Abstract
Objectives: the different analytical methods for measurement of serum 25-hydroxyvitamin D (25(OH)D) are not yet fully harmonized and no consensus exists on a threshold of 25(OH)D defining a deficiency status. In this study, we compared the results from the assays of serum 25(OH)D performed with three different methods to evaluate the presence of potential biases and how much these biases can influence the assignment of patients to specific 25(OH)D deficiency/sufficiency categories. Design and Methods: Liaison 25(OH) Vitamin D Total (DiaSorin Liaison XL), Elecsys Vitamin D Total II (Roche Elecsys) and Lumipulse G25(OH) Vitamin D (Fujirebio Lumipulse G1200) were used. Methods comparability was established performing Passing-Bablok regression and Bland-Altman analysis to prove whether the differences found were lower than the preliminarily pre-established maximum acceptable bias. Results: all Passing-Bablok regressions exhibited the presence of a proportional and constant systematic error. Bland-Altman analysis revealed biases well above the maximum acceptable bias, so the 25(OH)D concentrations measured were not comparable. To evaluate whether the three methods had the same ability to classify patients into different categories of vitamin D levels, we categorized results obtained by each method in reference classes. Lumipulse categorized most patients into the class with the lowest 25(OH)D concentrations (<20 ng/mL) whereas Elecsys ranked the lowest number. Conclusions: Liaison XL and Elecsys have shown good accuracy compared to Lumipulse in measuring 25(OH)D levels. Nevertheless, the assays were not interchangeable due to the lack of comparability of results as well as to the disagreement in classification of hormone deficiency or sufficiency.
Keywords: 25(OH)Vitamin D, Method comparison, Method clinical validation
Highlights
-
•
Three immunometric assays for the measuring of serum 25(OH)Vitamin D were compared.
-
•
Two of three assay show good accuracy whereas the third only fair.
-
•
Due to the lack of comparability of the results, the assays were not exchangeable.
-
•
Assays are at odds in the classification of hormone deficiency or sufficiency.
-
•
Efforts will be required in the future to improve the immunoassays harmonization.
1. Introduction
Vitamin D has a pivotal role in calcium metabolism and in the preservation of skeletal health and it is associated with several human disorders, including cardiovascular diseases, cancer, and infectious diseases, among others [[1], [2], [3], [4]]. The international scientific community recognizes the measurement of 25 OH total Vitamin D (25(OH)D) as the best parameter to evaluate the reserves of this hormone [5,6]. The 25(OH)D deficiency is spread worldwide [7] and the routine assessment of 25(OH)D status in patients has grown exponentially in recent years [8].
The clinical laboratory may use either mass spectrometry (HPLC/MSMS) or immunometric methods to assay serum levels of 25(OH)D [6]. However, the different analytical methods are not yet fully harmonized [9]. To date, in fact, despite technological innovations and its complete automation, the assay of 25(OH)D represents a challenge for the laboratory. The analytical inaccuracy is mainly due to the existence of different forms of 25(OH)D with similar biological activity and to the lipophilic nature of these molecules, as well as to the presence of various metabolic interferents and epimers [10]. Furthermore, 25(OH)D can be transported both from the Vitamin D Binding Protein (VDBP) and from albumin and lipoproteins. The concentration of these 25(OH)D binding proteins varies according to pathophysiological conditions of the organism, such as pregnancy or kidney disease. Moreover, 25(OH)D binds these proteins with different affinity constants and must be separated from them before the execution of the measurement, therefore introducing a further variable on 25(OH)D determination [10].
To address the lack of assays standardization, in 2010 several national and international organizations, together with the Office of Dietary Supplements (ODS) of the National Institutes of Health (NIH), have organized the Vitamin D Standardization Program (VDSP) aimed to: i) identify HPLC/MSMS as the reference procedure measurement; ii) provide reference sera to the manufacturers of analytical assays to calibrate their commercial systems; and, iii) promote two external quality assessment programs based on accuracy [11,12]. Within the VDSP, the US Center for Disease Control and Prevention (CDC) also certifies and publishes annually the list of manufacturers whose analytical method has successfully met the requirements for standardization [13].
However, although the accuracy of the various analytical methods has improved, much work remains to be done [10,14,15]. Numerous studies of automated immunoassays comparison demonstrated how, despite the excellent correlation with the reference procedure made them valid for the 25(OH)D measurement in the routine clinical laboratory, the incomplete standardization resulted in significant bias when the methods were compared to each other [[15], [16], [17]].
The significant variability in 25(OH)D measure obtained by different methods has also complicated the definition of a universal deficiency threshold for 25(OH)D [10,18]. Currently, in fact, no universal consensus exists on threshold of 25(OH)D defining a vitamin D deficiency status. The US Institute of Medicine (IOM), on the basis of predefined outcomes of mineral metabolism and bone health, recommends a serum 25(OH)D concentration of 20 ng/mL, while the Endocrine Society, after a deep systemic review of literature, proposes a 30 ng/ml concentration as threshold. Essentially, one cause of the controversial threshold could be the lack of vitamin D methods standardization [5,19].
This study describes the comparison of the results obtained by measurement of serum concentration of 25(OH)D performed with three different tests: Liaison 25(OH) Vitamin D Total test (DiaSorin Liaison XL) (DiaSorin, Saluggia, Italy), Elecsys Vitamin D Total II test (Roche Elecsys) (Roche Diagnostics Gmbh, Mannheim, Germany), and Lumipulse G 25OH Vitamin D test (Fujirebio Lumipulse G1200) (Fujirebio inc, Tokyo, Japan). The aim of study was: i) evaluate the presence of a potential bias among the different methods and ii) how much this bias can influence the assignment of patients to the specific 25(OH)D deficiency/sufficiency categories.
