Highlights
-
•
Left ventricular global longitudinal strain (LV GLS) is a sensitive parameter that correlates with myocardial scar burden and fibrosis with potential value in CRT candidates.
-
•
First systematic review evaluating the existing evidence for the prognostic value of LV GLS in patients undergoing CRT implantation.
-
•
Despite significantly abnormal baseline GLS at CRT implantation, there is still a significant association between incrementally worse LV GLS at CRT implantation and prognostic outcomes on long-term follow-up.
Keywords: Global longitudinal strain, Cardiac resynchronisation therapy, Speckle tracking echocardiography
Abstract
Purpose
Cardiac resynchronisation therapy (CRT) has proven mortality benefits for heart failure patients with moderate to severe systolic left ventricular dysfunction and evidence of a left bundle branch block. Determining responders to this therapy can be difficult due to the presence of myocardial fibrosis and scar. Left ventricular global longitudinal strain (LV GLS) is a robust and sensitive measure of myocardial function and fibrosis that has significant prognostic value for a plethora of cardiac pathologies. Our aim was to perform a systematic review of the value of LV GLS for predicting outcomes in patients undergoing CRT.
Methods
A systematic review of the literature was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) protocol for reporting on systematic reviews and meta-analyses. An electronic search of all English, adult publications in EMBASE, MEDLINE/PubMed and the Cochrane Database of Systematic reviews was undertaken.
Results
The search yielded, 9 studies that included 3,981 patients with symptomatic heart failure, undergoing CRT implantation with LV GLS utilised as a predictor of all-cause mortality, cardiovascular death, rehospitalisation, LVAD implantation/ heart transplantation or left ventricular reverse remodelling. Significant heterogeneity was observed in study outcome measures, included populations, LV-GLS cut-offs and follow-up definitions, resulting in the inability to reliably conduct a meta-analyses. Overall, pre-CRT LV GLS was found to be a predictor of outcome post CRT insertion.
Conclusions
In conclusion, all studies implied that incrementally abnormal baseline LV GLS pre-CRT implantation was associated with a long term poorer outcome.
1. Introduction
Cardiac resynchronisation therapy (CRT) is an important adjunct to optimal medical therapy (OMT) for reducing morbidity and mortality in symptomatic heart failure patients with significant conduction delay [1], [2]. A complex milieu of electromechanical factors including myocardial substrate composition, CRT lead positioning, medical therapy and underlying patient characteristics are known to modulate the level of response to CRT [3]. Importantly, only two thirds of patients meeting guideline criteria for CRT implantation garner optimal benefits from this device therapy [4]. Furthermore, myocardial fibrosis and scar burden are principal factors that adversely affect the response from CRT at a substrate level [5], [6]. Cardiac MRI is the reference standard for detecting and quantifying myocardial fibrosis and scar [5]. However, this modality may not be technically feasible in a significant proportion of patients due to pre-existing device implantation, poor renal function and limited access to this investigation in many healthcare systems.
Speckle-tracking echocardiography utilising left ventricular global longitudinal strain (LV GLS) is a robust, cheaper, safer and more accessible non-invasive imaging modality that is not limited by pre-existing devices or renal function. Furthermore, there is a direct correlation between LV GLS and the presence and extent of myocardial scar and fibrosis [7], [8]. The supporting evidence for LV GLS as a prognostic predictor of outcome in varying cardiomyopathic and ischemic aetiologies is well established [9], [10], [11]. Its role in predicting the long-term prognostic outcomes in appropriate patients undergoing CRT implantation is not as well defined. The objective of this systematic review was to therefore consolidate the existing evidence for LV GLS as a prognostic marker in heart failure patients undergoing CRT implantation, independent of traditional indicators of response, through a systematic analysis of the existing literature.
2. Methods
2.1. Search strategy
We conducted a literature search with adherence to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) protocol for reporting on systematic reviews and meta-analyses. Three authors (VA, TM and ST) developed robust search terms to encompass all iterations of cardiac resynchronisation therapy and global longitudinal strain. Multiple broad search hedges were created including the terms “cardiac resynchronisation therapy”, “biventricular pacing”, “global longitudinal strain”, “speckle tracking echocardiography” and “myocardial deformation”. These were applied to EMBASE, MEDLINE/PubMed and the Cochrane Database of Systematic reviews. The search period was designated between January 2000 and December 2020 as 2-dimensional LV GLS was not being utilised prior to this period for prognostic purposes. Searches were limited to humans, patients above the age of 18 years, English language articles and full text articles.
2.2. Eligibility criteria
We assessed the suitability of articles along the guidance of the PRISMA protocol. Three authors (VA, TM and ST) independently assessed the initial search results via title and abstract. Selected articles were reviewed in full and reference lists were analysed for relevant articles that may not have been included in the initial search. The inclusion criteria developed by the authors and upon which studies were included were as follows: (1) heart failure patients with indications for cardiac resynchronisation therapy; (2) global longitudinal strain, applied peri-CRT insertion and utilised as a marker to predict outcome; (3) all-cause mortality, cardiovascular death, rehospitalisation, LVAD implantation/heart transplantation or left ventricular reverse remodelling as outcome measures; (4) available baseline characteristics for included participants.
2.3. Data extraction and reporting of outcomes
Data from selected articles were independently extracted by three investigators (VA, TM and ST). Data analysis were performed by two investigators (VA and ND) primarily and then processes and results were further reviewed by three investigators (TM, GS and JC). For the systematic review portion, study design, population inclusion criteria, demographic characteristics, echocardiographic results, methodology and study outcomes were recorded. Bias assessment of studies included in the final analysis were performed as per the Prediction model Risk Of Bias ASsessment Tool - PROBAST protocol by two authors (VA and TM) [12]. LV GLS values throughout the results were reported with a negative sign to maintain consistency across the studies analysed. Improvement in LV GLS or “better” LV GLS is indicative of a more negative LV GLS and decline or “worse” LV GLS is indicative of a more positive shift in the absolute LV GLS. Continuous variables were reported with up to 1 decimal place. Means were reported with standard deviations and medians reported with interquartile ranges. There was significant heterogeneity between study design, definition and reporting of LV GLS cut-offs and primary endpoints which resulted in the study investigators deciding against proceeding to a meta-analysis of outcome measures.
