-D administration (1 FR, 1 no FR)/ dietary treatment were used as serum blank controls as described in Baxter et al. (2017). Blood drawn from the brachial vein 1 h post-gavage was collected into serum separation tubes, allowed to clot at room temperature in the dark, and centrifuged at 1,000 × g for 15 min. Serum was transferred into amber tubes, diluted 1:5 in saline, and stored at −20˚C until analysis. Standard curves were prepared the day of analysis by dissolving FITC-D in blank serum at 6,400 ng/mL and performing a 2X serial dilution to 100 ng/mL with a minimum value of 0 ng/mL (blank serum only). All standards and diluted serum samples were plated on black 96-well plates in duplicate (100 µL/well). Plates were read at 485 and 528 nm excitation and emission wavelengths, respectively. Readings from blank serum (0 ng/mL FITC) was subtracted from all sample readings and the intercept on all standard curves set to 0. Resultant standard curve equations were used to calculate serum fluorescence (ng/mL).
Similar methods were used to perform the same assay in study 2 at 1, 3, 7, and 14 dpi using 40 birds/ treatment/ timepoint. The day prior to each assay, 4 birds/ pen were weighed individually to calculate FITC-D dosage. One hour post-gavage, blood was collected, including control samples collected from 10 birds/ inoculation status without FITC-D administration for serum blanks. At 1 and 3 dpi, birds were CO2-anesthetized for blood collection by cardiac puncture prior to cervical dislocation euthanasia. At 7 and 14 dpi, blood was collected from the brachial vein as in study 1. Assays to determine serum fluorescence were carried out as described above.
Oocyst Enumeration and Lesion Scoring
At 7 and 14 dpi, fresh excreta were collected from all pens and pooled by treatment for oocyst enumeration, with additional excreta and litter samples taken from the inoculated side of the barn at 8 dpi to confirm oocyst shedding and environmental presence. Pens were pooled by treatment due to the nonsynchronized nature of the oocyst cycling and shedding. Oocyst enumeration was conducted using McMaster chambers by the Iowa State University Veterinary Diagnostic Laboratory (Ames, IA). Briefly, 2 g excreta or bedding were diluted 15X in a glucose solution (1.2–1.25 specific gravity) and pipetted into both wells of a McMaster chamber. After 15 min, visible oocysts within both McMaster chamber grids were counted using a microscope at 10X objective. The sum of both counts was then multiplied by 50 to determine the number of oocysts/excreta g. General lesion scoring was done at 14 dpi in accordance with published criteria by one observer on the duodenum, jejunum, and ceca from 5 birds/treatment (Johnson and Reid, 1970). In this general scoring system, a score of 0 indicates no evidence of Eimeria lesions, while scores of 1–4 correspond with increasing lesion severity and phenotypic inflammation (altered content characteristics, ballooning, and intestinal wall thickening).
Histomorphology
The Coccivac-B52 vaccine comprises species that primarily invade the duodenum and/or jejunum (E. mivati, E. acervulina, and E. maxima) and the ceca (E. tenella; López-Osorio et al., 2020). Duodenum and jejunum segments collected at d 14 (baseline), 3, 7, 14, and 28 dpi were fixed for 24 h in neutral-buffered formalin (10%) before being transferred to 70% ethanol. Tissue sections were paraffin-embedded, mounted on microscope slides, and H&E stained. An Olympus BX 54/43 microscope with DP80 Olympus camera was used to image slides and VH and CrD measurements were taken using the Olympus Cell Sens Dimension software (version 1.16; Olympus Corporation, Tokyo, Japan). VH was defined as the distance from the villus-crypt junction to villus tip in sections with intact lamina propria and CrD was the invagination depth between adjacent villi. The average of 10 measurements for both VH and CrD were taken per section from 5 birds/treatment and the VH:CrD ratio was calculated from these measurements.
Spleen Immune Cell Profiles
Along with intestinal segments, spleens were collected from 5 birds/ treatment, homogenized in PBS, and passed through a 70-μm sterile cell strainer. Four splenocyte aliquots were frozen in chicken serum with 7.5% DMSO at −80°C until analysis. Prior to extracellular staining, cells from each spleen were thawed, enumerated by hemocytometer, and aliquoted into 7 polystyrene flow cytometry tubes (approximately 3 million cells/ tube). Extracellular marker staining was done by diluting 0.5 μL fluorochrome-conjugated antibody in 50 μL PBS (0.08 μg/106 cells and 0.02 μg/106 cells for antibody stock concentrations at 0.5 mg/mL and 0.1 mg/mL, respectively) and incubating cells at 4°C in the dark for 30 min. The staining panel comprised mouse anti-chicken CD1.1 FITC (0.5 mg/mL; clone CB3; mouse IgG1κ), CD3 Pacific Blue (0.5 mg/mL; clone CT-3; mouse IgG1κ), CD4 Alexa Fluor 700 (0.5 mg/mL; clone CT-4; mouse IgG1κ), CD8α SPRD (0.1 mg/mL; clone CT-8; mouse IgG1κ), TCRγδ PE (0.1 mg/mL; clone TCR-1; mouse IgG1κ), and monocyte/macrophage biotin (0.5 mg/mL; clone KUL01; mouse IgG1κ). All primary antibodies were purchased from Southern Biotech (Birmingham, AL). Fluorescence-minus-one controls in which the antibody for each extracellular marker is excluded from the stain mixture and replaced with the associated isotype (0.2 μL/50 μL PBS; 0.007 μg/106 cells) were used to account for nonspecific binding by each antibody. Following extracellular staining, cells were washed in PBS and a Brilliant Violet (BV) 785-conjugated streptavidin stain (BioLegend, San Diego, CA) was applied (0.3 μL/50 μL PBS) to fluorescently label biotin-conjugated monocyte/macrophage antibody. Cells were incubated at 4°C in the dark for 30 min, washed, and resuspended in PBS prior to analysis. Cell measurements were collected by BD FACSCanto cytometer (BD Biosciences, San Jose, CA) and individual populations were gated using FlowJo software (version 10.5.0). Cells were initially gated based on forward scatter to identify singlets and exclude cells that were adhered or conjugated together before additional gating based on live cells. Each population of interest was gated based on fluorochrome signals within the population of singlet live cells.
Statistical Analysis
In study 1, data were assessed for normality using PROC UNIVARIATE and analyzed using a mixed linear model (PROC MIXED, SAS version 9.4, Cary, NC). Data between replicate experiments within study 1 were not determined to be significantly different, hence were analyzed as one dataset. Performance data were analyzed with the fixed effect of dietary treatment, and FITC-D fluorescence data were analyzed with the fixed effects of dietary treatment, FR, and the dietary treatment × FR interaction. The following statistical model was used to analyze serum fluorescence, histomorphology, and immune cell population data in study 2:
In this model, yijk is the dependent variable, μ is the overall mean, Di is the main effect of diet at the ith level (± algae additive; i=2), Cj is the main effect of Eimeria at the jth level (uninoculated or inoculated; j=2), (D × J)ij is the interaction effect of diet at the ith level and inoculation at the jth level, and eijk is the random error.
Study 2 performance was analyzed with initial BW (iBW) as a covariate using the following statistical model:
Terms used in this model are the same as outlined previously. Outliers were identified and excluded using the UNIVARIATE procedure and data were analyzed using the MIXED procedure in SAS 9.4 (SAS Institute, Cary, NC). LSmeans were separated by the PDIFF option and the Tukey adjustment to account for multiple comparisons. For data in healthy birds at d 14, only diet effects were included in the model as birds had not been inoculated at this timepoint. Significant results were denoted at P ≤ 0.05 for all parameters.
