Highlights
-
•
Blood flow restriction at resting periods of high-intensity load exercise increases Pax7 expression.
-
•
miR-206 levels significantly decreased in the blood flow restriction leg compared to the control.
-
•
Blood flow restriction can cause DNA damage, judging from the increase in messenger RNA levels of Ku70.
Keywords: Blood flow restriction, High-intensity resistance training, microRNA, Satellite cells
Graphical abstract
Abstract
Backgroud
Blood flow restriction (BFR) with low-intensity resistance training has been shown to result in hypertrophy of skeletal muscle. In this study, we tested the hypothesis that BFR during the rest periods between acute, high-intensity resistance exercise sessions (70% of 1 repetition maximum, 7 sets with 10 repetitions) enhances the effects of the resistance training.
Methods
A total of 7 healthy young men performed squats, and between sets BFR was carried out on one leg while the other leg served as a control. Because BFR was applied during rest periods, even severe occlusion pressure (approximately 230 mmHg), which almost completely blocked blood flow, was well-tolerated by the participants. Five muscle-specific microRNAs were measured from the biopsy samples, which were taken 2 h after the acute training.
Results
Doppler data showed that the pattern of blood flow recovery changed significantly between the first and last BFR. microRNA-206 levels significantly decreased in the BFR leg compared to the control. The mRNA levels of RAC-β serine/threonine-protein kinase v22, nuclear respiratory factor 1, vascular endothelial growth factor, lupus Ku autoantigen protein p70 genes (p < 0.05), and paired box 7 (p < 0.01) increased in the BFR leg. The protein levels of paired box 7, nuclear respiratory factor 1, and peroxisome proliferator-activated receptor γ coactivator 1α did not differ between the BFR leg and the control leg.
Conclusion
BFR, during the rest periods of high-load resistance training, could lead to mRNA elevation of those proteins that regulate angiogenesis, mitochondrial biogenesis, and muscle hypertrophy and repair. However, BFR also can cause DNA damage, judging from the increase in mRNA levels of lupus Ku autoantigen protein p70.
1. Introduction
It has been repeatedly shown that low-intensity resistance training with blood flow restriction (BFR) results in increased skeletal muscle hypertrophy.1, 2, 3, 4 The molecular background of BFR training has been intensively studied, and data reveal that ribosomal S6 kinase 1 phosphorylation, a downstream target of mammalian target of rapamycin (mTOR), is elevated, with a decreased eukaryotic translation elongation factor 2 phosphorylation.2 Gundermann et al.5 confirmed that BFR during low-intensity resistance training leads to increases in the phosphorylation of mTOR, S6 kinase 1, ribosomal protein S6, extracellular signal-regulated kinases1/2, and Mnk1-interacting kinase 1. It has also been reported that BFR training causes a decrease in creatine phosphate and intramuscular pH.6 The efficiency of BFR can be further increased with low-intensity neuromuscular electrical stimulation.7 There are a number of studies in which the effects of high-intensity resistance training and low-intensity resistance training with BFR8, 9, 10, 11 have been compared. Generally, it seems that high-intensity resistance training is more effective for strength generation11 and results in greater surface electromyographic activity.8 Indeed, due to the low intensity, BFR training provides greater benefit to endurance athletes than to sprinters.12 Therefore, because of the different thresholds of fast- and slow-twitch fibers and because of the pain that could take place when BFR is carried out during exercise,13, 14 this sort of training is not often used by elite athletes.
Recently we have shown that microRNA (miR) plays an important role in the regulation of muscle hypertrophy.15 miRs are small regulatory transcripts capable of post-transcriptional silencing of mRNA messages by entering a cellular bimolecular apparatus called the RNA-induced silencing complex.16 However, the possible role of miR in BFR training is not unequivocally known. It is well-accepted that the adaptive response to exercise training in skeletal muscle depends on intensity and takes place in the resting period after the training session.17 However, during BFR training, even at low loads, intensity can cause adaptive responses to muscle mass, similar to those caused by high-intensity resistance loading. We assumed that if BFR were applied during rest periods between exercise sessions, the associated pain would be significantly decreased, and, more important, the exercise could be done at a higher load by permitting the utilization of fast-twitch fibers.
Therefore, we tested the hypothesis that BFR, between sets, during resistance training would beneficially impact the effects of a single bout of exercise in human subjects. We also contemplated that miRs are involved in the adaptive response.
2. Materials and methods
2.1. Participants
A total of 7 healthy young male individuals voluntarily participated in the present study (24.5 ± 4.7 years old, body height 182.9 ± 7.7 cm, body mass 78.8 ± 6.7 kg, mean ± SD). All participants carefully read and signed the detailed protocol of the study. Written and informed consent was obtained from all participants. This study was carried out according to the Helsinki Declaration and was approved by the local Science Research Ethical Committee, Budapest, Hungary.
2.2. Acute exercise protocol
The 1st participant started the exercise protocol at 8:20 a.m. Participants were instructed to avoid food consumption 10 h prior to the exercise protocol. The maximal load of squatting was measured as 1 repetition maximum (RM). The 1RM measurements were conducted 1 week prior to the occlusion protocol. All participants were familiar with the squatting exercise. Briefly, after a 10-min warm-up on a cycling ergometer, participants performed 10 repetitions at a load one-half their body weight and 4–6 repetitions at total body weight. In the testing phase, load was gradually increased to reach 1RM by following the National Strength and Conditioning Association (USA) guidelines.18 In 4 trials, all participants reached 1RM. Between sets there was a 2-min rest period.
