Abstract
Nexrutine (NX), a marketable herbal extract from a traditional Chinese herbal plant, Phellodendron amurense, is majorly used for the resolution of inflammation, gastroenteritis, and some tissue-specific cancer. Strategies for the identification of the safety of anticancer solutions of plant origin are an important area of study. The present investigation assesses the single and repeated dose (28 days) toxicity of NX following OECD guidelines 425 and 407, respectively. Briefly, to identify acute toxic properties of NX, a dose of 2000 mg/kg b. wt was administered once orally. Simultaneously, repeated dose toxicity was evaluated through daily administration of the three different doses (250, 500, 750 mg/kg b. wt) of NX for 28days. The single administration of NX showed no signs of toxicity and morbidity, suggesting LD50 of NX more than 2000 mg/kg b. wt. Furthermore, repeated dose exposure of NX for 28 days did not show any sign of toxicity. Hematology, serum biochemistry, and histopathological analysis also did not show any significant abnormalities. However, a marginal decrease in triglyceride, cholesterol, and glucose levels along with mild tubular degeneration in the kidney was also noticed in the high dose NX treatment group. Overall, the findings of the study suggest that NX is safe for use up to 500 mg/kg b.wt.
Keywords: Safety evaluation, Nexrutine, Single dose toxicity, Repeated dose toxicity
Highlights
-
•
Single dose toxicity confirms LD50 of NX to be greater than 2000 mg/kg b. wt.
-
•
Repeated dose toxicity study used three doses of NX (250, 500, 750 mg/kg b. wt).
-
•
Minimal aberrations in hematology and biochemical parameters.
-
•
Histopathology depicts mild tubular degeneration at a high dose in the kidney.
-
•
No morbidity or mortality was recorded in both the experimental setups.
Safety evaluation; Nexrutine; Single dose toxicity; Repeated dose toxicity.
1. Introduction
Nexrutine (NX) is a bark extract of the Chinese medicinal plant, Phellodendron amurense, which is a species of the Rutaceae family. Traditionally, NX has been an important member of the traditional Chinese medicinal string used as medicaments for relief from meningitis, pneumonia, bacillary dysentery liver cirrhosis, and tuberculosis [1]. Moreover, oral treatment of NX is effective in abdominal pain, urinary tract infections, diarrhea, and gastroenteritis [2, 3, 4]. Fingerprinting analysis of NX suggests that isoquinoline alkaloids such as phellodendrine, berberine, jatrorrhizine, palmatine, and limonin are the major biologically active components of this extract [2, 3, 5]. Several studies have demonstrated the anticancer property of NX tested in breast, melanoma, liver, multiple myeloma, colon, prostate, pancreatic, nonmelanoma, and skin cancer [6, 7, 8, 9, 10].
NX has been used as a natural alternative dietary supplement for pain and inflammation. One of our previous findings confirms that NX inhibits the body's production of COX-2 without any side effects; unlike mainstream pain killers such as naproxen/ibuprofen [11]. Few studies provide evidence that NX does not impose deleterious effects that damage the liver, kidneys, and heart [12]. The mechanistic investigation depicts that NX modulates cell survival by the modulation of a survival pathway i.e. PI3K/AKT and STAT3/NF-κB signaling [3, 6, 8, 13, 14]. NX mitigates phosphorylation and DNA binding activity of CREB, a transcription factor of PI3K/AKT signaling [3, 13]. NX treatment showed inhibition of NF-κB reporter and DNA interacting ability in androgen-independent cells [6]. Similarly, a low dose of radiation combined with NX inhibits survival fragment rather than high-dose, radiation-exposed, androgen-independent cells. Swanson et al. demonstrated the extract benefit of NX in PCA patients undergoing prostatectomy or radiation therapy. In a patient trial study, oral administration of NX for 1–2 months before the therapeutical procedures (radiation/surgery) or with radiation reduced PSA levels in 81 % of patients was with a negligible amount of grade 3 toxicity [15]. NX also decreases COX-2 and PPARγ in breast cancer cells [16]. A study also suggests that the combination of NX with an autophagy inhibitor enhanced its therapeutic benefit [9]. Recently, in our laboratory, it has been demonstrated that NX inhibits anchorage-independent growth of colon adenocarcinoma cells (COLO 205, HCT-15), reduced the over expression of COX-2 in the 2AAF/DEN lover cancer model, and in AOM-induced colon carcinogenesis [7, 17, 18].