3. Results
3.1. Study selection
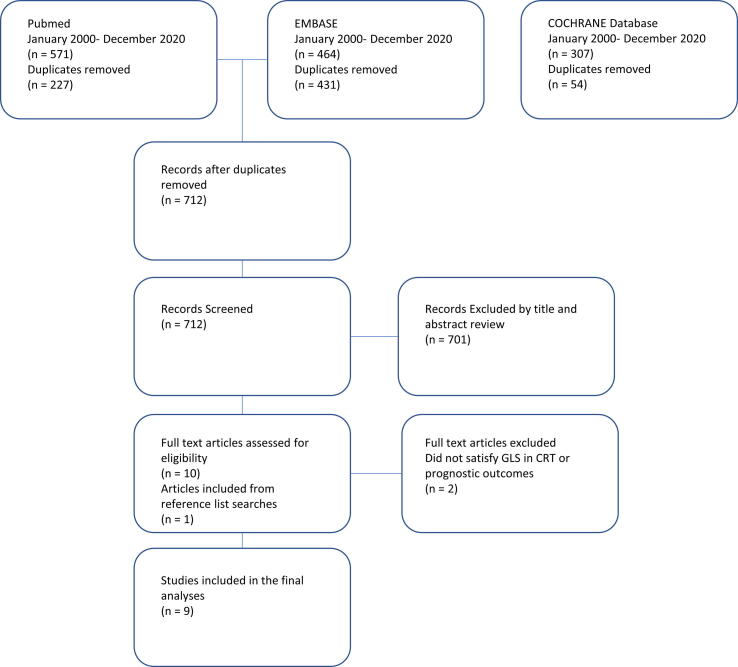
The literature search yielded 1,342 results, after removal of duplicates there were 712 articles. Articles unrelated to the topic, review articles, abstracts without full texts and articles with no prognostic outcomes were excluded (701 articles), leaving 10 studies suitable for full text review. There was a further 1 study identified through review of selected studies reference lists. Of these 11 studies, 9 studies met the predefined inclusion criteria for the systematic review, including 3,981 patients with symptomatic heart failure, undergoing CRT implantation, with complete results for GLS and the primary inclusion endpoint (see Fig. 1) [13], [14], [15], [16], [17], [18], [19], [20], [21]. The remaining 2 studies were excluded due to no clear data on the prespecified primary combined outcome.
Fig. 1.
PRISMA flow diagram of study search and selection outcomes.
The following analysis of results of the included studies will be divided into: (1) overall design of studies and methodology of strain assessment, (2) baseline patient characteristics, (3) definitions of outcome measures, (4) prognostic outcomes reported by studies.
-
(1)
Overall design of studies:
There was variation between the design of included studies, with 2 being randomised controlled trials [15], [18], 3 prospective cohort studies [14], [16], [19] and 4 retrospective cohort studies [13], [17], [20], [21] of which 2 studies shared similar patient populations from the same site [13], [21]. Table 1 provides a complete description of the included study designs. The size of the patient populations varied significantly between 57 and 1185 individuals [17], [21] with an overall analysed population of 3,981 patients. The follow-up duration was reported as a mean in 4 studies varying between 19.4 and 28.8 months [14], [15], [18], [20] and as median values in 5 studies varying between 32 and 66 (IQR 25–88.8) months [13], [16], [17], [19], [21]. 6 studies were single site recruitment [13], [16], [17], [19], [20], [21], 1 study was a national multi-centre recruitment [14] and the remaining 2 studies were international multi-centre recruitment [15], [18]. Inclusion criteria across the studies was relatively homogenous, in terms of adhering to guideline-based recommendations for CRT implantation [4], with heart failure patients in NYHA class II-IV status, QRS duration ≥120 ms, an LVEF ≤35% and being on optimal medical therapy [13], [14], [16], [17], [18], [19], [20], [21]. One of the randomised controlled trials inclusion criteria was the same as the aforementioned values except it recruited patients with a QRS duration <130 ms as part of its study design [15].
Table 1.
Summary of included studies: CRT – Cardiac Resynchronisation Therapy; OMT – optimal medical therapy; LVAD – Left ventricular assist device; NYHA – New York Heart Association; LVESV – Left ventricular end-systolic volume; GE – General electric.
| Study | Design | Sample Size | Single centre vs multi-centre | Inclusion criteria | Exclusion criteria | Objective | Primary endpoint | Secondary endpoint | Follow-up Duration (months) | Echocardiography Machines |
|---|---|---|---|---|---|---|---|---|---|---|
| Khidir et al. (2018) [13] | Retrospective Cohort Study | N = 829 | Single centre registry recruitment | Heart failure patients managed with CRT | Pacemaker upgrade to CRT, history of LV reconstruction, heart transplantation, atrial fibrillation, congenital heart disease, inflammatory or infiltrative heart disease. | Evaluate the prognostic value of LV GLS in HF patients managed with CRT | Combination of all-cause mortality, heart transplantation and LVAD implantation | Occurrence of ventricular arrhythmia or appropriate ICD therapy. | Median: 66 (IQR 36–88.8) | GE Vivid 5/7, E9 |
| Bax et al. (2017) [15] | Randomised control trial | N = 755 (in total study) N = 374 (CRT-ON) |
International multi-centre recruitment | >18 year old with heart failure symptoms NYHA III-IV, on OMT, LVEF ≤ 35%, QRS < 130 ms, LVEDD ≥ 55 mm, echo evidence of LV dyssynchrony and indication for ICD. | Acute decompensated heart failure (hemodynamically unstable or need for inotropic support), atrial fibrillation within the previous month or bradycardia requiring pacing | Investigate the prognostic value of LVGLS in patients with a narrow QRS complex recruited into the Echo-CRT trial | All-cause mortality and heart failure hospitalisation. | Ventricular arrhythmias defined as appropriate ICD therapy, arrhythmic death and atrial tachyarrhythmias | Mean: 19.4 | GE Vivid 7, E9 |
| Hasselberg et al. (2016) [14] | Prospective cohort study | N = 170 | Multi-centre site recruitment | Heart failure patients with NYHA II-IV heart failure, OMT, QRS width ≥ 120 ms and LVEF ≤ 35% | Patients with < 90% biventricular pacing | Investigate echocardiographic predictors of ventricular arrhythmias and fatal outcome in heart failure patients with BiV pacing. | Composite of all cause death, heart transplantation, and LVAD implantation. | Arrhythmic end point – first sustained ventricular arrhythmic event following CRT placement – VF, anti-tach pacing, Defib therapy, SCA | Mean: 24 (±1 month) | GE Vivid 7, E9 |
| Delgado-Montero et al. (2016) [16] | Prospective cohort study | N = 205 | Single centre recruitment | Heart failure patients with NYHA II-IV heart failure, OMT, QRS width ≥ 120 ms and LVEF ≤ 35% | Chronic RV pacing or failed CRT implant. Atrial fibrillation | Determine prognostic value of baseline GLS/GCS to long-term clinical outcomes after CRT; prognostic value of GLS/GCS in ICM and NICM; determine additive prognostic value of GLS/GCS in intermediate ECG criteria for CRT | Composite endpoint of death, LVAD implant, heart transplantation. | First heart failure hospitalisation or death during follow-up | Median: 47 | GE Vivid 7 |
| Van Der Bijl et al. (2019) [21] | Retrospective cohort study | N = 1185 | Single centre registry recruitment | Heart failure patients who received CRT implantations based on ESC guideline recommendations. | If no 6 month follow-up echocardiogram after CRT implantation. | Investigate LVESV and LV GLS changes and prognostic implications of improvement in LVESV and LVGLS compared to no improvement in either. | All-cause mortality. | NR | Median: 53 (25–80) | GE Vivid 7 or E9 |