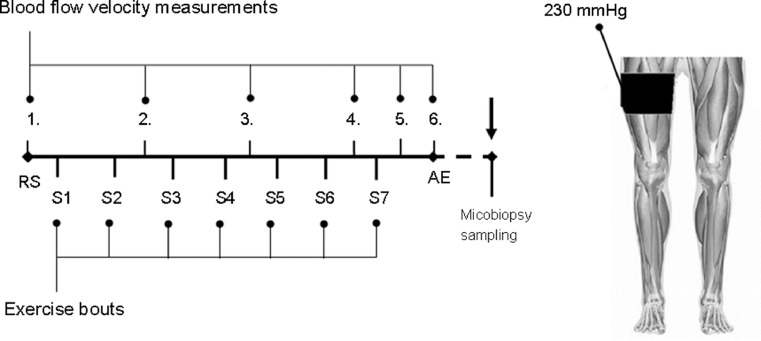
All participants performed the squat exercises at 70% 1RM in 7 sets with 10 repetitions. Between sets, during the 2-min rest period, right leg BFR was performed using the Mizuho BFR system (Tourniquet #8; Mizuho, Tokyo, Japan) with an 11-cm-wide cuff. The left leg served as a control. During the 2-min rest periods between sets, immediately after the last squat, BFR was applied with repeated pumping, which increased the pressure of the cuff to 230 mmHg19 for 1 min. The cuff was then removed. Between each set, subjective discomfort feelings were recorded using the conventional 20-point Borg scale.20 Fig. 1 shows the protocol for the study.
Fig. 1.
The experimental protocol. Participants performed squats at 70% of 1 repetition maximum, with 7 sets and 10 repetitions. BFR was applied to the right leg for 1 min of the 2-min rest periods between sets: S1–S7. Blood flow characteristics were measured by Doppler at RS 1., after every 2 sets (RS 2., RS 3., and RS 4.), after the last set (RS 5.), and 5 min after the last set (AE, RS 6.) exercise bout. Microbiopsy samples were taken 2 h after the last exercise set. AE = after exercise; BFR = blood flow restriction; RS = rested state; S = set.
2.3. Blood flow measurement
The occlusion pressure was approximately 230 mmHg using the standard cuff.21 Doppler 2-dimensional real-time ultrasound examination (General Electric, Boston, MA, USA) was used to detect blood flow in the restricted leg during the rest periods. The results from our pilot study previously demonstrated that this pressure was well-tolerated by the participants and also suggested that greater levels of restriction during the resting periods could enhance the effects of BFR.
Blood flow velocity was measured before, during, and immediately after depressurizing the cuff, using 7.5–10.0 MHz frequency, with a linear transducer placed at the popliteal artery (at 60°), by an experienced physician. The blood flow was detected by Doppler before starting the exercise, after every 2 sets of squats, after the last set, and 5 min after the last exercise bout (Fig. 1). Resting diastolic diameter (mm) was averaged over 30 cardiac cycles. Blood flow (mL/min) was calculated as (time-average mean velocity × πr2) × 60, where r is the radius of the artery lumen. Resting blood flow was averaged over 20 cardiac cycles. Synchronized diameter and velocity data enabled calculation of blood flow.
2.4. Muscle biopsy samples
To determine the expression level of the corresponding mRNA, micro biopsy samples were taken for miR and protein fractions using a semiautomatic needle (EASY-RAM 14G (gauge) × 100 mm (length); RI.MOS., Mirandola, MO, Italy). Local anesthetic was applied (20 mg/mL lidocaine-hydrochloride; EGIS, Budapest, Hungary) to the vastus lateralis muscle of both legs 2 h after the last exercise set.22 The biopsy resulted in about 10 mg of muscle samples. The samples were divided in half, frozen in liquid nitrogen, and kept at –80°C for subsequent RNA and protein extraction procedures.
2.5. RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR) of the mRNA and miR transcripts
Expression levels of target genes were measured by qRT-PCR. The cycle threshold (Ct) values of each PCR run were recorded. The housekeeping gene was selected by using the RefFinder23 online tool, which uses the common methods for GeNorm,24 Normfinder,25 BestKeeper,26 and the comparative ΔCt method27 for identifying housekeeping genes. Comprehensive gene stability (gene geomean of ranking values) was calculated. Of the 5 candidate genes, the 28S RNA showed the best expression stability (mean = 20 Ct, SD = 0.38 Ct, coefficient of variation = 1.9).
RNA extraction (NucleoSpin RNA mini; Macherey-Nagel, Düren, Germany) and cDNA synthesis (SensiFAST™ cDNA Synthesis Kit; Bioline, London, UK) were performed according to the manufacturers’ instructions. Specific gene products were amplified by qRT-PCR using primer pairs (Appendix). The following applied primers were also used: paired box 7 (Pax7), Pax3,28 mTOR,29 silent mating type information regulation 2 homolog 1,30 nuclear respiratory factor 1 (NRF1),31 vascular endothelial growth factor (VEGF), insulin-like growth factor 1, peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), forkhead box protein O1, 28S RNA,32 mitochondrial transcription factor A,31 hypoxia-inducible factor 1α,33 lupus Ku autoantigen protein p70 (Ku70),34 RAC-β serine/threonine-protein kinase 1(Akt1), Akt2,35 superoxide dismutase 1, superoxide dismutase 2,36 and U6 small nuclear RNA (U6).37
The most stable gene expression was found to be 28S RNA. Consequently, this gene expression was used as the reference gene (BestKeeper).26 The mRNA samples were annualized using the SYBR Green real-time system (Thermo Fisher Scientific Inc., Waltham, MA, USA).