2. Materials and methods
2.1. Samples collection
Within a week, 250 serum samples were analysed for the 25(OH)D concentration by DiaSorin Liaison XL (DiaSorin, Saluggia, Italia) at Clinical Biochemistry Laboratory of Mater Domini University Hospital (Catanzaro, Italy) in February 2020, as part of daily work routine. A total of 51 samples, ranging of serum 25(OH)D concentrations from 4.0 to 103.0 ng/mL, were selected, anonymized, aliquoted into 2 tubes and 25(OH)D concentration assayed by Roche Elecsys and Fujirebio Lumipulse G1200 tests.
Ethical approval was not required because the samples are anonymized before to be subjected to further analysis. The study was conducted according to Helsinki declaration on human experimentation.
2.2. 25(OH)D methods
The 25(OH)D (ng/ml) determinations were performed with the following automated immunoassays: Liaison 25(OH) Vitamin D Total assay (DiaSorin, Saluggia Italy; lot 134858B); Elecsys Vitamin D Total II test (Roche Diagnostics, Mannheim, Germany; lot 424,995), and Lumipulse G 25OH Vitamin D test (Fujirebio Inc., Tokyo, Japan; lot 8XX0074). All three methods are CDC certified [13].
DiaSorin Liaison XL is a competitive chemiluminescent immunoassay (CLIA) and Roche Elecsys is based on a competing protein-binding assay for 25(OH)D and uses electro chemiluminescent detection (ECLIA). Finally, Fujirebio Lumipulse G 1200 is a two-step non-competitive sandwich assay based on chemiluminescent enzyme immunoassay (CLEIA). The 25(OH)D measurement range was 4–150 ng/ml for DiaSorin Liaison XL and Fujirebio Lumipulse G 1200 tests and 3–100 ng/ml for Roche Elecsys test. As reported by manufactures, the 25 OH Vitamin D2 and 25 OH Vitamin D3 cross reactivity is equal to 100 % for DiaSorin Liaison XL whereas of 97 % and 100 % for Roche Elecsys, respectively. No data were reported for Fujirebio Lumipulse G 1200. All 25(OH)D measurements were performed according to the manufacturer's instructions in our laboratory.
2.3. Methods imprecision
Imprecision for each method was carried out according to the classic 3 × 5 scheme during the routine analytic session [20]. The quality control sera, provided by respective manufacturers, were used to calculate imprecision as coefficient of variation (CV%).
Briefly, for each method, two levels of internal quality control serum were determined in triplicate (at the beginning, in the middle and at the end of the analytical session) for 5 consecutive days and then was calculated the CV% [21].
2.4. Methods comparisons
The methods comparison was carried out following the operating procedures suggested by documents of Clinical Biochemistry and Clinical Molecular Biology Italian Society SiBioC [20,22]. In particular, each method was compared with other methods by Bland-Altman analysis to verify if the maximum bias was minor the maximum acceptable error (Tea%), that for 25OH(D) is 25 % [23].
Briefly, the maximum acceptable bias was calculated according to the following formula: bias max % = TEa% - 1.65 CVa where CVa is the highest analytical imprecision within the laboratory expressed in % and calculated using the precision control sera.
2.5. Methods accuracy
The accuracy of DiaSorin Liaison XL, Roche Elecsys, and Fujirebio Lumipulse G 1200 methods was evaluated using 10 International Vitamin D External Quality Assessment Scheme (DEQAS) control sera. DEQAS is an external quality control scheme “accuracy based”. The “true” value of 25(OH)D in DEQAS samples were assigned using the LC–MS/MS assay, the Reference Measurement Procedure (RMP) for 25(OH)D measurement. Then, the accuracy of the participants’ results is assessed verifying how far they deviates from the true value indicated by DEQAS program. The biases among results obtained with the three methods were calculated and then compared with the limit threshold of 25 % proposed by the organizer [24,25].
2.6. Statistical analysis
The mean, CV, variance, standard deviation, Passing Bablok regression and Bland-Altman analysis were calculated by MetComp software module vers. 1.0 [20].
To assess whether DiaSorin Liaison XL, Roche Elecsys, and Fujirebio Lumipulse G 1200 methods homogeneously classified the patients in the 25(OH)D deficiency/sufficiency categories, the Cohen's kappa coefficient, a measure of the strength of the agreement between methods, was calculated [26]. The values obtained were evaluated according to this scheme:
-
•
if κ assumes values between 0.01 and 0.40, the concordance is poor.
-
•
if κ assumes values between 0.41 and 0.60, the concordance is fair.
-
•
if κ assumes values between 0.61 and 0.80, the concordance is good.
-
•
if κ assumes values between 0.81 and 1.00, the concordance is very good.
3. Results
3.1. Evaluation of analytical precision
The precision of DiaSorin Liaison XL, Roche Elecsys, and Fujirebio Lumipulse G 1200 methods was calculated as intra-laboratory CV according to 3 × 5 scheme (Table 1). All methods showed a CV% falling within the limits of acceptability declared by manufacturers: 9.8 %, 7.2 %, and 6 % for DiaSorin Liaison XL, Roche Elecsys, and Fujirebio Lumipulse G 1200, respectively. The only exception was Roche Elecsys control serum level 1 with a CV = 11.41 % (Table 1).
Table 1.