These preclinical observations suggested NX as a potential chemopreventive agent for the management of inflammation-associated malignancies. However, in accordance with Campbell-Tofte et al. (2012), WHO has called up to lay down international standards and procedures for evaluating the safety and medicinal potency of conventional medicine providing remedies for numerous infectious and long-term illnesses; although pharmacological and toxicological assessments are necessary for drug/standardization herbal solution development [19]. Safety evaluation of plant-derived medicine especially to speculate the consequences of usage for an extended period, are pivotal for identification and formulations of the standardized herbal alternatives and the acceptance in health care for therapeutic application.
Although the pharmacological properties of NX are well-recognized, adequate data with regard to toxicological profiles are scarce. The present investigation was focused to determine the single and repeated oral toxicity assessment of NX by using rat as an animal model. The aim of the present study was to determine the MTD and the safety index (SI) of NX in a rat model.
2. Materials and methods
2.1. Chemical and reagents
CM Cellulose, hematoxylin, and eosin were purchased from Sigma (St. Louis, MO, USA), Nexrutine® (NX) was purchased from Next Pharmaceuticals (Irvine, CA). The chemicals and reagents engaged in the entire study were of the purest commercially available options.
2.2. Dose preparation
Different concentrations of NX were prepared in phosphate-buffered saline (PBS) containing 0.01 % CM Cellulose.
2.2.1. Animals
Adult male Wistar rats used for the present study were procured from the GLP (Good Laboratory Practices) certified animal breeding facility of the CSIR-Indian Institute of Toxicology Research, Lucknow, India. The experimental animals were housed in a random group of 5 animals in each group and appropriate care, maintenance, and hygienic conditions were maintained following the guidelines as reported earlier [11]. Prior approval for conducting the study was obtained from the Institutional Animal Ethics Committee of CSIR-IITR, Lucknow (IITR/IAEC/22/2017).
2.3. Animals and treatment protocol
2.3.1. Single dose toxicity
For the determination of MTD, the study was performed in accordance with the Organization for Economic Cooperation and Development (OECD) guidelines 425 [20]. A dose of 2000 mg/kg b.wt of NX was used with five rats per group to ascertain any toxic effects. Each experimental subject was administered with a sole oral dose of 2000 mg/kg b. wt of NX at periodic intervals of 24-h. All experimental animals were under keen observation for any signs of notable toxicity and symptoms were recorded twice daily. All animals were sacrificed humanely under ethical norms after 14 days and particular vital organs were excised, weight was blotted, and processed for necessary microscopic examination.
2.3.2. Repeated dose toxicity assessment
The toxicity study of NX was carried out in accordance with OECD guidelines 407 [21] with minimal modifications. From the single dose toxicity study results, three distinct doses of extract, i.e. 250, 500, and 750 mg/kg b.wt were opted for oral administration for 28 days to three different groups of rats (n = 5) on a daily basis. In the control group (n = 5), the fourth group of the study received only vehicle-injected control for the same duration. After every single administration of NX, all the experimental animals were monitored daily for any abnormalities and mortality. The record of the body weight of each animal was jotted on the first day followed by weekly recodings for the entire treatment module (28 days) and on the day of terminal sacrifice. The food intake was recorded for 28 days. The amount of food was measured daily from the quantity of food remaining after 24-h.
After the conclusion of the 28 days observation period, the animals were sacrificed as mentioned in the guideline for the care and use of laboratory animals of CSIR-IITR. The heart, liver, lung, spleen, and kidney were isolated for relative organ weight assessment and fixation was carried out in a 10 % neutral buffered formalin solution for histological evaluation. The relative organ weight was calculated by using formula 1 [22]:
| (1) |
2.4. Specimen collection and procession
Blood was collected in a BD Vacationer® Tube K2-EDTA (Dipotassium Ethylene Diamine Tetra Acetic acid) (BD Franklin Lakes, NJ, USA) for hematological indices. For serum extraction, blood was allowed to clot in a BD Vacutainer® Tube with clot activator (BD Franklin Lakes, NJ, USA) for 5 min at room temperature followed by 30 min in an ice bath. Then the blood sample was centrifuged at 3000xg for 10 min for serum separation, and subsequently, stored at -80 °C for future analysis.
2.5. Hematological indices
Blood samples were obtained by a cardiac puncture procedure from each animal under the influence of anesthesia induced by intraperitoneal injection of ketamine HCl solution (30–40 mg/kg b.wt). Several hematology parameters were analyzed by using the Sysmex XT 1800i hematology analyzer (Sysmex Corporation, Japan). The parameters considered were hemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), hematocrit (MCT), total white blood cells (WBC), total red blood cell (RBC), mean corpuscular hemoglobin (MCH), lymphocytes, platelet count (PLT), eosinophils, neutrophils, monocytes, and basophils.
