Abstract
A large number of heavy metals are generated in tailings of precious metal extractive operations, which could cause high levels of water contamination. Because of the environmental and health concerns, many conventional technologies have been applied to capture heavy metals from mining-polluted streams with limited performance in terms of effectiveness and immobilization efficiency. In this context, this study evaluates the retention of mine-generated heavy metals using Technosols prepared with iron-rich soils and multicomponent nanoparticles of Fe/FeS (MCNPs). Firstly, nanoparticles were synthesized using orange-peel extract and sodium borohydride (NaBH4) as reductant agents and FeCl3.6H2O and Na2SO4 as metal precursors. The TEM and SEM images showed nanoparticles with roughly spherical morphology with a size in the range of 35.9 ± 11.7 nm arranged in a kind of filamentous structure. Secondly, Soils were dosed with 1% and 3% (w/w) of multicomponent nanoparticles and then used to capture heavy metals present in mine tailings using batch and fixed-bed column tests. The Technosol prepared with 97% soil, and 3% MCNPs reached on average 70% retention of heavy metals for fixed-bed setups. While, in batch experiments using the same Technosol, the capture of heavy metals was 80% after 6 min of treatment, and upon reaching 30 min, 90% removal was attained. This suggests that tailored Technosols might be part of a promising technology to treat contaminated mine tailings with reasonable spending.
Keywords: Technosols, Orange peel, Fe/FeS nanoparticles, Tailings
Technosols, Orange peel, Fe/FeS nanoparticles, Tailings
1. Introduction
Goldrush in Southern Ecuador began in the early 1980s, when artisanal miners started to work in old mines of Zaruma-Portovelo, Ecuador (Appleton et al., 2001). Zaruma-Portovelo is located over the Paleozoic metamorphic complex of El Oro, covered by Cretaceous volcanic rocks and subsequently intruded by Cenozoic igneous complexes. The ore at this site consists mainly of gold and silver, included in quartz veins associated with sulfides arsenopyrite, chalcopyrite, galena, sphalerite. Benefit plants of Portovelo have extracted gold and silver, but also a few metals such as copper, lead, cadmium, germanium, and indium have been generated as residuals. Typically, wastewaters of this artisanal process have been stored in tailings pools and their effluents have discharged to the surrounding Amarillo and Calera rivers. Unfortunately, the effluents handling has spread the metallic pollution and has become worse downstream. Chemical analyses of waters of the Amarillo and Calera rivers showed that chromium, lead, cadmium, copper, zinc, nickel, selenium, and arsenic, exceeded the values of the environmental regulations and are categorized as very toxic (Guerrero, 2013).
Processing plants have also used mercury, sodium cyanide, activated carbon, and zinc powder to extract gold, which has caused a variety of negative impacts, including land degradation, soil erosion, loss of biodiversity, and carbon accumulation (Ahirwal and Maiti, 2018). However, one of the most troublesome environmental impacts is the degradation of freshwater quality due to the mine tailings discharges into water bodies. In Portovelo town, these discharges are released into Amarillo and Calera rivers (Adler et al., 2013) (Tarras-Wahlberg, 2002), as a result, there is no life in certain stretches of the rivers, and in some places, this water is used for human consumption or irrigation (Ministerio de Energía y Minas del Ecuador, 1999). Therefore, precious-metal extractive operations, especially artisanal ones, generate high levels of river water contamination; although there are very few benefit plants that include remediation strategies at the end of its mining activity (Parra, 2009).
Heavy metals are considered the principal anthropogenic pollutants in the environment. They are a serious threat to human health, natural ecosystems, and living organisms due to their toxicity, persistence, and bioaccumulation characteristic (Sreenivasulu et al., 2018). Because of the environmental and health concerns, many conventional technologies have been applied to capture heavy metals from polluted streams even though with limited performance in terms of effectiveness and immobilization efficiency. For example, adsorption on solid sorbents (Han et al., 2013; Lu et al., 2018) and chemical precipitation (Zhang et al. 2010; Li, 2019) has been tried but with limited effectiveness. Technosols, a new group of soils strongly influenced by the activities done by humans, are prepared with local soils mixed with uncontaminated wastes and are used for the regeneration of degraded or polluted soils. These materials are no longer considered waste rather it is a value-added product (Macía et al., 2014). As an example, Technosols were prepared with anaerobic-digested sewage sludge and a CaO-treated aerobic sludge mixed with fly ash, slag, foundry sand, shot-blasting machine scrap, fettling, and barley straw (Arbestain and Camps, 2008) have been used for immobilizing heavy metals. Unfortunately, these materials cannot permanently immobilize heavy metals because the organic content will end up biodegraded and would release metals. The only study reported to remove metals from aqueous residuals of the Zaruma-Portovelo mining environmental liability, is a passive system consisting of Ca–Mg dispersed alkaline substrate. This was run during the 8 months in the laboratory, to remove metals from acid mine drainage (AMD). Chemical analysis demonstrated that treatment effectively increased water pH and promoted the retention of about 80% of Fe, Al, Mn, and Cu. Under acid conditions As, Cr and Pb concentrations decreased with Fe and possible precipitation, but further investigations need to be carried out to improve Zn retention.
On the other hand, nanoparticles have been already tested in the removal of heavy metals from polluted waters. Also, nanoparticles have been used to immobilized heavy metals in contaminated soils (Wang et al., 2010; Xin et al., 2012; Ge et al., 2012; Sounthararajah et al., 2015). Nevertheless, almost all procedures rely on the functionalization of nanoparticles (NPs) to provide them with specific capability to capture and retain heavy metals. In addition, fabrication methodologies are complex and use some toxic chemicals.
In this study, we exploit green nanoscience to reduce the risks of using nanomaterials on humans and environment. Synthesis of NPs using plant extracts is cost-effective and; therefore, can be used as an economical and valuable alternative for large-scale production of nanoparticles. Plant extracts can act as reducing agents and stabilizers in the synthesis of nanoparticles. Moreover, waste from fruit processing contains beneficial nutrients and biomass, which can be converted into a value-added waste such as the natural antioxidant extracts which is considered a promising option from an economic and environmental perspective (Kumar et al., 2018; Ozturk et al., 2018). Orange peel has a higher concentration of phenolic compounds and greater antioxidant potential compared to the fruit pulp. Hence, the citrus peel is the richest source of bioactive phenolic compounds, especially flavonoids with a higher polyphenol content compared to edible parts (Safdar et al., 2017). Therefore, our attention was towards: 1) the green synthesis of Fe/FeS multicomponent nanoparticles (MCNPs) using orange peel extract as the partial reducing and stabilizing agent that reacts with the iron and sulfur precursors, 2) the fabrication of Technosols using different weights of the as-fabricated nanoparticles and a local iron-rich soil, and. 3) the evaluation of the removal of heavy metals from mine tailings of Portovelo-Ecuador using the tailored Technosols.