| Knappe et al. (2011) [18] | Randomised controlled trial | N = 661 of 1077 | International multi-centre recruitment | MADIT-CRT enrolled patients with IHD & NYHA class I-II or non-IHD patients with NYHA Class II: QRS ≥ 130 ms and LVEF ≤ 30%; Divided into ICD only vs CRT-D. | MI within last 90 days, implanted PPM, Implanted ICD/CRT device, NYHA class III or IV in past 90 days, reversible non-ischemic cardiomyopathy, chronic AF, any concurrent disease that would reduce survival duration, pregnancy, 2nd or 3rd degree AV block, significant coronary artery disease requiring revascularisation or revascularisation in last 90 days. Insufficient image quality or obtained imaging windows, frame rate < 30 Hz. | Identify those would benefit from to CRT through strain-based assessments of LV dyssynchrony and contractile function | All-cause death or non-fatal heart failure events | NR | Mean: 28.8 | NR (110 hospital sites) |
| Menet et al. (2016) [19] | Prospective cohort study | N = 170 | Single centre recruitment | Heart failure patients: LVEF ≤ 35%, NYHA II-IV despite OMT, QRS duration > 120 ms in LBBB or > 150 ms if no-LBBB. |
Myocardial infarction, acute coronary syndrome, or coronary revascularization during the previous 3 months; primary mitral or aortic valvular disease; uncontrolled rapid atrial fibrillation; poor echocardiographic windows | Evaluate value of changes in LV reverse remodelling (LVESV) vs LV performance improvement (LVEF or LVGLS) in predicting long-term outcome in patients undergoing CRT implantation | All-cause mortality and/or congestive heart failure hospitalisation. | NR | Median: 32 | GE Vivid E9 |
| Kalogeropoulos et al. (2011) [17] | Retrospective cohort study | N = 57 | Single centre recruitment | Heart failure patients meeting ESC guidelines for CRT-D implantation in 2004 | Patients participating in RCTs or had congenital heart disease. | Assess long term LV response to CRT with strain-based echocardiography | Death, LVAD or urgent heart transplant | All cause and heart failure readmissions | Median: 42 (27–48) | NR |
| Park et al. (2013) [20] | Retrospective cohort study | N = 330 | Single centre recruitment | Heart failure patients: LVEF ≤ 35%, ≥NYHA II despite OMT, QRS duration ≥ 120 ms. |
Patients excluded if no longitudinal follow-up echo, Insufficient echo image quality, patients outside review period having previously implanted CRT replaced or battery changed. | Develop a multiparametric echocardiographic score for predicting CRT response | Composite of death from any cause, heart transplantation, LVAD or heart failure hospitalisation. | LV reverse remodelling defined as a ≥ 15% reduction in LVESV | Mean: 57 (22) | NR |
Echocardiographic hardware utilised within the studies was predominantly GE Vivid 7 or E9s, Horton, Norway [19], [21], [13], [14], [15], [16], however, one study covered multiple sites internationally and there was no information regarding the ultrasound platforms used [18] while the remaining other two studies made no mention to echocardiography hardware [17], [20]. For the studies that employed GE hardware, vendor specific software in the form of EchoPAC (GE Healthcare, Horten, Norway) software with versions varying from BT111 to BT113 were used [19], [21], [13], [14], [15], [16] for speckle tracking analysis. There were 2 studies [17], [18], that presumably used multiple ultrasound platforms, which performed their speckle tracking analysis on a vendor independent software by TomTec Imaging Systems (Unterschleissheim, Germany) with no versions specified and the final study [20] utilised Velocity Vector Imaging (Axius, Siemens Medical Solutions, Mountain View, CA).
Left ventricular GLS was measured from standard apical 2-chamber, 4-chamber and apical long axis windows in 8 of the studies [13], [14], [15], [16], [17], [19], [20], [21] over 3 cardiac cycles and with one study performing strain only on the apical 4 chamber and 2 chamber windows [18] over one cardiac cycle on TomTec Imaging Systems (Unterschleissheim, Germany). There were 7 studies reported on GLS interobserver correlation coefficients [13], [14], [15], [16], [19], [20], [21], while one utilised coefficient of variation for this assessment [18] and one study did not report on this at all [17].
Several studies incorporated other forms of speckle tracking assessment including global circumferential strain [14], [16], [17], and mechanical dispersion [18], along with GLS in evaluating predictors of outcome. Cardiac remodelling as defined by a reduction in LVESV was also reported in outcomes in several studies [19], [20], [21]. There was minimal bias amongst studies as assessed by the PROBAST tool (see supplementary table 1).
-
(2)
Patient characteristics:
Clinical characteristics:
Complete details of patient characteristics can be found in Table 2, Table 3. The age of patients ranged from 52 (±15) years to 70 (±11) years [17], [19] with between 67.1 and 76% of the populations being male [14], [20]. Only 3 studies [14], [18], [19] reported on heart rate with the range of patients having a history of AF being 0% [13], [15], [16], [17] to 19% [19] of the studied populations. The range of patients included in studies with an ischemic aetiology to their cardiomyopathy was between 26.3 and 60% [13], [17] with only one study not mentioning this characteristic [16]. All patients within all the studies were heart failure patients with ranges of NYHA classes completely reported in 6 of the studies [13], [15], [17], [19], [20], [21], incompletely in 2 of the studies [16], [18] and a median value reported in one study [14]. In the studies that included a reported breakdown of NYHA class, NYHA class ≥ III comprised between 50 and 97% of the study populations [15], [19]. QRS duration was reported in 8 studies [13], [14], [15], [16], [18], [19], [20], [21] and ranged between 149 (±30)ms to 165 (±22)ms [13], [14]. For a complete summary of medication usage reported amongst included patients refer to Table 3. Briefly, 7 studies [20], [21], [13], [14], [15], [16], [17] reported on beta-blocker usage ranging from 70.3% to 92%, ACE-I or ARB usage ranged between 80.7% and 94% and 6 studies [17], [20], [21], [13], [14], [15] reported on MRA usage ranging between 29.9% and 56% of studied patients.
Echocardiographic characteristics:
Table 2.
Summary of baseline demographics NR – Not reported; SD – Standard Deviation; CM- Cardiomyopathy; HTN – Hypertension; BMI – Body Mass Index; AF- Atrial Fibrillation; HR – Heart Rate; IHD – Ischemic Heart Disease; HF- Heart failure; CKD – Chronic Kidney Disease; eGFR – estimated glomerular filtration rate; NYHA – New York Heart Association; MWT – Metre Walk Test; BB- Beta-blocker; ARB – Angiotensin receptor Blocker; ACE-I – Angiotensin Converting Enzyme-Inhibitor; CCB – Calcium Channel Blocker; MRA- Mineralocorticoid receptor antagonist.