2.6. Serum biochemical assay
Alkaline phosphatase (ALP), serum alanine aminotransferase (ALT), total bilirubin, aspartate aminotransferase (AST), uric acid, urea, triglycerides, creatinine, glucose, and total protein were estimated using a fully automated biochemical analyzer (Rx Daytona, Randox Laboratories Ltd, UK).
2.7. Histological processing
Formalin-fixed tissues were processed for histopathological analysis as described earlier [23].
2.8. Statistical analysis
All the obtained results i.e. average body weight, feed intake, hematology, clinical chemistry, and absolute organ weights were analyzed by using the certified copy of SPSS-10 software. Statistical significance between the groups was determined by one-way analysis of variance (ANOVA) followed by the Student t-test which was used for paired data. A value of ∗p < 0.05 was considered as statistically significant.
3. Results
3.1. Single dose toxicity
3.1.1. Clinical signs and mortality
Oral administration of a single dose (2000 mg/kg b.wt) of NX to five rats did not develop notable clinical indications of toxicity or mortality; either immediately or in the duration of the observation period (14 days). Wistar rats that were treated with NX did not exhibit differences in body weight when compared with the control group as depicted in Table 1. No histological changes were observed in the collected tissues of animals treated with NX (data not demonstrated).
Table 1.
Body weights (g) of rats treated with Nexrutine in single dose toxicity study.
| Dose (mg/kg b.wt./day) | Average Animal Weight (gm) |
||
|---|---|---|---|
| 1 Day | 7 Day | 15 Day | |
| 0 | 191.25 ± 18.20 | 206.65 ± 16.99 | 234.55 ± 27.79 |
| 2000 | 191.26 ± 9.23 | 208.62 ± 9.62 | 236.42 ± 10.62 |
Data represent mean ± SD of five animals. Animals treated with 0.01 % CM cellulose serve as negative control and are comparable to untreated control group animals.
3.2. Repeated dose toxicity
In the repeated dose toxicity tests, 28 days of repeated oral administration of 250, 500, and 750 mg/kg/day doses of NX to three groups of rats revealed no visual indications of toxicity or mortality in animals during the entire experimental protocol and observation period.
3.3. Effect on body weight gain and food consumption
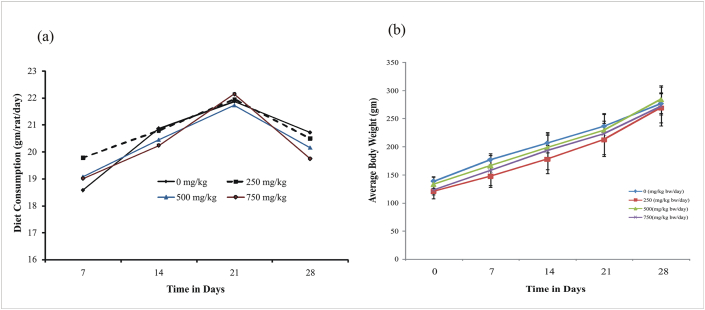
The animal orally gavages with different doses of NX for 28 days and exhibits a normal trend of weight gain during the experimental schedule as given in Figure 1a. Total weight gain after 28 days of treatment shows normal and it is comparable to the control group. The effect of NX on food consumption patterns did not show any significant change. The consumption of food increases from the first to the third week in all the groups and slightly decreases in the fourth week in all the treated and control group Figure 1b.
Figure 1.
(a) Effect of repeated dose of NX on the food consumption pattern of treated rats. The details of the treatment of animals are described under the materials and method section. Animals treated with 0.01 % CM cellulose serve as negative control and are comparable to untreated control group animals. (b) Effect on body weight of experimental animals following the repeated administration of NX. The details of the treatment of animals are described under the materials and method section. Animals treated with 0.01 % CM cellulose serve as negative control and are comparable to untreated control group animals.
3.4. Effect on relative organ weight
The effect of oral administration of NX on the terminal relative organ weight is marked in Table 2. Compared with the vehicle control, the relative weight of the liver did not show any significant change at any dose of NX. Similarly, the lungs, spleen, and heart also did not show an increase in weight. However, a significant decrease in relative organ weight of the kidney was observed at a higher dose (750 mg/kg b.wt) (Table 2).
Table 2.