The qPCRs for each miRNA (10 μL total volume) were performed in triplicate, and each 10-μL reaction mixture included 2.4 μL of 10 × diluted reverse transcriptase product. Reactions were run on a PRISM 7900HT Fast Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Twofold dilution series were performed for all target miRNAs to verify the linearity of the assay. To account for possible differences in the amount of starting RNA, all samples were normalized to U6, which proved to be the most stable (mean = 23.1 Ct, SD = 0.44 Ct, coefficient of variation = 1.88). All reactions were run singularly and quantified using the Ct (ΔΔCt) method.38
2.6. Immunoblot
The frozen samples were measured and homogenized in a lysis buffer (137 mmol/L NaCl, 20 mmol/L Tris-HCl pH 8.0, 2% Nonidet P40 substitute, 10% glycerol, 1 mmol/L phenylmethylsulfonyl fluoride, protease inhibitor cocktail). Protein levels were determined by the Lowry method. Samples containing 40 μg of protein were loaded on sodium dodecyl sulfate-Polyacrylamide gel electrophoresis. Separated protein bands were immobilized on polyvinylidene difluoride membrane (Immobilon P, 0.45 μm pore size; Merck Millipore, Burlington, MA, USA) membranes and incubated with target-specific antibodies overnight at 4°C. Primary antibodies and dilution factors were the following: Pax7 (1:500, sc-81648; Santa Cruz, Dallas, TX, USA), NRF1 (1:1000, sc-33771, Santa Cruz), PGC-1α (1:3000, KP9803; Calbiochem, San Diego, CA, USA), and α-tubulin (1:5000, T6199; Sigma-Aldrich, St. Louis, MO, USA).
2.7. Statistical analyses
All variables were subjected to the Shapiro–Wilk test to assess if the variables were normally distributed. Data gathered from qPCR measurements and in the miR gene expression experiment data showed normal distribution. The gene expression levels were normalized to the housekeeping genes (28S for mRNA, U6 for miR) and normalized values were compared. For Western blot, α-tubulin was used for normalization.
To determine differences between the BFR and the control limbs, either Student's paired t test (two-tailed) or the Mann-Whitney U test was used for qPCR and Western blot variables, as appropriate. For time course data, repeated measures of analysis of variance were evaluated using the Greenhouse–Geisser correction and Tukey's HSD post hoc analysis. Borg scale data were analyzed by Friedman's analysis of variance. To determine the strength of correlation between Pax7 and miR-206 expression, Pearson's r was calculated. The graphs show fold-difference values for gene-expression levels of the BFR and the control limbs. Data are presented as mean ± SE. The significance level was set at p < 0.05.
3. Results
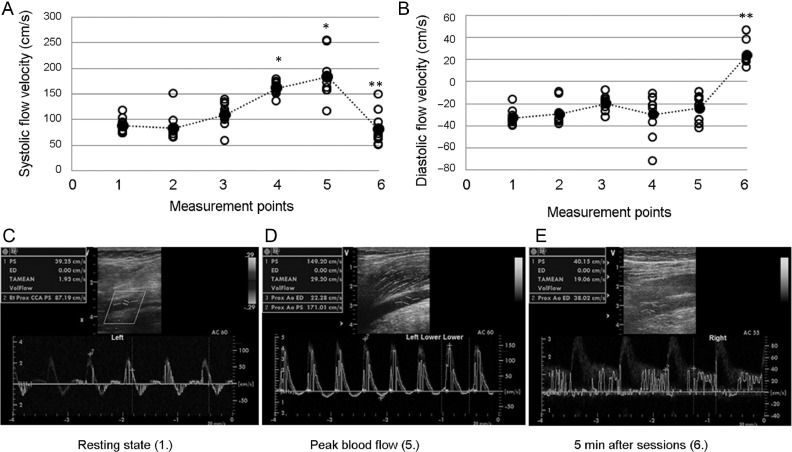
Doppler measurement of popliteal artery blood flow was done before exercise, after every 2 sets of squats and last set, and 5 min after the last exercise bout. Systolic and diastolic flow velocities were registered before and after cuffing. With 230 mmHg of pressure, the blood flow was almost completely blocked. After the 4th and 5th measurements, systolic blood flow increased sharply when the cuff pressure was released, compared to the 3rd basal values (4th measurement: 108.88 ± 28.48 mmHg vs. 157.94 ± 13.89 mmHg; 5th measurement: 108.88 ± 28.48 mmHg vs.176.81 ± 42.3 mmHg). Pressure dropped back to the initial values 5 min after the cuff pressure was released (86.56 ± 35.79 mmHg). However, the diastolic blood flow increased during the 5th and 6th measurements (–23.28 ± 11.72 mmHg vs. 25.37 ± 12.13 mmHg; Fig. 2). After the last trial, the normal 3-phase Doppler blood flow curve changed to a biphasal curve with increased blood flow speed, suggesting decreased peripheral resistance (Fig. 2). The Borg scale data did not show significant differences in pain perception during the BFR (7 sets: 4.9 ± 2.5, 4.6 ± 1.5, 6.9 ± 1.9, 5.9 ± 2.9, 5.4 ± 2.0, 5.3 ± 2.9, and 5.6 ± 3.4; p = 0.296).
Fig. 2.