Precision, calculated as CV%, for DiaSorin Liaison XL, Roche Elecsys, and Fujirebio Lumipulse G1200 methods.
| 25(OH)D (ng/mL) |
DiaSorin Liaison XL |
Roche Elecsys |
Fujirebio Lumipulse G1200 |
|||
|---|---|---|---|---|---|---|
| Control serum |
||||||
| level 1 | level 2 | level 1 | level 2 | level 1 | level 2 | |
| Meantot | 14.12 | 45.73 | 14.81 | 36.30 | 32.46 | 49.67 |
| Stdtot | 0.98 | 2.32 | 1.69 | 2.30 | 1.44 | 2.15 |
| Variancetot | 0.99 | 5.37 | 2.86 | 5.31 | 2.10 | 4.61 |
| CVtot (%) | 6.99 | 5.10 | 11.41 | 6.35 | 4.45 | 4.32 |
Std = standard deviation; CV = coefficient of variation.
mean, std, variance and CV were calculated on total data obtained during five days of 3 × 5 precision scheme [19].
3.2. Method comparisons
Relationship and agreement among the three methods were performed by comparing them two by two using the Passing - Bablok regression and the Bland – Altman analysis to verify whether the differences between the sample values were below a pre-established beforehand maximum deviation limit, the “maximum acceptable bias” (Bias max). The formula: Bias max = TEa % - 1.65 CVa was used to calculate the Bias max, where TEa is the total allowable error and CVa the coefficient of variation that express the within-laboratory analytical inaccuracy calculated using precision control sera as reported above. Starting from the total acceptable error (TEa%) that for vitamin D, based on the evidence in the literature [23] is 25 %, and choosing the CVa with the highest value among those obtained with precision tests, we calculated 1.65 CVa max that is 11.41 × 1.65 = 18.83 %. Consequently, the bias max % that not to be exceeded was: 25 % –18.83 % = 6.17%.
First, the 25(OH)D results obtained by DiaSorin Liaison XL were compared with those obtained by Roche Elecsys and then Fujirebio Lumipulse G 1200, respectively. Moreover, the Passing - Bablok regression and the Bland - Altman analyses were applied also to results obtained by Roche Elecsys and Fujirebio Lumipulse G 1200.
3.2.1. DiaSorin Liaison XL vs Roche Elecsys
The Passing - Bablok regression between DiaSorin Liaison XL and Roche Elecsys exhibited a linear relation (y = 1.4924 + 1.1592 x; 95 % C.I. intercept = −0.41 to 4.55; 95 % C.I. slope = 1.26 to 1.03 p > 0.05) (Fig. 1A) and the presence of a proportional systematic error. The results of Bland-Altman analysis are shown in Fig. 1B. The mean difference ± standard deviation of 25(OH)D was −6.16 ± 8.12 ng/mL (95 % CI = −8.49 to −3.83) and the limits of agreement carried out were −22.24 to + 9.91 ng/mL (Fig. 1B). The analysis of Bland - Altman on percentage differences showed a mean percentage difference or bias% equal to −22.5 % (95 % C.I. = −29.0 % to −16.0 %) (Fig. 1C). Since the previously calculated maximum acceptable bias between the methods (6.17 %) was less than the absolute lower limit value of 95 % C.I. bias%, the results provided by DiaSorin Liaison XL and Roche Elecsys were not comparable.
Figs. 1.
25(OH)D (ng/mL) determination by DiaSorin Liaison XL and Roche Elecsys.
(A) Passing – Bablok regression analysis; (B) Bland - Altman analysis of agreement on absolute values of mean differences (ng/mL) between methods; (C) Bland - Altman analysis of agreement on percentage values of mean differences (%) between methods.