Body weights (g) of rats treated with Nexrutine in repeated dose toxicity study.
| Dose | Relative Organ Weight (g/100 body weight) |
||||
|---|---|---|---|---|---|
| Liver | Lungs | Kidney | Spleen | Heart | |
| Control | 4.8 ± 0.46 | 0.86 ± 0.08 | 0.82 ± 0.02 | 0.47 ± 0.08 | 0.39 ± 0.03 |
| 250 mg/kg | 4.3 ± 0.30 | 0.87 ± 0.21 | 0.74 ± 0.09 | 0.48 ± 0.10 | 0.36 ± 0.03 |
| 500 mg/kg | 4.2 ± 0.25 | 0.87 ± 0.06 | 0.75 ± 0.09 | 0.43 ± 0.08 | 0.35 ± 0.01 |
| 750 mg/kg | 4.06 ± 0.27 | 0.84 ± 0.11 | 0.65 ± 0.16 | 0.50 ± 0.14 | 0.53 ± 0.28 |
Data represent mean ± SD of five animals. Animals treated with 0.01 % CM cellulose serve as negative control and are comparable to untreated control group animals.
3.5. Effect on hematological indices
The hematological analysis is demonstrated in Table 3. No significant changes were observed in RBC and WBC count in all the NX-treated groups; however, a slight decrease in the level of hemoglobin and hematocrit was noticed compared with control. Other parameters such as MCHC, MCH, HCT, RDW-CV, MCV, RDW-SD, MPV, PCT, PDW, and P-LCR did not show any significant changes.
Table 3.
Effect of oral administration of Nexrutine on hematological parameter in Wistar rats.
| Parameter | Control | Nexrutine (mg/kg bw) |
||
|---|---|---|---|---|
| 250 | 500 | 750 | ||
| WBC | 18.90 ± 1.93 | 11.66 ± 8.00 | 15.99 ± 1.95 | 7.47 ± 3.9 |
| RBC | 8.34 ± 0.41 | 7.62 ± 0.44∗ | 7.54 ± 0.30∗ | 8.26 ± 0.23 |
| HGB | 15.10 ± 0.82 | 13.76 ± 0.52∗ | 13.88 ± 0.17∗ | 14.84 ± 0.66 |
| HCT | 45.35 ± 0.75 | 41.5 ± 1.56∗ | 41.75 ± 0.28∗ | 44.5 ± 1.49 |
| MCV | 54.4 0 ± 2.27 | 54.52 ± 2.22 | 55.4 ± 2.19 | 53.66 ± 1.84 |
| MCH | 18.08 ± 0.53 | 18.08 ± 0.55 | 18.4 ± 0.62 | 17.98 ± 0.77 |
| MCHC | 33.27 ± 1.32 | 33.18 ± 0.40 | 33.25 ± 0.35 | 33.36 ± 0.66 |
| RDW-SD | 30.08 ± 1.69 | 31.3 ± 0.71 | 31.57 ± 1.71 | 30.08 ± 0.88 |
| RDW-CV | 18.20 ± 0.31 | 17.48 ± 1.48 | 17.4 ± 1.81 | 17.66 ± 0.8 |
| PDW | 8.10 ± 0.29 | 8.02 ± 0.25 | 8.00 ± 0.53 | 8.04 ± 0.42 |
| MPV | 7.27 ± 0.15 | 7.32 ± 0.17 | 7.26 ± 0.40 | 7.26 ± 0.32 |
| P-LCR | 7.32 ± 1.38 | 7.48 ± 1.20 | 7.10 ± 2.32 | 7.22 ± 1.56 |
| PCT | 0.58 ± 0.01 | 0.50 ± 0.04 | 0.50 ± 0.01 | 0.45 ± 0.07 |
Data represent mean ± SD of five animals. Animals treated with 0.01 % CM cellulose serve as negative control and are comparable to untreated control group animals. ∗p < 0.05, significant with respect to control group.
Abbreviations- HGB- Hemoglobin; WBC-White blood cells; RBC- Red blood cell; HCT- Hematocrit; MCV- Mean corpuscular volume; MCH- Mean corpuscular hemoglobin; MCHC- Mean corpuscular hemoglobin concentration; RDW-SD- Red cell distribution width-standard deviation; RDW-CV- Red cell distribution width-coefficient of variation; PDW- Platelet distribution width; MPV- mean platelet volume; P-LCR- Platelet large cell ratio; PCT- Procalcitonin.
3.6. Effect on biochemical parameters
The biochemical analysis revealed no significant change in hepatic functioning in terms of ALP, ALT, total protein, and total bilirubin as compared to control, but a mild reduction in the level of triglyceride, cholesterol, and glucose along with slight augmentation in AST level was recorded (Table 4). NX treatment did not cause any alteration in creatinine level, whereas a slight reduction in the uric acid and urea level, an indicator of renal function was observed.