2. Materials and methods
Soil samples were taken in two different dates and places. First one (S001) was carried out on August 3, 2018, located at 9585779 m S, 651958 m E, in the surroundings of Portovelo. The second sampling (S002) was taken on October 4, 2018; 12 km from the first point, on Loja-Catamayo-Portovelo road and located at 9576342.1 m S, 659329 m E (Figure 1). Mining tailings for this study came from the mineral processing plants located in the Zaruma-Portovelo mining district, Ecuador. The associated mineralization is polymetallic, of hydrothermal origin, with quartz veins with gold, sulfides and sulfosalts of Cu, Pb and Zn. Tailing samples were collected in two sedimentation pools (Pool 1 and Pool 2) within the Santa Monica processing plant, Portovelo-Ecuador (Figure 1). Before water sample collection, the plastic containers were cleaned with 1:1 (V:V) HNO3 and then rinsed with deionized water. Next, 10 gallons of each mine tailing was collected, treated with NO3H to pH < 2 (Zhang et al. 2010), stored in a freezer at 5 °C of temperature and transported to the laboratory for chemical analysis.
Figure 1.
Locations of the sites where soil and mine tailings samples were taken.
Soils samples (S001 and S002) were tested using the method ASTM-D2787-11 (2007) (Unified Soil Classification System) to estimate the content of gravel, sand, silt or clay. To measure the cation exchange capacity of soil, ammonium acetate, sodium chloride, formaldehyde, phenolphthalein and sodium hydroxide were used (Jaramillo, 2002). Exchangeable or macro-metallic cations were obtained using the ammonium acetate method (Jaramillo, 2002). While for carbonates, sulfuric acid, phenolphthalein and ethanol were used (USDA, 1969). Moisture was determined by the method ASTM-D 4318 (2005) and the granulometry by the ASTM-D422 (2007). The liquid and plastic limits following the ASTM D-4318 (2005). Electrical conductivity was measured using the ISO 11265 Standard (1994) and the Zagal and Zadzwaka protocol (2007). The organic content and pH were measured by the AASHTO Standards (2008) and ISO 10390 Standard (2005), respectively. Concentrations of mg/L and μg/L were converted to mg/kg according to Jankiewicz et al. (2002); Eq. (1).
| (1) |
Soil samples (S001 and S002) were exposed to sequential extraction tests with different extractant solutions to determine the content of heavy metals in the different soil fractions [i.e., exchangeable, reducible (bound to organic matter), oxidizable (bound to Mn and Fe oxides), etc.) (Tessier et al., 1979). The extraction was consecutively performed with an initial weight of 1.0 g of soil following a five-step procedure: Step 1: for the exchangeable-weakly sorbed, 8 mL of 1 M MgCl2 adjusted to pH 7 was added to 1.0 g of soil sample placed in a Falcon tube and was shaken for 1.0 h at room temperature. The extract was then separated from the solid residue by centrifugation at 3000 rpm for 20 min, and the supernatant liquid was filtrated with a 0.45 μm membrane. The solid residue was washed by adding 20 mL of deionized water, shaken for 15 min, and centrifuged for 20 min at 3000 rpm. Step 2: for carbonate sorbed heavy metals, 8 mL of NaAC adjusted to pH 5 was added to the residue from step 1 and resuspended by shaking for 5h at ambient temperature. Step 3: heavy metals bound to Fe/Mn oxides were extracted by adding 40 mL of 0.1 M hydroxylamine hydrochloride to the residue from step 2, and resuspended by mechanical shaking for 16 h at room temperature. Step 4: heavy metals strongly bound or incorporated into organic matter or other oxidizable species. The residue from the Step 3 was treated twice with 10 mL of 8.8 M hydrogen peroxide (H2O2). Then, the digestion was allowed to proceed at room temperature for 1.0 h with occasional manual agitation, followed by a digestion for another hour at 85 ± 1 °C in a water bath. During the digestion, the Falcon tube was loosely capped to avoid loss of hydrogen peroxide. Next, the tube was uncapped and heating was continued until the volume decreased to approximately 2–3 mL. Additionally, 10 mL of peroxide was added to the tube, capped, and digested at 85 ± 1 °C for 1.0h. Heating continued as before until the volume was reduced to 2–3 mL. Finally, 25 mL of 1.0 M ammonium acetate was added to the cold mixture and shaken for 16 h at room temperature. Step 5: for the heavy metals bound to residual fraction, the residue from step 4 was digested with 8 mL of aqua regia (HCl + HNO3), at 70 °C during 1.5 h. The separation of the extract; filtration of supernant; and the rinsing, shaking and centrifuging of residues for steps 2, 3, 4, and 5 were carried out the same as in Step 1.
For analysis of heavy metals (Cu, Pb, Cd, Zn, Cr) the mine tailings were filtered through 0.45 μm syringe filters. Filtrates of the mine tailings and of the soil extracted fractions were analyzed using a flame Perkin Elmer AA800 atomic absorption spectrometer, equipped with air-acetylene burner, hollow cathode lamps coupled with FIAS 100. Standardized procedures were used for the chemical analysis of metals and arsenic (Greenberg et al., 2017). For example, to measure total copper: First, standard solutions of 1, 5, and 10 mg/L Cu were prepared from a stock solution of 1000 mg/L. Second, 1 mL of the filtered sample was added to a test tube and diluted with deionized water as needed. Third, the AA equipment was ignited and fixed the wave-length to 324.7 nm. Thereafter, an equipment calibration was carried out using the standards prepared before. Finally, the liquid samples were measured for copper. For As, 1 mL of tailing or the filtrate, 1 mL of pure hydrochloric acid and 1 mL of the solution of 5% potassium iodide plus 5% ascorbic acid were added in each test tube, stirred and after 45 min, 7 mL of distilled water was added. Reading of total arsenic was performed in the AA-FIAS system. For equipment calibration 500, 1000, and 2000 μg/L of arsenic solutions were used.
For preparing the organic extract to be used as a reductant, it was used a protocol developed by López (2017) with modifications. Oranges brought from Portovelo were washed with tap water and dried at ambient temperature. Then, peels of oranges were carefully removed with a stainless-steel knife, collected and dried in oven at 60 °C during 90 min. Next, dried peels were ground using an electronic mill RM 200 and macerated at a 2:1 ratio (ethanol: peel) under stirring for 48 h. The resulting content was sonicated, centrifuged and filtered. Afterwards, the permeated liquid was concentrated using a BUCHI R-210 rotary evaporator for 6 h until the extract showed a semi-solid consistency. Finally, it was lyophilized and a green-yellowish gummy extract was obtained.