Blood flow analysis (n = 7). The blood flow pattern was measured by Doppler at rested state, after every 2 sets, and last set, and 5 min after the last squat. Systolic and diastolic blood flow was measured on the popliteal artery. (A) Systolic blood flow increased during the 4th and 5th measurements and decreased at the 6th. (B) The diastolic blood flow velocity increased in the 5th and 6th measurements. Representative Doppler images of (C) the resting state, (D) peak blood flow, and (E) 5 min after the last bout of exercise. Filled dots represent mean values and open circles show individual values. * p < 0.05 for the 4th- and 5th- vs. 3rd- measurement point; ** p < 0.01 for the 6th- vs. 5th measurement point.
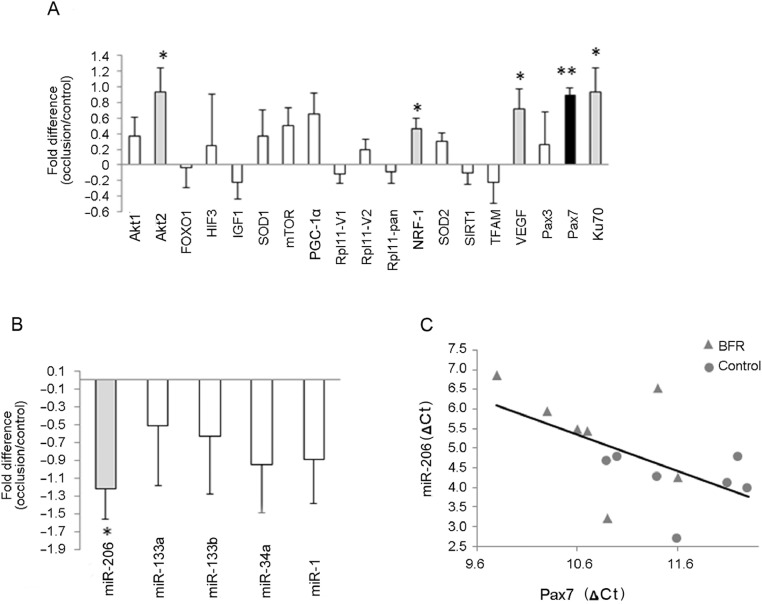
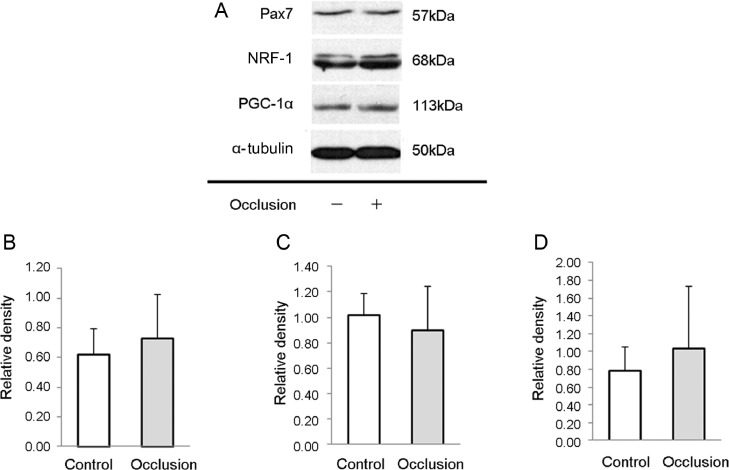
The levels of miR-1, miR-34a, miR-133a, and miR-133b did not change with BFR. However, the concentration of miR-206 (–1.22 ± 1.02 fold, p = 0.0192) significantly decreased as a result of BFR training (p < 0.05) (Fig. 3). Key signaling proteins involved in protein synthesis, vascularization, mitochondrial biogenesis, and the antioxidant system were selected. Resistance exercise with BFR increased the mRNA levels of Akt2 (0.929 ± 0.923 fold, p = 0.037), NRF1 (0.457 ± 0.409 fold, p = 0.025), VEGF (0.721 ± 0.748 fold, p = 0.0433), and Ku70 genes (0.929 ± 0.923 fold, p = 0.037) (Fig. 3). However, the most intriguing finding was the robust increase in the mRNA levels of Pax7 (0.886 ± 0.318 fold, p = 0.0003). Significant correlations were found between the levels of miR-206 and Pax7 (r2 = 0.332, r = −0.577, p = 0.0310) (Fig. 3). The protein levels of Pax7, NRF1, and PGC-1α measured 2 h after the exercise bouts did not demonstrate differences between the BFR and control legs (Fig. 4).
Fig. 3.
mRNA and miR levels from BFR and control legs. (A) Biopsy samples were taken 2 h after the last exercise bout, and the mRNA of key signaling proteins was measured. BFR induced Akt2, NRF1, Pax7, VEGF, and Ku70 levels. (B) A total of 5 myomiRs were detected but only miR-206 changed significantly. (C) Correlation was found between miR-206 and Pax7. The ΔCt values were normalized to the corresponding reference gene. The mRNA and miR results are expressed as fold changes of BFR vs. control (mean ± SE; n = 7). * p < 0.05 (highlighted in gray), ** p < 0.01 (highlighted in black), significant difference marked between the treated and control limbs. Akt1 = RAC-β serine/threonine-protein kinase 1; Akt2 = RAC-β serine/threonine-protein kinase 2; BFR = blood flow restriction; Ct = cycle threshold; FOXO1 = forkhead box protein O1; HIF-3 = hypoxia-inducible factor 3; IGF1 = insulin-like growth factor 1; Ku70 = lupus Ku autoantigen protein p70; miR = microRNA; mTOR = mammalian target of rapamycin; myomiR = skeletal muscle microRNA; NRF-1 = nuclear respiratory factor 1; Pax3 = paired box protein 3; Pax7 = paired box protein 7; PGC-1α = peroxisome proliferator-activated receptorγcoactivator 1α; Rpl11-pan = ribosomal protein L11 v1 and v2; Rpl = ribosomal protein L; SIRT1 = silent mating type information regulation 2 homolog 1; SOD1 = superoxide dismutase 1; SOD2 = superoxide dismutase (Mn); TFAM = mitochondrial transcription factor A; VEGF = vascular endothelial growth factor A.