RESULTS
Study 1: Performance
In brooder caged-birds, no growth differences were detected during the starter period, but the algae-fed group showed increased BWG in the grower period (d 14–27) by 7% (0.05 kg; P = 0.005), and overall weight gain (d 0–27) by 4.5% (0.05 kg; P = 0.034, Table 2). This impact of algae inclusion was also reflected in significantly increased average daily gain in the grower period. No significant differences were detected in body weight at d 14 or d 27. Feed intake nor FCR were affected by diet in either performance period.
Table 2.
Study 1: Dietary treatment effects on straight-run Ross 308 broiler performance outcomes averaged per bird including feed intake, weight gain, ADG, and FCR by each 2-wk performance period1 and overall2.
| Performance outcome | Control | 0.175% Algae | SEM | P-value |
|---|---|---|---|---|
| Feed intake, kg | ||||
| Starter | 0.46 | 0.45 | 0.02 | 0.73 |
| Grower | 1.21 | 1.24 | 0.04 | 0.65 |
| Overall | 1.68 | 1.69 | 0.04 | 0.80 |
| Weight gain, kg | ||||
| Starter | 0.38 | 0.39 | 0.01 | 0.62 |
| Grower | 0.72b | 0.77a | 0.01 | 0.01 |
| Overall | 1.11b | 1.16a | 0.02 | 0.03 |
| ADG, g | ||||
| Starter | 27.3 | 27.6 | 0.45 | 0.62 |
| Grower | 55.5b | 57.4a | 0.79 | 0.05 |
| Overall | 41.4 | 42.5 | 0.35 | 0.09 |
| Body weight, kg | ||||
| d 0 | 0.04 | 0.04 | 0.0003 | 0.31 |
| d 14 | 0.43 | 0.43 | 0.01 | 0.89 |
| d 27 | 1.15 | 1.18 | 0.02 | 0.20 |
| FCR | ||||
| Starter | 1.27 | 1.25 | 0.03 | 0.56 |
| Grower | 1.61 | 1.57 | 0.03 | 0.34 |
| Overall | 1.42 | 1.40 | 0.02 | 0.44 |
Starter period indicates wk d 0–14, grower wk d 14–28. Values presented are LSMeans (pooled SEM) averaged per bird.
Means within a row lacking a common superscript differ (P ≤ 0.05).
Study 2: Performance
Feeding the algae ingredient did not affect bird performance in the starter period prior to Eimeria inoculation. Throughout the post-inoculation period, algae inclusion did not impact performance outcomes during the grower and finisher periods. The main effect of Eimeria inoculation decreased BWG by 20.4% (0.20 kg) and FI by 10.7% (0.17 kg) with a 20-point (12.7%) less efficient FCR compared to uninoculated birds (P < 0.0001, = 0.002, and 0.003, respectively). Weekly performance during this time showed reduced BW by 19.2% (0.16 kg), BWG by 34.1% (0.14 kg), and FI by 18.2% (0.13 kg) resulting in a 43-point (24.0%) less efficient FCR during week 3 (P < 0.0001). In wk 4, inoculated birds weighed 15.7% (0.22 kg) less, gained 10.5% (0.06 kg) less BW, and had a 13-point (8.5%) less efficient FCR compared to uninoculated birds (P < 0.0001, P = 0.03, respectively). While no differences in finisher performance were observed, Eimeria-inoculated birds weighed 9.7 and 6.1% (0.20 and 0.17 kg) less than uninoculated birds in wk 5 and 6, respectively (P < 0.001 and = 0.004, respectively; Tables 3 and 4). During the entire 42 d study, Eimeria-inoculated birds gained 6.2% (0.17kg) less BW and had a 4-point (3.0%) less efficient FCR compared to uninoculated birds, regardless of algae inclusion (main effect: P = 0.004 and 0.01, respectively; Table 4).
Table 3.
Study 2: Weekly performance of healthy and Eimeria-inoculated Ross 308 broilers fed basal diet ± 0.175% algae ingredient for 42 d represented on a per bird basis.
| Treatment |
SEM |
P-values |
||||||
|---|---|---|---|---|---|---|---|---|
| Performance outcome | control | Algae | Diet | Eimeria | Diet × Eimeria | |||
| d 0 BW, kg1 | 0.05 | 0.05 | 0.0004 | 0.83 | N/A | N/A | ||
| Wk 1 | ||||||||
| BW, kg | 0.16 | 0.16 | 0.002 | 0.46 | N/A | N/A | ||
| BWG, kg | 0.11 | 0.11 | 0.002 | 0.52 | N/A | N/A | ||
| FI, kg | 0.13 | 0.13 | 0.002 | 0.95 | N/A | N/A | ||
| FCR | 1.14 | 1.15 | 0.01 | 0.16 | N/A | N/A | ||
| Wk 2 | ||||||||
| BW, kg | 0.40 | 0.41 | 0.009 | 0.20 | N/A | N/A | ||
| BWG, kg | 0.24 | 0.26 | 0.007 | 0.52 | N/A | N/A | ||
| FI, kg | 0.33 | 0.34 | 0.007 | 0.95 | N/A | N/A | ||
| FCR | 1.37 | 1.34 | 0.01 | 0.16 | N/A | N/A | ||
| Treatment |
SEM |
P-values |
||||||
|---|---|---|---|---|---|---|---|---|
| Performance outcom | Control | Algae | Control + Eimeria | Algae + Eimeria | Diet | Eimeria | Diet × Eimeria | |
| Wk 3 | ||||||||
| BW, kg | 0.83 | 0.83 | 0.67 | 0.67 | 0.03 | 0.89 | <0.0001 | 0.98 |
| BWG, kg | 0.42 | 0.42 | 0.29 | 0.26 | 0.02 | 0.31 | <0.0001 | 0.47 |
| FI, kg | 0.75 | 0.72 | 0.63 | 0.58 | 0.03 | 0.03 | <0.0001 | 0.66 |
| FCR | 1.79 | 1.74 | 2.19 | 2.19 | 0.10 | 0.70 | <0.0001 | 0.68 |
| Wk 4 | ||||||||
| BW, kg | 1.39 | 1.41 | 1.18 | 1.19 | 0.06 | 0.62 | <0.0001 | 0.89 |
| BWG, kg | 0.56 | 0.58 | 0.50 | 0.52 | 0.04 | 0.39 | 0.03 | 0.85 |
| FI, kg | 0.85 | 0.85 | 0.80 | 0.83 | 0.03 | 0.54 | 0.22 | 0.49 |
| FCR | 1.55 | 1.46 | 1.64 | 1.62 | 0.08 | 0.30 | 0.03 | 0.51 |
| Wk 5 | ||||||||
| BW, kg | 2.11 | 2.10 | 1.92 | 1.88 | 0.06 | 0.52 | <0.0001 | 0.69 |
| BWG, kg | 0.72 | 0.69 | 0.75 | 0.69 | 0.04 | 0.10 | 0.59 | 0.68 |
| FI, kg | 1.09 | 1.10 | 1.11 | 1.07 | 0.03 | 0.64 | 0.83 | 0.25 |
| FCR | 1.52 | 1.61 | 1.51 | 1.56 | 0.08 | 0.23 | 0.66 | 0.73 |
| Wk 6 | ||||||||
| BW, kg | 2.84 | 2.82 | 2.70 | 2.62 | 0.08 | 0.34 | 0.004 | 0.62 |
| BWG, kg | 0.74 | 0.72 | 0.77 | 0.74 | 0.03 | 0.27 | 0.21 | 0.67 |
| FI, kg | 1.29 | 1.29 | 1.33 | 1.32 | 0.04 | 0.78 | 0.23 | 0.67 |
| FCR | 1.76 | 1.80 | 1.73 | 1.80 | 0.06 | 0.17 | 0.73 | 0.53 |
Abbreviations: BW, body weight; BWG, body weight gain.