For the multicomponent nanoparticles (MCNPs) synthesis, 5 mL of 1M FeCl3.6H2O and 3.5 mL of 1M Na2SO4 were used, purged with nitrogen gas for 15 min, then 20 mL of 0.8 M NaBH4 and 20 mL of concentrated orange peel extract, previously adjusted to pH 9, were added. The reaction was stopped when it was observed that nanoparticles were precipitated and attracted to a magnetic field. Several washes were made to remove impurities contained on nanoparticles, then the content was frozen with liquid nitrogen and lyophilized for 18 h at -60 °C.
Transmission electron microscope (TEM) images were digitally recorded for morphological studies (Tecnai G2 Spirit TWIN, FEI, Netherlands). The size and morphology of the nanostructures were characterized using a field emission gun scanning electron microscope (FEG-SEM, Mira3 Tescan). The operation voltage ranged between 3 and 5 kV. In a classic procedure, low-magnification (Å~2000) micrographs were obtained to determine the size distribution.
For batch tests, 10 g of Technosols were used: A Technosol containing 99.5% soil + 0.5% of MCNPs and another Technosol with 99% soil + 1% MCNPs. In all treatments, 50 mL of tailings were added into plastic bottles and capped without adjusting the pH. All bottles were stirred for 17 h in a rotary shaker at 40 rpm. Tests with each Technosol were performed by triplicate as well as a negative control was used (only soil). At the end of the experiment, 10 mL of the supernatant were centrifuged and filtered through 0.45 μm membrane filter. Finally, the filtrate was used for chemical analysis using the atomic absorption equipment.
Tests in fixed-bed columns at laboratory scale were carried out to evaluate heavy metals retention using Technosols. Columns were packed with a metal mesh base, filter paper and Technosol. For these tests, 2 g of Technosols were employed: the first one containing 97% of soil and 3% of MCNPs, was labeled as (2T2), the second with 99% of soil and 1% of NPs, was named as 2T1. Tests were performed by triplicate. Also, a negative control (only soil) was used in each test. For the tests, 100 mL of tailings were passed through columns at a controlled flow rate of 0.50 mL/min and a contact time of 4.24 min.
Additional fixed-bed column tests were carried out using 10 g of Technosol at ratios of 1% MCNPs:99% soil and 3% MCNPs:97% soil, named as 10T1 and 10T2. Volumes of 200 and 400 mL of tailings were fed into the columns at a flow rate of 2.5 mL/min. These tests were also carried out by triplicate plus a negative control (only soil) were carried out. Because arsenic was not detected in the tailing samples, for these tests, As(V) was spiked to the liquid phase.
Furthermore, adsorption kinetics tests were performed in Boeco bottles using 2 g of Technosol (97% soil + 3 % MCNPs) and 200 mL of tailing containing different initial concentration of metal ions. Bottles were sealed, placed on a rotary shaker and shaken at 40 rpm for 63 h. One milliliter aliquots were taken every 3 min until reaching 15 min and then every 5 min up to 45 min. Aliquots were centrifuged, filtered and then measured the heavy metals concentration by atomic absorption. The following equations were used to adjust experimental results of the kinetic studies:
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
where, qe is the calculated adsorption of the metal at equilibrium in Technosol (mg/g), qt is adsorption of the metal at time t in Technosol (mg/g), Co refers to the initial concentration of the metal in solution (mg/L) and Ct is the concentration of the metal at time t (mg/L), t is the time (min), k is a constant for first, second, and third order kinetics for liquid phase and k1 and k2 are constants for pseudo-first and pseudo-second order kinetics for the Technosol phase.
For adsorption isotherm tests, Eq. (8) was used to calculate the adsorption of the metal at equilibrium, qeq (mg/g) (Gong et al., 2011):
| (8) |
where V is volume of the tailing (L), m is mass of the Technosol (g), Ci refers to the initial concentration of metal in solution (mg/L) and Ceq is the equilibrium concentration of the metal in solution.
Langmuir and Freundlich adsorption models were applied to fit data of the adsorption isotherm tests.
| (9) |
| (10) |
where Qmax (mg/g) and b (L/mg) are Langmuir constants and K and n are Freundlich constants of metal adsorption on the Technosols.
3. Results
3.1. Physical characterization of soils
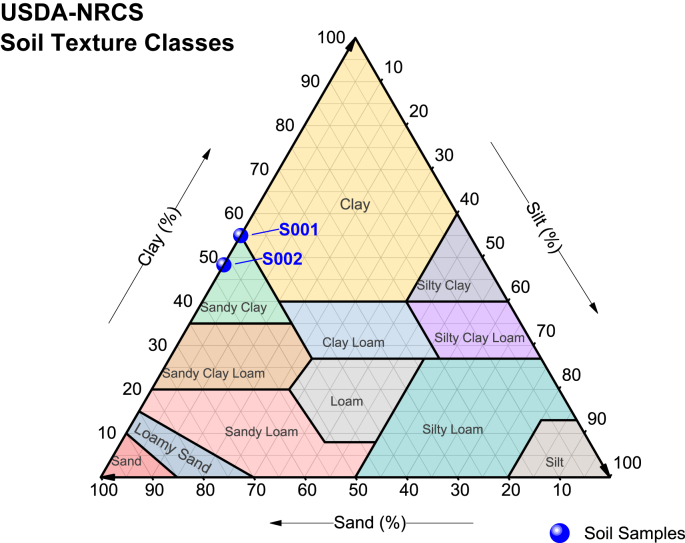
Moisture content of soils S001 and S002 is 25% and 4%, respectively. The organic matter content is 8.55% for soil S001 and for S002 4.35%. According to Jaramillo (2002) the organic content is directly related to moisture. If soil contains a high humidity its capacity to store water increases, as is indicated with both studied soils. For S001, the minimum humidity limit is 26% and the maximum is 38% and humidity of S002 ranges between 15% and 22%, which means that soil S001 is more plastic than soil S002. Regarding to electrical conductivity (EC), S001 has 13.12 μS/cm and S002 33.95 μS/cm, which means both are saline soils. Table 1 and Table S1 show the granulometry results. Based on Unified Soil Classification System (USCS) (ASTM D-2778-11, 1985), both soils (S001 and S002) are sandy-clay and do not contain silt (Figure 2).
Table 1.
Granulometry of soils S001 and S002 based on Unified Soil Classification System.
| Soil | Gravel | Sand | Clay |
|---|---|---|---|
| S001 | 1% | 45% | 54% |
| S002 | 10% | 47% | 44% |
Figure 2.
Classes of soil textures.
3.2. Chemical characterization of soil
Copper, cadmium, zinc, lead, arsenic and iron concentrations were determined using the sequential extraction supernatants. It is observed in Table 2 that iron concentration of S001 is higher compared to S002 (7744.14 vs. 6293.18 mg/kg), lead is also higher in S001 (43.74 mg/kg) while zinc is lower in S001 contrasted to S002 (10.20 vs. 40.18 mg/kg).
Table 2.