| Study | Age (SD) | Male (%) | HR (SD) bpm | BMI (SD) kg/m2 | AF (%) | HTN (%) | Ischemic CM (%) | HF (%) | CKD (%) | eGFR (SD) mL/min/1.73 m2 | NYHA (%) | QRS duration (SD) ms | 6MWT (SD) metres |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Khidir et al. (2018) [13] | 64.6 (10.4) | 600 (72) | NR | 26.7 (4.5) | Excluded | NR | 495 (60) | 829 (100) | NR | 69 (25) | I-II: 270 (31) III-IV: 559 (69) |
149 (30) | 323 (117) |
| Bax et al. (2017) [15] | 58.5 (12.5) | 547 (72) | NR | NR | Excluded | 496 (66.3) | 413 (54.8) | 755 (100) | 103 (13.8) | NR | I-II: 23 (3) III-IV: 732 (97) |
105.7 (12.7) | NR |
| Hasselberg et al. (2016) [14] | 66 (10) | 130 (76) | 70 (14) | NR | 30 (17.6) | NR | 81 (47.6) | 170 (100) | NR | NR | Median: 2.8 ± 0.5 | 165 (22) | NR |
| Delgado-Montero et al. (2016) [16] | 65 (11) | 150 (73) | NR | NR | Excluded | NR | NR | 205 (100) | NR | NR | I: 0 II: NR III: 148 (72) IV: NR |
157 (26) | NR |
| Van Der Bijl et al. (2019) [21] | 65 (10) | 861 (73) | NR | NR | 179 (15.1) | NR | 665 (56.1) | 1185 (100) | 442 (37.3) | NR | I: 53 (4.5) II: 299 (25.2) III/IV: 833 (70.3) |
154.6 (34.8) | 332.8 (120.2) |
| Knappe et al. (2011) [18] | 62 (11) −66 (11) | 809 (75) | 58 (9) – 70 (11) | NR | NR | 663 (61.6) | 601 (55.8) | 1077 (100) | NR | 68 (20) – 71 (21) | I: NR II: 906 (84) III: NR IV: NR |
152 (17) – 165 (19) | NR |
| Menet et al. (2016) [19] | 70 (11) | 121 (71) | 72 (13) | 28 (5.4) | 32 (19) | 71 (42) | 66 (39) | 170 (100) | NR | NR | I-II: 85 (50) III-IV: 85 (50) |
162 (26) | NR |
| Kalogeropoulos et al. (2011) [17] | 52 (15) | 40 (70) | NR | NR | Excluded | 26 (45.6) | 15 (26.3) | 57 (100) | NR | NR | I: 0 II: 7 (12.3) III: 35 (61.4) IV: 15 (26.3) |
NR | NR |
| Park et al. (2013) [20] | 65 (12) | 224 (67.1) | NR | NR | NR | NR | 176 (52.7) | 334 (100) | NR | NR | I-II: 37 (11.1) III-IV: 297 (88.9) |
158 (31) | NR |
Table 3.
Summary of baseline demographics NR – Not reported; SD – Standard Deviation; CM- Cardiomyopathy; HTN – Hypertension; BMI – Body Mass Index; AF- Atrial Fibrillation; HR – Heart Rate; IHD – Ischemic Heart Disease; HF- Heart failure; CKD – Chronic Kidney Disease; eGFR – estimated glomerular filtration rate; NYHA – New York Heart Association; MWT – Metre Walk Test; BB- Beta-blocker; ARB – Angiotensin receptor Blocker; ACE-I – Angiotensin Converting Enzyme-Inhibitor; MRA- Mineralocorticoid receptor antagonist.
| Study | BB (%) | ACE/ARB (%) | MRA (%) | Statin (%) | Diuretics (%) | Antiplatelet (%) | Digoxin (%) |
|---|---|---|---|---|---|---|---|
| Khidir et al. (2018) [13] | 632 (76) | 733 (88) | 364 (44) | 517 (62) | 652 (79) | 672 (81%) | NR |
| Bax et al. (2017) [15] | 728 (90) | 719 (89) | 451 (56) | NR | NR | NR | NR |
| Hasselberg et al. (2016) [14] | 157 (92) | 160 (94) | 67 (39) | NR | 142 (84) | NR | 18 (11) |
| Delgado-Montero et al. (2016) [16] | 179 (87) | 186 (91) | NR | NR | NR | NR | NR |
| Van Der Bijl et al. (2019) [21] | 833 (70.3) | 994 (83.9) | 493 (41.6) | NR | 891 (75.2) | NR | 168 (14.2) |
| Knappe et al. (2011) [18] | NR | NR | NR | NR | NR | NR | NR |
| Menet et al. (2016) [19] | NR | NR | NR | NR | NR | NR | NR |
| Kalogeropoulos et al. (2011) [17] | 44 (77.2) | 46 (80.7) | 22 (38.6) | NR | NR | NR | NR |
| Park et al. (2013) [20] | 283 (84.7) | 275 (82.3) | 100 (29.9) | NR | 267 (79.9) | NR | NR |
The mean LVEDV (SD) was reported in 7 studies [13], [15], [17], [18], [21], [22] ranging from 188 (59) mL to 284 (74) mL. The mean LVEF (SD) amongst the studies [13], [14], [15], [17], [18], [19], [20], [21], [22] was within the severe systolic dysfunction category and ranged between 22% and 31.1%. The mean LV GLS amongst the studies [13], [14], [15], [17], [18], [19], [20], [21], [22] ranged between −6.5% and −9.5%. The interobserver and intraobserver agreements for the reporting of LV GLS were generally excellent, ranging from 0.92 to 0.97[13], [14], [15], [19], [20], [21], [22] and 0.94 to 0.99 [20], [21], [13], [14], [15], [16] respectively (see Table 4).
-
(3)
Definitions of outcome measures:
Table 4.
Summary of echocardiography parameters NR – Not reported; SD – Standard Deviation; LVEDV – Left ventricular end diastolic volume; LVESV – Left ventricular end systolic volume; LVEF - left ventricular ejection fraction; GLS – Global longitudinal strain (presented in whole numbers as opposed to negatives); MR – Mitral regurgitation.
| Study | Echocardiography Machines | Software for GLS analysis | LVEDV (mL) (SD) (range = IQR) |
LVESV (mL) (SD) (range = IQR) |
LVESV Index (mL/m2) (SD) |
LVEF (%) (SD) |
GLS (%) (SD) |
GLS Interobserver correlation Coefficient | GLS Intraobserver correlation Coefficient |
MR Grades (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Khidir et al. (2018) [13] | GE Vivid 5 or 7, E9 | EchoPAC v.113 GE Healthcare, Horten, Norway | 210 (78) | 156 (68) | NR | 27 (8) | −7.9 (2.7) | 0.95 | 0.99 | 0–2: 710 (86) 3–4: 118 (14) |
| Bax et al. (2017) [15] | GE Vivid 7, E9 | EchoPAC version BT 11–12 GE Heathcare, Horten, Norway | 188 (59) | 139 (50) | NR | 27 (5.5) | −8.2 (2.8) | 0.92 | 0.97 | 0–2: 670 (83) 3–4: 77 (10) |
| Hasselberg et al. (2016) [14] | GE Vivid 7, E9 | EchoPAC GE Healthcare, Horten, Norway | NR | NR | 70 (30) | 26 (9) | −8.2 (3.9) | 0.92 | 0.94 | NR |
| Delgado-Montero et al. (2016) [16] | GE Vivid 7 | EchoPAC BT11 or BT12, GE Vingmed, Horten, Norway |
198 (72) | 152 (62) | NR | 24 (6) | −8.9 (3.1) | 0.92 | 0.97 | NR |
| Van Der Bijl et al. (2019) [21] | GE Vivid 7 or E9 | EchoPAC v.113, GE Healthcare, Horten, Norway | 204 (76) | 151 (66) | NR | 27 (8) | −7.3 (3.4) | 0.92 | 0.97 | NR |
| Knappe et al. (2011) [18] | NR (110 hospital sites) | TomTec Imaging Systems, Unterschleissheim, Germany | 219 (39) − 284 (74) | 151 (31) – 207 (60) | NR | 28.8 (3.4) – 31.1 (3.3) | −8.5 (2.9) to −9.5 (3) | Correlation coefficient NR. Coefficient of variation = 8% | Correlation coefficient NR. Coefficient of variation = 7.7% | NR |
| Menet et al. (2016) [19] | GE Vivid E9 | EchoPAC BT12, GE Vingmed, Horten, Norway |
250 (68) | NR | NR | 26 (5) | −8 (2.8) | 0.9 | NR | NR |
| Kalogeropoulos et al. (2011) [17] | NR | 2D Cardiac Performance Analysis Image Arena TomTec Imaging systems (version not specified) | 209 (171–301) | 180 (112–253) | NR | 22 (17–25) | −6.5 (5–8.4) | NR | NR | NR |
| Park et al. (2013) [20]) | NR | Velocity Vector Imaging (Axius, Siemens Medical Solutions, Mountain View, CA) | NR | NR | 74 (33) | 24 (7) | −7.2 (2.6) | 0.97 (95% CI, 0.9–0.99) | 0.97 (95% CI, 0.88–0.99) | 2–4: 62 (39) |
All the included studies incorporated mortality, predominantly all-cause mortality, into their primary composite endpoint. For a complete summary of the primary and secondary outcomes of the analysed studies, refer to Table 5 and Table 6. 5 studies [13], [14], [16], [17], [20] reported on the composite of all-cause mortality along with heart transplantation and/or LVAD implantation in the primary endpoint, 3 studies [15], [18], [19] reported on all-cause mortality and/or heart failure events/hospitalisations in the primary endpoint and the remaining study [21] only reported on all-cause mortality as the primary endpoint.