The first 2 weeks of the trial represent a period of time before administration of 10X Coccivac-B52 (Merck, Kenilworth, NJ). The main effects of Eimeria status and the interaction were omitted from analysis.
Table 4.
Study 2: Performance of healthy and Eimeria-inoculated Ross 308 broilers fed basal diet ± 0.175% algae ingredient for 42 d divided into 14-d starter, grower, and finisher periods represented on a per bird basis.
| Treatment |
SEM |
P-values |
||||||
|---|---|---|---|---|---|---|---|---|
| Performance outcom | control | Algae | Diet | Eimeria | Diet × Eimeria | |||
| d 0 BW, kg1 | 0.05 | 0.05 | 0.0005 | 0.83 | N/A | N/A | ||
| Starter (d 0–14) | ||||||||
| BWG, kg | 0.35 | 0.37 | 0.009 | 0.20 | N/A | N/A | ||
| FI, kg | 0.46 | 0.47 | 0.009 | 0.21 | N/A | N/A | ||
| FCR | 1.30 | 1.29 | 0.009 | 0.38 | N/A | N/A | ||
| Treatment |
SEM |
P-values |
||||||
|---|---|---|---|---|---|---|---|---|
| Performance outcom | Control | Algae | Control + Eimeria | Algae + Eimeria | Diet | Eimeria | Diet × Eimeria | |
| Grower (d 14–28) | ||||||||
| BWG, kg | 0.98 | 1.00 | 0.79 | 0.78 | 0.05 | 0.83 | <0.0001 | 0.66 |
| FI, kg | 1.63 | 1.61 | 1.45 | 1.45 | 0.06 | 0.73 | 0.0002 | 0.75 |
| FCR | 1.68 | 1.61 | 1.85 | 1.86 | 0.07 | 0.50 | 0.0003 | 0.46 |
| Finisher (d 28–42) | ||||||||
| BWG, kg | 1.46 | 1.41 | 1.51 | 1.43 | 0.06 | 0.11 | 0.32 | 0.63 |
| FI, kg | 2.38 | 2.39 | 2.45 | 2.39 | 0.07 | 0.60 | 0.45 | 0.37 |
| FCR | 1.64 | 1.70 | 1.62 | 1.68 | 0.05 | 0.11 | 0.62 | 0.93 |
| Overall (d 0–42) | ||||||||
| BWG, kg | 2.80 | 2.77 | 2.65 | 2.58 | 0.08 | 0.34 | 0.004 | 0.63 |
| FI, kg | 4.48 | 4.47 | 3.34 | 4.30 | 0.12 | 0.76 | 0.08 | 0.82 |
| FCR | 1.60 | 1.61 | 1.64 | 1.67 | 0.02 | 0.19 | 0.01 | 0.57 |
The starter phase represents a period of time before administration of 10X Coccivac-B52 (Merck, Kenilworth, NJ). The main effects of Eimeria status and the interaction were omitted from analysis.
Study 1: Intestinal Permeability During Feed Restriction Challenge
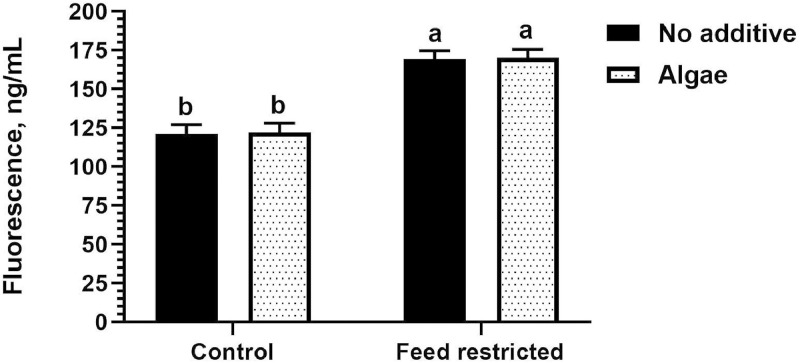
In the 4-wk caged replicates, the algae feed ingredient did not display a protective effect on intestinal barrier function following 12 h FR challenge. While FR was successful in stressing the gut and triggering translocation of FITC-d beyond the no FR control (P < 0.0001), there were no differences detected based on diet (P = 0.87), nor was there a diet by FR interaction (P = 0.98, Figure 1).
Figure 1.
Values are expressed as mean fluorescence (ng/mL) of serum fluorescein isothiocyanate dextran (FITC-D) using a 12-h feed restriction model with control and algae-supplemented dietary treatments fed to Ross 308 broiler chickens. Bars with different superscripts denote means that are significantly different (P ≤ 0.05).
Study 2: Intestinal Permeability Following Eimeria Challenge
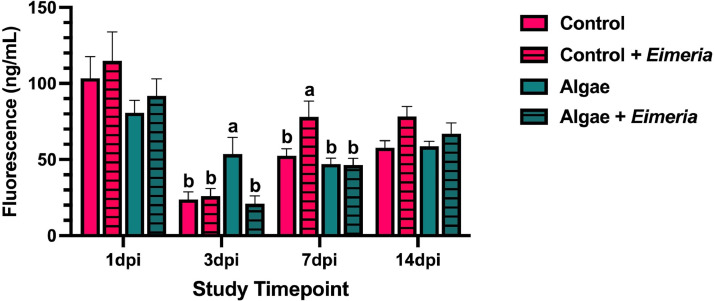
Between 1 and 3 dpi, serum fluorescence generally decreased across all treatments, indicating increased intestinal barrier function. At 7 dpi, Eimeria-inoculated and uninoculated birds fed the algae ingredient had similar levels of serum fluorescence, while inoculated birds fed the control diet had a 1.5-fold increase in serum fluorescence compared to their uninoculated counterparts (P = 0.04). Although the main effect of Eimeria-inoculation increased serum fluorescence 1.2-fold at 14 dpi, no differences between individual treatments were observed at this timepoint (P = 0.01; Figure 2).
Figure 2.
The serum fluorescence of uninoculated and Eimeria-inoculated birds fed a corn-soybean meal basal diet ± 0.175% algae ingredient and orally gavaged with fluorescein isothiocyanate-dextran (FITC-D; 8.32 mg/kg of body weight). Values represent the mean serum fluorescence of 40 birds/ treatment ± SEM. Bars with differing superscripts are considered significant at P ≤ 0.05.
Study 2: Oocyst Counts and Lesion Scores
Oocyst enumeration was completed to confirm inoculation and monitor oocyst shedding. As excreta samples were pooled by treatment due to the nonsynchronized nature of shedding in a floor pen environment and high degree of variability between pens, results for oocyst shedding are based on numerical observations. At 7 dpi, Eimeria-inoculated birds fed the control diet shed 2.5-fold greater oocysts compared to inoculated birds fed the algae ingredient. At 8 dpi, detected oocysts in the litter were similar between control and algae-fed pens on the inoculated side of the barn; however, inoculated birds fed the algae ingredient had a 3.9-fold increase in shed oocysts. At the conclusion of study 2, Eimeria-inoculated, algae-fed birds shed 2.3-fold more oocysts compared to inoculated birds fed the control diet (Table 5). Such variable responses in oocyst shedding indicate that this measure may not be representative of feed ingredient efficacy in an experiment with non-synchronous Eimeria cycling.
Table 5.