Content of iron and heavy metals in different fractions of soils.
| Fractions | Cu (mg/kg) | Cd (mg/kg) | Zn (mg/kg) | Pb (mg/kg) | As (mg/kg) | Fe (mg/kg) |
|---|---|---|---|---|---|---|
| F1: Exchangeable | ||||||
| S001 | 0.39 | 0.57 | 0.61 | 2.51 | 0.002 | 3.85 |
| S002 | 0.47 | 0.54 | 1.21 | 7.14 | 0.005 | 3.99 |
| F2: linked to carbonates | ||||||
| S001 | 0.95 | 0.54 | 1.33 | 4.96 | 0.002 | 4.42 |
| S002 | 0.84 | 0.50 | 2.57 | 6.25 | 0.010 | 3.73 |
| F3: linked to oxides of Fe and Mn | ||||||
| S001 | 4.59 | 0.00 | 5.62 | 18.58 | 0.001 | 6397.00 |
| S002 | 3.57 | 0.00 | 14.03 | 13.21 | 0.041 | 1863.80 |
| F4: linked to organic matter | ||||||
| S001 | 3.33 | 0.03 | 1.16 | 11.81 | 0.002 | 124.48 |
| S002 | 1.39 | 0.00 | 5.26 | 0.00 | 0.007 | 269.91 |
| F5: residual | ||||||
| S001 | 2.29 | 0.05 | 1.49 | 5.88 | 0.002 | 1214.40 |
| S002 | 6.91 | 0.00 | 17.11 | 1.46 | 0.151 | 4151.76 |
| Total S001 | 11.56 | 1.19 | 10.20 | 43.74 | 0.01 | 7744.14 |
| Total S002 | 13.18 | 1.05 | 40.18 | 28.06 | 0.21 | 6293.18 |
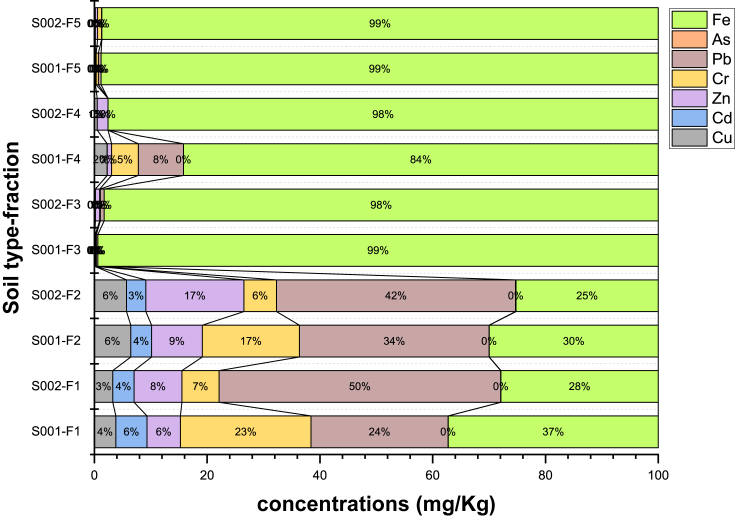
It is also seen in Table 2 that the majority of studied heavy metals are linked to metal oxides and residual phases, few of them are contained in the exchangeable phase and the remaining part is in the organic matter and carbonates (Figure 3). The resulting heavy metal concentrations contrasted to the Ecuadorian background limits for soils, provide the following information: Cd and Pb exceed the background limits. Cd equals to 1.19 mg/kg for S001 and 1.05 mg/kg for S002 and are above the allowed limit (0.50 mg/kg). S001 and S002 soils have 43.74 mg/kg Pb and 28.06 mg/kg Pb, respectively, these values are above the regulated concentration (25 mg/kg). Results of Energy Dispersive Spectroscopy (EDS) analysis are shown in Table 3. Based on these data, the soil with the highest iron content is S001 with 10.8%. In addition, the cation exchange capacity (CEC), for soil S001 is 3.12 (meq/100g) and 9.88 (meq/100g) for soil S002, which are considered very low and low; respectively, according to Gasim et al. (2014).
Figure 3.
Content of iron and heavy metals in (Figure 4a) the different fractions of soils.
Table 3.
Content of macro elements obtained by EDS analysis.
| Percentages in normalized mass | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrometals | O | Na | Mg | Al | Si | P | S | K | Ca | Ti | Fe |
| Soil S001 | 51.1 | 0.5 | 0.5 | 19.0 | 16.1 | 0.3 | 0.3 | 0.5 | 0.2 | 0.6 | 10.8 |
| Soil S002 | 49.3 | 1.3 | 1.7 | 16.3 | 19.1 | 0.2 | 0.3 | 1.9 | 0.3 | 0.5 | 9.0 |
3.3. Chemical characterization of liquid tailings
Hydrogen potential (pH) of liquid tailings measured in situ was 3.6 for pool 1 and 3.34 for pool 2. These values are considered very acidic according to Acuerdo Ministerial 097-A (2015). In addition, concentrations of heavy metals of liquid tailings (pool 1 and pool 2) are shown in Table 4. Compared to the regulated limits for the discharge of tailings into freshwater bodies (Acuerdo Ministerial 097-A, 2015), it is verified that for pool 1, Cu = 3.51, Cd = 0.17, Zn = 23.61, and Pb = 1.18 mg/L exceed the allowed limits (1.0, 0.02, 5.0, and 0.20 mg/L, respectively). In pool 2, metals that exceed levels are Cr = 0.52 mg/L and Pb = 1.59 mg/L.
Table 4.
Concentrations of heavy metals in liquid tailings.
| Pools | Cu (mg/L) | Cd (mg/L) | Zn (mg/L) | Pb (mg/L) | As (mg/L) |
|---|---|---|---|---|---|
| Pool 1 | 3.51 | 0.17 | 23.61 | 1.18 | 0.05 |
| Pool 2 | 0.16 | 0.02 | 3.33 | 1.59 | 0.00 |
3.4. Characterization of nanoparticles
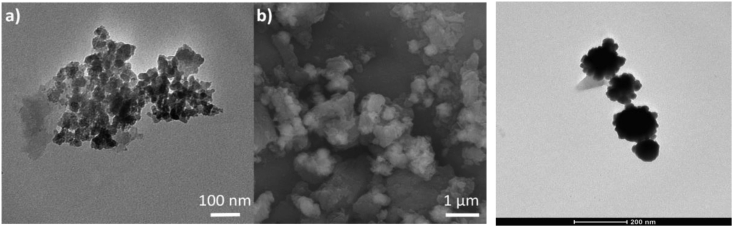
TEM and SEM images are shown in Figures 4a, 4b, 4c. Zero-valence iron nanoparticles in the core and iron sulfide as precipitated granules around its superficial area are observed (Figure 4c). Nanoscale size particles are roughly spherical with sizes of 35.9 ± 11.7 as provided by a DLS analysis (Fig. S1). These tiny particles show a minimal agglomeration but they are arranged in a morphological structure similar to a filament (Figure 4c).