3.2.2. DiaSorin Liaison XL vs Fujirebio Lumipulse G 1200
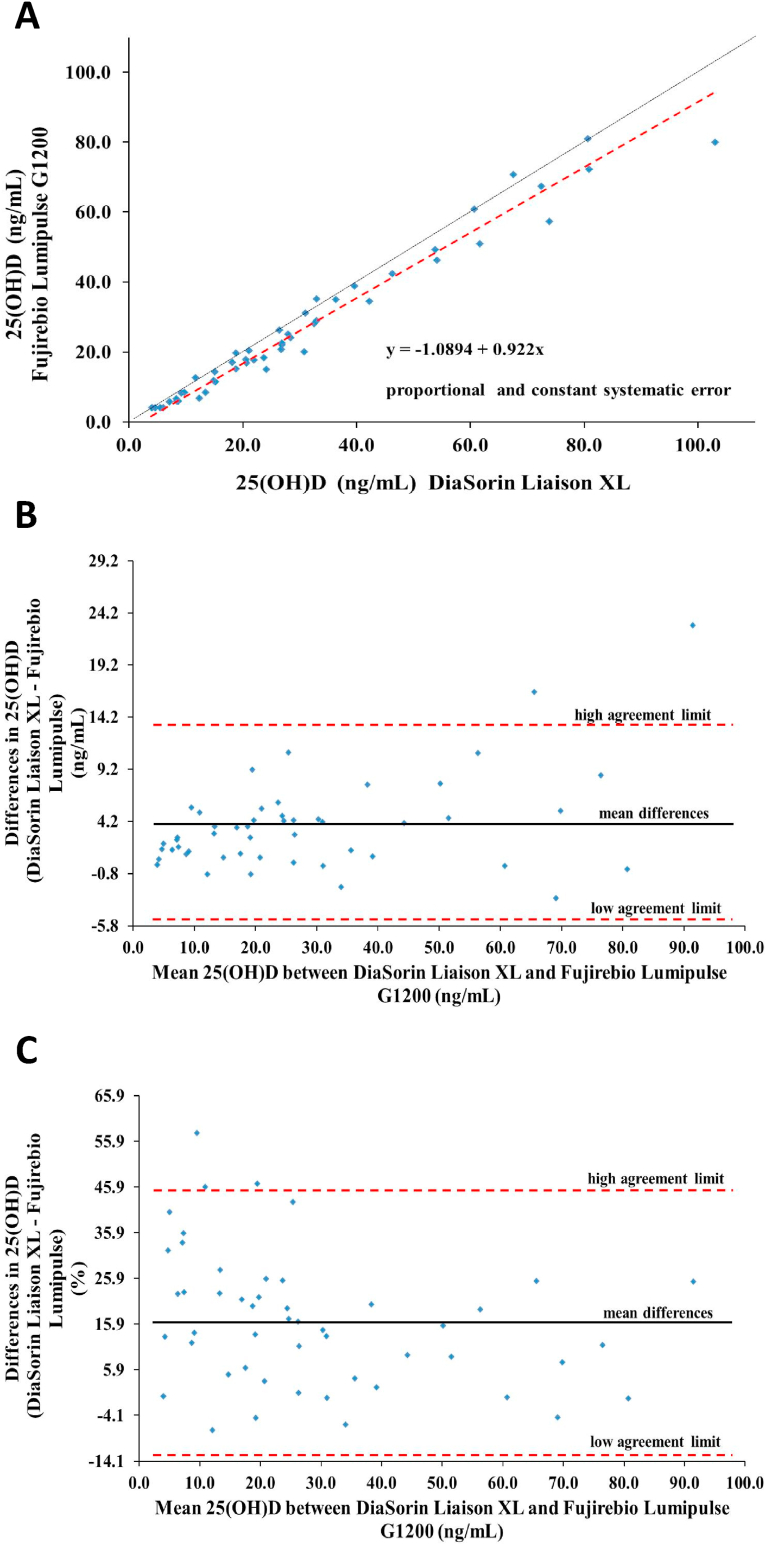
The Passing - Bablok regression analysis between DiaSorin Liaison XL and Fujirebio Lumipulse G 1200 showed a close relation (y = −1.0894 + 0.922 x; 95 % C.I. intercept = -2.48 to 0.05; 95 % C.I. slope = 0.87 to 0.98; p > 0.05) (Fig. 2A) but both proportional and constant systematic error was present. Moreover, Bland – Altman analysis revealed a mean difference ± standard deviation of 25(OH)D equal to 3.67 ± 4.53 ng/mL (95 % CI = 2.39 to 4.35) and the limits of agreement carried out were −5.25 to 12.59 ng/mL (Fig. 2B) whereas the mean bias% was 16.2 % (95 % C.I. bias% = 12.01 % to 20.35 %) (Fig. 2C) above the maximum acceptable bias so the 25(OH)D concentration measured with DiaSorin Liaison XL and Fujirebio Lumipulse G 1200 are not comparable.
Fig. 2.
25(OH)D determination by DiaSorin Liaison XL and Fujirebio Lumipulse G1200. (A) Passing – Bablok regression analysis; (B) Bland - Altman analysis of agreement on absolute values of mean differences (ng/mL) between methods; (C) Bland - Altman analysis of agreement on percentage values of mean differences (%) between methods.
3.2.3. Roche Elecsys vs Fujirebio Lumipulse G 1200
The Passing - Bablok regression between Roche Elecsys and Fujirebio Lumipulse G 1200 showed a proportional and constant systematic error even in the presence of a good relation (y = −2.7262 + 0.8224 x; 95 % C.I. intercept = −4.10 to −1.67; 95 % C.I. slope = 0.77 to 0.87; p > 0.05) (Fig. 3A). Moreover, Bland – Altman analysis revealed a mean difference ± standard deviation of 25(OH)D equal to 9.45 ± 7.20 ng/mL (95 % CI = 7.38 to 11.51) and the limits of agreement carried out were −4.80 to 23.70 ng/mL (Fig. 3B). The mean bias% was 38.2 % (95 % C.I. bias% = 32.47 % to 43.93 %) (Fig. 3C) well above the maximum acceptable bias (6.17 %) meaning that the 25(OH)D concentration measured with Roche Elecsys and Fujirebio Lumipulse G 1200 were not comparable.
Fig. 3.
25(OH)D determination by Roche Elecsys and Fujirebio Lumipulse G1200. (A) Passing - Bablok regression analysis; (B) Bland - Altman analysis of agreement on absolute values of mean differences (ng/mL) between methods; (C) Bland - Altman analysis of agreement on percentage values of mean differences (%) between methods.
3.3. Accuracy
The accuracy was evaluated by determining 25(OH)D concentration in 10 DEQAS sera. Samples 561–565 belong to the DEQAS October 2019 distribution and samples 566–570 were from the DEQAS January 2020 distribution. The calculation of the bias % between the 25(OH)D “true value” provided by DEQAS and those determined by DiaSorin Liaison XL, Roche Elecsys, and Fujirebio Lumipulse G 1200 methods was performed applying the following formula: [(25(OH)DMETHOD X - 25(OH)DDEQAS)/25(OH)DDEQAS] x 100. Next, the mean bias % was calculated for each method. In Table 2 are reported the target values and the bias obtained. DEQAS Advisor Panel considers satisfactory the performances obtained if the mean bias % is less than 25 % at least in 3 of the first 4 samples of each distribution. Since for all three methods the % of bias in at least 3 of the 4 samples of each distribution is less than 25 %, it can therefore be said that all methods have achieved the performance target set by DEQAS program.
Table 2.