Oocyst counts1 from pooled excreta collected from 10 healthy and Eimeria-inoculated pens per dietary treatment consisting of basal diet ± 0.175% algae ingredient.
| Treatment |
||||
|---|---|---|---|---|
| Sample type | Control | Algae | Control + Eimeria | Algae + Eimeria |
| 7 dpi | ||||
| Excreta, oocysts/ g | 0 | 50 | 48,350 | 19,100 |
| 8 dpi2 | ||||
| Litter, oocysts/ g | N/A | N/A | 8,750 | 8,700 |
| Excreta, oocysts/ g | N/A | N/A | 5,800 | 22,650 |
| 14 dpi | ||||
| Excreta, oocysts/ g | 200 | 50 | 20,700 | 48,500 |
Oocyst counts enumerated using McMaster chambers. Two g of pooled excreta were mixed into 28 mL of float solution (1.2–1.25 specific gravity). Oocysts were enumerated under a microscope and multiplied by 50 to obtain the number of oocysts per gram of excreta.
Samples were collected at 8dpi to confirm fecal shedding and the presence of oocysts in the bedding of birds on the inoculated side of the barn.
Lesion scoring at 14 dpi showed no lesion evidence in the uninoculated birds. Generally, inoculated birds had low average scores ranging from 1.2 to 2.2. Average lesion scores were similar in inoculated birds across all tissues (Table 6).
Table 6.
Average lesion scores in the intestinal tract of healthy and Eimeria-inoculated Ross 308 broilers from 5 birds per treatment consisting of basal diet ± 0.175% algae ingredient.
| Location1 | Treatment |
|||
|---|---|---|---|---|
| Control | Algae | Control + Eimeria | Algae + Eimeria | |
| Duodenum | 0 | 0 | 1.2 | 1.4 |
| Jejunum | 0 | 0 | 1.4 | 1.2 |
| Ceca | 0 | 0 | 2.2 | 1.8 |
Scores of 0 = no observed signs of disease.
Lesion scores done by one observer in accordance with parameters published by Johnson and Reid, 1970.
Study 2: Intestinal Histomorphology
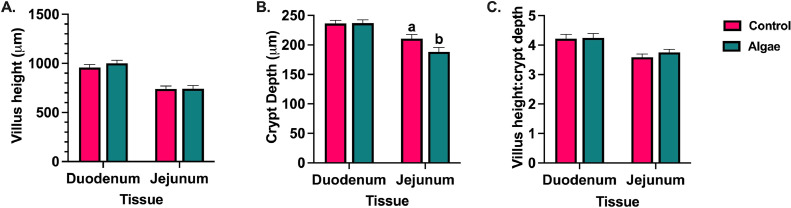
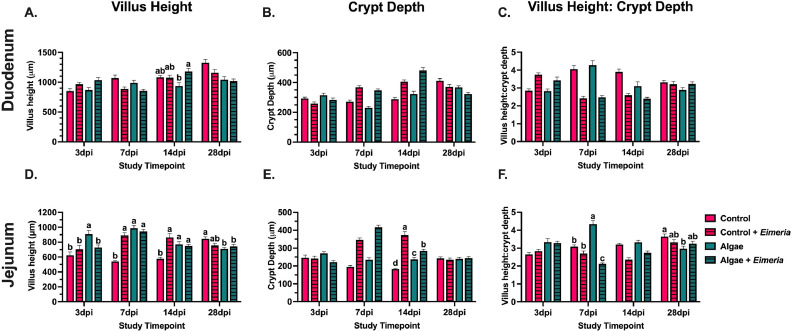
In 14-day-old broilers, dietary algae did not alter duodenal morphology, but reduced jejunal CrD by 10.7% compared to the control (P = 0.03; Figure 3). The Eimeria inoculation main effect resulted in 16.2% increased duodenal VH at 3 dpi before showing a 15.5% reduction at 7 dpi compared to uninoculated birds (P = 0.007 and 0.0001, respectively). Though responses in VH fluctuated across timepoints, Eimeria-inoculated birds showed 43.0 increased CrD at 7 dpi (P < 0.0001). Changes in CrD relative to VH ultimately resulted in Eimeria-inoculated birds having VH:CrD ratios 41.1% below those of their uninoculated counterparts at 7 dpi (P < 0.0001). At 14 dpi, Eimeria-inoculated birds fed algae had 25.7% increased VH compared to their uninoculated counterparts, while these measures were similar in control-fed animals (P = 0.008). Though no interaction effects were observed at 28 dpi, the main effect of algae inclusion reduced VH and CrD by 17.1 and 11.9%, respectively (P < 0.0001 and = 0.0008) in algae-fed birds compared to the control at 28 dpi, ultimately resulting in an unchanged VH:CrD ratio (Figures 4A–4C).
Figure 3.
Histomorphological measurements (A) villus height, (B) crypt depth, and (C) villus height:crypt depth ratio in the duodenum and jejunum of pre-inoculation 14-day-old broilers fed a corn-soybean meal basal diet ± 0.175% algae ingredient. Data represent the average measurement from 10 birds/ diet ± SEM. Bars with differing superscripts are considered significant at P ≤ 0.05.
Figure 4.
Histomorphological measurements in the (A–C) duodenum and (D–F) jejunum of uninoculated and Eimeria-inoculated broilers fed a corn-soybean meal basal diet ± 0.175% algae ingredient at 3, 7, 14, and 28 d post-inoculation. Data represent the mean histomorphological measurement from 5 birds/ treatment/ study timepoint ± SEM. Bars with differing superscripts are considered significant at P ≤ 0.05.
In the jejunum, comparable VH was observed between uninoculated and Eimeria-inoculated birds fed algae diets at 7 dpi, while inoculated control-fed birds had 64.9% taller VH compared to their uninoculated counterparts (P < 0.0001). While CrD was similar across treatments at 7 dpi, uninoculated birds fed the algae ingredient had 51.1% increased VH:CrD ratio compared to their Eimeria-inoculated counterparts (P < 0.0001; Figures 4D–4F). At 14 dpi, Eimeria-inoculated birds fed the control diet had 49.8% greater VH compared to their uninoculated counterparts while VH measurements were similar between uninoculated and inoculated algae-fed birds (P < 0.001; Figure 4D). Simultaneously, Eimeria-inoculated birds fed the control diet had 51.2% deeper crypts than their uninoculated counterparts, while CrD was reduced 16.6% in inoculated vs. uninoculated birds fed algae (P < 0.0001; Figure 4E). By 28 dpi, jejunal histomorphology was similar across all treatments, suggesting resolution of Eimeria-induced damage.
Study 2: Spleen Immune Cell Profiles
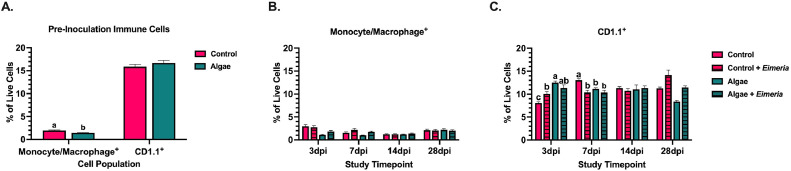
Two innate/antigen presenting cell (APC) populations analyzed in this study were monocyte/macrophage+ and CD1.1+ innate lipid, glycolipid, and lipopeptide antigen presenting cells. In pre-inoculated birds (d 14), monocyte/macrophage+ cells were present at low percentages in the spleen (≤2%) and were reduced 26.6% in birds fed the algae ingredient (P < 0.0001). This pattern of algae-fed birds having fewer monocyte/macrophage+ cells was maintained until 14 dpi when both groups showed similar percentages of this population within the spleen (Figure 5B). Splenic monocyte/macrophage+ cell populations were minimally affected by Eimeria, with inoculated birds showing a 35.1% increase in this cell population compared to uninoculated at 7 dpi only, regardless of algae inclusion (P = 0.001; Figure 5B). By the 14dpi all treatments had similar splenic populations of monocyte/macrophage+ cells.