Table 5.
Primary outcomes reported in included studies. LV GLS – left ventricular global longitudinal strain; LVAD –Left ventricular assist device; HR – Hazard Ratio; CI – Confidence Interval; HF – heart failure; LVEDD – Left ventricular end diastolic diameter; LVESV – Left ventricular end systolic volume; LA – Left atrium; RA – Right atrium; RVEDA – Right ventricular end diastolic area; RFAC – Right ventricular fractional area change; ICD – Implantable cardiac defibrillator.
| Study | Primary endpoint | Design of analysing LV GLS significance in predicting endpoint | Primary endpoint reached during follow-up (%) | All-cause mortality (%) | LVAD (%) | Heart transplantation (%) | Heart failure admissions (%) | ROC analysis | Findings of LV GLS and association with outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Khidir et al. (2018) [13] | Combination of all-cause mortality, heart transplantation and LVAD implantation | LVGLS divided into quartiles (≤-9.8%; −9.7% to −7.8%; −7.7% to −5.9%; ≥-5.8%) and best GLS quartile assessed against worst for outcome | 332 (40%) | 328 (39.5) | 2 (0.2) | 2 (0.2) | NR | NR |
|
| Bax et al. (2017) [15] | All-cause mortality or heart failure hospitalisation. | Cut-off of LV GLS of > −6.2% and < −6.2% were used to divide out cohort and then further subdivided into CRT-ON group vs CRT-OFF group | 206 (27) – total population 111 (29.7) – CRT ON group |
NR | NR | NR | NR | NR |
|
| Hasselberg et al. (2016) [14] | Composite of all cause death, heart transplantation, and LVAD implantation. | Multivariate analysis of LVGLS, LVEF and LVESV prior to CRT implant and association with primary and secondary endpoint | 24 (14) | 16 (9) | 2 (1) | 6 (3.5) | NR | GLS before CRT worse or equal −8.3% detected fatal endpoint: sensitivity 88% (95% CI 68–97%) and specificity 55% (95% CI 47–64%) – C-statistics 0.73 (95% CI 0.64–0.82). |
|
| Delgado-Montero et al. (2016) [16] | Composite endpoint of death, LVAD implant, heart transplantation. | Predefined GLS cut-off of > −9% vs ≤ −9% was utilised | 81 (39.5) | 60 (29.2) | 8 (3.9) | 13 (6.3) | NR | NR |
|
| Van Der Bijl et al. (2019) [21] | All-cause mortality. | Defined absolute improvement in LVGLS as ≥ 5% and relative reduction of LVESV of ≥ 15% - divided cohort into ≥ 5 %LVGLS + ≥15 %LVESV; ≥5%LVGLS OR ≥ 15 %LVESV; <15% LVESV and < 5 %LVGLS | 323 (27) | 323 (27) | NR | NR | NR | NR |
|
| Knappe et al. (2011) [18] | All-cause death or non-fatal heart failure events | Assessed quartiles of contractile function as measured by LVGLS −19.0 to −10.6; −10.59 to −8.7; −8.69 to −6.94; −6.93 to −2.0) | 213 (19.8) (in ICD + CRT-D arms) 108 (10) (in CRT-D arm alone) |
30 (2.8) - within total cohort |
NR | NR | 158 (14.6) - within total cohort |
NR |
|
| Menet et al. (2016) [19] | All-cause mortality and/or congestive heart failure hospitalisation. | Analysed the affect of change in LVGLS to outcome | 47 (27.6) | 20 (11.8) | NR | NR | 27 (15.9) | NR |
|
| Kalogeropoulos et al. (2011) [17] | Death, LVAD or urgent heart transplant | Assessed LVGLS as relative improvement in LVGLS of > 15% when compared to < 15% improvement in LVGLS across follow-up period and impact on outcome. | 18 (31.6) | 15 (26) | NR | 3 (5.3) | Annualized rates of HF admission = 59.7%. | NR |
|
| Park et al (2013) [20] | Composite of death from any cause, heart transplantation, LVAD or heart failure hospitalisation. | Utilised LVGLS as one of 6 variables in an echocardiographic model for predicting LV reverse remodelling | 245 (74%) | 134 (40.6) | 7 (2.1) | 11 (3.3) | 93 (28.2) | NR |
|
Table 6.
Secondary outcomes reported in studies. LV GLS – left ventricular global longitudinal strain; LVAD –Left ventricular assist device; HR – Hazard Ratio; CI – Confidence Interval; HF – heart failure; LVEDD – Left ventricular end diastolic diameter; LVESV – Left ventricular end systolic volume; LA – Left atrium; ICD – Implantable cardiac defibrillator.
| Study | Secondary endpoint | Design of analysing LV GLS significance in predicting endpoint | Secondary endpoint reached during follow-up (%) | Findings of LV GLS and association with outcomes |
|---|---|---|---|---|
| Khidir et al. (2018) [13] | Occurrence of ventricular arrhythmia or appropriate ICD therapy. | LVGLS divided into quartiles (≤-9.8%; −9.7% to −7.8%; −7.7% to −5.9%; ≥-5.8%) and best GLS quartile assessed against worst for outcome | 233 (28.1) |
|
| Bax et al. (2017) [15] | Ventricular arrhythmias defined as appropriate ICD therapy, arrhythmic death and atrial tachyarrhythmias | Cut-off of LV GLS of > −6.2% and < −6.2% were used to divide out cohort and then further subdivided into CRT-ON group vs CRT-OFF group | 72 (9.5) – total population 38 (10.1) – CRT ON group |
|
| Hasselberg et al. (2016) [14] | Arrhythmic end point – first sustained ventricular arrhythmic event following CRT placement – VF, anti-tach pacing, Defib therapy, SCA | Multivariate analysis of LVGLS, LVEF and LVESV prior to CRT implant and association with primary and secondary endpoint | 18 (11) |
|
| Delgado-Montero et al. (2016) [16] | First heart failure hospitalisation or death during follow-up | Predefined LVGLS cut-off of > −9% vs ≤ −9% was utilised | 110 (53.7) |
|
| Kalogeropoulos et al. (2011) [17] | All cause and heart failure readmissions | Assessed LVGLS as relative improvement in LVGLS of > 15% when compared to < 15% improvement in LVGLS across follow-up period and impact on outcome | Annualized rates of HF admission = 59.7%. |
|
| Park et al (2013) [20] | LV reverse remodelling defined as a ≥ 15% reduction in LVESV | Utilised LVGLS as one of 6 variables in an echocardiographic model for predicting LV reverse remodelling and broken up into quartiles based on score (0–7; 8–19; 20–31; 32–37) | Reduction ≥ 15% in LVEDV – 110 (33) patients. |
|
In terms of secondary endpoints, 3 studies [13], [14], [15] reported on the occurrence of ventricular arrhythmias and/or appropriate ICD therapies or sudden cardiac death with one of these studies including atrial arrhythmias within this endpoint [15]. 2 studies [16], [17] included heart failure hospitalisations into their secondary endpoints with one of these studies including death during follow-up as part of this endpoint, the remaining study [20] that reported on a secondary endpoint, incorporated LV reverse remodelling after CRT implantation as defined by a ≥15% reduction in LVESV.