Fig. 4.
Western blot analysis of some key proteins from BFR and control legs. Representative images of protein bands of (A) Pax7, NRF-1, PGC-1α, and α-tubulin. The protein content of (B) Pax7, (C) NRF-1, and (D) PGC-1α was measured from the biopsy samples. Results are expressed as mean ± SE (n = 7). BFR = blood flow restriction; NRF-1 = nuclear respiratory factor 1; Pax7 = paired box protein 7; PGC-1α = peroxisome proliferator-activated receptor γ coactivator 1α.
4. Discussion
BFR with low-intensity resistance training is widely used to increase muscle hypertrophy, especially in older individuals.39, 40, 41, 42, 43, 44, 45 However, due to the low resistance, this kind of training mostly targets slow-twitch fibers, since fast-twitch fibers have higher thresholds and are activated by high resistance or high speed. Indeed, Suga et al.6 reported that low-intensity BFR training resulted in recruitment of fast-twitch fibers, evaluated by inorganic phosphate splitting, but occurred in only 31% of the participants, while high-intensity resistance training caused 70% recruitment of these fibers. However, it has been suggested that BFR increases the recruitment of fast-twitch fibers,46 and high-intensity exercise is more appropriate to activate them.6 Hence, BFR training is not regularly used by top athletes. We tested whether BFR used during rest periods of resistance training sets (with an intensity of 70% of 1RM) could be an alternative to BFR done during exercise. Although the acute effects of BFR during rest periods are encouraging, the chronic effects need to be mechanistically studied.
It has been reported that high-intensity resistance training without BFR increases the levels of miR-206.47 Our experimental conditions (high-intensity resistance exercise with BFR during the rest periods) resulted in down regulation of miR-206. It has been shown that repression of miR-206 can promote hypertrophy associated with increased protein synthesis in cultured myotubes, but not in skeletal muscle.48 This effect is probably mediated by the interaction of miR-206 and histone deacetylase 4.48 miR-206 plays a crucial role in muscle development, since transforming growth factor β can inhibit myogenic differentiation by suppressing levels of miR-206 and miR-29.49 The myogenesis-related transcription factors myoblast determination protein 1, myogenin, and myocyte-specific enhancer factor 2 bind to the upstream regions of the miR-206 locus, thus ensuring and maintaining its skeletal muscle tissue-specific expression.50 miR-206 levels are sharply up-regulated during satellite cell differentiation and down-regulated after muscle injury.51
Inhibition of miR-206 substantially enhances satellite cell proliferation and increases Pax7 protein levels in vivo.51 The present study found that BFR during the resting periods of high-intensity resistance training resulted in down-regulation of miR-206 and increased expression of Pax7, suggesting that BFR could induce muscle damage and the related adaptive response. Although the adaptive capacity is very limited after acute exercise and BFR,52 it was found that the mRNA levels of Akt2, NRF1, VEGF, and Pax7 increased significantly when performed during the rest periods of resistance training. This could indicate wide range of cellular adaptive responses. Significant alteration was not detected in the content of measured signaling proteins. This partly could be due to the time of sampling, which was 2 h post-exercise. Although 2–3 h is often used for post-exercise sampling in BFR studies, it is possible that this period is too short to detect changes in protein content.10, 45, 53
It is estimated that the DNA in a typical mammalian cell suffers approximately 2 × 105 lesions per day,54 and double-strand break is one of the most toxic lesions. Ku70 is the key protein for the repair of double-strand break. The increase in mRNA levels of Ku70 could mean that the applied BFR induces DNA damage and as a result cells respond by rapid induction of Ku70.
One of the novel results in this study of BFR during rest periods of resistance training relates to the induction of Pax7. Pax7 regulates myogenesis through the regulation of muscle precursor cell proliferation. The present results suggest that the applied BFR activates satellite cells, which could lead to a rise in daughter myogenic precursor cells and enhanced regeneration.55 In a recent study, we found that compensatory hypertrophy is associated with increased levels of Pax7.15 Therefore, it is suggested that BFR during rest periods of resistance training enhances satellite cell proliferation, which could lead to repair and hypertrophy of skeletal muscle. It is important to note that Pax7 expression peaks 24–48 h after a single bout of eccentric exercise56; but even after 1 h following a bout of eccentric exercise, a 15% increase in mRNA levels of Pax7 has been reported.56, 57
The present study has several limitations. Biopsy samples were taken just once to limit the discomfort of the participants, who previously had agreed that only 1 sample would be taken from each leg. The amount of sampled muscle tissue was inadequate to study all of the important proteins that accompany hypertrophy and vascularization changes. In addition, the contralateral leg (which was involved in training but did not have blood flow restricted to it) was used as a control. It cannot be confidently concluded that BFR did not have systemic effects that could have reached the contralateral leg as well. Moreover, since this study only evaluated the effects of acute exercise bouts with BFR during rest periods, the chronic effects of this training are unknown.
5. Conclusion
Overall, it is suggested that BFR, used during rest periods of resistance training, appears to enhance the gene expression of angiogenesis, mitochondrial biogenesis, muscle repair, and hypertrophy. BFR during rest periods decreases the levels of miR-206, which could be involved with the adaptive response to this exercise training. However, the present results are only valid for healthy young men, and further investigation is needed to validate their applicability to other populations.