Figure 5.
Populations of innate/antigen-presenting immune cell populations in the spleens of (A) pre-inoculation 14-day-old broilers fed a corn-soybean meal basal diet ± algae-based feed ingredient. Examined populations in uninoculated or Eimeria-inoculated broilers on the same diets include (B) monocyte/macrophage+ cells and (C) CD1.1+ lipid antigen-presenting cells. Data represent the mean population of cells staining positive for either marker within the live cell gate from 10 birds/ diet (Panel A) or 5 birds/ treatment/ timepoints (Panels B-C) ± SEM. Bars with different letter superscripts are significantly different at P ≤ 0.05.
CD1.1+ lipid APCs were also analyzed in the broiler spleen with this cell population comprising 15 to 17% of analyzed live cells and were unaffected by dietary algae in 14-day-old broilers (Figure 5A). At 3 dpi, CD1.1+ populations were similar between uninoculated and inoculated birds on algae diets while inoculated control-fed birds had 20.0% more CD1.1+ cells than their uninoculated counterparts (P = 0.0008). Between 3 and 7 dpi, only uninoculated control-fed birds showed a 61.9% expansion in CD1.1+ cells to levels 14.4 to 20.5% greater than all other treatments (P = 0.02; Figure 5C). While early changes to splenic CD1.1+ populations were observed in control-fed animals, these cells remained at consistent levels in algae-fed birds for the first 14 dpi. At 28 dpi, the main effect of Eimeria inoculation increased CD1.1+ cells by 23.5% compared to uninoculated animals (P < 0.0001).
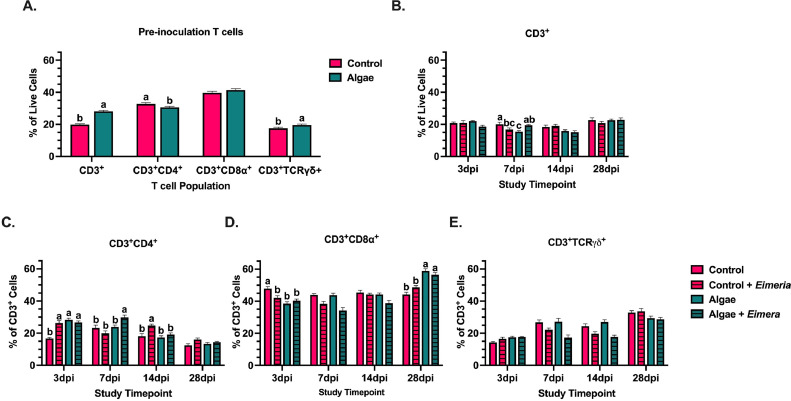
As a lymphatic organ, the spleen is home to a significant lymphocyte population. Therefore, T cells and their subpopulations were analyzed to determine alterations in the systemic adaptive immune response to Eimeria. At baseline (d 14), CD3+ T cell populations accounted for about 20 to 30% of analyzed splenocytes and algae inclusion increased this population by 29.6% compared to the control diet (P < 0.0001; Figure 6A). At 7 dpi, Eimeria-inoculated algae-fed birds had 20.9% more T cells than uninoculated birds fed the same diet while inoculated birds fed the control diet had 16.7% fewer T cells than their uninoculated counterparts (P < 0.0001). Uninoculated birds fed the algae diet showed a 29.9% reduction in splenic T cells between 3 and 7 dpi, while their Eimeria-inoculated counterparts maintained this cell population. Between these same timepoints, uninoculated control-fed birds maintained T cell populations while their Eimeria-inoculated counterparts displayed a 19.6% reduction in this cell type (Figure 6B). No differences were observed between healthy and Eimeria-inoculated birds on either diet beyond 7 dpi.
Figure 6.
T cell populations in the spleens of (A) pre-inoculation 14-day-old broilers fed a corn-soybean meal basal diet ± algae-based feed ingredient. Examined populations in uninoculated or Eimeria-inoculated broilers on the same diets include (B) overall CD3+ cells within the live cell gate and underlying subpopulations of (C) CD3+CD4+ helper T cells, (D) CD3+CD8α+ cytotoxic T cells, and (E) CD3+TCRγδ+ cells within the CD3+ cell gate. Data represent the mean population from 10 birds/ diet (panel A) or 5 birds/ treatment/ timepoints (panels B-E) ± SEM. Bars with different letter superscripts are significantly different at P ≤ 0.05.
Within overall populations of CD3+ T cells, different subpopulations were analyzed to gain better insight into cell types contributing to observed changes. Of these subpopulations, CD3+CD4+ helper T cells (TH), made up 20 to 30%, CD3+CD8α+ cytotoxic T (TC) cells made up approximately 40%, and CD3+TCRγδ+ (γδ) T cells comprised 15 to 20% of analyzed T cells in the pre-inoculation 14-day-old broiler spleen (Figure 6A). Prior to Eimeria inoculation, algae inclusion in healthy birds reduced TH cells by 6.5% and increased γδ T cells by 10.3% compared to the control (P = 0.03 and 0.04, respectively).
Between 3 and 7 dpi, Eimeria-inoculated birds fed the control diet showed a 24.2% reduction in splenic TH while these populations increased 10.9% in inoculated algae-fed birds to levels approximately 33.3% above all other treatments without displacing TC or γδ T cells (P = 0.002; Figures 6A–6C). Between 7 and 14 dpi, Eimeria-inoculated birds fed algae-containing diets showed a 34.6% TH cell reduction to levels similar to their uninoculated counterparts. In contrast, Eimeria-inoculated, control-fed birds showed a 19.7% TH cell increase to levels 22.9 to 30.2% above all other treatments (P = 0.03; Figure 6C). Changes in TH from 7 to 14 dpi in inoculated birds fed both diets occurred without impacting TC or γδ T cells; however, the main effect of Eimeria reduced TC cells by 17.2 and 7.5% at 7 and 14 dpi, respectively, in inoculated vs. uninoculated birds (P < 0.0001, = 0.01, respectively). Likewise, populations of γδ T cells in inoculated birds were reduced 26.4 and 27.1% compared to uninoculated birds at 7 and 14 dpi, respectively (P < 0.0001). Algae likely did not protect against Eimeria-induced changes to splenic TC or γδ T cells as no differences were observed between inoculated control and algae-fed birds at 7 and 14 dpi. In the last 14 d of the study (14–28 dpi) TC cells in birds fed algae-containing diets increased to levels 19.5% greater than control-fed birds (P < 0.0001). Populations of γδ T cells in inoculated birds fed either diet also increased between 14 and 28 dpi to levels similar to their corresponding controls, suggesting recovery of Eimeria-induced losses of splenic γδ T cells at this time. However, birds fed algae-containing diets had 12.8% fewer γδ T-cells than birds fed the control diet (P < 0.0001; Figures 6D and 6E).
DISCUSSION
The 2 studies described here implemented separate approaches to examine the effects of algae on challenges to poultry intestinal health and systemic immune responses. The importance of employing multiple experimental approaches relates to the fact that birds are exposed to numerous challenge types over the growth cycle, including pathogen, environment, temperature, and conspecific stress, and it is important to understand which types of stressors may or may not be improved by feeding novel ingredients. Study 1 utilized an acute physiological challenge and emphasized changes to intestinal integrity. Specifically, acute feed restriction challenges occur commonly in industry settings from basic overnight dark hours, to raising feed lines during heat stress (Liew et al., 2003), to feed restriction prior to slaughter. In contrast, study 2 used an Eimeria pathogen challenge to model a commercially-relevant poultry disease with continued environmental cycling. This allowed the implementation of assays to evaluate changes to intestinal integrity and systemic immunity over a comparatively longer period of time (28 d vs. 12 h), as well as understand potential mechanisms for health maintenance or improvement. Data show that responses to algae inclusion in broiler diets did vary between the different challenges conditions used. Despite these differences, the results of studies 1 and 2 suggest that feeding the algae ingredient at inclusion levels as low as 0.175% may benefit broiler production.