-
(4)
Prognostic outcomes reported by studies:
Primary outcomes – mortality:
The incidence of the primary endpoint occurring within the follow-up duration of the studies varied significantly between 14% and 74% [20]. All-cause mortality, more specifically, varied between 2.8% of the total cohort to 40.6%. The incidence of LVAD implantation and heart transplantation during follow-up was reported completely in 4 studies [20], [14], [15], [16] and varied between 0.2–3.9% and 0.2–6.3%, respectively. The prevalence of heart failure hospitalisation during follow-up was reported in 4 studies ranging from 14.6% to 28.2% of the populations.
The reporting of outcomes was quite heterogenous amongst the included studies with 6 studies utilising predefined cut-offs of LV GLS to subgroup the populations for analysis. Within these cut-offs 2 studies utilised LV GLS quartiles that equally divided the analysed populations, generally finding the lowest quartiles of ≥−5.8%, in Khidir et al.’s [13] study, and worse than the absolute median cut-off of −8.7% (instead of the lowest quartile; −6.93% to −2.0%), in Knappe et al.’s [18] study, to have a significantly higher risk of reaching the primary endpoint. In addition, Khidir et al. [13], Bax et al. [15] and Hasselberg et al. [14], individually demonstrated in multivariate analysis that an incremental decline in LVGLS was significantly associated with their primary endpoints HR 1.08 (95% CI 1.02–1.13; p 0.007), HR 1.11 (95% CI 1.04–1.17, p < .001) and 1.16 (95% CI 1.05–1.30; p 0.006), respectively. The caveat to Bax et al.’s [15] study being that this incremental decline in LVGLS also included patients with a CRT in place that was randomised in their trial to being turned off. In sub-group analysis for this study, LVGLS provided value in discriminating between patients that would benefit from a CRT-ON vs CRT-OFF at a predefined LVGLS cut-off of −6.2%. Similarly, Delgado-Montero et al. [16] described a LVGLS cut-off of −9% upon which their population was divided, and multi-variate analyses demonstrated a significantly higher risk of reaching the primary endpoint if LVGLS was >−9% at the time of CRT implantation. Kalogeropoulos et al. [17] deviated slightly from an absolute cut-off value of LV GLS and instead focused on a relative improvement in LV GLS of >15% on follow-up echocardiograms post CRT implantation at >12 months post implantation, interestingly there was no mention of the significance of this on the primary endpoint in their results. Regardless, none of these studies mentioned their rationale for determining their individual cut-off values.
Of note, Khidir et al. [13] and Van der Bijl et al. [21], shared a similar patient population, with analysis of a CRT-implanted population occurring at different time points within the same institution, with different numbers of patients and different methods of utilising LV GLS to assess slightly different endpoints. This in turn affected the ability to reliably meta-analyse these populations given the selection bias and the overlap of data.
One study incorporated LV GLS into an echocardiographic scoring system to predict LV reverse remodelling and investigate the combined scores performance in predicting a composite endpoint [20].
Secondary outcomes – arrhythmia:
Overall, 3 studies reported secondary endpoints predominantly focusing on ventricular arrhythmias and appropriate device therapy with the occurrence ranging between 10.1 and 28.1% of the populations [13], [14], [15]. In all 3 studies, on multi-variate analysis, declining LV GLS was not significantly associated with the secondary endpoint. Heart failure hospitalisation was reported in 2 studies as a secondary endpoint with 53.7% prevalence in one study and an annualized rate of admission of 59.7% in the other study [16], [17]. In both these studies worse LV GLS at predefined cut-offs was significantly associated with the secondary outcome.
4. Discussion
The objective of this systematic review was to analyse the existing literature on the prognostic value of LV GLS in heart failure patients being managed with cardiac resynchronisation therapy. Of the 9 studies reviewed, including 3,981 heart failure patients undergoing CRT implantation, there can be several conclusions drawn. Foremost, in all but one study (which failed to report on their predetermined primary outcome [17]), worse LV GLS at the time of CRT implantation was associated with a higher incidence of reaching the primary endpoint, which in all studies included all-cause mortality. Secondly, lower LV GLS at implantation was not significantly associated with the occurrence of ventricular arrhythmias in CRT populations. Finally, predominantly one ultrasound platform and its corresponding vendor-specific software was utilised in the majority of studies. Complicating analyses of results was the presence of significant heterogeneity in the variation of pre-determined LV GLS “cut-off” values. Furthermore, there was clinical overlap between the reviewed populations in 2 studies [13], [21], poor delineation of the impact of LV GLS on purely CRT implanted patients in one study [18] and variation in the inclusion criteria of QRS duration in one study [15] resulting in some of the included studies analysed not being suitable for a meta-analysis.
Pathophysiologic mechanisms of CRT benefit
It is evident that left ventricular electro-mechanical dyssynchrony results in inefficient myocardial contraction and impaired cardiac output [23]. The premise behind CRT is its ability to minimise the electrical dyssynchrony and conduction delay observed in symptomatic heart failure patients, in a bid to restore mechanical synchrony and improve myocardial function. The Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) randomised controlled trial supported the role of CRT in improving morbidity and mortality through improving mechanical synchrony by demonstrating a significant 34–40% reduction in the combined end point of death from or hospitalisation for heart failure in the CRT-P and CRT-D arms over the optimal medical therapy arm for heart failure patients with a QRS duration >120 ms [24]. Similar reductions in mortality and symptomatic improvements in heart failure were observed in the CARE-HF and MADIT-CRT trial populations [25], [26] and are among the landmark trials that have shaped our use of CRT for the last 20 years.
Despite this, nearly a third of patients treated with CRT enjoy no benefit. The burden of myocardial fibrosis is an important independent predictor of response to CRT [5], with extensive LV myocardial scar contributing to impaired LV reverse remodelling. This may explain why individuals with underlying ischemic cardiomyopathy respond less frequently to CRT compared to individuals with non-ischemic cardiomyopathy [27].