Accuracy study using DEQAS samples.
| Target (ng/mL) | DiaSorin |
Elecsys |
Lumipulse |
||||
|---|---|---|---|---|---|---|---|
| 25(OH)D (ng/mL) | Bias (%) | 25(OH)D (ng/mL) | Bias (%) | 25(OH)D (ng/mL) | Bias (%) | ||
| DEQAS 561 | 26.6 | 29.2 | 9.8 | 30.0 | 12.8 | 22.1 | −16.9 |
| DEQAS 562 | 19.2 | 21.0 | 9.6 | 19.6 | 2.5 | 13.6 | −29.0 |
| DEQAS 563 | 35.5 | 34.9 | −1.7 | 41.2 | 16.0 | 31.1 | −12.7 |
| DEQAS 564 | 17.4 | 19.4 | 11.8 | 18.8 | 8.3 | 13.3 | −23.8 |
| DEQAS 565 | 30.5 | 35.1 | 15.1 | 30.8 | 0.9 | 26.4 | −13.5 |
| DEQAS 566 | 27.4 | 25.2 | −7.9 | 30.4 | 11.0 | 22.7 | −16.9 |
| DEQAS 567 | 26.8 | 25.6 | −4.6 | 30.8 | 15.0 | 23.3 | −13.1 |
| DEQAS 568 | 12.7 | 12.6 | −0.6 | 15.8 | 24.9 | 9.2 | −27.0 |
| DEQAS 569 | 33.0 | 35.0 | 5.9 | 38.4 | 16.2 | 29.8 | −9.8 |
| DEQAS 570 | 54.0 | 54.8 | 1.5 | 65.6 | 21.5 | 50.3 | −6.8 |
| Mean | 3.9 | 12.9 | −16.9 | ||||
| SD | 7.6 | 7.6 | 7.4 | ||||
DEQAS, Vitamin D External Quality Assurance Scheme, bias (%) = [(method – target)/target] x 100. Samples 561–565 belong to the DEQAS October 2019 exercise and samples 566–570 were from the DEQAS January 2020 exercise.
3.4. Clinical validation
To evaluate whether the three methods had a comparable ability in classifying patients into different categories of vitamin D levels according both to Endocrine Society's guidelines [5] and IOM study [19], we first categorized the results obtained by each method in a reference class (Table 3, Table 4).
Table 3.
Classification of patients into different categories of vitamin D levels according to Endocrine Society's Guidelines. Green denotes, in each category, the method that categorizes the least patients into that particular category, while red denotes the method which categorizes most patients into that particular category.
| 25(OH)D (ng/mL) |
DiaSorin |
Elecsys |
Lumipulse |
|---|---|---|---|
| N (%) | |||
| <10 | 12 (23.5) | 11 (21.2) | 14 (27.4) |
| 11–20 | 11 (21.5) | 6 (11.8) | 15 (29.4) |
| 21–29 | 10 (19.6) | 13 (25.5) | 7 (13.7) |
| 30–103 | 18 (35.3) | 21 (41.2) | 15 (29.4) |
n, numbers of patients for each level; %, percentage of patients for each level.
Table 4.
Classification of patients into different categories of vitamin D levels according to IOM categories. Green denotes the instrument in each category that categorizes the least patients into that particular category, while red denotes the instrument which categorizes most patients into that particular category.
| 25(OH)D (ng/mL) | DiaSorin | Elecsys | Lumipulse |
|---|---|---|---|
| N (%) | |||
| <20 | 23 (45.1) | 17 (33.3) | 29 (56.8) |
| >20 | 28 (54.9) | 34 (66.7) | 22 (43.1) |
n, numbers of patients for each level; %, percentage of patients for each level.
According to the Endocrine Society's, Fujirebio Lumipulse G 1200 method categorizes most patients into the class with the lower 25(OH)D concentration (<10 ng/mL and 11–20 ng/mL) whereas Roche Elecsys categorizes the smaller number of patients into the same class (Table 3). Conversely, Roche Elecsys categorizes most patients into the 21–29 ng/mL and 30–100 ng/mL categories and Fujirebio Lumipulse G 1200 categorizes the smaller number of patients into the same class.
According to IOM, Roche Elecsys categorizes the lower number of patients into <20 ng/mL and the higher in >20 ng/mL class and Fujirebio Lumipulse G 1200 vice versa (Table 4).
Finally, we assessed the strength of the agreement between the results obtained with the different methods by calculating Cohen's κ index, a concordance index that considers the probability that the concordance is random (Table 5).
Table 5.
κ values for the strength of agreement between assays according to Endocrine Society's Guidelines. κ < 0.20 (poor), 0.21–0.4 (fair), 0.41–0.60 (moderate), 0.61–0.80 (good), 0.81–1.00 (very good). Figures in bold represent very good agreement.
| 25(OH)D | DiaSorin vs Elecsys |
DiaSorin vs Lumipulse |
Elecsys vs Lumipulse |
|---|---|---|---|
| Κ (% agreement) | |||
| <10 ng/mL | 0.94 (98.1) | 0.90 (96.2) | 0.84 (94.3) |
| 11–20 ng/mL | 0.66 (90.5) | 0.79 (92.4) | 0.49 (83.0) |
| 21–29 ng/mL | 0.83 (94.4) | 0.79 (94.3) | 0.64 (88.7) |
| 30–100 ng/mL | 0.88 (94.3) | 0.87 (94.3) | 0.77 (88.7) |
κ, Cohen index.
When the Endocrine Society's reference values were used, the strength of the agreement between DiaSorin Liaison XL and Roche Elecsys was very good for each class, except for 11–20 ng/mL category. DiaSorin Liaison XL and Fujirebio Lumipulse G 1200 showed a very good agreement only in the lower and the higher 25(OH)D concentration classes, Roche Elecsys and Fujirebio Lumipulse G 1200 only in <10 ng/mL category (Table 5).
Conversely, when the IOM reference values were used, the strength of the agreement between DiaSorin Liaison XL and Roche Elecsys and DiaSorin Liaison XL and Fujirebio Lumipulse G 1200 were good whereas between Roche Elecsys and Fujirebio Lumipulse G 1200 was moderate (Table 6).