In both study 1 and 2, regardless of housing conditions (battery cage vs. floor pen), algae did not alter performance in the starter period. Impacting performance in the initial growth period where birds are at a low BW and eating the least amount of feed may be difficult, yet feeding additives during this stage may set up positive benefits in later growth periods. Alterations in growth or feed intake may become more apparent as birds grow and consume more feed and therefore are exposed to the test ingredient at a higher rate. These outcomes are consistent with other reports showing unchanged performance in broiler chickens fed algae-derived ingredients compared to control (Waldenstedt et al., 2003; Alfaia et al., 2021). In study 1, algae inclusion contributed to 4.5% (0.05 kg) greater weight gain observed over the 27 d grow-out period (Table 2). In study 2, stocking density was reduced from 20 birds to approximately 14 birds/pen as animals were euthanized for FITC-D serum and tissue collection over the inoculation time course. Therefore, performance changes were more difficult to accurately assess in study 2 during the grower period due to regular reductions in stocking density and overall n/treatment over time. Future work could place additional birds in order to assess performance and immune outcomes simultaneously.
Importantly, the objectives of study 2 were not to examine performance as a main indicator of ingredient efficacy, but rather to understand physiological outcomes in response to a challenge. Therefore, bird performance over time, especially during heavy sampling periods, was understood to likely not reflect a true performance-based trial. Though not statistically significant, uninoculated birds fed the algae additive weighed 1.7% (0.02 kg) more and gained 2.2% (0.02 kg) more BW than the uninoculated control group during the grower period. In wk 3 and 4, algae-fed uninoculated birds had a 5- and 9-point numerically improved FCR, respectively, compared to those fed the control diet, culminating in a 6.7-point (4.0%) more efficient grower FCR (Tables 3 and 4). Despite not being statistically significant, this level of FCR improvement may be valuable to producers and supports future research into this algae ingredient's effects on performance in a floor pen model. The BW and BWG changes in the algae-fed groups in study 1 correspond with other published reports showing improved weight gain and average daily gain in unchallenged algae-fed broilers (Evans et al., 2015; Long et al., 2018; Park et al., 2018).
Study 2 implemented a pathogen challenge requiring confirmation of successful inoculation, which was accomplished by enumerating shed oocysts in pooled excreta samples at 7 dpi (Table 5). Despite variations in oocyst counts throughout the study, intestinal health and immunological outcomes between control and algae-fed birds starting at 7 dpi suggest that shed oocysts are not direct indicators of host responses. Due to the comparatively longer challenge period, study 2 measured intestinal health as changes to both intestinal structure and permeability over time. Histomorphological responses to coccidiosis are characterized by reduced VH and increased CrD ultimately resulting in a reduced VH:CrD occurring around 7 dpi (Assis et al., 2010; Gautier et al., 2020). Duodenal histomorphology changes attributed to Eimeria inoculation in this study were in accordance with these previously reported outcomes. In the jejunum, CrD increased in Eimeria-inoculated birds as expected, but VH was significantly increased at 7 and 14 dpi in Eimeria-inoculated birds fed the control diet compared to their uninoculated counterparts. This response is in contrast to previous reports and may be the result of utilizing a less virulent vaccine strain of Eimeria rather than a wild strain. The low average lesion scores (<2) observed in this study are similar to those observed in other studies utilizing high-dose CocciVac-B52 (Johnson et al., 2019; Savary et al., 2020) and suggest that vaccine-strain Eimeria cause low-grade tissue inflammation compared to other studies utilizing wild-type strains (lesion score >2; Barrios et al., 2017; Teng et al., 2020). Intestinal morphology roughly corresponds with intestinal health, with both VH and CrD being positively associated with increased surface area for nutrient absorption (Amat et al., 1996; Bogucka et al., 2019). This, in addition to the comparatively low-grade inflammation, suggests that increased jejunal VH in inoculated birds fed the control diet may have been a compensatory response to duodenal alterations rather than a typical pathological outcome.
Results in study 2 additionally showed that the algae ingredient's effect on intestinal structures differed between intestinal segments. Eimeria-induced changes to duodenal histomorphology were not impacted by feeding algae, whereas its inclusion protected jejunal VH as early as 7 dpi, as evidenced by comparable measurements recorded between uninoculated and Eimeria-inoculated birds fed diets with the algae ingredient (Figure 4A). In accordance with expected outcomes, CrD increased in Eimeria-inoculated birds at 7 and 14 dpi; however, the magnitude of Eimeria’s effect differed between diets. While feeding algae did not appear to have the same protective effect on jejunal CrD at 7 dpi, it reduced the magnitude of change in CrD at 14 dpi relative to control when comparing uninoculated vs. Eimeria-inoculated birds fed similar diets (Figure 4E). Increased jejunal VH in algae-fed birds translates to increased available surface area for nutrient absorption. Further work is needed to determine what degree of alterations significantly contributes to physiologically improved absorptive capacity and functionality.
While histomorphology is one method to assess intestinal health, the FITC-D intestinal permeability assay provides in vivo insight into gut barrier function. This assay has been optimized for use in broiler chickens with increased serum fluorescence corresponding to FITC-D paracellular movement across the intestine as an indicator of permeability/gut leakage (Tellez et al., 2014; Vicuña et al., 2015). Feed restriction has been shown to increase this translocation (Baxter et al., 2017), an outcome validated in study 1 (Figure 1B). While intestinal permeability after 12 h FR was not altered by the algae ingredient in study 1, there was a protective effect on gut barrier function at 7 dpi in the algae-fed groups, a timepoint associated with heightened Eimeria-induced damage (Williams 2005; Figure 2). This outcome, combined with the protective effects on jejunal histomorphology observed in algae-fed inoculated birds, suggests that this ingredient beneficially modulates the intestine to reduce Eimeria-associated damage to intestinal structure and integrity. Outcome differences in studies 1 and 2 may be due to the challenge used (physiological stress vs. pathogen), challenge duration, and acute vs. continuous challenge exposure.
In both challenges used herein, intestinal functions provide site-specific responses while immunological assessments in study 2 provide insight into systemic outcomes. Prior to Eimeria inoculation, algae-fed groups had reduced splenic monocytes/macrophages and augmented T cell subpopulations, enhancing γδ T cells over TH cells. This supports an anti-inflammatory function of the algae ingredient in unchallenged broilers, as increased monocytes/macrophages are associated with inflammatory responses. γδ T cells function in regulating inflammatory responses while TH subtypes are associated with effector functions in inflammatory responses (Masson and Belz, 2010; Vantourout and Hayday, 2013; Yang et al., 2014). Combined with performance outcomes, pre-inoculation immunological observations correspond with reports that algae-based feed ingredients have anti-inflammatory effects in unchallenged broilers without negatively impacting starter performance (Qureshi et al., 1996; Toyomizu et al., 2001; Kang et al., 2013).
Algae also altered systemic immune cell responses following Eimeria inoculation, primarily in T cells. Innate CD1.1+ APC, cells responsible for presenting antigens to T and B lymphocytes, were increased, as expected due to Eimeria vaccination. CD1.1 is a marker on B cells and a variety of antigen-presenting cells including dendritic cells, natural killer cells, and some macrophages. Overall, algae ingredient ± inoculation increased CD1.1+ cells by 3 dpi compared to unchallenged control and this likely account for the enhanced T cell response (Figure 5C). The macrophage response was not altered by feeding the algae ingredient or due to inoculation (Figure 5B); however, the CD1.1 alteration is more remarkable, as these cells were likely digesting and presenting lipid bilayers and other lipid-containing cellular walls and components from the oocysts.