Figure 4.
Images (a) TEM, (b) SEM of NPs and (c) SEM close up of nanoparticles.
3.5. Batch tests for retention of heavy metals using technosols
Technosols prepared with 99.5% of soil and 0.5% of MCNPs and 99% of soil and 1% of MCNPs were used for the tests. Table 5 shows percentages of heavy metals retention for both Technosols. There is around 1% on an average difference on the adsorption of heavy metals using both Technosols. It is remarkable to observe that retention of Cu and Pb in batch tests were 98% and 100% for both prepared materials. Although, the treatment with only soil (negative control) also retains heavy metals.
Table 5.
Retention of heavy metals after 17 h of batch tests.
| Elements | Initial concentration of heavy metals, mg/L | Retention on Technosols, % |
||
|---|---|---|---|---|
| Technosol 1 99.5% soil + 0.5% MCNPs |
Technosol 2 99% soil + 1% MCNPs |
Negative Control (only soil) | ||
| Cu | 3.51 | 98 | 98 | 95 |
| Cd | 0.17 | 83 | 82 | 82 |
| Zn | 23.61 | 97 | 98 | 93 |
| Pb | 1.18 | 100 | 100 | 100 |
3.6. Retention tests using technosols in fixed-bed columns
For the first set of fixed-bed column tests, 2 g of Technosols with 1% and 3% of MCNPs were used (2T1 and 2T2). As observed in Table 6, Cu is efficiently retained using 2T2 (93%) and a better adsorption occurs when immobilizing the Pb (100%). The retention of Zn using 2T1 and 2T2 is 48 and 47%, respectively. Similarly, Cd shows a better adsorption when treated with 2T1 (46%) while with 2T2 the uptake is 43%. In addition, columns packed with only soil also adsorb the four studied heavy metals; nevertheless, the column with 99% (1.98 g) of soil retains more heavy metals. On an average basis, MCNPs improve the retention of pollutants on the as-prepared Technosols.
Table 6.
Heavy metal retention with 2 g Technosol.
| Treatments | Retention of heavy metals on Technosols, % |
|||
|---|---|---|---|---|
| Cu | Cd | Zn | Pb | |
| Initial concentration of heavy metals in tailings, mg/L |
3.48 |
0.20 |
20.06 |
0.57 |
| 2T1: 99% soil + 1% MCNPs | 86 | 46 | 48 | 99 |
| 2T2: 97% soil + 3% MCNPs | 93 | 43 | 47 | 100 |
| Negative Control (99% soil) | 44 | 16- | 21 | 79 |
| Negative Control (97% soil) | 29 | 9 | 13 | 66 |
For the second set of fixed-bed columns, 10 g of Technosols were used. Treatment 10T1 fed with 200 mL of tailings showed, an average retention percentage of 61.67% while with 400 mL, the Technosol captured 42.17% of heavy metals. For 10T2 the retention percentages decreased from 70% to 58.33% for 200 and 400 mL of tailings, respectively (Table 7). Note that columns packed with only soil also retained on an average 42% of heavy metals but for an individual metal it can be higher. For example: 93% and 85% of Pb were adsorbed for columns packed with 9.9 g soil and fed with 200 and 400 mL of tailings, respectively but for columns filled with 9.7 g soil and fed with 200, 400 mL of tailings, 36% and 48% of Pb were retained.
Table 7.
Heavy metal retention with 10 g Technosol.
| Treatments | Volume of tailings | Cu | Cd | Zn | Pb | As |
|---|---|---|---|---|---|---|
| 10T1 | 200 mL | 57 | 45 | 36 | 87 | 69 |
| Negative Control (9.9 g only soil) | 43 | 33 | 25 | 93 | 60 | |
| 10T1 | 400 mL | 63 | 30 | 18 | 64 | 78 |
| Negative control (9.9 g only soil) | 47 | 26 | 23 | 85 | 66 | |
| 10T2 | 200 mL | 86 | 58 | 50 | 92 | 88 |
| Negative control (9.7 g only soil) | 22 | 20 | 36 | 57 | ||
| 10T2 | 400 mL | 75 | 39 | 33 | 89 | 89 |
| Negative control (9.7 g only soil) | 25 | 18 | 48 | 55 |
Results of statistical analysis show that according to Tukey test, average doses of Technosol and volume of tailings are significantly different (Table S2)) while the ANOVA test establishes that 10T2 (Technosol with 3% MCNPs + 97% soil) fed with 200 mL of tailings is more efficient (Table S3). Using this treatment, metals with the highest retention were 86% Cu, 58% Cd, 50% Zn, 92% Pb and 89% As. For Cu there is a discrepancy between averages at a significance level of 0.05, and through the Tukey test, it is determined that the two Technosols differ from the negative control. For Cd there is no variation among averages of the three treatments. For Zn there is a difference between means and through the Tukey test, it is proven that all treatments have a different means, being Technosol with 3% MCNPs the one with the lowest concentration, followed by the Technosol with 1% MCPNs and finally the negative control.
3.7. Heavy metal adsorption kinetics
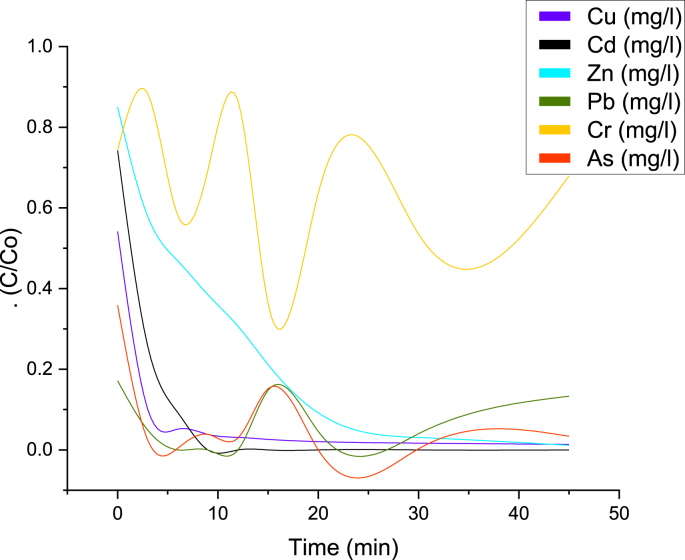
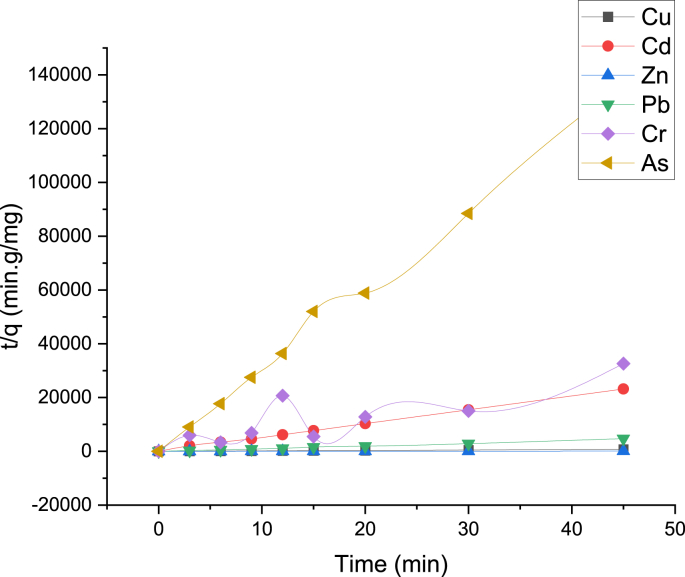
Because the best results of heavy metals adsorption were obtained with 2T2 Technosols (97% soil + 3 % nanoparticles) in fixed-bed columns and in batch tests, adsorption kinetics were run with only 2T2 treatment. Figure 5 shows the adsorption of heavy metal ions dissolved in the tailings versus time, using 2 g of Technosol 2. Up to 3 min of kinetic test, the uptake of heavy metals shows a sharp slope except for Zn. However, with the progress of time, the adsorption rate for all metallic species becomes insignificant due to saturation of reactive sites and complete steady state is reached after 45 min of testing. Also, there is no a significant change in the concentration of heavy metals in the period of 20–45 min.