Table 4.
Effect of oral intake of Nexrutine on serum biochemical parameter in rats.
| Parameter | Control | Nexrutine (mg/kg bw) |
||
|---|---|---|---|---|
| 250 | 500 | 750 | ||
| ALT (U/L) | 93.4 ± 6.50 | 96.6 ± 10.66 | 100.2 ± 11.32 | 102.8 ± 9.57 |
| AST (U/L) | 230.4 ± 8.01 | 228.6 ± 10.23 | 241.4 ± 8.96∗ | 261.4 ± 5.41∗ |
| ALP (U/L) | 477.4 ± 20.16 | 448.4 ± 31.33 | 486.6 ± 10.50∗ | 479.6 ± 15.97∗ |
| Creatinine (mg/dL) | 0.52 ± 0.04 | 0.5 ± 0.07 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| Uric acid (mg/dL) | 2.04 ± 0.88 | 1.92 ± .14 | 1.8 ± 0.2 | 1.34 ± 0.18∗ |
| Total Protein (g/dL) | 6 ± 0.21 | 5.96 ± 0.31 | 6.02 ± 0.34 | 6.18 ± 0.20 |
| Cholestrol (mg/dL) | 48.4 ± 5.59 | 40.2 ± 6.05 | 46.08 ± 3.88 | 50.4 ± 5.59 |
| Total Bilirubin (mg/dL) | 0.1 ± 0.0 | 0.12 ± 0.04 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Triglycerides (mg/dL) | 75 ± 10.51 | 67.6 ± 7.7∗ | 80.8 ± 5.54 | 61.2 ± 8.58∗ |
| Urea (mg/dL) | 58.2 ± 2.94 | 48.4 ± 4.56∗ | 49.2 ± 6.97∗∗ | 43.4 ± 5.17∗ |
| Glucose (mg/dL) | 190.4 ± 4.61 | 175 ± 7.64∗∗ | 182.8 ± 10.68 | 169.6 ± 6.65∗ |
Data represent mean ± SD of five animals. Animals treated with 0.01 % CM cellulose serve as negative control and are comparable to untreated control group animals. ∗p < 0.05, ∗∗p < 0.01 indicates significant difference with respect to control.
Abbreviations: ALT- Alanine aminotransferase; AST- Aspartate aminotransferase; ALP- Alkaline phosphatase.
3.7. Effect on histopathology of tissue
All necropsy observations for animals sacrificed at the end of the study were normal. In histopathological assessment, the presence of dispersed occasional lymphocytes was recorded and congested blood vessels without any edema were noted in sections of the heart of the NX- treated group. Furthermore, a small number of prebronchial lymphocytic infiltrations and light congestion without edema were also noted in the lung tissue. The histopathological assessment of the liver revealed little lymphatic infiltration surrounding the central vein, in the portal tract and the parenchyma of both control and treated animals. Histological examinations of the spleen and intestine showed no pathological appearance, whereas the kidney at 750 mg/kg b. wt dose shows slight tubular degeneration Figure 2.
Figure 2.
Cross section area of liver, colon, kidney, heart, lung, spleen of control and NX-treated rat in repeated dose toxicity study. After 28 days of repeated exposure of three different doses of NX (250, 500, 750 mg/kg b.wt.) through oral route, rats were sacrificed and tissue sections were fixed in 10 % buffer formalin for paraffin sectioning. The tissue sections were stained with hematoxylin-eosin and visualized with a bright microscope at 20X magnification.
4. Discussion
NX is used as a herbal remedy in various inflammation-associated diseases and in the management of some tissue-specific cancer. Therefore, it is important to provide an adequate amount of data for their safety. In the single exposure toxicity study, single exposure with 2000 mg/kg b. wt dose of NX to experimental Wistar rats did not disclose any signs of toxicity/mortality in any animal in the entire duration of the study. Therefore, it can be deduced that the LD50 (lethal dose 50) of the extract is greater than 2000 mg/kg. According to the Globally Harmonized System (GHS) of Classification and Labelling of Chemicals, the substances with an LD50 value greater than 2000 mg/kg b. wt are considered relatively safe [24]. Similarly, in the repeated toxicity study, daily administration of NX (250, 500, and 750 mg/kg b.wt) for 28 days in rats did not show any sign of toxicity. No significant differences were observed in ROW of the liver, lung, spleen, and heart, whereas a slight decrease in ROW of the kidney was noticed in the 750 mg/kg b.wt. treatment group. During the entire experiment, weight gain and food consumption showed no observed adverse effect level (NOAEL).