From 3 to 7 dpi, Eimeria-inoculated birds fed the control diet showed a 19.6% reduction in T cells while their algae-fed counterparts maintained these cell populations (Figure 6B). At the same time, Eimeria-inoculated birds fed the control diet had a 24.2% reduction in TH cells without corresponding expansion of TC or γδ T cells (Figures 6C–6E). Combined, this suggests early recruitment of T cells, particularly TH cells, from the spleens of control-fed birds in response to Eimeria that may not have been necessary for algae-fed birds. The extracellular staining panel used herein and available reagents for poultry did not provide insight into the maturity or proliferation rate of splenic T cell populations; however, the relatively constant population of this cell type in Eimeria-inoculated algae-fed birds could indicate that T cells were not recruited from the spleen in the same manner as control-fed inoculated birds. Although T cell recruitment from the spleen in algae-fed birds during coccidiosis was not observed, the main effect of Eimeria status suppressed age-related expansion of γδ T cells at 7 and 14, requiring compensation in the last 14 dpi, regardless of diet type (Figure 6E). The implications of this finding are unclear but may suggest an altered immune response to commercially impactful secondary infections that requires additional research. Despite these alterations, T cell populations and their subtypes at 28 dpi are consistent with published values observed in 42-day-old broilers, indicating that systemic T cell populations were recovered by 28 dpi (Meyer et al., 2019).
Overall, algae-associated intestinal integrity improvements were specific to Eimeria challenge, suggesting that algae may exert protective effects over time, especially in pathogen-challenged environments. While intestinal health was improved during Eimeria challenge, systemic immune responses were characterized by reduced reliance on splenic T cells in the first 7 dpi. Changes to T cell subpopulations suggests the algae ingredient induces a faster cytotoxic T cell response that lasts into later post-inoculation timepoints, while the helper T subsets remain improved over time. Future research to elucidate the functions of each augmented T cell population is warranted to further characterize this response. Because the algae ingredient is predominantly protein (~50%) with approximately 9% fat, it is unlikely that the very minor nutritional differences due to 0.175% algae inclusion were the driving force behind physiological results, rather, bioactive component(s) within the ingredient. These outcomes show promising health benefits associated with this algae ingredient, specifically during Eimeria challenge, and require further investigation into the ingredient's effects in commercial-scale broiler production.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to thank ZIVO Biosciences Inc. for funding this research and Ivan Alvarado Ortiz and Merck Animal Health (USA) for donating the Coccivac®-B52 vaccine used in this study. Additional thanks to staff at the Iowa State University Poultry Research and Teaching Farm, Sally Howard, and Emily Kurtz for assistance with animal care and sample collection.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Alfaia C.M., Pestana J.M., Rodrigues M., Coelho D., Aires M.J., Ribeiro D.M., Major V.T., Martins C.F., Santos H., Lopes P.A., Lemos J.P.C., Fontes C., Lordelo M.M., Prates J.A.M. Influence of dietary Chlorella vulgaris and carbohydrate-active enzymes on growth performance, meat quality and lipid composition of broiler chickens. Poult. Sci. 2021;100:926–937. doi: 10.1016/j.psj.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat C., Planas J.M., Moreto M. Kinetics of hexose uptake by the small and large intestine of the chicken. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996;271:1085–1089. doi: 10.1152/ajpregu.1996.271.4.R1085. [DOI] [PubMed] [Google Scholar]
- Assis R.C.L., Luns F.D., Beletti M.E., Assis R.L., Nasser N.M., Faria E.S.M., Cury M.C. Histomorphometry and macroscopic intestinal lesions in broilers infected with Eimeria acervulina. Vet. Parasitol. 2010;168:185–189. doi: 10.1016/j.vetpar.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Austic R.E., Mustafa A., Jung B., Gatrell S., Lei X.G. Potential and limitation of a new defatted diatom microalgal biomass in replacing soybean meal and corn in diets for broiler chickens. J. Agric. Food Chem. 2013;61:7341. doi: 10.1021/jf401957z. [DOI] [PubMed] [Google Scholar]
- Baba E., Furata T., Arakawa A. Establishment and persistence of Salmonella typhimurium infection stimulated by Eimeria tenella in chickens. Res. Vet. Sci. 1982;33:95–98. [PubMed] [Google Scholar]
- Barrios M.A., Da Costa M., Kimminau E., Fuller L., Clark S., Pesti G., Beckstead R. Relationship between broiler body weights, Eimeria maxima gross lesion scores, and microscores in three anticoccidial sensitivity tests. Avian Dis. 2017;61:237–241. doi: 10.1637/11518-102116-Reg.1. [DOI] [PubMed] [Google Scholar]
- Baxter M.F.A., Merino-Guzman R., Latorre J.D., Mahaffey B.D., Yang Y., Teague K.D., Graham L.E., Wolfenden A.D., Hernandez-Velasco X., Bielke L.R., Hargis B.M., Tellez G. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front. Vet. Sci. 2017;4 doi: 10.3389/fvets.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.P., Tomley F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014;30:12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Bogucka J., Ribeiro D.M., Bogusławska-Tryk M., Dankowiakowska A., Costa R.P.R., Bednarczyk M. Microstructure of the small intestine in broiler chickens fed a diet with probiotic or synbiotic supplementation. J. Anim. Physiol. Anim. Nutr. (Berl). 2019;103:1785–1791. doi: 10.1111/jpn.13182. [DOI] [PubMed] [Google Scholar]
- Calder P.C. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem. Soc. Trans. 2017;45:1105–1115. doi: 10.1042/BST20160474. [DOI] [PubMed] [Google Scholar]
- Christaki E., Florou-Paneri P., Bonos E. Microalgae: a novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011;62:794. doi: 10.3109/09637486.2011.582460. [DOI] [PubMed] [Google Scholar]
- Collier C.T., Hofacre C.L., Payne A.M., Anderson D.B., Kaiser P., Mackie R.I., Gaskins H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Eggersdorfer M., Wyss A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018;652:18–26. doi: 10.1016/j.abb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Evans A.M., Smith D.L., Moritz J.S. Effects of algae incorporation into broiler starter diet formulations on nutrient digestibility and 3 to 21 d bird performance. J. Appl. Poult. Res. 2015;24:206–214. [Google Scholar]
- Gautier A.E., Latorre J.D., Matsler P.L., Rochell S.J. Longitudinal characterization of coccidiosis control methods on live performance and nutrient utilization in broilers. Front. Vet. Sci. 2020;6:468. doi: 10.3389/fvets.2019.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.N., Kogut M.H., Genovese K., He H., Kazemi S., Arsenault R.J. Administration of a postbiotic causes immunomodulatory responses in broiler gut and reduces disease pathogenesis following challenge. Microorganisms. 2019;7:268. doi: 10.3390/microorganisms7080268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kang H.K., Park S.B., Kim C.H. Effects of dietary supplementation with a Chlorella by-product on the growth performance, immune response, intestinal microflora and intestinal mucosal morphology in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2017;101:208–214. doi: 10.1111/jpn.12566. [DOI] [PubMed] [Google Scholar]