Table 6.
κ values for the strength of agreement between assays according to IOM categories. κ < 0.20 (poor), 0.21–0.4 (fair), 0.41–0.60 (moderate), 0.61–0.80 (good), 0.81–1.00 (very good).
| 25(OH)D |
DiaSorin vs Elecsys |
DiaSorin vs Lumipulse |
Elecsys vs Lumipulse |
|---|---|---|---|
| Κ (% agreement) | |||
| >20 ng/mL | 0.78 (88.7) | 0.76 (88.7) | 0.56 (77.3) |
| <20 ng/mL | 0.78 (88.7) | 0.76 (88.7) | 0.56 (77.3) |
κ, Cohen index.
4. Discussions
The presence of some DEQAS samples with % of bias >25 % (the cut-off proposed by DEQAS program for accuracy) makes fair the Fujirebio Lumipulse G 1200 test performance, but the other two methods show good performance fullfilling the criteria of DEQAS external quality control scheme “accuracy based”. This could be explained with the presence in DEQAS samples of metabolites of vitamin D as 25(OH)D2, 3-epi-25-hydroxyvitamin D and 24,25-dihydroxyvitamin D that could influence 25(OH)D determination [25]. Despite the fact that the three tests are accurate, they are not interchangeable as demonstrated by the lack of comparability between the results obtained by the single method.
A possible explanation could be that the three analytical procedures measure 25(OH)D concentration with different mechanisms. The Roche Elecsys and Liaison methods are competitive immunoassays while the Lumipulse test is non-competitive. The Elecsys test is an electrochemiluminescent binding assay in which the labelled 25(OH)D competes with the native for binding to a recombinant VDBP; the DiaSorin method is a chemiluminescent assay in which the labelled 25(OH)D competes with the sample's 25(OH)D for antibody binding. Finally, the Lumipulse method, in turn, is a non-competitive chemiluminescence assay in which the sandwich is formed between a capture antibody for 25(OH)D and a second labelled monoclonal antibody directed against the immunocomplex 25(OH)D - first antibody.
These are therefore different methods which overlap only partially even if are traceable to the same standard (Standard Reference Material (SRM) 972, National Institute of Standard and Tecnology (NIST)). The standardization effort carried out by the Vitamin D Standardization Program should have overcome the differences in the design of the analytical procedures [11,12]. As several authors have pointed out, however, we are still far from a complete standardization [10,14,15].
The need to maintain the serum 25(OH)D concentrations at acceptable levels is recognized by the scientific community as a public health emergency.
A complex discussion is underway, aimed at examining the available data on the methods used in determining the concentration of vitamin D and identifying the persistent problems associated with them [27].
It is now universally accepted that, in the general population without problems of chronic kidney disease, the measurement of 25(OH)D is the best parameter to evaluate the reserves of the active form of the hormone 1,25 OH vitamin D (1,25(OH)2D) [5,6]. The 1,25(OH)2D, with other factors including Parathormone (PTH) and Fibroblast Growth Factor 23 (FGF 23), plays a key role in controlling bone metabolism in the human body while maintaining the homeostasis of calcium and phosphorus. Therefore, vitamin D directly influences the mineral density of bone, and its determination can provide important indications on the risk of falls and fractures [[28], [29], [30], [31]]. Several studies have confirmed the diagnostic importance of vitamin D concentration in diseases affecting bone metabolism. In recent years, numerous experimental and clinical studies have evaluated, with mixed results, the influence of vitamin D deficiency in disorders such as diabetes and cardiovascular, oncological and autoimmune diseases [[32], [33], [34], [35], [36]]. Furthermore, the role of 25 (OH)D in viral infection has recently been clarified [37] and the use of vitamin D as a possible adjuvant therapy to fight infections such as SARS Covid-2 has been proposed [[38], [39], [40]]. In fact, there are studies showing that 25(OH)D counteracts viral replication of rotavirus both in vitro and in vivo [41] and that supplementation with 4000 IU/day of vitamin D reduces dengue virus infection [42]. On the other hand, it has been shown that 25(OH)D deficiency correlates with increased levels of IL-6, a marker of inflammation infection associated with dysregulated production of hematopoietic cells in patients with HIV [43].
Taken together, these findings have led to an exponential increase in vitamin D determinations. Efforts in the future to improve the standardization policy of the immunoassays will be required. If successful, it will allow us to use the same values of cut off and to recognize with the same effectiveness the subjects deficient and those with sufficient 25(OH)D.
5. Conclusions
Two out of three compared immunoassays have shown good accuracy and therefore can be considered as reliable in measuring the 25(OH)D in the routine clinical laboratory. Nevertheless, we showed that the assays are not interchangeable due the lack of comparability of the results. Moreover, these three immunological tests do not agree in classifying patients with either the method proposed by the Endocrine Society or the IOM.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Credit authorship contribution statement
FT: Methodology, Software, Validation and Writing - Review & Editing. GC: Supervision, Review & Editing. EA: Conceptualization, Methodology, Software, Writing - Original Draft and Supervision. SR: Data Curation. SR, SA, SM, NM: Formal analysis. LF, MG: Conceptualization and Resources. GCA, AC, MG, FG, LGC, DI, SM, MM, CT: Investigation.
Acknowledgments
We thank Prof. Francesco S. Costanzo and Prof. Daniela P. Foti for their valuable advice and encouragement.
Contributor Information
Francesca Trimboli, Email: trimboli@unicz.it.
Giovanni Cuda, Email: cuda@unicz.it.