Theoretically, quantifying the burden of myocardial fibrosis prior to CRT implantation could be desirable in both selecting appropriate recipients for CRT, and optimising outcomes. To this point, LV GLS as measured through speckle-tracking echocardiography has the potential to be an important modality for quantitative myocardial assessment as a predictor of response. LV GLS has been utilised in various cardiomyopathic processes as a tool for quantifying fibrosis burden [7], [8], [28] and indeed has demonstrated clinical utility as a predictor of LV reverse remodelling in patients post CRT implantation.
GLS as a predictor of outcome in CRT implantation:
Based on the current review of the literature, the prognostic value of LV GLS in heart failure patients undergoing CRT implantation comprised predominantly of cohort reviews and post-hoc sub-study analyses of completed randomised controlled trials. The inclusion of ischemic cardiomyopathy patients, the ranges of LVEF and the composition of heart failure functional classes within the analysed populations for this review were comparable to those in landmark CRT trials such as CARE-HF, MUSTIC and COMPANION, providing some degree of validity for the applicability of their results to the broader CRT population [2]. More importantly, the value of predictive markers is dependent on their efficacy in application to pre-intervention assessments to produce optimal outcomes. From this perspective, predictive scoring systems such as EAARN, CRT score and ScREEN have been developed to assist with the selection of patients prior to CRT implantation to enhance the likelihood of benefit from CRT [29], [30], [31]. These scoring systems have included traditional variables such as age, gender, presence of AF, NYHA class, QRS width and renal function [29], [30], [31]. LVEF has also been included as the only echocardiographic parameter to be incorporated. Depending on the score incremental % changes in LVEF as well as cut-off value of LVEF have been utilised. It is well established that LV GLS is more sensitive and reproducible than LVEF with narrower margins of inter-observer and intra-observer variability.
Considering this, in 2 of the reviewed studies [16], [18], LV GLS worse than a predefined cut-off ranging between −8.7% and −9% pre-CRT implantation, was an independent predictor of all-cause mortality, heart transplantation or LVAD implantation, compared to an LV GLS better than these values. Furthermore, in 3 of the reviewed studies [13], [14], [15], an incremental % decline in pre-CRT implant LV GLS was significantly associated with all-cause mortality independent of the aforementioned clinical variables included in the traditional CRT scoring algorithms [29], [30], [31].
Consequently, the application of LV GLS within a predictive scoring system was attempted by Park et al. [20]. Their study generated a validation cohort for an LV GLS derived cut-off of −7% for pre-implant CRT candidates, in conjunction with 5 other echocardiographic parameters of varying weights in their scoring system. These other parameters were not previously validated as part of CRT scoring systems [29], [30], [31]. Park et al. [20] demonstrated that an incrementally better echocardiographic score, which incorporated LV GLS, was significantly associated with a declining risk of mortality, lending weight to the value of LV GLS as a clinically meaningful predictor of outcome in a CRT population model.
Considerations in clinical application:
From a technical standpoint, the majority of LV GLS analyses performed in the reviewed studies [19], [21], [13], [14], [15], [16] were vendor-specific with legacy versions of EchoPAC (GE Healthcare, Horten, Norway) software. The variability experienced in inter-vendor, inter-hardware and inter-version software analysis for LV GLS should be taken into consideration during interpretation of these values as absolutes for translation to clinical practice [32].
Furthermore, although there have been significant associations with poorer LV GLS and an increase in reaching the primary endpoints, and to some extent secondary endpoints, a consensus cut-off value of LV GLS at which clinical utility can be extrapolated was not possible. This prevailing issue can be attributed to a combination of factors identified in this review, specifically: the small study sample sizes, the retrospective nature of the majority of studies and most importantly the heterogenous pre-specified LV GLS values upon which comparisons have been drawn. The lack of explanation as to how these LV GLS values were settled upon in individual studies and the absence of independent validation cohorts for fidelity, in all but one study [20], compounds the inability to utilise these absolute values in clinical practice.
In light of this, the value of LV GLS in the CRT population carries significant value given its clear incremental predictive capacity for all-cause mortality, rehospitalisation and heart transplantation which was clearly demonstrated in 3 of the studies with representative CRT populations on multivariate regression analysis [13], [14], [15]. It is the authors’ conclusion that the true clinical implementation of LV GLS in the CRT population may more clearly be realised in a prospective randomised controlled trial designed to incorporate it into the randomisation arms for predicting responders to CRT. Future prospective studies are still needed in this area preferably utilising vendor-neutral software for cross-applicability in multi-vendor laboratories, before LV GLS can effectively be used as a discriminating factor for predicting response and outcome in CRT implantation.
Limitations:
This is the most comprehensive assessment of the literature regarding LV GLS and its prognostic value in patients undergoing CRT implantation to date. However, there are several limitations that affect the applicability of the results. The first is the small number of studies on the topic including the relative overlap of study population in 2 papers [13], [21] and the poor delineation of the predictive effect of LV GLS on CRT specific patients in 2 other papers [15], [18]. The second major issue is the observational nature of the majority of the reviewed studies which would add significant selection bias into the interpretation of the results. Finally, the limitation of the searches to only the English language may have resulted in a degree of publication bias.
5. Conclusion
This systematic review of 3,981 patients undergoing CRT implantation demonstrated that there is a significant association between significantly abnormal baseline LV GLS at CRT implantation and the occurrence of all-cause mortality, rehospitalisation, LVAD implantation or heart transplantation on long-term follow-up. Future research into this area should incorporate LV GLS into prospective trial design to provide more robust and consistent LV GLS values to guide the appropriate use of CRT and monitoring of outcomes.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100849.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Wells G., Parkash R., Healey J.S., Talajic M., Arnold J.M., Sullivan S. Cardiac resynchronization therapy: a meta-analysis of randomized controlled trials. CMAJ. 2011;183(4):421–429. doi: 10.1503/cmaj.101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao P., Faddis M. Cardiac resynchronisation therapy: current indications, management and basic troubleshooting. Heart. 2017;103(24):2000–2007. doi: 10.1136/heartjnl-2016-310656. [DOI] [PubMed] [Google Scholar]