Figure 5.
Adsorption kinetics for heavy metals contained in mine tailings.
Pseudo second order kinetics provides the best correlation of data for the analyzed metals. All fitting curves exhibit good linearity with correlation factors (R2 ~ 1.0). See (Table 8) and (Figure 6).
Table 8.
Correlation coefficients (R2) for kinetic fitting models after 45 min of treatment.
| Metal | Zero order | First order | Second order | Third order | Pseudo first order | Pseudo second order |
|---|---|---|---|---|---|---|
| Cu | 0.254 | 0.599 | 0.947 | 0.992 | 0.752 | 1.000 |
| Cd | 0.303 | 0.365 | 0.174 | 0.091 | 0.434 | 0.999 |
| Zn | 0.747 | 0.985 | 0.845 | 0.694 | 0.922 | 0.979 |
| Pb | 0.030 | 0.120 | 0.082 | 0.062 | 0.142 | 0.995 |
| As | 0.158 | 0.002 | 0.039 | 0.065 | 0.082 | 0.997 |
Figure 6.
Pseudo second order kinetic model for the uptake of heavy metals from mine tailings.
3.8. Adsorption isotherms study
Isothermal tests were conducted to describe the equilibrium adsorption of the Technosol prepared with 97% soil and 3% MCNPs to remove zinc (a heavy metal model) from the mine tailings. Results of adsorption tests fit fairly accurate the Langmuir isotherm model, with a correlation coefficient, R2 = 0.9703. Constants of Langmuir isotherm were estimated as Qmax = 4.67 mg Zn/g of Technosol and b = 0.43 L/mg (Figure 7a). To compare, data were also fitted to the Freundlich isothermal model (Figure 7b). It is observed that its R2 = 0.826, which means that Freundlich is far low than the Langmuir model.
Figure 7.
Linear fit of isothermal adsorption models: (a) Langmuir and (b) Freundlich.
4. Discussion
Granulometry of the soil used to prepare Technosols (S001) corresponds to a sandy-clay type. Thus, this soil exhibits good properties for preparing Technosols, such as a large surface area, light impermeability, compactness, high content of iron (10.8% w/w) and a yellowish-brown color. Moisture of this soil is 25% so it contains enough water to hydrate metal oxides (a very reactive component). Furthermore, this soil contains background concentrations of heavy metals, which might be associated to the high concentration of iron. It is well known that hydrated iron oxides behave as soft acids and link heavy metals selectively through Lewis's acid-base (LAB) interactions (Cumbal and SenGupta, 2005a).
Technosols prepared with an iron-rich soil and two doses of MCNPs showed good retention of heavy metals in batch tests, on an average basis 94.5% and 92.5% for Technosol 1 and Technosol 2, respectively; being 100% and 98% for Pb and Cu, in that order. This high removal of heavy metals from liquid tailings, could be related to the turbulent flow created inside of the reactor by agitation, which in turn increase collisions with particles of Technosols. As a result, a high adsorption of metallic ions occurs. Hossain et al. (2012) reported that increasing the agitation speed and temperature, the percentage of heavy metal adsorption on a banana-peel sorbent was also increased. Nevertheless, less retention of toxic metals was obtained in fixed-bed columns packed with 2 g and 10 g of Technosols. The best treatment resulted using 10 g of Technosol 2, fed with 200 mL of a polluted tailing, it retained around 75% of heavy metals. Also, a column packed with only soil retained an average of 34% of toxic metals. It is believed that Technosols containing 97% soil + 3% MCNPs show an enhanced adsorption of metals because its reactivity is improved due to the involvement of the nanoparticles. Sizes of these tiny particles that are in the range of 35.9 ± 11.70 nm (Fig. S1) certainly influence in its physical and chemical properties and therefore the reactivity (Wang & Herron, 1991; Grassian, 2008). The combination of Fe0 and FeS in the nanoparticle provides of a synergetic reactivity for the selective capture of heavy metals from mine tailings. The oxidized iron retains heavy metals forming stable inner-sphere complexes (Cumbal and SenGupta, 2005a; Cumbal and Sengupta, 2005b). In addition, FeS on the shell of the nanoparticle once contacted with free heavy metals can promptly react with them, forming chemically stable sulfides (Dean at al. 1972; Ludwing et al. 2005). Solubility products of the metal sulfides favor their precipitation (Ksp,Cu = ~10−47; Ksp,Cd = ~10−29; and Ksp,Pb = ~10−28) (Chang and Goldsby, 2014). In addition, iron-rich soil also uptakes heavy metals by the interaction with the hydrated iron oxides.
On the other hand, results of kinetic tests suggest that during the first 3 min there exists a physisorption because the heavy metal uptake is fast and the electronic structure of the sorbent is scarcely perturbed upon adsorption (Amendola et al., 2011). However, as the test time progresses a chemisorption can occur. This retention mechanism can be stimulated by inner-sphere complexes formation between the metallic ions and the iron oxides of soil and of the nanoparticles and also the metal-sulfide precipate formation. If physisorption were the only adsorption mechanism in the treatment, there would be desorption of the metallic pollutants in the aqueous media as the test goes to completion. In the tests, leakage of heavy metals was not observed after 20 min of the test. Hence, chemical adsorption plays an important role on the heavy metals’ adsorption using Technosols. In previous research, it has been demonstrated that chemisorption of heavy metals is quite permanent and no leakage has been observed in leachate tests using multicomponent nanoparticles and soils with different aqueous extractants (Abril et al., 2018). The best correlation for all of the kinetic systems studied, is the pseudo-second order model. The correlation coefficients for the linear plots of t/qt against time from the pseudo-second order rate law are greater than 0.979 for all tests for contact times of 45 min. This suggests that this sorption system is not a first, second, third, and pseudo-first order reactions and that the pseudo-second order model, based on the assumption that the rate-limiting step could be chemisorption involving valency forces through sharing or exchange of electrons between heavy metal and Technosol, provides the best correlation of the data (Ho and McKay, 1999). Prior studies reported similar behavior for fitting results of adsorption of heavy metals from artificial mine tailings (Flores et al., 2015). Zhou et al., 2013 informed the same adsorption pattern when these authors studied the removal of heavy metal ions dissolved in metallurgical wastewater using sepolite.