Hematological studies easily explain the abnormalities in metabolic processes in one's body, and the blood profile most often displays a clear picture by revealing the responses of the body to any foreign assault, deprivation, and/or stress [25]. The hematological analysis of NX-treated animals showed no significant changes in RBC count, WBC count, HCT, HGB, PCT, MCV, MCH, MCHC, MPV, PDW, and RDW. These results rule out the possibility of occurrence of an anemic condition or other disorders such as hypothyroidism, thalassemia, liver disease, and polycythemia. Platelets are a crucial biomarker for the early detection of thromboembolic diseases. Platelet indices such as MPV and PDW are simple platelets and they increase during platelet activation [26]. NX treatment for 28 days did not show any significant change in MPV and PDW levels. Besides, P-LCR is also an indicator of risk factors associated with thromboembolic ischemic events [27]. A high PCT was indicative of inflammation in the body. Here, no significant differences were observed on PCT after NX treatment, which reveal that NX did not cause any infectious diseases or thromboembolic disorders, and collectively suggest that tested doses are safe for a hematopoietic system of the body.
The clinical biochemistry of serum assesses a series of chemicals, enzymes, and proteins that have indispensable roles in the body's chemical reactions in the blood to reflect the general information about the status/functioning of an organ (especially the liver, kidneys, and pancreas). Urea and uric acid are the biomarkers of renal function and retention of these in the body is an indicator of renal damage [28]. In the study, NX causes no significant change in the levels of urea and uric acid. Similarly, there is no demarcating effect on serum ALT, AST, and ALP levels as compared with control. Since ALT is a cytoplasmic enzyme whose highest activity and concentration are found in the liver, an increase of this specific enzyme suggests possible hepatocellular damage as a consequence of the extract treatment in rats [29]. According to Rhiouani et al. (2008), in the Moroccan traditional medicinal plant, Herniariaglabra, the highest dose of the extract caused a significant increase in ALT [30]. However, there is no altered effect on other hepatic parameters such as total protein and total bilirubin in NX-treated animals as compared with the control group. Further, NX treatment reduced triglycerides, cholesterol, and glucose levels. A high level of serum triglyceride and cholesterol is a risk factor for cardiovascular diseases, hypertension, and obesity. Similarly, high levels of glucose may lead to diabetes mellitus in the participants [31, 32]. Our findings suggest that NX exposure may reduce the probability of any cardiovascular disorder and diabetes mellitus.
The histopathological assessment is the benchmark for evaluating treatment-related pathological changes. In the current investigation, histopathological evaluation of repeated dose of NX did not cause any adverse effect on lung, liver, heart, colon, and spleen; however, a minor alteration in tubular degeneration in kidney tissue was observed at the higher dose (750 mg/kg b.wt.). Looking at the entire landscape, it can be noted that the highest dose of Nx i.e. 750 mg/kg b.wt. shows a few alterations in the tested parameters esp on hepatic and renal functions. A decreased ROW-kidney is noted along with tubular degeneration is observed in histopathological analysis. Few of the biochemical parameters are observed to be altered. Therefore, taken all results together viz body weight changes, relative organ weights, hematological, serum biochemistry, and histological findings indicate that single or repeated dose treatment of NX did not produce any significant sign of toxicity and is safe as a supplement for dietary consumption up to the dose of 500 mg/kg b.wt. However, long-term toxicity effect of NX needs further evaluation and validation.
5. Conclusion
These results altogether provide evidence for single and repeated dose toxicity of NX, which may facilitate the researcher for a suitable dose selection. The findings of the study suggest that bark extract of Phellodendron amurense, NX is safe for use up to 500 mg/kg b.wt.
Declarations
Author contribution statement
Shamshad Alam; Payal Mandal: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Pankaj R Jagdale: Performed the experiments.
Anjaneya Ayanur: Analyzed and interpreted the data; Wrote the paper.
Kausar Mahmood Ansari: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Council for Scientific and Industrial Research, New Delhi, India (Mega Laboratory Project – MLP-23).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the Director of our institute for his keen interest in this present study. S. A. is thankful to the Council of Scientific and Industrial Research, New Delhi for the award of Senior Research Fellowship. The CSIR-IITR communication number for this manuscript is 3724.