- Kang H.K., Salim H.M., Akter N., Kim D.W., Kim J.H., Bang H.T., Kim M.J., Na J.C., Hwangbo J., Choi H.C., Suh O.S. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Res. 2013;22:100–108. [Google Scholar]
- Kuttappan V.A., Vicuña E.A., Latorre J.D., Wolfenden A.D., Téllez G.I., Hargis B.M., Bielke L.R. Evaluation of gastrointestinal leakage in multiple enteric inflammation models in chickens. Front. Vet. Sci. 2015;2:66. doi: 10.3389/fvets.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R., Horst G., Tonda R., Lumpkins B., Mathis G. Evaluation of the effects of feeding dried algae containing beta-1,3-glucan on broilers challenged with Eimeria. Poult. Sci. 2018;97:3494. doi: 10.3382/ps/pey227. [DOI] [PubMed] [Google Scholar]
- Liew P.K., Zulkifli I., Hair-Bejo M., Omar A.R., Israf D.A. Effects of early age feed restriciton and heat conditioning on heat shock protein 70 expression, resisitance to infection bursal disease, and growth in male broiler chickens subjected to heat stress. Poult. Sci. 2003;82:1879–1885. doi: 10.1093/ps/82.12.1879. [DOI] [PubMed] [Google Scholar]
- Long S.F., Kang S., Wang Q.Q., Xu Y.T., Pan L., Hu J.X., Li M., Piao X.S. Dietary supplementation with DHA-rich microalgae improves performance, serum composition, carcass trait, antioxidant status, and fatty acid profile of broilers. Poult. Sci. 2018;97:1881–1890. doi: 10.3382/ps/pey027. [DOI] [PubMed] [Google Scholar]
- López-Osorio S., Chaparro-Gutiérrez J.J., Gómez-Osorio L.M. Overview of poultry Eimeria life cycle and host-parasite interactions. Front. Vet. Sci. 2020;7:384. doi: 10.3389/fvets.2020.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira M.S., Cardoso C., Lopes P.A., Coelho D., Afonso C., Bandarra N.M., Prates J.A.M. Microalgae as feed ingredients for livestock production and meat quality: a review. Livest. Sci. 2017;205:111–121. [Google Scholar]
- Maguey-Gonzalez J.A., Michel M.A., Baxter M.F.A., Tellez G., Moore P.A., Solis-Cruz B., Hernández-Patlan D., Merino-Guzman R., Hernandez-Velasco X., Latorre J.D., Hargis B.M., Gomez-Rosales S., Tellez-Isaias G. Effect of humic acids on intestinal viscosity, leaky gut and ammonia excretion in a 24 hr feed restriction model to induce intestinal permeability in broiler chickens. Anim. Sci. J. 2018;89:1002–1010. doi: 10.1111/asj.13011. [DOI] [PubMed] [Google Scholar]
- Mariey Y., Samak H., Abou-Khashba H., Sayed M., Abou-Zeid A. Effect of using Spirulina platensis algae as feed additives for poultry diets. 2. Productive performance of broiler. Egypt Poult. Sci. J. 2014;34:245–258. [Google Scholar]
- Masson F., Belz G.T. Mobilizing forces–CD4+ helper T cells script adaptive immunity. Cell Res. 2010;20:1–3. doi: 10.1038/cr.2010.1. [DOI] [PubMed] [Google Scholar]
- Meyer M.M., Fries-Craft K.A., Bobeck E.A. Composition and inclusion of probiotics in broiler diets alter intestinal permeability and spleen immune cell profiles without negatively affecting performance. J. Anim. Sci. 2019;98 doi: 10.1093/jas/skz383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaie S., Zirak-Khattab M.F., Hosseini H.S.A., Donyaei-Darian H. Effects of dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian-Australas. J. Anim. Sci. 2018;31:556–563. doi: 10.5713/ajas.17.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullenix G.J., Greene E.S., Emami N.K., Tellez-Isaias G., Bottje W.G., Erf G.F., Kidd M.T. Spirulina platensis inclusion reversed circulating pro-inflammatory (chemo)cytokines profiles in broilers fed low-protein diets. Front. Vet. Sci. 2021;8:640968. doi: 10.3389/fvets.2021.640968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 9th Rev. Ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Park J.H., Lee S.I., Kim I.H. Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poult. Sci. 2018;97:2451–2459. doi: 10.3382/ps/pey093. [DOI] [PubMed] [Google Scholar]
- Pieniazek J., Williams M.P., Latham R., Walters H., Wickersham T.A., Levine R., Lebrun J., Caldwell D., Lee J.T. Evaluation of an algal beta-1,3-glucan on broiler growth performance and immune response. Int. J. Poult. Sci. 2016;15:201–210. [Google Scholar]
- Qureshi M.A., Garlich J.D., Kidd M.T. Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharmacol. Immunotoxicol. 1996;18:465–476. doi: 10.3109/08923979609052748. [DOI] [PubMed] [Google Scholar]
- Savary R.K., Fiss T.A., Abbott D.A., Nicholds J.A., Van Kessel A.G., Classen H.L. Development of a coccidiosis disease challenge model using a commerically available live oocyst vaccine. Avian Dis. 2020;65:149–158. doi: 10.1637/aviandiseases-D-20-00105. [DOI] [PubMed] [Google Scholar]
- Światkiewicz S., Arczewska-Włosek A., Józefiak D. Application of microalgae biomass in poultry nutrition. Worlds Poult. Sci. J. 2015;71:663–672. [Google Scholar]
- Tavernari F.D.C., Roza L.F., Surek D., Sordi C., Silva M.L.B.D., Albino L.F.T., Migliorini M.J., Paiano D., Boiago M.M. Apparent metabolisable energy and amino acid digestibility of microalgae Spirulina platensis as an ingredient in broiler chicken diets. Br. Poult. Sci. 2018;59:562. doi: 10.1080/00071668.2018.1496401. [DOI] [PubMed] [Google Scholar]
- Tellez G., Latorre J.D., Kuttappan V.A., Kogut M.H., Wolfenden A., Hernandez-Velasco X., Hargis B.M., Bottje W.G., Bielke L.R., Faulkner O.B. Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Front. Genet. 2014;5:339. doi: 10.3389/fgene.2014.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P.-Y., Fuller A.L., Kim W.K. Evalution of nitro compounds as feed additives in diets of Eimeria-challenged brolers in vitro and in vivo. Poult. Sci. 2020;99:1320–1325. doi: 10.1016/j.psj.2019.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomizu M., Sato K., Taroda H., Kato T., Akiba Y. Effects of dietary Spirulina on meat colour in muscle of broiler chickens. Br. Poult. Sci. 2001;42:197–202. doi: 10.1080/00071660120048447. [DOI] [PubMed] [Google Scholar]
- Vantourout P., Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicuña E.A., Kuttappan V.A., Galarza-Seeber R., Latorre J.D., Faulkner O.B., Hargis B.M., Tellez G., Bielke L.R. Effect of dexamethasone in feed on intestinal permeability, differential white blood cell counts, and immune organs in broiler chicks. Poult. Sci. 2015;94:2075–2080. doi: 10.3382/ps/pev211. [DOI] [PubMed] [Google Scholar]
- Waldenstedt L., Inborr J., Hansson I., Elwinger K. Effects of astaxanthin-rich algal meal (Haematococcus pluvalis) on growth performance, caecal campylobacter and clostridial counts and tissue astaxanthin concentration of broiler chickens. Anim. Feed Sci. Technol. 2003;108:119–132. [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian. Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Yaakob Z., Ali E., Zainal A., Mohamad M., Takriff M. An overview: biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. (Thessalon.) 2014;21:6. doi: 10.1186/2241-5793-21-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhang L., Yu C., Yang X.-F., Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014;2:1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Sun Z., Sun P., Chen T., Chen F. Microalgal carotenoids: beneficial effects and potential in human health. Food Funct. 2014;5:413–425. doi: 10.1039/c3fo60607d. [DOI] [PubMed] [Google Scholar]