Acknowledgments
Acknowledgments
This study was supported by Országos Tudományos Kutatási Alapprogramok (112810) and National Excellence Program (126823) grants awarded to ZR. FT was supported by Új Nemzeti Kiválóság Program-17-3, New National Excellence Program of the Ministry of Human Capacities.
Authors’ contributions
ZR participated in the design of the study and contributed to data collection and data reduction/analysis; FT, ZG, MF, GL, and ZT participated in the design of the study; ZM, HN, NIS, and PO participated in the design of the study and contributed to data collection; MT contributed to data reduction/analysis; BM contributed to data analysis and interpretation of results. All authors contributed to the manuscript writing. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Appendix
| Target mRNA | Fw. primer | Re. primer |
|---|---|---|
| 28S | AGCCGATCCATCATCCGCAA | CAGCCAAGCTCAGCGCAAC |
| Akt1 | TCTATGGCGCTGAGATTGTG | CTTAATGTGCCCGTTCCTTGT |
| Akt2 | TGAAAACCTTCTGTGGGACC | TGGTCCTGGTTGTAGAAGGG |
| FOXO1 | AAGAGCGTGCCCTACTTCAA | CATCCCCTTCTCCAAGATCA |
| HIF-1α | TTCCAGTTACGTTCCTTCGATCA | TTTGAGGACTTGCGCTTTCA |
| IGF1 | CGAAGTCTCAGAGAAGGAAAGG | ACAGGTAACTCGTGCAGAGC |
| Ku70 | CTGTCCAAGTTGGTCGCTTC | CTGCCCCTTAAACTGGTCAA |
| mTOR | TCGCTGAAGTCACACAGACC | CTTTGGCATATGCTCGGCAC |
| NRF-1 | CGCTCTGAGAACTTCATGGAGGAACAC | GCCACATGGACCTGCTGCACTT |
| Pax3 | CTCACCTCAGGTAATGGGACT | CGTGGTGGTAGGTTCCAGAC |
| Pax7 | CCCCCGCACGGGATT | TATCTTGTGGCGGATGTGGTT |
| PGC-1α | GTGAAGACCAGCCTCTTTGC | CACGTCTCCATCTGTCAGC |
| Rpl11-pan | AACTTCGCATCCGCAAACTC | CTCCGGATGCCAAAGGATCT |
| Rpl11-V1 | ATCATGGCGCAGGATCAAGGT | CTCCGGATGCCAAAGGATCT |
| Rpl11-V2 | GCTCTCCATCATGGCGGATCA | TGGAAAACACAGGGGTCTGC |
| SIRT1 | TGCGGGAATCCAAAGGATAATTCAGTGTC | CTTCATCTTTGTCATACTTCATGGCTCTATG |
| SOD2 | GCAGAAGCACAGCCTCCCCG | CCTTGGCCAACGCCTCCTGG |
| TFAM | TGCCTCATCCACCGGAGCGA | CACAAAACTGAAGGGGGAGCGCA |
| VEGF | AGGAGGAGGGCAGAATCATCA | CTCGATTGGATGGCAGTAGCT |
Abbreviations: 28S = 28S ribosomal RNA; Akt1 = RAC-β serine/threonine-protein kinase 1; Akt2 = RAC-β serine/threonine-protein kinase 2; FOXO1 = forkhead box protein O1; Fw. = forward; HIF-1α = hypoxia-inducible factor 1α; IGF1 = insulin-like growth factor 1; Ku70 = lupus Ku autoantigen protein p70; mTOR = mammalian target of rapamycin; NRF-1 = nuclear respiratory factor 1; Pax3 = paired box protein 3; Pax7 = paired box protein 7; PGC-1α = peroxisome proliferator-activated receptor γ coactivator 1α; Re. = reverse; Rpl11-pan = ribosomal protein L11 v1 and v2; Rpl11-V1 = ribosomal protein L11 v1; Rpl11-V2 = ribosomal protein L11 v2; SIRT1 = silent mating type information regulation 2 homolog 1; SOD2 = superoxide dismutase (Mn); TFAM = mitochondrial transcription factor A; VEGF = vascular endothelial growth factor A.