References
- 1.Hsu K. Chinese Pharmaceutical Science and Technology Publication Co.; Beijing, China: 1996. Chinese Traditional Medicine; p. 802. [Google Scholar]
- 2.Cuellar M., Giner R., Recio M., Manez S., Rıos J. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia. 2001;72(3):221–229. doi: 10.1016/s0367-326x(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 3.Garcia G.E., Nicole A., Bhaskaran S., Gupta A., Kyprianou N., Kumar A.P. Akt-and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract. Neoplasia. 2006;8(6):523–533. doi: 10.1593/neo.05745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xian Y.-F., Lin Z.-X., Ip S.-P., Su Z.-R., Chen J.-N., Lai X.-P. Comparison the neuropreotective effect of Cortex Phellodendri chinensis and Cortex Phellodendri amurensis against beta-amyloid-induced neurotoxicity in PC12 cells. Phytomedicine. 2013;20(2):187–193. doi: 10.1016/j.phymed.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Ryuk J.A., Zheng M.S., Lee M.Y., Seo C.S., Li Y., Lee S.H., Moon D.C., Lee H.W., Lee J.-H., Park J.Y. Discrimination of Phellodendron amurense and P. chinense based on DNA analysis and the simultaneous analysis of alkaloids. Arch Pharm. Res. (Seoul) 2012;35(6):1045–1054. doi: 10.1007/s12272-012-0612-y. [DOI] [PubMed] [Google Scholar]
- 6.Muralimanoharan S.B., Kunnumakkara A., Shylesh B., Kulkarni K.H., Haiyan X., Ming H., Aggarwal B.B., Rita G., Kumar A.P. Butanol fraction containing berberine or related compound from Nexrutine® inhibits NFκB signaling and induces apoptosis in prostate cancer cells. Prostate. 2009;69(5):494–504. doi: 10.1002/pros.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar R., Das M., Ansari K.M. Nexrutine® inhibits tumorigenesis in mouse skin and induces apoptotic cell death in human squamous carcinoma A431 and human melanoma A375 cells. Carcinogenesis. 2012;33(10):1909–1918. doi: 10.1093/carcin/bgs219. [DOI] [PubMed] [Google Scholar]
- 8.Gong J., Xie J., Bedolla R., Rivas P., Chakravarthy D., Freeman J.W., Reddick R., Kopetz S., Peterson A., Wang H. Combined targeting of STAT3/NF-κB/COX-2/EP4 for effective management of pancreatic cancer. Clin. Canc. Res. 2014;20(5):1259–1273. doi: 10.1158/1078-0432.CCR-13-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong J., Muñoz A.R., Chan D., Ghosh R., Kumar A.P. STAT3 down regulates LC3 to inhibit autophagy and pancreatic cancer cell growth. Oncotarget. 2014;5(9):2529. doi: 10.18632/oncotarget.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh R., Graham H., Rivas P., Tan X.J., Crosby K., Bhaskaran S., Schoolfield J., Banu J., Fernandes G., Yeh I.-T. Phellodendron amurense bark extract prevents progression of prostate tumors in transgenic adenocarcinoma of mouse prostate: potential for prostate cancer management. Anticancer Res. 2010;30(3):857–865. [PubMed] [Google Scholar]
- 11.Alam S., Pal A., Singh D., Ansari K.M. Topical application of Nexrutine inhibits ultraviolet B-induced cutaneous inflammatory responses in SKH-1 hairless mouse. Photodermatol. Photoimmunol. Photomed. 2018;34(1):82–90. doi: 10.1111/phpp.12348. [DOI] [PubMed] [Google Scholar]
- 12.Patel D.I., Wallace D., Abuchowski K., Rivas P., Gallegos A., Musi N., Kumar A.P. Nexrutine® preserves muscle mass similar to exercise in prostate cancer mouse model. Physiol. Rep. 2019;7(16) doi: 10.14814/phy2.14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh R., Garcia G.E., Crosby K., Inoue H., Thompson I.M., Troyer D.A., Kumar A.P. Regulation of Cox-2 by cyclic AMP response element binding protein in prostate cancer: potential role for Nexrutine. Neoplasia. 2007;9(11):893–899. doi: 10.1593/neo.07502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A.P., Bhaskaran S., Ganapathy M., Crosby K., Davis M.D., Kochunov P., Schoolfield J., Yeh I.-T., Troyer D.A., Ghosh R. Akt/cAMP-responsive element binding protein/cyclin D1 network: a novel target for prostate cancer inhibition in transgenic adenocarcinoma of mouse prostate model mediated by Nexrutine, a Phellodendron amurense bark extract. Clin. Canc. Res. 2007;13(9):2784–2794. doi: 10.1158/1078-0432.CCR-06-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson G.P., Jones W.E., III, Ha C.S., Jenkins C.A., Kumar A.P., Basler J. Tolerance of Phellodendron amurense bark extract (Nexrutine®) in patients with human prostate cancer. Phytother Res. 2015;29(1):40–42. doi: 10.1002/ptr.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan G., Lanza-Jacoby S., Wang C. Nexrutine inhibits survival and induces G1 cell cycle arrest, which is associated with apoptosis or autophagy depending on the breast cancer cell line. Nutr. Canc. 2014;66(3):506–516. doi: 10.1080/01635581.2013.780627. [DOI] [PubMed] [Google Scholar]