References
- 1.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Targher G., Pichiri I., Lippi G. Vitamin D, thrombosis, and hemostasis: more than skin deep. Semin. Thromb. Hemost. 2012;38:114–124. doi: 10.1055/s-0031-1300957. [DOI] [PubMed] [Google Scholar]
- 3.Bjelakovic G., Gluud L.L., Nikolova D., Whitfield K., Wetterslev J., Simonetti R.G., Bjelakovic M., Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev. 2014:CD007470. doi: 10.1002/14651858.CD007470.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pludowski P., Holick M.F., Pilz S., Wagner C.L., Hollis B.W., Grant W.B., Shoenfeld Y., Lerchbaum E., Llewellyn D.J., Kienreich K., Soni M. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—a review of recent evidence. Autoimmun. Rev. 2013;12:976–989. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J.Clin. Endocrinol. Metabol. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 6.Heureux N. 2017. Vitamin D Testing—Where Are We and what Is on the Horizon? pp. 59–101. [DOI] [PubMed] [Google Scholar]
- 7.Amrein K., Scherkl M., Hoffmann M., Neuwersch-Sommeregger S., Köstenberger M., Tmava Berisha A., Martucci G., Pilz S., Malle O. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur. J. Clin. Nutr. 2020;74:1498–1513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiljer J., Friedel R., Herr T., Rausch F., Roos D.A., Wahl Denys A., Dominique D Pierroz, Weber Peter, Hoffmann Kristina. A systematic review of the vitamin D status worldwide. Br. J. Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 9.le Goff C., Cavalier E., Souberbielle J.-C., González-Antuña A., Delvin E. Measurement of circulating 25-hydroxyvitamin D: a historical review. Prac. Lab. Med. 2015;2:1–14. doi: 10.1016/j.plabm.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann M., Farrell C.-J.L., Pusceddu I., Fabregat-Cabello N., Cavalier E. Assessment of vitamin D status – a changing landscape. Clin. Chem. Lab. Med. 2017;55:3–26. doi: 10.1515/cclm-2016-0264. [DOI] [PubMed] [Google Scholar]
- 11.Sempos C.T., Vesper H.W., Phinney K.W., Thienpont L.M., Coates P.M. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand. J. Clin. Lab. Investig. 2012;243:32–40. doi: 10.3109/00365513.2012.681935. [DOI] [PubMed] [Google Scholar]
- 12.Binkley N., Sempos C.T. Standardizing vitamin D assays: the way forward. J. Bone Miner. Res. 2014;29:1709–1714. doi: 10.1002/jbmr.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laboratory Quality Assurance and Standardization Programs, VDSCP List of Certified Participants | CDC, (n.d.). https://www.cdc.gov/labstandards/vdscp.html (accessed January 29, 2021).
- 14.Wise S.A., Phinney K.W., Tai S.S.-C., Camara J.E., Myers G.L., Durazo-Arvizu R., Tian L., Hoofnagle A.N., Bachmann L.M., Young I.S., Pettit J., Caldwell G., Liu A., Brooks S.P.J., Sarafin K., Thamm M., Mensink G.B.M., Busch M., Rabenberg M., Cashman K.D., Kiely M., Kinsella M., Galvin K., Zhang J.Y., Oh K., Lee S.-W., Jung C.L., Cox L., Goldberg G., Guberg K., Prentice A., Carter G.D., Jones J., Brannon P.M., Lucas R.M., Crump P.M., Cavalier E., Merkel J., Betz J.M., Sempos C.T. Baseline assessment of 25-hydroxyvitamin D assay performance: a vitamin D standardization program (VDSP) interlaboratory comparison study. J. AOAC Int. 2017;100:1244–1252. doi: 10.5740/jaoacint.17-0258. [DOI] [PubMed] [Google Scholar]
- 15.Altieri B., Cavalier E., Bhattoa H.P., Pérez-López F.R., López-Baena M.T., Pérez-Roncero G.R., Chedraui P., Annweiler C., della Casa S., Zelzer S., Herrmann M., Faggiano A., Colao A., Holick M.F. Vitamin D testing: advantages and limits of the current assays. Eur. J. Clin. Nutr. 2020;74:231–247. doi: 10.1038/s41430-019-0553-3. [DOI] [PubMed] [Google Scholar]
- 16.Lippi G., Salvagno G.L., Fortunato A., Dipalo M., Aloe R., da Rin G., Giavarina D. Multicenter comparison of seven 25Oh vitamin D automated immunoassays/multicentrično poređenje sedam automatizovanih imunoeseja za 25Oh vitamin D. J. Med. Biochem. 2015;34:344–350. doi: 10.2478/jomb-2014-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavalier E., Lukas P., Crine Y., Peeters S., Carlisi A., le Goff C., Gadisseur R., Delanaye P., Souberbielle J.-C. Evaluation of automated immunoassays for 25(OH)-vitamin D determination in different critical populations before and after standardization of the assays. Clin. Chim. Acta. 2014;431:60–65. doi: 10.1016/j.cca.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Bjerg L.N., Halgreen J.R., Hansen S.H., Morris H.A., Jørgensen N.R. An evaluation of total 25-hydroxyvitamin D assay standardization: where are we today? J. Steroid Biochem. Mol. Biol. 2019;190:224–233. doi: 10.1016/j.jsbmb.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., Kovacs C.S., Mayne S.T., Rosen C.J., Shapses S.A. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of medicine: what clinicians need to know. J.Clin. Endocrinol. Metabol. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidali M., Tronchin M., Dittadi R. SIBioC Documents, Premessa Protocollo per la comparazione di due metodi analitici di laboratorio. Biochim. Clin. 2016;40:129–142. [Google Scholar]
- 21.Clinical and Laboratory Standard Institute (CLSI) third ed. 2014. EP15 User Verification of Precision and Estimation of Bias; pp. 1–106. [Google Scholar]
- 22.Vidali M., Padoan A., Dittadi R., Brugnoni D., Carobene A., Mattioli S., Sciacovelli L., Cerriotti F. Protocol to verify the comparability of quantitative laboratory results obtained with different measurement procedures. Biochim. Clin. 2019;43:228–243. [Google Scholar]
- 23.J. Westgard, What's the TEa for D? Allowable Error for vitamin D, (n.d.). https://www.westgard.com/vitamin-d-allowable-error.htm (accessed February 2, 2021).