In terms of costs, the Technosol is not an expensive sorbent material. As been described, the best Technosol contains 97% of an iron-rich soil and 3% of nanoparticles. In the local market, the cost of a ton of Technosol would be approximately $4.900; however, this amount of material can retain on average 10.000 meq of heavy metals or treat 20.500 L of mine tailings with an average concentration of 0.74 meq/L of heavy metals. Compared to other sorbent materials such as carbon-based (Di Natale et al., 2009), the cost is approximately three-fold but this Technosol due to the properties explained above, can operate in a broad range of pH (3–8.9), which secures the removal of heavy metals from acidic (pH = 3–4). and basic (pH = 6–8) mine tailings.
5. Conclusion
Technosols were successfully prepared by a simple and cost-effective process, mixing iron-rich local soils and small percentages of multicomponent nanoparticles. The most efficient treatment in fixed-bed columns is that one using 10 g of Technosols 2 (97% soil + 3% NPs) fed with 200 mL of tailings. It captures on average 75% of heavy metals with a retention time of 4.24 min; while in batch test, the average removal was 80% in 6 min and after 30 min, 90% of average removal was obtained. Adsorption of Cu, Cd, Pb and As in Technosol rapidly increased up to 3 min, thereafter the adsorption speed decreases. Hence, based on the retention percentages, it is obvious that the batch reactor provides the best results although it could be less applicable in the field. It is unquestionable that adsorption of heavy metals by Technosols is due to a combination of coulombic and Lewis's acid-base interactions and precipitation of metal sulfides and its uptake obeys a pseudo-second order model, in which physisorption followed by chemisorption mechanisms are those what control the process. The latter suggests that adsorption of heavy metals from mine tailings using Technosols could be permanent and these may not leachate from the solid phase under heavy rains. Therefore, this sorbent material can be used in the retention of heavy metals dissolved in mine tailings and what is most important it can be prepared locally.
Declarations
Author contribution statement
Dario Bolaños-Guerrón: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jacqueline Capa: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Luis Cumbal Flores: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the support given by Universidad de las Fuerzas Armadas ESPE, Center for Nanoscience and Nanotechnology (CENCINAT) and Dr. Brajesh Kumar for language review.
Footnotes
Featured Application: Combination of the indigenous soils with nanoparticles to efficiently retain heavy metals to enhance the environmental remediation of metallic-mining polluted processes.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary material
References
- AASHTO Standards . 2008. Determination of Organic Content in Soils by Loss on Ignition. [Google Scholar]
- Abril M., Ruiz H., Cumbal L.H. Biosynthesis of multicomponent nanoparticles with extract of mortiño (Vaccinium floribundum Kunth) berry: application on heavy metals removal from water and immobilization in soils. J. Nanotechnol. 2018;2018 [Google Scholar]
- Acuerdo Ministerial 097-A . 2015. Refórmese el Texto Unificado de Legislación Secundaria, Quito. [Google Scholar]
- Adler R., Bergquist B., Adler S., Davée J., Lees P., Niquen W., Velasquez-López M. Veiga y P. Challenges to measuring, monitoring, and addressing the cumulative impacts of artisanal and small-scale gold mining in Ecuador. Resour. Pol. 2013 [Google Scholar]
- Ahirwal J., Maiti S. Development of Technosol properties and recovery of carbon stock after 16 years of revegetation on coal mine degraded lands, India. Catena. 2018;166:114–123. [Google Scholar]
- Amendola V., Riello P., Polizzi S., Fiameni S., Innocenti C. Magnetic iron oxide nanoparticles with tunable size and free surface obtained via a “green” approach based on laser irradiation in water. J. Mater. Chem. 2011;21(46):18665–18673. [Google Scholar]
- Appleton J., Williams T., Orbea H., Carrasco M. Fluvial contamination associated with artisanal gold mining in the ponce enríquez, portovelo-zaruma and nambija areas, Ecuador. Water Air Soil Pollut. 2001;131(1-4):19–39. [Google Scholar]
- Arbestain M., Camps Extractability and leachability of heavy metals in Technosols prepared from mixtures of unconsolidated wastes. Waste Manag. 2008;28(12):2653–2666. doi: 10.1016/j.wasman.2008.01.008. [DOI] [PubMed] [Google Scholar]
- ASTM D-422. AASHTO T88, Bowles J.E. 2007. Análisis granulométrico de suelos por tamizado. [Google Scholar]
- ASTM D-4318 . 2005. Los métodos estándar de ensayo para límite líquido, límite plástico y el índice de plasticidad de los suelos. [Google Scholar]
- ASTM D2787-11 . American Society for Testing and Materials; 1985. Classification of Soils for Engineering Purposes: Annual Book of Standards. [Google Scholar]
- Chang R., Goldsby K.A. seventh ed. McGraw-Hill; New York, NY, USA: 2014. General Chemistry: the Essential Concepts. [Google Scholar]
- Cumbal L., SenGupta A.K. Arsenic removal using polymer-supported hydrated iron (III) oxide nanoparticles: role of Donnan membrane effect. Environ. Sci. Technol. 2005;39(17):6508–6515. doi: 10.1021/es050175e. [DOI] [PubMed] [Google Scholar]
- Cumbal L.H., Sengupta A.K. Preparation and characterization of magnetically active dual-zone sorbent. Ind. Eng. Chem. Res. 2005;44(3):600–605. [Google Scholar]
- Dean J.G., Bosqui F.L., Lanouette K.H. Removing heavy metals from waste water. Environ. Sci. Technol. 1972;6(6):518–522. [Google Scholar]
- Di Natale F., Erto A., Lancia A., Musmarra D. A descriptive model for metallic ions adsorption from the equeous solutions onto activated carbons. J. Hazard Mater. 2009;169(1-3):360–369. doi: 10.1016/j.jhazmat.2009.03.105. [DOI] [PubMed] [Google Scholar]
- Flores L.C.H., Debut A., Delgado D., Bastidas C., Stael C. Synthesis of multicomponent nanoparticles for immobilization of heavy metals in aqueous phase. NanoWorld J. 2015;1(4):105–111. [Google Scholar]