- 17.Alam S., Pal A., Kumar R., Mir S.S., Ansari K.M. Nexrutine inhibits azoxymethane-induced colonic aberrant crypt formation in rat colon and induced apoptotic cell death in colon adenocarcinoma cells. Mol. Carcinog. 2016;55(8):1262–1274. doi: 10.1002/mc.22368. [DOI] [PubMed] [Google Scholar]
- 18.Alam S., Yadav R.S., Pal A., Purshottam S.K., Chaudhari B.P., Das M., Ansari K.M. Dietary administration of Nexrutine inhibits rat liver tumorigenesis and induces apoptotic cell death in human hepatocellular carcinoma cells. Toxicol. Rep. 2015;2:1–11. doi: 10.1016/j.toxrep.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell-Tofte J.I., Mølgaard P., Winther K. Harnessing the potential clinical use of medicinal plants as anti-diabetic agents. Botanics Targets Ther. 2012;2:7–19. [Google Scholar]
- 20.Hazarika I., Geetha K., Sundari P.S., Madhu D. Acute oral toxicity evaluation of extracts of Hydrocotyle sibthorpioides in wister albino rats as per OECD 425 TG. Toxicol. Rep. 2019;6:321–328. doi: 10.1016/j.toxrep.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higashihara N., Shiraishi K., Miyata K., Oshima Y., Minobe Y., Yamasaki K. Subacute oral toxicity study of bisphenol F based on the draft protocol for the “Enhanced OECD Test Guideline no. 407”. Arch. Toxicol. 2007;81(12):825–832. doi: 10.1007/s00204-007-0223-4. [DOI] [PubMed] [Google Scholar]
- 22.Porwal M., Khan N.A., Maheshwari K.K. Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdenia tenacissima leaves in experimental rats. Sci. Pharm. 2017;85(3):29. doi: 10.3390/scipharm85030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagdale P.R., Dev I., Ayanur A., Singh D., Arshad M., Ansari K.M. Safety evaluation of Ochratoxin A and Citrinin after 28 days repeated dose oral exposure to Wistar rats. Regul. Toxicol. Pharmacol. 2020;115:104700. doi: 10.1016/j.yrtph.2020.104700. [DOI] [PubMed] [Google Scholar]
- 24.Konan N.A., Bacchi E.M., Lincopan N., Varela S.D., Varanda E.A. Acute, subacute toxicity and genotoxic effect of a hydroethanolic extract of the cashew (Anacardium occidentale L.) J. Ethnopharmacol. 2007;110(1):30–38. doi: 10.1016/j.jep.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G., Lilly P., Sanders J., Sipes G., Bracken W. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32(1):56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 26.Vagdatli E., Gounari E., Lazaridou E., Katsibourlia E., Tsikopoulou F., Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14(1):28. [PMC free article] [PubMed] [Google Scholar]
- 27.Grotto H., Noronha J. Platelet larger cell ratio (P-LCR) in patients with dyslipidemia. Clin. Lab. Haematol. 2004;26(5):347–349. doi: 10.1111/j.1365-2257.2004.00634.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson R.J., Nakagawa T., Jalal D., Sánchez-Lozada L.G., Kang D.-H., Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol. Dial. Transplant. 2013;28(9):2221–2228. doi: 10.1093/ndt/gft029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.B.C. Tennant, Hepatic Function, Clinical biochemistry of domestic animals, Elsevier1997, pp. 327-352.
- 30.Rhiouani H., El-Hilaly J., Israili Z.H., Lyoussi B. Acute and sub-chronic toxicity of an aqueous extract of the leaves of Herniaria glabra in rodents. J. Ethnopharmacol. 2008;118(3):378–386. doi: 10.1016/j.jep.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 31.McBride P. Triglycerides and risk for coronary artery disease. Curr. Atherosclerosis Rep. 2008;10(5):386–390. doi: 10.1007/s11883-008-0060-9. [DOI] [PubMed] [Google Scholar]
- 32.Shen G.X. Lipid disorders in diabetes mellitus and current management. Curr. Pharmaceut. Anal. 2007;3(1):17–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.