- 24.Carter G.D., Berry J., Durazo-Arvizu R., Gunter E., Jones G., Jones J., Makin H.L.J., Pattni P., Sempos C.T., Twomey P., Williams E.L., Wise S.A. Hydroxyvitamin D assays: an historical perspective from DEQAS. J. Steroid Biochem. Mol. Biol. 2018;177:30–35. doi: 10.1016/j.jsbmb.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 25.DEQAS participant portal, (n.d.). www.deqas.org (accessed January 21, 2021).
- 26.Bottarelli E., Ostanello F. 2011th ed. 2011. Epidemiologia-teoria ed esempi di medicina veterinaria. Bologna. [Google Scholar]
- 27.Giustina A., Adler R.A., Binkley N., Bollerslev J., Bouillon R., Dawson-Hughes B., Ebeling P.R., Feldman D., Formenti A.M., Lazaretti-Castro M., Marcocci C., Rizzoli R., Sempos C.T., Bilezikian J.P. Consensus statement from 2nd international conference on controversies in vitamin D. Rev. Endocr. Metab. Disord. 2020;21:89–116. doi: 10.1007/s11154-019-09532-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brent G.A., Leboff M.S., Seely E.W., Conlin P.R., Brown E.M. Relationship between the concentration and rate of change of calcium and serum intact parathyroid hormone levels in normal humans*. J.Clin. Endocrinol. Metabol. 1988;67:944–950. doi: 10.1210/jcem-67-5-944. [DOI] [PubMed] [Google Scholar]
- 29.Turner A., Morris H., Anderson P. Vitamin D and bone health. Scand J Clin Lab Invest Supl. 2012;243:65–72. doi: 10.3109/00365513.2012.681963. [DOI] [PubMed] [Google Scholar]
- 30.Vervloet M. Renal and extrarenal effects of fibroblast growth factor 23. Nat. Rev. Nephrol. 2019;15:109–120. doi: 10.1038/s41581-018-0087-2. [DOI] [PubMed] [Google Scholar]
- 31.Quarles L.D. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sciacqua A., Perticone M., Grillo N., Falbo T., Bencardino G., Angotti E., Arturi F., Parlato G., Sesti G., Perticone F. Vitamin D and 1-hour post-load plasma glucose in hypertensive patients. Cardiovasc. Diabetol. 2014;13:48–56. doi: 10.1186/1475-2840-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Zhou S., Lei S., Hao L., Sun D., Hu H. Vitamin D deficiency and cardiovascular diseases. Sci. Adv. Mater. 2020;12:27–37. doi: 10.1166/sam.2020.3715. [DOI] [Google Scholar]
- 34.Zhang Y., Fang F., Tang J., Jia L., Feng Y., Xu P., Faramand A. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366 doi: 10.1136/bmj.l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouillon R., Marcocci C., Carmeliet G., Bikle D., White J.H., Dawson-Hughes B., Lips P., Munns C.F., Lazaretti-Castro M., Giustina A., Bilezikian J. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr. Rev. 2019;40:1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manson J.E., Cook N.R., Lee I.-M., Christen W., Bassuk S.S., Mora S., Gibson H., Gordon D., Copeland T., D'Agostino D., Friedenberg G., Ridge C., Bubes V., Giovannucci E.L., Willett W.C., Buring J.E. Vitamin D Supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teymoori-Rad M., Shokri F., Salimi V., Marashi S.M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019;29 doi: 10.1002/rmv.2032. [DOI] [PubMed] [Google Scholar]
- 38.Kakodkar P., Kaka N., Baig M. Cureus; 2020. A Comprehensive Literature Review on the Clinical Presentation, and Management of the Pandemic Coronavirus Disease 2019 (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12 doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakovac H. COVID-19 and vitamin D—is there a link and an opportunity for intervention? Am. J. Physiol. Endocrinol. Metab. 2020;318 doi: 10.1152/ajpendo.00138.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y., Ran Z., Jiang Q., Hu N., Yu B., Zhu L., Shen L., Zhang S., Chen L., Chen H., Jiang J., Chen D. Vitamin D alleviates rotavirus infection through a microrna-155-5p mediated regulation of the TBK1/IRF3 signaling pathway in vivo and in vitro. Int. J. Mol. Sci. 2019;20:3562. doi: 10.3390/ijms20143562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martínez-Moreno J., Hernandez J.C., Urcuqui-Inchima S. Effect of high doses of vitamin D supplementation on dengue virus replication, Toll-like receptor expression, and cytokine profiles on dendritic cells. Mol. Cell. Biochem. 2020;464:169–180. doi: 10.1007/s11010-019-03658-w. [DOI] [PubMed] [Google Scholar]
- 43.Manion M., Hullsiek K.H., Wilson E.M.P., Rhame F., Kojic E., Gibson D., Hammer J., Patel P., Brooks J.T., Baker J.v., Sereti I. Vitamin D deficiency is associated with IL-6 levels and monocyte activation in HIV-infected persons. PloS One. 2017;12 doi: 10.1371/journal.pone.0175517. e0175517-undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]