- 4.Brignole M., Auricchio A., Baron-Esquivias G., Bordachar P., Boriani G., Breithardt O.A. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Eur. Heart J. 2013;34(29):2281–2329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 5.Harb S.C., Toro S., Bullen J.A., Obuchowski N.A., Xu B., Trulock K.M. Scar burden is an independent and incremental predictor of cardiac resynchronisation therapy response. Open Heart. 2019;6(2):e001067. doi: 10.1136/openhrt-2019-001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsan N.A., Westenberg J.J.M., Ypenburg C., van Bommel R.J., Roes S., Delgado V. Magnetic resonance imaging and response to cardiac resynchronization therapy: relative merits of left ventricular dyssynchrony and scar tissue. Eur. Heart J. 2009;30(19):2360–2367. doi: 10.1093/eurheartj/ehp280. [DOI] [PubMed] [Google Scholar]
- 7.Saito M., Okayama H., Yoshii T., Higashi H., Morioka H., Hiasa G. Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging. 2012;13(7):617–623. doi: 10.1093/ejechocard/jer318. [DOI] [PubMed] [Google Scholar]
- 8.Haland T.F., Almaas V.M., Hasselberg N.E., Saberniak J., Leren I.S., Hopp E. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging. 2016;17(6):613–621. doi: 10.1093/ehjci/jew005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalam K., Otahal P., Marwick T.H. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100(21):1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 10.Diao K.-Y., Yang Z.-G., Ma M., He Y., Zhao Q., Liu X. The Diagnostic Value of Global Longitudinal Strain (GLS) on Myocardial Infarction Size by Echocardiography: A Systematic Review and Meta-analysis. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-09096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertini M., Ng A.C.T., Antoni M.L., Nucifora G., Ewe S.H., Auger D. Global longitudinal strain predicts long-term survival in patients with chronic ischemic cardiomyopathy. Circ. Cardiovasc. Imaging. 2012;5(3):383–391. doi: 10.1161/CIRCIMAGING.111.970434. [DOI] [PubMed] [Google Scholar]
- 12.Wolff R.F., Moons K.G.M., Riley R.D., Whiting P.F., Westwood M., Collins G.S. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019;170(1):51. doi: 10.7326/M18-1376. [DOI] [PubMed] [Google Scholar]
- 13.Khidir M.J.H., Abou R., Yilmaz D., Ajmone Marsan N., Delgado V., Bax J.J. Prognostic value of global longitudinal strain in heart failure patients treated with cardiac resynchronization therapy. Heart Rhythm. 2018;15(10):1533–1539. doi: 10.1016/j.hrthm.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Hasselberg N.E., Haugaa K.H., Bernard A., Ribe M.P., Kongsgaard E., Donal E. Left ventricular markers of mortality and ventricular arrhythmias in heart failure patients with cardiac resynchronization therapy. Eur. Heart J. Cardiovasc. Imaging. 2016;17(3):343–350. doi: 10.1093/ehjci/jev173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bax J.J., Delgado V., Sogaard P., Singh J.P., Abraham W.T., Borer J.S. Prognostic implications of left ventricular global longitudinal strain in heart failure patients with narrow QRS complex treated with cardiac resynchronization therapy: a subanalysis of the randomized EchoCRT trial. Eur Heart J. 2017;38(10):720–726. doi: 10.1093/eurheartj/ehw506. [DOI] [PubMed] [Google Scholar]
- 16.Delgado-Montero A., Tayal B., Goda A., Ryo K., Marek J.J., Sugahara M. Additive Prognostic Value of Echocardiographic Global Longitudinal and Global Circumferential Strain to Electrocardiographic Criteria in Patients With Heart Failure Undergoing Cardiac Resynchronization Therapy. Circ. Cardiovasc. Imaging. 2016;9(6) doi: 10.1161/CIRCIMAGING.115.004241. [DOI] [PubMed] [Google Scholar]
- 17.Kalogeropoulos A., Savoye L.P., Georgiopoulou V., Raj L., Lloyd M.S., Chiladakis J. Long-term response of the left ventricle to cardiac resynchronization therapy: insights from standard and strain echocardiography. Congest Heart Fail. 2011;17(2):71–79. doi: 10.1111/j.1751-7133.2011.00212.x. [DOI] [PubMed] [Google Scholar]
- 18.Knappe D., Pouleur A.-C., Shah A.M., Cheng S., Uno H., Hall W.J. Dyssynchrony, contractile function, and response to cardiac resynchronization therapy. Circ. Heart Fail. 2011;4(4):433–440. doi: 10.1161/CIRCHEARTFAILURE.111.962902. [DOI] [PubMed] [Google Scholar]
- 19.Menet A., Guyomar Y., Ennezat P.-V., Graux P., Castel A.L., Delelis F. Prognostic value of left ventricular reverse remodeling and performance improvement after cardiac resynchronization therapy: A prospective study. Int. J. Cardiol. 2016;204:6–11. doi: 10.1016/j.ijcard.2015.11.091. [DOI] [PubMed] [Google Scholar]
- 20.Park J.-H., Negishi K., Grimm R.A., Popovic Z., Stanton T., Wilkoff B.L. Echocardiographic predictors of reverse remodeling after cardiac resynchronization therapy and subsequent events. Circ. Cardiovasc. Imaging. 2013;6(6):864–872. doi: 10.1161/CIRCIMAGING.112.000026. [DOI] [PubMed] [Google Scholar]
- 21.van der Bijl P., Khidir M.J.H., Leung M., Yilmaz D., Mertens B., Ajmone Marsan N. Reduced left ventricular mechanical dispersion at 6 months follow-up after cardiac resynchronization therapy is associated with superior long-term outcome. Heart Rhythm. 2018;15(11):1683–1689. doi: 10.1016/j.hrthm.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Delgado V., Ypenburg C., Zhang Q., Mollema S.A., Fung J.-H., Schalij M.J. Changes in global left ventricular function by multidirectional strain assessment in heart failure patients undergoing cardiac resynchronization therapy. J. Am. Soc. Echocardiogr. 2009;22(6):688–694. doi: 10.1016/j.echo.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Linde C., Ellenbogen K., McAlister F.A. Cardiac resynchronization therapy (CRT): clinical trials, guidelines, and target populations. Heart Rhythm. 2012;9(8):S3–S13. doi: 10.1016/j.hrthm.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Bristow M.R., Saxon L.A., Boehmer J., Krueger S., Kass D.A., De Marco T. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004;350(21):2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 25.Cleland J.G.F., Daubert J.-C., Erdmann E., Freemantle N., Gras D., Kappenberger L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 26.Moss A.J., Hall W.J., Cannom D.S., Klein H., Brown M.W., Daubert J.P. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 2009;361(14):1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 27.Ypenburg C., Roes S.D., Bleeker G.B., Kaandorp T.A.M., de Roos A., Schalij M.J. Effect of total scar burden on contrast-enhanced magnetic resonance imaging on response to cardiac resynchronization therapy. Am. J. Cardiol. 2007;99(5):657–660. doi: 10.1016/j.amjcard.2006.09.115. [DOI] [PubMed] [Google Scholar]
- 28.Cameli M., Mondillo S., Righini F.M., Lisi M., Dokollari A., Lindqvist P. Left Ventricular Deformation and Myocardial Fibrosis in Patients With Advanced Heart Failure Requiring Transplantation. J. Card. Fail. 2016;22(11):901–907. doi: 10.1016/j.cardfail.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Providencia R., Marijon E., Barra S., Reitan C., Breitenstein A., Defaye P. Usefulness of a clinical risk score to predict the response to cardiac resynchronization therapy. Int. J. Cardiol. 2018;260:82–87. doi: 10.1016/j.ijcard.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Khatib M., Tolosana J.M., Trucco E., Borras R., Castel A., Berruezo A. EAARN score, a predictive score for mortality in patients receiving cardiac resynchronization therapy based on pre-implantation risk factors. Eur. J. Heart Fail. 2014;16(7):802–809. doi: 10.1002/ejhf.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoke U., Mertens B., Khidir M.J.H., Schalij M.J., Bax J.J., Delgado V. Usefulness of the CRT-SCORE for Shared Decision Making in Cardiac Resynchronization Therapy in Patients With a Left Ventricular Ejection Fraction of </=35. Am. J. Cardiol. 2017;120(11):2008–2016. doi: 10.1016/j.amjcard.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Shiino K., Yamada A., Ischenko M., Khandheria B.K., Hudaverdi M., Speranza V. Intervendor consistency and reproducibility of left ventricular 2D global and regional strain with two different high-end ultrasound systems. Eur. Heart J. Cardiovasc. Imaging. 2017;18(6):707–716. doi: 10.1093/ehjci/jew120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.