References
- 1.Takarada Y., Sato Y., Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol. 2002;86:308–314. doi: 10.1007/s00421-001-0561-5. [DOI] [PubMed] [Google Scholar]
- 2.Fujita S., Abe T., Drummond M.J. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol (1985) 2007;103:903–910. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- 3.Bahreinipour M.A., Joukar S., Hovanloo F. Mild aerobic training with blood flow restriction increases the hypertrophy index and MuSK in both slow and fast muscles of old rats: Role of PGC-1α. Life Sci. 2018;202:103–109. doi: 10.1016/j.lfs.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda T., Loenneke J.P., Thiebaud R.S., Abe T. Effects of blood flow restricted low-intensity concentric or eccentric training on muscle size and strength. PLoS One. 2012;7:e52843. doi: 10.1371/journal.pone.0052843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gundermann D.M., Fry C.S., Dickinson J.M. Reactive hyperemia is not responsible for stimulating muscle protein synthesis following blood flow restriction exercise. J Appl Physiol (1985) 2012;112:1520–1528. doi: 10.1152/japplphysiol.01267.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suga T., Okita K., Morita N. Intramuscular metabolism during low-intensity resistance exercise with blood flow restriction. J Appl Physiol (1985) 2009;106:1119–1124. doi: 10.1152/japplphysiol.90368.2008. [DOI] [PubMed] [Google Scholar]
- 7.Natsume T., Ozaki H., Saito A.I., Abe T., Naito H. Effects of electrostimulation with blood flow restriction on muscle size and strength. Med Sci Sports Exerc. 2015;47:2621–2627. doi: 10.1249/MSS.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 8.Fatela P., Reis J.F., Mendonca G.V. Acute neuromuscular adaptations in response to low-intensity blood-flow restricted exercise and high-intensity resistance exercise: Are there any differences? J Strength Cond Res. 2018;32:902–910. doi: 10.1519/JSC.0000000000002022. [DOI] [PubMed] [Google Scholar]
- 9.Martín-Hernández J., Ruiz-Aguado J., Herrero A.J. Adaptation of perceptual responses to low-load blood flow restriction training. J Strength Cond Res. 2017;31:765–772. doi: 10.1519/JSC.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 10.Conceição M.S., Chacon-Mikahil M.P., Telles G.D. Attenuated PGC-1α isoforms following endurance exercise with blood flow restriction. Med Sci Sports Exerc. 2016;48:1699–1707. doi: 10.1249/MSS.0000000000000970. [DOI] [PubMed] [Google Scholar]
- 11.Lixandrão M.E., Ugrinowitsch C., Laurentino G. Effects of exercise intensity and occlusion pressure after 12 weeks of resistance training with blood-flow restriction. Eur J Appl Physiol. 2015;115:2471–2480. doi: 10.1007/s00421-015-3253-2. [DOI] [PubMed] [Google Scholar]
- 12.Takada S., Okita K., Suga T. Blood flow restriction exercise in sprinters and endurance runners. Med Sci Sports Exerc. 2012;44:413–419. doi: 10.1249/MSS.0b013e31822f39b3. [DOI] [PubMed] [Google Scholar]
- 13.Brandner C.R., Warmington S.A. Delayed onset muscle soreness and perceived exertion after blood flow restriction exercise. J Strength Cond Res. 2017;31:3101–3108. doi: 10.1519/JSC.0000000000001779. [DOI] [PubMed] [Google Scholar]
- 14.Souza D.B., Duncan M., Polito M.D. Improvement of lower-body resistance-exercise performance with blood-flow restriction following acute caffeine intake. Int J Sports Physiol Perform. 2019;14:216–221. doi: 10.1123/ijspp.2018-0224. [DOI] [PubMed] [Google Scholar]
- 15.Koltai E., Bori Z., Chabert C. SIRT1 may play a crucial role in overload-induced hypertrophy of skeletal muscle. J Physiol. 2017;595:3361–3376. doi: 10.1113/JP273774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radak Z., Ishihara K., Tekus E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017;12:285–290. doi: 10.1016/j.redox.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levinger I., Goodman C., Hare D.L., Jerums G., Toia D., Selig S. The reliability of the 1RM strength test for untrained middle-aged individuals. J Sci Med Sport. 2009;12:310–316. doi: 10.1016/j.jsams.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Kacin A., Strazar K. Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Sports. 2011;21:e231–e241. doi: 10.1111/j.1600-0838.2010.01260.x. [DOI] [PubMed] [Google Scholar]
- 20.Noble B.J., Borg G.A., Jacobs I., Ceci R., Kaiser P. A category-ratio perceived exertion scale: Relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc. 1983;15:523–528. [PubMed] [Google Scholar]
- 21.Layne A.S., Larkin-Kaiser K., MacNeil R.G. Effects of blood-flow restriction on biomarkers of myogenesis in response to resistance exercise. Appl Physiol Nutr Metab. 2017;42:89–92. doi: 10.1139/apnm-2016-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrick H.L., Pignanelli C., Barbeau P.A. Blood flow restricted resistance exercise and reductions in oxygen tension attenuate mitochondrial H2O2 emission rates in human skeletal muscle. J Physiol. 2019;597:3985–3997. doi: 10.1113/JP277765. [DOI] [PubMed] [Google Scholar]
- 23.Xie F., Xiao P., Chen D., Xu L., Zhang B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol. 2012;80:75–84. doi: 10.1007/s11103-012-9885-2. [DOI] [PubMed] [Google Scholar]
- 24.Vandesompele J., De Preter K., Pattyn F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multipleinternal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen C.L., Jensen J.L., Ørntoft T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 26.Pfaffl M.W., Tichopad A., Prgomet C., Neuvians T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: Best Keeper—Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 27.Silver N., Best S., Jiang J., Thein S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol. 2006;7:33. doi: 10.1186/1471-2199-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shelton M., Kocharyan A., Liu J., Skerjanc I.S., Stanford W.L. Robust generation and expansion of skeletal muscle progenitors and myocytes from human pluripotent stem cells. Methods. 2016;101:73–84. doi: 10.1016/j.ymeth.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi K., Tabata S., Piras V., Tomita M., Selvarajoo K. Systems biology strategy reveals PKCσ is key for sensitizing TRAIL-resistant human fibrosarcoma. Front Immunol. 2014;5:659. doi: 10.3389/fimmu.2014.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie L., Feng H., Li S. SIRT3 mediates the antioxidant effect of hydrogen sulfide in endothelial cells. Antioxid Redox Signal. 