- Gasim M., Ismail B., Mir S., Abd Rahim S., Toriman M. The physico-chemical properties of four soil series in Tasik Chini, Pathang, Malaysia. Asian J. Earth Sci. 2014;4(3):75. [Google Scholar]
- Ge F., Li M.-M., Ye H., Zhao B.-X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard Mater. 2012;211–212:366–372. doi: 10.1016/j.jhazmat.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Gong J., Liu T., Wang X., Hu X., Zhang L. Efficient removal of heavy metals from aqueous phase with the assembly of anisotropic layered double hydroxide nanocrystals@carbon nanosphere. Environ. Sci. Technol. 2011;45(14):6181–6187. doi: 10.1021/es200668q. [DOI] [PubMed] [Google Scholar]
- Grassian V.H. When size really matters: size-dependent properties and surface chemistry of metal and metal oxide nanoparticles in gas and liquid phase environments. J. Phys. Chem. C. 2008;112(47):18303–18313. [Google Scholar]
- Greenberg A.E., Clesceri L.S., Eaton A.D. American Public Health Association, American Water Works Association, and Water Environment Federation; 2017. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Guerrero D. 2013. Diagnostico ambiental de las descargas mineras liquidas y solidas, en los recursos hidricos superficiales y subterraneos del Canton Portovelo, Quito. [Google Scholar]
- Han C., Li H., Pu H. Synthesis and characterization of mesoporous alumina and their performances for removing arsenic(V) Chem. Eng. J. 2013;20:1–9. [Google Scholar]
- Hossian M.A. Removal of copper from water by adsorption onto banana peel as bioadsorbent. Int. J. Geom. 2012;2(2):227–234. [Google Scholar]
- SO 10390 . Determination of pH; Genéve, Switzerland: 2005. Soil Quality. [Google Scholar]
- SO 11265 . 1994. Soil Quality–Determination of the Specific Electrical Conductivity, Genéve, Switzerland. [Google Scholar]
- Jankiewicz B., Ptaszyński B., Turek A. Spectrophotometric determination of iron (II) in the soil of selected allotment gardens in lódź. Pol. J. Environ. Stud. 2002;11(6):745–749. [Google Scholar]
- Jaramillo D. Medellín; 2002. Introducción a la ciencia del suelo. [Google Scholar]
- Ho Y., McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999;34(5):451–465. [Google Scholar]
- Kumar U., Ankamwar B., Karmakar S., Halder A., Das P. Green synthesis of Silver nanoparticles using the plant extract of Shikakai and Reetha. Materials. 2018 [Google Scholar]
- Li J. Removal and immobilization of heavy metals in contaminated soils by chlorination and thermal treatment on an industrial-scale. Chem. Eng. J. 2019;359:385–392. [Google Scholar]
- López N. 2017. Biosíntesis de nanopartículas multicomponente mediante el extracto de Citrus sinensis para inmovilización de metales pesados en aguas contaminadas, Sangolquí. [Google Scholar]
- Lu H.P. Use of magnetic biochars for the immobilization of heavy metals in a multi-contaminated soil. Sci. Total Environ. 2018;622:892–899. doi: 10.1016/j.scitotenv.2017.12.056. [DOI] [PubMed] [Google Scholar]
- Ludwig R.D., McGregor R.G., Blowes D.W., Benner S.G., Mountjoy K. A permeable reactive barrier for Treatment of heavy metals. Ground Water. 2005;40(1):59–66. doi: 10.1111/j.1745-6584.2002.tb02491.x. [DOI] [PubMed] [Google Scholar]
- Macía P., Fernández-Costas C., Rodríguez E., Sieiro P., Pazos M., Sanromán M. Technosols as a novel valorization strategy for an ecological management of dredged marine sediments. Ecol. Eng. 2014;67:182–189. [Google Scholar]
- Ministerio de Energía y Minas del Ecuador . 1999. Monitoreo ambiental de las áreas mineras en el sur del Ecuador 1996-1998, Quito. [Google Scholar]
- Ozturk B., Parkinson C., Gonzalez-Miquel M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Separ. Purif. Technol. 2018;206:1–13. [Google Scholar]
- Parra H. Cuenca; 2009. La responsabilidad ambiental de las empresas mineras conforme a la legislación del Ecuador. [Google Scholar]
- Safdar M., Kausar T., Jabbar S., Mumtaz M., Ahad K., Saddozai A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017;25(3):488–500. doi: 10.1016/j.jfda.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounthararajah D.P., Loganathan P., Kandasamy P., Vigneswaran S. Adsorptive removal of heavy metals from water using sodium titanate nanofibres loaded onto GAC in fixed-bed columns. J. Hazard Mater. 2015;287:306–316. doi: 10.1016/j.jhazmat.2015.01.067. [DOI] [PubMed] [Google Scholar]
- Sreenivasulu G., Jayaraju N., Sundara R., Lakshmanna B., Rajasekhar M., Nirmala K., Lakshm P. Assessment of heavy metal pollution from the sediment of Tupilipalem Coast, southeast coast of India. Int. J. Sediment Res. 2018;33(3):294–302. [Google Scholar]
- Tarras-Wahlberg N. Environmental management of small-scale and artisanal mining: the Portovelo-Zaruma gold mining area, southern Ecuador. J. Environ. Manag. 2002;65(2):165–179. doi: 10.1006/jema.2002.0542. [DOI] [PubMed] [Google Scholar]
- Tessier A., Campbell P., Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979;51(7):844–850. [Google Scholar]
- Richards L., editor. Diagnosis and Improvement of Saline and Alkali Soils. 1969. United States Salinity Laboratory Staff (USDA) p. 98. [Google Scholar]
- Wang J., Zheng S., Shao Y., Liu J., Xu Z. Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 2010;349(1):293–299. doi: 10.1016/j.jcis.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Wang Y., Herron N. Nanometer-sized semiconductor clusters. J. Phys. Chem. 1991;95(2):525–532. [Google Scholar]
- Xin X., Wej O., Yang J., Yan L., Feng R. Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe3O4 nanoparticles. Chem. Eng. J. 2012;184:132–140. [Google Scholar]
- Zagal E., Sadzawka A. 2007. Protocolo de métodos de análisis para suelos y lodos. [Google Scholar]
- Zhang W. Influence of soil washing with a chelator on subsequent chemical immobilization of heavy metals in a contaminated soil. J. Hazard Mater. 2010;278(1-3):578–587. doi: 10.1016/j.jhazmat.2010.01.124. [DOI] [PubMed] [Google Scholar]
- Zhou X.Y., Xue X.X., Xiang X. Study on adsorption of heavy metal ion in metallurgical wastewater by sepiolite. Adv. Mater. Res. 2013;726:2585–2588. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data included in article/supplementary material/referenced in article.