2016;24:329–343. doi: 10.1089/ars.2015.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bori Z., Zhao Z., Koltai E. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Exp Gerontol. 2012;47:417–424. doi: 10.1016/j.exger.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koltai E., Bori Z., Osvath P. Master athletes have higher miR-7, SIRT3 and SOD2 expression in skeletal muscle than age-matched sedentary controls. Redox Biol. 2018;19:46–51. doi: 10.1016/j.redox.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glover L.E., Irizarry K., Scully M. IFN-γ attenuates hypoxia-inducible factor (HIF) activity in intestinal epithelial cells through transcriptional repression of HIF-1β. J Immunol. 2011;186:1790–1798. doi: 10.4049/jimmunol.1001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acs Z., Bori Z., Takeda M. High altitude exposure alters gene expression levels of DNA repair enzymes, and modulates fatty acid metabolism by SIRT4 induction in human skeletal muscle. Respir Physiol Neurobiol. 2014;196:33–37. doi: 10.1016/j.resp.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Chock K.L., Allison J.M., Shimizu Y., ElShamy W.M. BRCA1-IRIS overexpression promotes cisplatin resistance in ovarian cancer cells. Cancer Res. 2010;70:8782–8791. doi: 10.1158/0008-5472.CAN-10-1352. [DOI] [PubMed] [Google Scholar]
- 36.Radak Z., Bori Z., Koltai E. Age-dependent changes in 8-oxoguanine-DNA glycosylase activity are modulated by adaptive responses to physical exercise in human skeletal muscle. Free Radic Biol Med. 2011;51:417–423. doi: 10.1016/j.freeradbiomed.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Q., Chen Y., Guo L., Jiang T., Lin Z. miR-23a/b regulates the balance between osteoblast and adipocyte differentiation in bone marrow mesenchymal stem cells. Bone Res. 2016;4:16022. doi: 10.1038/boneres.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Iida H., Nakajima T., Kurano M. Effects of walking with blood flow restriction on limb venous compliance in elderly subjects. Clin Physiol Funct Imaging. 2011;31:472–476. doi: 10.1111/j.1475-097X.2011.01044.x. [DOI] [PubMed] [Google Scholar]
- 40.Karabulut M., Bemben D.A., Sherk V.D., Anderson M.A., Abe T., Bemben M.G. Effects of high-intensity resistance training and low-intensity resistance training with vascular restriction on bone markers in older men. Eur J Appl Physiol. 2011;111:1659–1667. doi: 10.1007/s00421-010-1796-9. [DOI] [PubMed] [Google Scholar]
- 41.Fry C.S., Glynn E.L., Drummond M.J. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol (1985) 2010;108:1199–1209. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarkson M.J., Conway L., Warmington S.A. Blood flow restriction walking and physical function in older adults: A randomized control trial. J Sci Med Sport. 2017;20:1041–1046. doi: 10.1016/j.jsams.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda T., Fukumura K., Tomaru T., Nakajima T. Thigh muscle size and vascular function after blood flow-restricted elastic band training in older women. Oncotarget. 2016;7:33595–33607. doi: 10.18632/oncotarget.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vechin F.C., Libardi C.A., Conceição M.S. Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J Strength Cond Res. 2015;29:1071–1076. doi: 10.1519/JSC.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 45.Centner C., Wiegel P., Gollhofer A., König D. Effects of blood flow restriction training on muscular strength and hypertrophy in older individuals: A systematic review and meta-analysis. Sports Med. 2019;49:95–108. doi: 10.1007/s40279-018-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krustrup P., Söderlund K., Relu M.U., Ferguson R.A., Bangsbo J. Heterogeneous recruitment of quadriceps muscle portions and fibre types during moderate intensity knee-extensor exercise: Effect of thigh occlusion. Scand J Med Sci Sports. 2009;19:576–584. doi: 10.1111/j.1600-0838.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 47.D'Souza R.F., Markworth J.F., Aasen K.M.M., Zeng N., Cameron-Smith D., Mitchell C.J. Acute resistance exercise modulates microRNA expression profiles: Combined tissue and circulatory targeted analyses. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winbanks C.E., Beyer C., Hagg A., Qian H., Sepulveda P.V., Gregorevic P. miR-206 represses hypertrophy of myogenic cells but not muscle fibers via inhibition of HDAC4. PLoS One. 2013;8:e73589. doi: 10.1371/journal.pone.0073589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winbanks C.E., Wang B., Beyer C. TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J Biol Chem. 2011;286:13805–13814. doi: 10.1074/jbc.M110.192625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao P.K., Kumar R.M., Farkhondeh M., Baskerville S., Lodish H.F. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci U S A. 2006;103:8721–8726. doi: 10.1073/pnas.0602831103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J.F., Tao Y., Li J. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010;190:867–879. doi: 10.1083/jcb.200911036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radak Z., Zhao Z., Koltai E., Ohno H., Atalay M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18:1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor C.W., Ingham S.A., Ferguson R.A. Acute and chronic effect of sprint interval training combined with postexercise blood-flow restriction in trained individuals. Exp Physiol. 2016;101:143–154. doi: 10.1113/EP085293. [DOI] [PubMed] [Google Scholar]
- 54.Barnes D.E., Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet. 2004;38:445–476. doi: 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- 55.Motohashi N., Asakura A. Muscle satellite cell heterogeneity and self-renewal. Front Cell Dev Biol. 2014;2:1. doi: 10.3389/fcell.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snijders T., Nederveen J.P., McKay B.R. Satellite cells in human skeletal muscle plasticity. Front Physiol. 2015;6:283. doi: 10.3389/fphys.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toth K.G., McKay B.R., De Lisio M., Little J.P., Tarnopolsky M.A., Parise G. IL-6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS One. 2011;6:e17392. doi: 10.1371/journal.pone.0017392. [DOI] [PMC free article] [PubMed] [Google Scholar]